Abstract
Members of the heme peroxidase family play an important role in host defense. Myeloperoxidase (MPO) is expressed in phagocytes and is the only animal heme peroxidase previously reported to be capable of using chloride ion as a substrate to form the highly microbicidal species hypochlorous acid (HOCl) at neutral pH. Despite the potent bacterial killing activity of HOCl, individuals who fail to express MPO typically show only a modest increase in some fungal infections. This may point to the existence of redundant host defense mechanisms. Vascular peroxidase 1 (VPO1) is newly discovered member of the heme peroxidase family. VPO1 is expressed in cells of the cardiovascular system and is secreted into the bloodstream. In the present study, we investigate whether VPO1 is capable of generating HOCl and its role in host defense. Like MPO, VPO1 in the presence of H2O2 and chloride generates HOCl. VPO1-dependent HOCl generation was demonstrated by chlorination of taurine and tyrosine using mass spectrometry. In addition, the VPO1/H2O2/Cl− system can cause the chlorination of monochlorodimedone and the oxidation of 5-thio-2-nitrobenzoic acid. Purified VPO1 and VPO1 in plasma mediate bacterial killing that is dependent on chloride and H2O2; killing is inhibited by peroxidase inhibitors and by the H2O2 scavenger catalase. In the presence of erythrocytes, bacterial killing by VPO1 is slightly reduced. Thus, VPO1, in addition to MPO, is the second member of the heme peroxidase family capable of generating HOCl under physiological conditions. VPO1 is likely to participate in host defense, with bactericidal activity mediated through the generation of HOCl.
INTRODUCTION
Microbiota are frequent visitors to open luminal structures of organ systems such as the gastrointestinal tract and the airways of the respiratory system in humans; there, they are dealt with by a variety of host defense mechanisms, including phagocytic cells and luminally secreted microbicidal components (14, 29). The circulatory system, however, represents a fluid-containing closed luminal structure that must be kept sterile for host survival. Nevertheless, minor and major traumas, including surgery and indwelling catheters, can cause transient or prolonged disruption in the integrity of this compartment, allowing the entry of microbes, which must be killed or removed from circulation, as well as microbial contamination at the site of injury.
The primary mechanism for maintenance of sterility in tissues involves professional phagocytes, especially neutrophils, which migrate from circulation into tissues where they utilize both oxidative and nonoxidative mechanisms to kill invading microbes. Oxidative mechanisms center around the phagocyte Nox2-containing NADPH-oxidase system (28). Upon phagocytosis of microbes, Nox2, the catalytic subunit of the oxidase which is initially present in specific granule and secretory vesicle membranes, relocates to the phagosomal membrane where it produces superoxide directed into the lumen of the phagosome. Superoxide rapidly dismutes to form hydrogen peroxide (H2O2), which, like superoxide, is only weakly bactericidal. Azurophilic granules containing myeloperoxidase (MPO) fuse with the phagosome, releasing MPO into the phagosome. MPO in the presence of its substrates H2O2 plus chloride anion generates hypochlorous acid (HOCl) in a reaction that has been thought to be unique to MPO among the animal heme peroxidases (for a review, see reference 19). HOCl, unlike H2O2 or superoxide, is potently bactericidal. Despite the potent bacterial killing activity of HOCl, individuals who fail to express MPO show little or no phenotype, typically showing only a modest increase in some fungal infections (27). In contrast, individuals with chronic granulomatous disease who fail to show NADPH-oxidase activity and therefore fail to generate the weakly microbicidal superoxide and H2O2 show a profound phenotype characterized by frequent and severe infections (22). This conundrum might be explained by the existence of an unknown enzyme with a bactericidal function that is redundant to that of MPO.
The human heme peroxidase gene family comprises 8 members which primarily mediate host defense functions localized to specialized cells (e.g., phagocytic cells) and body fluids (saliva and breast milk) (19). VPO1 is a recently discovered heme-containing peroxidase that is homologous to other mammalian heme-containing peroxidases, including MPO, eosinophil peroxidase (EPO), lactoperoxidase (LPO), and thyroid peroxidase. VPO1 is unique among these peroxidases in that it contains not only a catalytic domain at its C terminus but also a large N-terminal domain that includes five leucine-rich repeats and four immunoglobulin-like domains (5). VPO1 is highly expressed in the cardiovascular system, lung, liver, pancreas, and spleen (5), but its biological function has not been established. Unlike MPO, which is mostly retained within phagocytes, most VPO1 proteins in vascular endothelial cells are secreted into plasma (4). We investigate here the hypothesis that VPO1 functions in a microbicidal role and that this is due to its generation of HOCl. We demonstrated for the first time that the purified recombinant VPO1 (rVPO1) and plasma VPO1 generate HOCl and potently mediate microbicidal activity. We propose that VPO1 represents part of an arm of the innate immune system that maintains sterility either in the bloodstream itself or in regions in which the integrity of the bloodstream is disrupted and that functions of VPO1 may overlap with those of MPO.
MATERIALS AND METHODS
Reagents.
Bovine LPO (bLPO), NaI, 3,3′,5,5′-tetramethylbenzidine (TMB) and TMB liquid system, taurine, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), aminobenzoic acid hydrazide (ABAH), NaN3, H2O2, catalase, methionine (Met), tyrosine (Tyr), chlorotyrosine (Cl-Tyr), and glutathione (GSH) were all purchased from Sigma-Aldrich (St. Louis, MO). MPO was obtained from the Elastin Product Company (Owensville, MO). L-012, a luminol derivative, was purchased from from Wako Chemicals USA, Inc. (Richmond, VA). The chemiluminescent substrate for the immunoblots was from Pierce Biotechnology (Rockford, IL). Monochlorodimedone (MCD; 1,1-dimethyl-4-chloro-3,5-cyclohexanedione) was obtained from Bio-Research Products (North Liberty, IA). Escherichia coli cells (strain Top10) were purchased from Invitrogen, Inc. (Carlsbad, CA). NaCl (catalog no. S9888, lot 128K012) was purchased from Sigma-Aldrich. In 140 mM NaCl solution, the oxidation of the trace Br− or I− was verified to be insufficient to mediate taurine oxidation or to kill E. coli, suggesting that the trace Br− and I− concentrations are negligible for the generation of HOBr and HOI. 5-Thio-2-nitrobenzoic acid (TNB) was prepared as follows. DTNB (0.08 g) was dissolved in 10 ml of 0.1 M potassium phosphate buffer (pH 7.4). The solution was mixed with 120 μl of 5 M NaOH, followed by incubation at room temperature for 5 min. A total of 43 μl of 5 M HCl was added into the reaction to adjust the pH to ∼7.4.
Preparation of rVPO1.
rVPO1 was produced from VPO1 stably expressing cells and purified as described previously (4).
Measurement of heme concentration.
The concentrations of MPO, bLPO, and rVPO1 per heme were determined using the extinction coefficients 91, 114, and 112 mM−1 cm−1 at 428, 412, and 410 nm, respectively.
Assay for peroxidase activity.
TMB oxidation was used to determine peroxidase activity, as described previously (5).
Spectrophotometry of heme peroxidase-mediated Cl-tau.
N-Chlorotaurine (Cl-tau) was generated in a reaction containing 50 mM phosphate buffer (pH 5.5), 0.8 mM H2O2, 140 mM NaCl, 10 mM taurine, as well as 750 nM VPO1, 750 nM bLPO, or 100 nM MPO at 37°C. The kinetic trace of Cl-tau generated was immediately monitored at A252 by using a UV-2450 UV-VIS spectrophotometer (Shimadzu). In dose-dependent experiments, the concentration of NaCl or rVPO1, respectively, was determined.
LC-MS analysis.
Liquid chromatography-mass spectrometry (LC-MS) was carried out on MDS-Sciex Applied Biosystems API-3200 equipped with a Waters Acquity UPLC system. Separations were performed on a Waters UPLC BEH-C18 column (1.0 by 100 mm) with a usable pH range from 1 to 12.
For the detection of Cl-tau, we used mobile phase A (10 mM tetrabutylammonium hydroxide in H2O, pH 11.0) and mobile phase B (methanol with no modifier). The gradient proceeded as follows: 0 to 2 min = 0% phase B, 2 to 6 min = 100% B, 6 to 7 min = 100% phase B, and 7 to 7.1 min = 0% phase B, with a hold at 0% phase B for 12 min to equilibrate the column. The flow rate was 0.125 ml/min. Detection was performed using negative multiple reaction monitoring (MRM) mode with mass transitions of 158/79.9 and 160/79.9 based on 35Cl and 37Cl, respectively. The injection volume was 10 μl. The ion spray voltage was 4,500 V, and nitrogen was the only gas used throughout the runs. Standard Cl-tau was prepared as described earlier (24). In brief, 20 mM HOCl in 100 mM potassium phosphate buffer (pH 7.4) was added in equal volumes of 100 mM taurine with vigorous stirring. The concentration of Cl-tau was determined from the A252 (ε = 415 M−1 cm−1). Heme peroxidase-mediated Cl-tau was generated in a 100-μl reaction containing 50 mM potassium phosphate buffer (pH 5.5), 5 mM taurine, 0.8 mM H2O2, 100 mM NaCl, and either 1,000 nM/heme VPO1, 100 nM/heme MPO, or 100 nM/heme LPO. The reaction was incubated at 37°C for 30 min. In some reactions, NaCl was omitted.
For the detection of Cl-Tyr, mobile phase A (0.1% formic acid in H2O) and mobile phase B (acetonitrile plus 0.1% formic acid) were used. The gradient was as follows: 0 to 0.5 min, 15% mobile phase B; 0.5 to 2.0 min, 70% mobile phase B; 2.0 to 2.5 min, 15% mobile phase B; with a stop after 5 min. The flow rate was 0.05 ml/min. Detection was performed in MRM mode with a mass transition of 216/170 for Cl-Tyr. The injection volume was 10 μl. Samples were injected onto a Waters Acquity BEH-C18 column (1.0 by 100 mm, 1.7 μm). The ion spray voltage was 5,500 V, and nitrogen was the only gas used throughout the runs. Cl-Tyr stock was prepared as 0.5 mM in ultrapure H2O using reagent Cl-Tyr from Sigma-Aldrich. VPO1-mediated Cl-Tyr was generated in a 100-μl reaction containing 50 mM potassium phosphate buffer (pH 5.5), 0.5 mM H2O2, 140 mM NaCl, 100 μM tyrosine, and 1,000 nM/heme VPO1. In a positive control, 50 μM HOCl was added to 100 μM tyrosine in 50 mM potassium phosphate buffer (pH 5.5). The reactions were incubated at 37°C overnight. In some reactions, the VPO1 or NaCl was omitted.
Taurine chlorination-TMB oxidation assay for HOCl generation.
HOCl generation was detected utilizing the taurine chlorination assay in combination with TMB oxidation in the presence of iodide as previously reported (8) with slight modifications. In brief, 50 μM H2O2 was added to 100 μl of 50 mM phosphate buffer (pH 7.4), 140 mM NaCl, 5 mM taurine, and 100 nM/heme rVPO1, followed by incubation at 37°C for 30 min. The reaction was stopped by adding catalase (25 μg/ml). The reaction mixture was then mixed with freshly made developing agent. The developing agent consisted of 400 mM acetate buffer (pH 5.4), 1 mM TMB (predissolved in 100% dimethylformamide), and 100 μM NaI. After 5 min, absorbance at 650 mm was recorded. For verification of this assay, reagent HOCl was added to replace the VPO1/H2O2/Cl− system. In some experiments, peroxidase inhibitors (ABAH and NaN3), H2O2 scavenger (catalase), and HOCl scavenger (Met) were added as indicated. To selectively detect Cl-tau, iodide was omitted in the reaction (8).
VPO1-mediated chlorination of MCD for detection of HOCl generation.
This experiment was slightly modified from that reported in reference 16. In brief, a 100-μl reaction containing 50 mM phosphate buffer (pH 5.0), 100 μM MCD, 140 mM NaCl, 500 nM VPO1, or 50 nM MPO was carried out at 37°C. A total of 10 μM H2O2 was added to start the reaction. The A290 was immediately monitored for 20 min using a UV-2450 spectrophotometer. In some experiments, the pH of the phosphate buffer was varied as indicated, whereas in Cl−-dependent experiments, the NaCl concentration was varied. GSH (0.1 mM) or Met (0.1 mM) was also added in some experiments.
TNB assay for detection of HOCl generation.
TNB has a chromophore that has maximal absorbance at 412 nm, while its reaction product with HOCl, DTNB, is colorless. By detecting a decrease in the absorbance at 412 nm, the VPO1 activity is measured. In brief, 500 nM VPO1 was added to 50 mM phosphate buffer (pH 6.5) containing 100 μM TNB and 100 mM NaCl. MPO or LPO at 20 nM was used as a positive or negative control, respectively. The reaction was initiated by adding 100 μM H2O2. The absorbance at 412 nm was recorded at 37°C every 30 s for 10 min by using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). In dose-dependent experiments, VPO1 was added into the reactions with 0, 50, 100, and 500 nM, respectively.
Bacterial killing experiments.
E. coli cells were incubated in 50 mM phosphate buffer (pH 6.2) containing 140 mM NaCl, 10 μM H2O2, and the indicated amounts of MPO, bLPO, or rVPO1 at 37°C for 1 h. Cell mixtures were plated on lysogeny broth medium (LB) agar plates (tryptone, 10 g/liter; yeast extract, 5 g/liter; NaCl, 10 g/liter; agar, 15 g/liter), followed by incubation at 37°C overnight. In control experiments, only H2O2 (10 μM) or Cl− (140 mM) was present. The CFU were counted. The percentage of CFU in the experimental group compared to the control group was calculated. In some experiments, peroxidases were omitted and replaced by plasma, dialyzed plasma, or fractions of plasma VPO1 or MPO. These experiments were carried out at pH 7.2.
For verification of HOCl-mediated bacterial killing, the reagent HOCl was utilized instead of peroxidase-generated HOCl. HOCl was prepared, and its concentration was determined as previously described (8). E. coli was incubated with 50 mM phosphate buffer (pH 6.2) containing the indicated amounts of HOCl at 37°C for 30 or 60 min. Cell mixtures were plated on LB agar plates, followed by incubation at 37°C overnight. The CFU were counted and analyzed. In Cl−- and H2O2-dependent experiments of bacterial killing, the NaCl or H2O2 concentration was varied as indicated.
VPO1-mediated bacterial killing in the presence of RBCs.
The experiment for examining VPO1-mediated bacterial killing in the presence of red blood cells (RBCs) was slightly modified from one reported earlier (1). In brief, human blood was withdrawn from the finger of a healthy volunteer. RBCs were washed with 1× phosphate-buffered saline (PBS) twice and resuspended in 1× PBS to 109 RBCs/ml. E. coli cells were incubated in 100 μl of 50 mM phosphate buffer (pH 6.2) containing 140 mM NaCl, the indicated amounts of H2O2 and 50 nM MPO, or 200 nM rVPO1 in the presence or absence of 107 RBCs at 37°C for 1 h. Cell mixtures were plated on LB agar plates as described above. The CFU were counted after incubation at 37°C overnight. The percentage of CFU in the experimental group compared to the control group (E. coli only) was calculated.
Measuring the effects of MPO or VPO1/H2O2/Cl− systems on RBCs.
A total of 100 μl of 50 mM phosphate buffer (pH 6.2) containing 107 RBCs, 140 mM NaCl, 10 μl or 20 μM H2O2, and 50 nM MPO or 200 nM rVPO1 was incubated at 37°C for 1 h. The RBCs were spun down by centrifugation at 1,000 rpm for 5 min. The supernatant was used for hemoglobin detection. RBCs were lysed in 100 μl of distilled water, and the released hemoglobin was detected. The detection of hemoglobin was determined as the absorbance at 415 nm. The concentrations of hemoglobin in the supernatant and pellet were calculated based on a hemoglobin extinction coefficient of 128 mM−1 cm−1 at 415 nm. The rate of RBC damage was assessed by analysis of hemoglobin distribution in the supernatant or pellet divided by total amount of hemoglobin (supernatant plus pellet) (1).
VPO1-E. coli binding experiments.
Approximately 108 E. coli cells were incubated with 20, 80, or 200 nM rVPO1 or 50 nM MPO in 1× PBS (pH 7.4) at 37°C for 1 h in a 50-μl reaction. The control groups contained 200 nM rVPO1 or 50 nM MPO alone, without E. coli. After 1 h, the E. coli cells were spun down at 5,000 rpm for 5 min and then washed twice with 1 ml of 1× PBS. The E. coli pellet was used for two types of approaches to verify the binding of VPO1 to E. coli. (i) The pellet was lysed in 50 μl of radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM EDTA) and sonicated. The lysate (25 μl of lysate/lane) was resolved by SDS–12% PAGE and transferred to polyvinylidene difluoride membranes. VPO1 and MPO were detected by using anti-VPO1 and anti-MPO antibody, respectively, and visualized by chemiluminescence as previously described (1). (ii) The pellet was resuspended in 300 μl of 1× PBS plus 5 μM L-012. Then, a 95-μl mixture was taken, and 5 μl of 1 mM H2O2 was added. The relative light units (RLU) were immediately recorded at room temperature every 30 s for 20 min using a SpectraMax luminescence microplate reader (Molecular Devices, Sunnyvale, CA).
Dialysis of plasma.
Human plasma (3 ml) was added to a Thermo Slid-A-Lyzer dialysis cassette (20 kDa [molecular mass cutoff]) and dialyzed against 20 mM phosphate buffer (pH 7.4) for 24 to 30 h at 4°C with four changes of the buffer.
Separation of plasma VPO1 from plasma MPO.
Portions (3 ml) of human plasma were loaded onto a Sephacryl S-300 column (1 by 110 cm) and eluted with phosphate buffer (pH 7.2). The eluents were collected (4 ml/tube), and the absorbance levels at 280 and 412 nm were determined. The peroxidase activity of the eluted fractions was also measured using TMB oxidation. Fractions 10 to 20 were subjected to analysis by SDS-PAGE and immunoblotting with antibodies to VPO1 or MPO.
RESULTS
VPO1 generates HOCl.
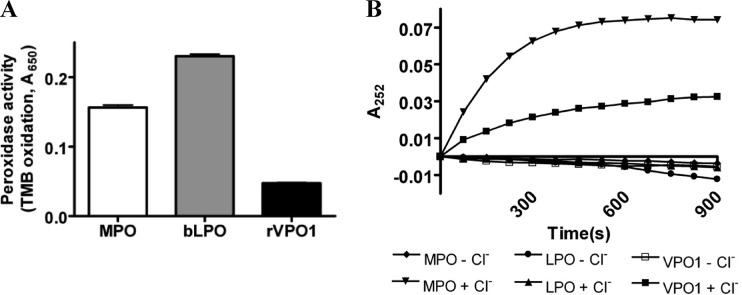
To demonstrate VPO1-mediated HOCl generation, we first compared the general peroxidase activities of MPO, bLPO, and rVPO1 using the oxidizable substrate TMB. Taking into account the difference in heme concentration, the peroxidase activity of rVPO1 was approximately one-tenth of the MPO activity and one-fifth of the bLPO activity (Fig. 1A), which is similar to the findings of a previous report (5). These data indicate that rVPO1 can catalyze TMB oxidation and its relative activities.
Fig 1.
HOCl generation by VPO1. (A) Comparison of the peroxidase activities of MPO, bLPO, and rVPO1 determined using TMB as a substrate as described in Materials and Methods. A 110-μl reaction contained 100 μl of TMB, 10 nM/heme MPO, 50 nM/heme bLPO, or 50 nM/heme rVPO1. The reaction (n = 9) was carried out at 37°C for 30 min, and the absorbance at 650 nm was recorded. (B) Detection of Cl-tau generation by the A252. Cl-tau generation was carried out in a 100-μl reaction that contained 50 mM potassium phosphate buffer (pH 5.5), 5 mM taurine, 0.8 mM H2O2, 100 mM Cl−, and either 100 nM/heme MPO, 100 nM/heme bLPO, or 1 μM/heme rVPO1. Reactions without Cl− were also performed. The absorbance at 252 nm was recorded every 30 s for 15 min.
We then examined whether VPO1 is capable of catalyzing HOCl production. HOCl is a potent oxidant and rapidly reacts with a variety of amino acid, proteins, lipids, and DNA. Taurine is an abundant free amino acid and exists in intercellular and intracellular spaces. HOCl readily reacts with taurine to generate Cl-tau. We detected VPO1-mediated HOCl generation by monitoring Cl-tau generation at A252 (25). The A252 increased in the reaction containing VPO1/H2O2/Cl−/taurine over the time as much as that in MPO/H2O2/Cl−/taurine, whereas no A252 was observed in the reaction containing LPO/H2O2/Cl−/taurine (Fig. 1B). Omitting Cl− from the reaction, no A252 was observed (Fig. 1B); the same occurred when either peroxidase or H2O2 was omitted (data not shown). These data strongly suggested that VPO1 catalyzes HOCl generation.
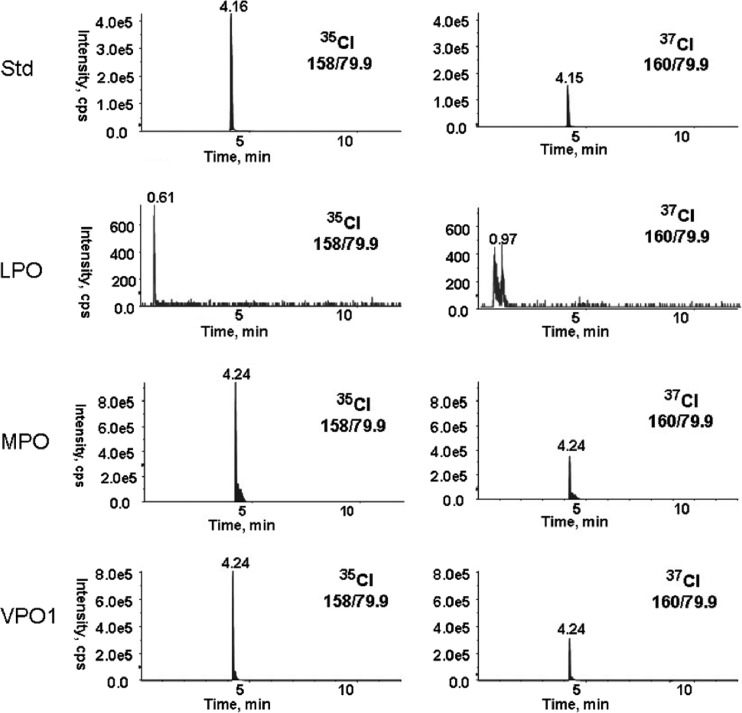
To directly demonstrate VPO1-mediated HOCl generation, we detected the Cl-tau production by using negative MRM mode LC-MS. Standard Cl-tau revealed the retention time at ∼4.16 min in mass transitions of 158/79.9 and 160/79.9 based on principally stable isotopes of 35Cl and 37Cl. The ratio of intensity of 35Cl-tau and 37Cl-tau was ∼2.8:1, a level similar to the mole fraction 0.7576 versus 0.2424 of 35Cl versus 37Cl found in nature (6). Generation of Cl-tau in a reaction of VPO1/H2O2/Cl−/taurine was observed at 4.24 min in mass transitions of 158/79.9 and 160/79.9. This is consistent with Cl-tau generated in positive-reaction MPO/H2O2/Cl−/taurine (Fig. 2). Both intensity ratios of 35Cl-tau and 37Cl-tau generated in VPO1 or MPO/H2O2/Cl−/taurine were ∼3:1, which is similar to the ratio of standard 35Cl-tau and 37Cl-tau Cl. The production of Cl-tau at 1,000 nM/heme rVPO1 versus 100 nM/heme MPO at the described conditions is 338 μM versus 456 μM, which is similar to the relevant activities measured by TMB oxidation (Fig. 1A). No Cl-tau was observed in LPO/H2O2/Cl−/taurine (Fig. 2), confirming a previous report (9) that concluded that bLPO does not produce HOCl. Using LC-MS, Cl-Tyr was detected in the reaction of VPO1/H2O2/Cl−/tyrosine and not in the reaction of VPO1/H2O2/tyrosine or H2O2/Cl−/tyrosine (Table 1). Cl-Tyr was also detected from the reaction of reagent HOCl and tyrosine (Table 1). However, the level of Cl-Tyr was relatively low compared to the concentration of tyrosine (0.0162% and 0.0275% for VPO1-mediated and reagent HOCl-mediated Cl-Tyr generation, respectively) (Table 1). These mass spectrometric data confirm the generation of Cl-tau and Cl-Tyr, further supporting the catalysis of chloride oxidation and HOCl production by VPO1. It was proposed that Cl-Tyr is a specific biomarker of MPO-catalyzed chlorination (13). Based on our observations above, VPO1 may contribute to Cl-Tyr formation in vivo and, presumably, Cl-Tyr is no longer a specific biomarker of MPO-catalyzed chlorination.
Fig 2.
Detection of VPO1-mediated Cl-tau by MS. Cl-tau generation and LC-MS were carried out as described in Materials and Methods. Standard Cl-tau (Std) was prepared as follows. First, 20 mM HOCl in 100 mM potassium phosphate buffer (pH 7.4) was added in equal volumes of 100 mM taurine with vigorous stirring. Then, 10 μl of Cl-tau at 80 μM was analyzed. Heme peroxidase-mediated Cl-tau was generated in a 100-μl reaction containing 50 mM potassium phosphate buffer (pH 5.5), 5 mM taurine, 0.8 mM H2O2, 100 mM NaCl, and either 1,000 nM/heme VPO1, 100 nM/heme MPO, or 100 nM/heme LPO. The reaction was incubated at 37°C for 30 min. The injection volume for LC-MS was 10 μl. The ion spray voltage was 4,500 V, and nitrogen was the only gas used throughout the runs. The data are representative of at least two independent experiments.
Table 1.
VPO1-mediated formation of Cl-Tyr detected by mass spectrometrya
| Sample | Cl-Tyr concn (μM) | Cl-Tyr/Tyr (%) |
|---|---|---|
| NaCl + H2O2 | 0 | 0 |
| H2O2 + VPO1 | 0 | 0 |
| NaCl + H2O2 + VPO1 | 0.0162 | 0.0162 |
| HOCl | 0.0275 | 0.0275 |
140 mM NaCl, 500 μM H2O2, and/or 1,000 μM VPO1 were added to 50 mM phosphate buffer (pH 5.5) containing 100 μM Tyr. The mixture was incubated at 37°C overnight. HOCl (50 μM) was added into 100 μM Tyr solution in 50 mM phosphate buffer (pH 5.5) as a positive control. The sample (10 μl) was analyzed by LC-MS. The Cl-Tyr concentration was calculated from the Cl-Tyr standard curve.
Properties of VPO1-catalyzing generation of HOCl.
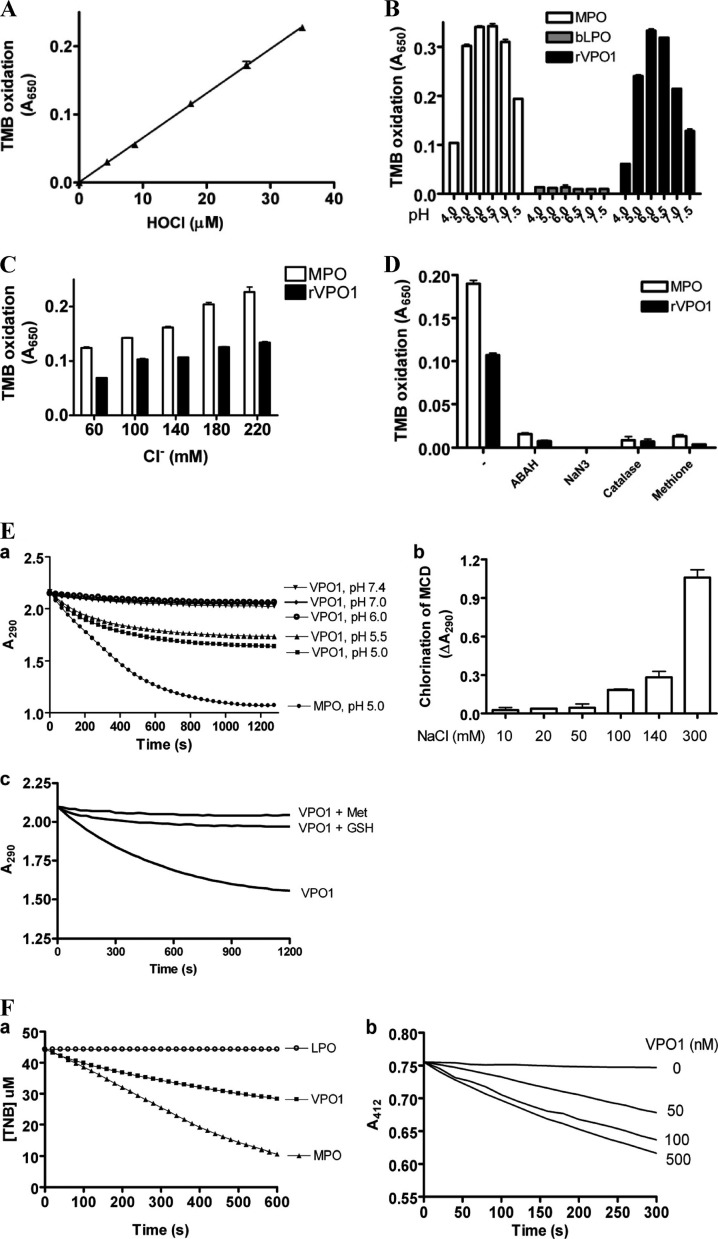
HOCl generation by VPO1 was further evaluated by an assay that combines chlorination of taurine and TMB oxidation in the presence of iodide ion, as previously reported (8). We first confirmed that the chlorination of taurine and TMB oxidation are dose dependent on reagent HOCl (Fig. 3A). We then measured HOCl generation by the VPO1/H2O2/Cl− system compared to that of MPO and LPO. As shown in Fig. 3B, VPO1 generated HOCl from pH 4.0 to 7.4, as did MPO, whereas LPO did not produce HOCl as expected. The optimal pH for HOCl generation by both VPO1 and MPO was at pH 6.0 (Fig. 3B). VPO1 at 100 nM/heme generated approximately two-thirds as much HOCl as MPO at 10 nM/heme, an observation consistent with the MS data above. At a physiological pH, HOCl generation by VPO1 was dose dependent on the chloride concentration (Fig. 3C). The taurine chlorination by VPO1, as well as by MPO, was impaired by a variety of peroxidase inhibitors (ABAH and NaN3), H2O2 scavenger (catalase), and HOCl scavenger (Met) (Fig. 3D).
Fig 3.
Detection of VPO1-mediated HOCl by multiple approaches. (A) Taurine chlorination-TMB oxidation assay for reagent HOCl. Details of the procedures are described in Materials and Methods. The data are from three independent experiments. (B) Optimal pH for HOCl generation by heme peroxidases. Reactions (100 μl) containing 50 mM phosphate buffer (with the pH as indicated), 140 mM NaCl, 50 μM H2O2, 5 mM taurine, and either 10 nM/heme MPO, 100 nM/heme LPO, or 100 nM/heme rVPO1 were carried out at 37°C for 30 min. The reaction was stopped by adding catalase (25 μg/ml). The stopped reaction solution was then mixed with a developing agent. After 5 min, the absorbance at 650 mm was recorded. (C) Dose-dependent HOCl generation by VPO1 compared to that by MPO. The experiments were carried out as in panel B at pH 7.4. The concentration of NaCl is indicated. (D) Inhibition of HOCl generation by inhibitory reagents. Experiments were carried out as described in panel B with reaction solution (pH 7.4) containing 140 mM chloride, ABAH (200 μM), NaN3 (1 mM), catalase (25 μg/ml), or Met (0.5 mM) as indicated. The data are representative from ≥3 independent experiments. Error bars indicate the standard deviations (SD). (E) VPO1-mediated MCD chlorination. A 100-μl reaction containing 50 mM phosphate buffer (pH 5.0), 100 μM MCD, 140 mM NaCl, and either 500 nM VPO1 or 50 nM MPO was carried out at 37°C. H2O2 was added to start the reaction. The absorbance at 290 nm was immediately monitored. (a) pH-dependent MCD chlorination. (b) NaCl dose-dependent MCD chlorination. (c) VPO1-mediated MCD chlorination in presence or absence of GSH (0.1 mM) or Met (0.1 mM). (F) VPO1-mediated TNB oxidation. VPO1 at 500 nM was added to 50 mM phosphate buffer (pH 6.5) containing 100 μM TNB and 100 mM NaCl; 20 nM MPO or LPO was used as a positive or negative control. The reaction was initiated by adding 100 μM H2O2. The absorbance at 412 nm was recorded at 37°C every 30 s. (a) Comparison of VPO1-mediated TNB oxidation to that of MPO and LPO. (b) VPO1 dose-dependent TNB chlorination. The data are representative of three independent experiments.
In addition, we carried out experiments with MCD and TNB as substrates for VPO1-mediated chlorination or oxidation compared to MPO and bLPO. As shown in Fig. 3E, VPO1 mediated Cl−- and pH-dependent chlorination of MCD, whereas the chlorination of MCD was inhibited by GSH or Met. A lower pH improved the chlorination of MCD. VPO1, like MPO, also mediated TNB oxidation, whereas LPO did not (Fig. 3Fa). The TNB oxidation was dependent on VPO1 (Fig. 3Fb).
Thus, in the present study, we provide several types of evidence to demonstrate that VPO1 can generate HOCl from H2O2 and Cl− at a physiological pH, and we show that MPO is not unique among peroxidases in generating this highly reactive species. Although the rate of chlorination is low compared to MPO, if sufficient quantities of VPO1 are present, the enzyme would be expected to have antibacterial properties.
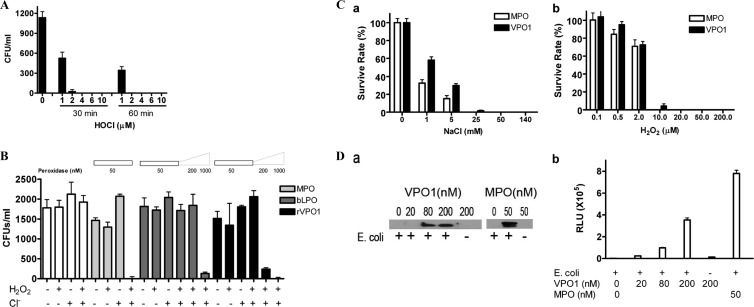
The VPO1/H2O2/Cl− system kills bacteria.
VPO1 in the presence or absence of H2O2 and Cl− was therefore investigated for its ability to kill bacteria and was compared to the MPO/H2O2/Cl− system (18, 19). We first evaluated the ability of reagent HOCl to kill E. coli. HOCl was generated from sodium hypochlorite as described in reference 8. E. coli cells were incubated at 37°C for 30 and 60 min, respectively. As shown in Fig. 4A, 4 μM HOCl completely killed E. coli at 60 min of incubation, whereas 98% of E. coli was killed at 30 min of incubation at a 4 μM HOCl concentration. Thus, HOCl is a potent oxidant for bacterial killing. E. coli was then incubated at 37°C for 1 h with or without H2O2 and/or Cl−, along with the indicated peroxidase. An aliquot was plated on LB plates, which were incubated overnight at 37°C prior to counting colonies. A low concentration of MPO (50 nM) in the presence of H2O2 and Cl− was highly effective in killing bacteria, whereas the omission of either H2O2 or Cl− resulted in the loss of bacterial killing (Fig. 4B). A 20-fold-higher concentration of rVPO1 also completely killed all of the bacteria; this was approximately the concentration seen in human plasma (see below), and this also required both H2O2 and Cl− (Fig. 4B). rVPO1 at 200 nM (approximately one-fifth of the plasma concentration) killed ∼90% of E. coli, dependent upon H2O2 and Cl− (Fig. 4B). In contrast, few if any E. coli cells were killed at the same concentration of LPO in the presence of H2O2 and Cl−, although ∼93% were killed at 1,000 nM LPO (Fig. 4B), possibly due to the direct action of LPO and H2O2 on the bacteria at this high concentration of enzyme. H2O2 or Cl− alone, as well as peroxidase/H2O2 or peroxidase/Cl−, did not significantly affect bacterial viability (Fig. 4B). VPO1-mediated E. coli killing is dependent on both Cl− and H2O2 concentrations (Fig. 4C).
Fig 4.
Bactericidal activity of rVPO1. (A) Bactericidal activity of reagent HOCl. E. coli was incubated in 50 mM phosphate buffer (pH 6.2) and the indicated amount of reagent HOCl at 37°C for 30 min and 1 h, respectively. The cell mixture was plated on LB plates. In control experiments, no HOCl was present. The data are from three independent experiments. (B) E. coli was incubated in 50 mM phosphate buffer (pH 6.2) containing 140 mM NaCl, 10 μM H2O2, and the indicated amount of MPO, bLPO, or rVPO1 at 37°C for 1 h. Cell mixtures were plated on LB plates. In control experiments, only H2O2 (10 μM) or Cl− (140 mM) was present. The data are representative of ≥3 independent experiments. Error bars indicate the SD. (C) NaCl- and H2O2-dependent bactericidal activities by VPO1. Experiments were carried out as described for panel B with 200 nM VPO1. In the NaCl dose-dependent experiments (a), H2O2 was maintained at 10 μM and the NaCl concentrations are indicated, whereas in the H2O2-dose dependent experiments (b), NaCl was maintained at 140 mM and the H2O2 concentrations are indicated. MPO at 50 nM was used as a control. (D) VPO1 binds to E. coli. E. coli cells were added into VPO1 or MPO as indicated in 1× PBS (pH 7.4). The mixtures were incubated at 37°C for 1 h. E. coli cells were spun down at 5,000 rpm for 5 min. The cells were washed twice and subjected to immunoblotting with anti-VPO1 or anti-MPO antibody (a) and chemiluminescence assay with L-012 (b).
It has been proposed that MPO binds to pathogens as a result of its higher cationic surface charge (pKa = 10) in the acidic environment of the phagosome (2). This binding has been demonstrated with some degree of selectivity and enhanced bacterial killing (1). Whether VPO1 is able to bind to bacteria has not yet been explored. We carried out experiments to investigate its binding ability. VPO1 or MPO was added to an E. coli suspension, followed by incubation at 37°C for 1 h. MPO binding to E. coli was confirmed by both immunoblotting and L-012 chemiluminescence (Fig. 4Da and b). Very interestingly, VPO1 also interacted with E. coli in a VPO1 dose-dependent manner (Fig. 4Da and 4Db). Thus, our data strongly suggest that VPO1 binds to bacteria.
Plasma contains heme peroxidases that kill bacteria in the presence of H2O2 and Cl−.
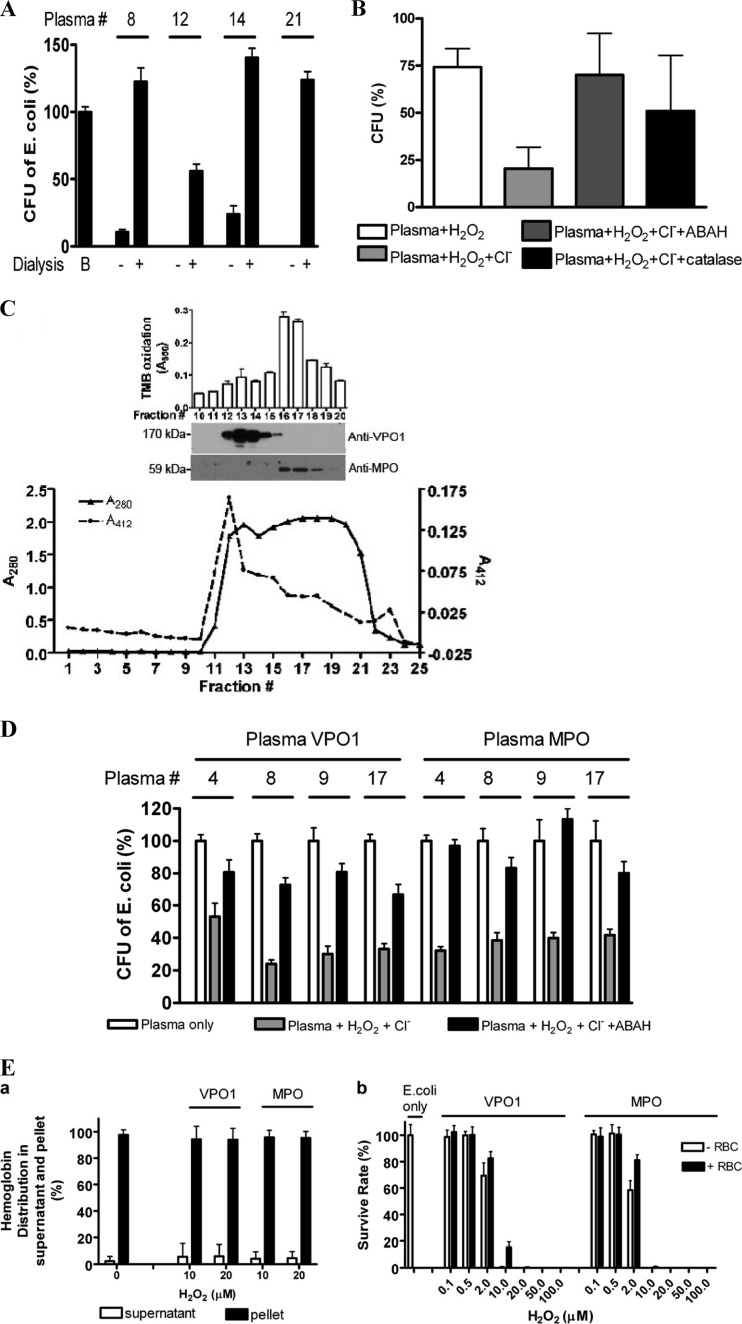
We next evaluated whether plasma contains heme peroxidases that may kill bacteria in the presence of H2O2 and Cl−. It is well known that plasma contains low-molecular-weight bactericidal species, including a variety of antimicrobial peptides (23, 30). To eliminate the low-molecular-weight bactericidal species, plasma was subjected to dialysis. Dialyzing plasma decreases its ability to kill bacteria spontaneously (Fig. 5A). Dialysis also removes Cl− and H2O2, which are required substrates for MPO and VPO1. To test whether a peroxidase in dialyzed plasma can kill bacteria, we investigated the ability of plasma at neutral pH to kill bacteria, its dependence on Cl− and H2O2, and the ability of the peroxidase inhibitor, ABAH, to inhibit bactericidal effects. Consistent with a role for one or more HOCl-generating peroxidases, the E. coli-killing ability of plasma depended upon chloride and was inhibited by ABAH (Fig. 5B; killing activity was characterized in plasma samples from 19 unrelated healthy individuals). Bacterial killing was also markedly inhibited by catalase, demonstrating the requirement for H2O2 (Fig. 5B). These data indicate that plasma contains at least one peroxidase capable of generating bactericidal HOCl.
Fig 5.
Bactericidal activity of plasma VPO1 and MPO. (A) Effect of dialysis on bactericidal activity of plasma. E. coli was incubated in 100 μl of 20 mM phosphate buffer (pH 7.2) containing 5 μl of plasma or dialyzed plasma at 37°C for 2 h. The cell mixtures were plated on LB plates. In control experiment B, the mixture contained only E. coli. The numbers are the plasma sample identifications. The data are representative of a minimum of three independent experiments for each group. (B) Bactericidal activity of plasma peroxidases. E. coli was incubated in 100 μl of 20 mM phosphate buffer (pH 7.2) containing 10 μl of dialyzed plasma sample each from 19 unrelated healthy individuals, H2O2 (10 μM), NaCl (140 mM), ABAH (500 μM), and 50 μg of catalase/ml as indicated at 37°C for 2 h. The cell mixtures were plated on LB plates and incubated at 37°C overnight. The colonies were counted, and the data are expressed as the percentages of colonies compared to the number seen with plasma plus E. coli alone. The data are representative of two independent experiments (n = 6) (means ± the SD). (C) Separation of plasma VPO1 from MPO. Portions (3 ml) of human plasma were loaded onto a Sephacryl S-300 column (1 by 110 cm) and eluted with phosphate buffer (pH 7.2). The eluent was collected (4 ml/tube), and the absorbance at 280 and 412 nm was monitored. The peroxidase activity of eluted fractions 10 to 20 was measured using TMB oxidation (inset). Fractions 10 to 20 were also subjected to analysis by SDS-PAGE and immunoblotting with antibodies against VPO1 and MPO (inset). These data are representative of at least three separations using different plasma samples. (D) Four human plasma samples were individually subjected to fractionation as described for panel C. The eluents with the strongest peroxidase activity (fractions 13 and 16 for VPO1 and MPO, respectively) were concentrated 10-fold to facilitate bacterial killing experiments. E. coli killing was carried out as described for panel B, except that 20 μl of fractionated plasma containing either VPO1 or MPO was added. The final concentrations of VPO1 and MPO were ca. 50% of that in native plasma. The numbers are plasma sample identifications. The data are representative of three independent experiments. Error bars indicate the SD. (E) VPO1-mediated bactericidal activity in the presence of RBCs. (a) Effects of VPO1 or MPO/H2O2/Cl− systems on RBCs. A total of 107 RBCs were incubated with 140 mM NaCl, 10 or 20 μM H2O2, and either 200 mM VPO1 or 50 nM MPO at 37°C for 1 h. Hemoglobin was determined by measuring the A415 of the supernatant and pellet, and the percentage of distribution was calculated using the A415 divided by the total A415 of the supernatant plus the pellet. (b) E. coli cells were incubated in 100 μl of 50 mM phosphate buffer (pH 6.2) containing 140 mM NaCl, the indicated amounts of H2O2, and either 200 nM rVPO1 or 50 nM MPO in the presence or absence of 107 RBCs at 37°C for 1 h. The cell mixtures were plated on LB agar plates, followed by incubation at 37°C overnight. The percentages of the CFU in the experimental group compared to the control group (E. coli only) were calculated.
Human plasma VPO1/H2O2/Cl− system kills bacteria.
Our previous studies showed that VPO1 was present in human plasma at concentrations ranging from 0.2 to 2.4 μM (average, 1.1 μM) and in murine serum at 1.7 to 3.7 μM (average, 2.6 μM) (4). In comparison, MPO has been reported to be present in serum at concentrations of 450 to 950 pM (26), which is 3 orders of magnitude less than plasma VPO1. Because both MPO and VPO1 are peroxidases that can produce significant quantities of HOCl, we sought to identify whether one or both of these enzymes is responsible for the bactericidal effects of dialyzed plasma. We first attempted to immunoprecipitate VPO1 from plasma using a polyclonal antibody; however, the antibody was not able to bind to VPO1 in the presence of other plasma components (G. Cheng, unpublished data). Therefore, we used gel filtration chromatography to fractionate plasma. Separation of VPO1 from MPO was verified by their different molecular masses on Western immunoblotting (Fig. 5C). TMB oxidation showed two peaks of activity corresponding to the peaks of VPO1 and MPO immunostaining. E. coli-killing experiments were performed using the separated plasma VPO1 and MPO fractions. Both VPO1- and MPO-containing fractions killed 50 to 75% of E. coli—an effect that was dependent on the presence of H2O2 and chloride—and it was inhibited by ABAH (Fig. 5D). The final concentrations of VPO1 and MPO in the reactions were ca. 50% of the concentration of these species in native plasma. These data indicate that the total bactericidal activity of VPO1 in plasma is at least as potent as that of MPO, combining their peroxidase activity and concentration.
RBCs have higher levels of catalase. Therefore, RBCs may provide catalase as well as serve as competitive substrates to consume H2O2 (1). RBCs thus reduce MPO-mediated bacterial killing; they may also be destroyed by the MPO/H2O2/Cl− system (1). The inhibition of MPO-mediated bacterial killing by RBCs is selective. The inhibition is decreased when MPO-bacterium binding increases. This is due to the proximity of the MPO-mediated oxidant in MPO-binding bacteria (1). As presented above, VPO1 is able to bind to E. coli. We then evaluated the effects of RBCs in the VPO1/H2O2/Cl− system. At 10 and 20 μM H2O2, the VPO1/H2O2/Cl− system did not destroy RBCs significantly (Fig. 5Ea). RBCs displayed only a slight inhibition of VPO1- and MPO-mediated E. coli killing (Fig. 5Eb), which is consistent with a previous report (1).
DISCUSSION
We demonstrate here that VPO1 is an enzymatic component in plasma that is capable of bacterial killing. Whereas MPO is primarily secreted (or “leaked”) from phagocytic cells, VPO1 is constitutively secreted by vascular endothelial cells, suggesting a primary role for VPO1 in maintaining the sterility of circulating plasma. This function of VPO1 may be critical in the clinical contexts of sepsis, traumatic injury, postsurgical wounds, or indwelling vascular catheters or devices. In the presence of its substrates, H2O2 and Cl−, VPO1 produces HOCl; bacterial killing is dependent upon the generation of this highly reactive species. Previously, MPO was thought to be uniquely capable among the animal heme-containing peroxidases of generating HOCl. However, genetic deficiency in MPO was not associated with an obvious defect in innate immune function, since affected individuals are not inordinately susceptible to infections (27). This is in marked contrast to individuals with chronic granulomatous disease in which mutation or absence in individual components of the phagocyte NADPH-oxidase results in a severely immunocompromised phenotype (28). The present study indicates that VPO1, like MPO, can generate HOCl. VPO1 is also likely to be present at sites of injury and infection; the absence of a phenotype in MPO-deficient individuals may be a result of a redundant role of VPO1. Taken together, our data strongly support a role for a novel heme peroxidase, VPO1, in supporting microbicidal activity and maintaining the sterility of circulating plasma.
VPO1 lacks a Met at the position that corresponds to Met409 in MPO and was therefore expected to lack chlorinating activity. Met at this position is a unique feature of MPO that has been proposed to be a key determinant of its unique ability to catalyze chlorination reactions (19). The covalent linkage of this residue to heme is responsible for the unusually red-shifted Soret band at 428 nm. MPO with Gln replacing Met409 shows decreased overall peroxidase activity, the loss of its chlorinating activity, and a blue shift of the Soret band from 428 to 411 nm, the latter being typical of heme-containing peroxidases (15, 20). However, substituting the corresponding Gln376 of bovine LPO with Met is not sufficient to confer either a red shift of the Soret band or chlorinating activity, underlining the complexity of interactions in the heme environment of mammalian peroxidases (31). The corresponding residue in VPO1 is Gln981, and the enzyme shows a Soret band at ∼410 nm (5), similar to LPO. In addition, EPO, with the corresponding residue Thr381, has chlorinating activity at pH 5 but is not considered to generate HOCl at neutral pH (10). These data demonstrate that VPO1 is a new member of this family with the ability to generate HOCl at physiological conditions. These findings strongly suggest that Met409 is not necessary but is able to enhance HOCl production in peroxidases and emphasize the complexity of interactions in the heme environment.
It was reported that the selective binding of MPO to bacteria benefited the bacterial killing and decreased damage of RBCs (1). Although there is no predicted structure motif in MPO binding to bacteria, there may be electrostatic binding. It has been proposed that MPO binds to bacteria as a result of its higher cationic surface charge (pKa = 10) (2). Our data show that VPO1 is able to bind to E. coli at a physiological pH (Fig. 4D). The pI value of VPO1 was predicted to be ∼7 (5). It seems that VPO1 is much less charged in physiological conditions. Analysis of the VPO1 amino acid sequence revealed that VPO1 possesses a unique N-terminal region containing five leucine-rich repeats and four immunoglobulin domains. These repeats and domains are frequently involved in protein-protein and protein-ligand interactions (3, 17). We predict that the N-terminal region of VPO1 may contribute to its binding to E. coli. The detailed mechanism of the interaction of VPO1 and E. coli is being investigated in an ongoing project.
In luminal structures such as the airway and oral cavity, invading pathogens are killed in part by LPO-generated hypothiocyanite, which is generated from H2O2 and thiocyanate in an LPO-dependent oxidation (11). Hypothiocyanite shows weaker antimicrobial activity than HOCl but is likely to be less toxic to host epithelial cells. Whereas neutrophils are effective in tissues, where they release HOCl primarily into their phagolysosomal vesicle, less is known about how organisms handle bacteria in blood. A small fraction of MPO from phagocytes that includes proMPO (the monomeric precursor of MPO, which contains heme and has peroxidase activity) is released into circulation (12), achieving serum levels ranging from 450 to 950 pM measured by enzyme-linked immunosorbent assay (26). H2O2 in blood is present at 1 to 7 μM (7, 21), as well as 100 mM Cl−. Thus, plasma itself possesses both the substrates and low levels of the enzyme MPO, which is capable of generating HOCl in blood. Other peroxidases including EPO and LPO are undetectable in plasma (X. Qiu and G. Cheng, unpublished data). Our finding demonstrates that VPO1 can be effective in physiological environments and plays an important role in innate immunity in plasma. Considering the growing threats posed by drug-resistant bacterial infection, our findings have realistic significance and merit further studies.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (NIH/NHLBI R01 HL086836) (to G.C.). V.J.T. was supported by the National Institutes of Health (NIH/NHLBI R01 HL067967 and R01 HL094230). J.D.L. was supported by the National Institutes of Health (NIH R01 CA 084138, R01 CA105116, and P01 ES011163). P.L.J. was supported by the Cystic Foundation (R464-CR11).
Footnotes
Published ahead of print 23 April 2012
REFERENCES
- 1. Allen RC, Stephens JT., Jr 2011. Myeloperoxidase selectively binds and selectively kills microbes. Infect. Immun. 79:474–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnhold J, Flemmig J. 2010. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 500:92–106 [DOI] [PubMed] [Google Scholar]
- 3. Chan JR, Hyduk SJ, Cybulsky MI. 2000. Alpha 4 beta 1 integrin/VCAM-1 interaction activates α-l-β2-integrin-mediated adhesion to ICAM-1 in human T cells. J. Immunol. 164:746–753 [DOI] [PubMed] [Google Scholar]
- 4. Cheng G, et al. 2011. Vascular peroxidase-1 is rapidly secreted, circulates in plasma, and supports dityrosine cross-linking reactions. Free Radic. Biol. Med. 51:1445–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng G, Salerno JC, Cao Z, Pagano PJ, Lambeth JD. 2008. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic. Biol. Med. 45:1682–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Laeteri JR, et al. 2003. Atomic weights of the elements: review 2000 (IUPAC technical report). Pure Appl. Chem. 75:683–800 [Google Scholar]
- 7. Deskur E, et al. 1998. Exercise-induced increase in hydrogen peroxide plasma levels is diminished by endurance training after myocardial infarction. Int. J. Cardiol. 67:219–224 [DOI] [PubMed] [Google Scholar]
- 8. Dypbukt JM, et al. 2005. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic. Biol. Med. 39:1468–1477 [DOI] [PubMed] [Google Scholar]
- 9. Furtmuller PG, et al. 2002. Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry 41:11895–11900 [DOI] [PubMed] [Google Scholar]
- 10. Furtmuller PG, et al. 2006. Active site structure and catalytic mechanisms of human peroxidases. Arch. Biochem. Biophys. 445:199–213 [DOI] [PubMed] [Google Scholar]
- 11. Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. 2003. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 17:1502–1504 [DOI] [PubMed] [Google Scholar]
- 12. Hansson M, Olsson I, Nauseef WM. 2006. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch. Biochem. Biophys. 445:214–224 [DOI] [PubMed] [Google Scholar]
- 13. Hazen SL, Heinecke JW. 1997. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 99:2075–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. 2003. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 4:269–273 [DOI] [PubMed] [Google Scholar]
- 15. Jacquet A, et al. 1994. Site-directed mutagenesis of human myeloperoxidase: further identification of residues involved in catalytic activity and heme interaction. Biochem. Biophys. Res. Commun. 202:73–81 [DOI] [PubMed] [Google Scholar]
- 16. Jakopitsch C, et al. 2001. Catalase-peroxidase from Synechocystis is capable of chlorination and bromination reactions. Biochem. Biophys. Res. Commun. 287:682–687 [DOI] [PubMed] [Google Scholar]
- 17. Karaulanov EE, Bottcher RT, Niehrs C. 2006. A role for fibronectin-leucine-rich transmembrane cell-surface proteins in homotypic cell adhesion. EMBO Rep. 7:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klebanoff SJ. 1968. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J. Bacteriol. 95:2131–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klebanoff SJ. 2005. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77:598–625 [DOI] [PubMed] [Google Scholar]
- 20. Kooter IM, et al. 1999. The sulfonium ion linkage in myeloperoxidase. Direct spectroscopic detection by isotopic labeling and effect of mutation. J. Biol. Chem. 274:26794–26802 [DOI] [PubMed] [Google Scholar]
- 21. Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. 2000. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36:878–884 [DOI] [PubMed] [Google Scholar]
- 22. Lambeth JD. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4:181–189 [DOI] [PubMed] [Google Scholar]
- 23. Levy O. 2000. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood 96:2664–2672 [PubMed] [Google Scholar]
- 24. Marcinkiewicz J, Grabowska A, Bereta J, Bryniarski K, Nowak B. 1998. Taurine chloramine downregulates the generation of murine neutrophil inflammatory mediators. Immunopharmacology 40:27–38 [DOI] [PubMed] [Google Scholar]
- 25. Marquez LA, Dunford HB. 1994. Chlorination of taurine by myeloperoxidase: kinetic evidence for an enzyme-bound intermediate. J. Biol. Chem. 269:7950–7956 [PubMed] [Google Scholar]
- 26. Meuwese MC, et al. 2007. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 50:159–165 [DOI] [PubMed] [Google Scholar]
- 27. Nauseef WM. 1988. Myeloperoxidase deficiency. Hematol. Oncol. Clin. N. Am. 2:135–158 [PubMed] [Google Scholar]
- 28. Rada B, Leto TL. 2008. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 15:164–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salzman NH, Underwood MA, Bevins CL. 2007. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 19:70–83 [DOI] [PubMed] [Google Scholar]
- 30. Shafer WM, Martin LE, Spitznagel JK. 1984. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect. Immun. 45:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watanabe S, et al. 1998. Recombinant bovine lactoperoxidase as a tool to study the heme environment in mammalian peroxidases. FEBS Lett. 441:476–479 [DOI] [PubMed] [Google Scholar]