Abstract
Pathogenic Yersinia species modulate host immune responses through the activity of a plasmid-encoded type III secretion system and its associated effector proteins. One effector, YopM, is a leucine-rich-repeat-containing protein that is important for virulence in murine models of Yersinia infection. Although the mechanism by which YopM promotes virulence is unknown, we previously demonstrated that YopM was required for the induction of high levels of the immunosuppressive cytokine interleukin-10 (IL-10) in sera of C57BL/6J mice infected with Yersinia pseudotuberculosis. To determine if IL-10 production is important for the virulence function of YopM, C57BL/6J or congenic IL-10−/− mice were infected intravenously with wild-type or yopM mutant Y. pseudotuberculosis strains. Analysis of cytokine levels in serum and bacterial colonization in the spleen and liver showed that YopM is required for IL-10 induction in C57BL/6J mice infected with either the IP32953 or the 32777 strain of Y. pseudotuberculosis, demonstrating that the phenotype is conserved in the species. In single-strain infections, the ability of the 32777ΔyopM mutant to colonize the liver was significantly increased by the delivery of exogenous IL-10 to C57BL/6J mice. In mixed infections, the competitive advantage of a yopM+ 32777 strain over an isogenic yopM mutant to colonize spleen and liver, as observed for C57BL/6J mice, was significantly reduced in IL-10−/− animals. Thus, by experimentally controlling IL-10 levels in a mouse infection model, we obtained evidence that the induction of this cytokine is an important mechanism by which YopM contributes to Y. pseudotuberculosis virulence.
INTRODUCTION
Yersinia pseudotuberculosis, like the other human-pathogenic Yersinia species (Y. pestis and Y. enterocolitica), shows a profound ability to modulate the host immune system via a virulence-plasmid-encoded type III secretion system (T3SS) (10). This T3SS is responsible for the translocation into host cells of a set of effector proteins that coopt or subvert a number of eukaryotic signaling pathways that are important for innate or adaptive immune responses to Yersinia (55). The T3SS thus contributes to the ability of these pathogens to cause human diseases, including bubonic, septicemic, and pneumonic plagues (Y. pestis) and terminal ileitis and mesenteric adenitis (Y. pseudotuberculosis and Y. enterocolitica). Y. pseudotuberculosis and Y. enterocolitica are also capable of causing bacteremia and sepsis in immunocompromised individuals. Y. pseudotuberculosis infection of mice is associated with bacterial dissemination from the intestinal tract to the spleen, liver, and lungs, causing a systemic plague-like disease. The systemic infection of mice with Y. pseudotuberculosis provides a useful model to study the mechanisms of T3SS effectors that promote virulence in pathogenic Yersinia spp.
Among the set of Yersinia T3SS effectors, the function of the YopM protein may be least understood. The three-dimensional structure of the Y. pestis YopM protein has been determined, and it consists of two antiparallel N-terminal α-helices that serve as the nucleation point for the subsequent folding of the leucine-rich-repeat (LRR) domain (14). A short C-terminal tail that was not resolved in the crystal structure extends beyond the last LRR in YopM. Depending on the particular YopM protein examined, the number of LRRs can vary from 13 to 21 (4). The functional consequence of this heterogeneity in LRR repeats for YopM function remains unknown. In mouse infection models, YopM is critical for the dissemination of Y. pseudotuberculosis and Y. enterocolitica from the intestinal tract to distal sites such as the spleen and for virulence (27, 29, 52). YopM is also important for the virulence of Y. pestis inoculated into mice via the intravenous (i.v.) (25, 35) or intradermal (57) route of infection. In spleens of mice infected with Y. pestis, YopM fused to a reporter protein (beta-lactamase) is translocated into polymorphonuclear leukocytes (PMNs), macrophages, and dendritic cells (26), suggesting that these cell types are key targets of the effector.
YopM has been shown to traffic to the nucleus of infected host cells (51). In addition, YopM has been shown to interact with members of the RSK and PRK (also known as PKN) families of eukaryotic serine/threonine kinases (18, 27–29). Recently, Hentschke et al. demonstrated that rather than being specific for the RSK1 and PRK2 kinase isoforms, YopM interacts with RSK1 to RSK4 as well as with PRK1 to PRK3 (18). The mechanism by which the nuclear localization of YopM or the YopM-RSK-PRK complex contributes to Yersinia virulence is unknown at present. Previous work by McDonald et al. demonstrated that upon binding to YopM, the kinase activities of RSK1 and PRK2 are increased (28). The binding of RSK1 to YopM prevents the dephosphorylation of the kinase, resulting in its sustained activation (18). The domain that interacts with RSK1 has been localized to the C-terminal tail of YopM, and this interaction is critical for virulence in Y. pseudotuberculosis (27, 29). The binding domain for PRK2 has been mapped to a region containing the C-terminal 10 LRRs of Y. pestis YopM, and this domain is required for virulence as well (29). YopM variants that do not interact with RSK1 due to the presence of amino acid substitutions or deletions in the C-terminal tail retain partial virulence function and are able to traffic to the nucleus of infected macrophages, indicating that there are RSK1-independent functions of this effector (27). Deletions within LRRs 8 to 15 prevent the entry of YopM into the nucleus, suggesting that this region is important for nuclear localization (27). Whether the YopM complex containing activated RSK1 and PRK2 phosphorylates other target proteins within the cytosol or nuclei of infected host cells remains to be determined.
The ability of YopM to alter the host innate immune response has been demonstrated with mouse models of infection with Yersinia spp. (21, 27, 29, 57, 58). Kerschen et al. showed previously by histopathology that in mice infected with a yopM+ Y. pestis strain, the initial sites of liver and spleen colonization develop into foci with neutrophilic inflammation and necrosis, while the same organs infected with a yopM mutant contain granuloma-like lesions with little evidence of necrosis (21). Similar results were reported for murine spleens infected by wild-type or yopM mutant Y. pseudotuberculosis strains (27). Additionally, Kerschen et al. demonstrated that larger amounts of mRNA for the proinflammatory cytokines interleukin-12 (IL-12), IL-18, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-1β were observed for spleens of mice infected with a Y. pestis yopM mutant than for spleens of controls infected with a wild-type strain (21). In partial concordance with these results, we previously observed significantly increased levels of IFN-γ in the sera of mice infected with a Y. pseudotuberculosis yopM mutant compared to levels in the sera of mice infected with the parental 32777 strain (29). We also observed a marked YopM-dependent induction of the anti-inflammatory cytokine IL-10 in the sera of Y. pseudotuberculosis-infected mice and showed that this induction required domains of YopM that are required for interactions with RSK1 and PRK2 (29).
The possibility that YopM induces IL-10 expression to promote virulence can be considered controversial for several reasons. First, several investigations have failed to detect increased IL-10 expression levels in mice infected with Yersinia spp. For example, Kerschen et al. (21) did not detect increased levels of IL-10 transcripts in their experiments with Y. pestis-infected mice, either in spleen or liver tissue or in spleen-derived macrophages. Other investigators observed little to no increase in IL-10 protein levels in sera (53) or lungs (7, 23) of mice infected with Y. pestis or in tissues of mice infected with Y. enterocolitica or Y. pseudotuberculosis (increased IL-10 levels were detected in spleens and livers of mice at late stages of infection with Y. pseudotuberculosis) (2). Second, when increased IL-10 levels have been detected in Yersinia-infected mice, they have been associated with the activity of another T3SS-associated virulence factor, LcrV (6, 13, 47), which comprises the tip complex of the T3SS needle (31). Third, a mechanism independent of the T3SS by which the systemic infection of mice with Y. pestis can lead to IL-10 expression in natural killer (NK) cells in blood and nonlymphoid organs has been reported (37). In this case, pathogen-associated molecular patterns such as lipopolysaccharide (LPS) activated IL-12 production in dendritic cells, which in turn caused IL-10 expression in NK cells (37). Fourth, the penetration of purified recombinant Y. enterocolitica YopM into human HL60-derived macrophage-like cells was not associated with increased transcription levels of IL-10 mRNA after LPS stimulation (43). Therefore, the possibility that the induction of IL-10 is an important virulence function of YopM remains to be established.
Here, we sought to test the model that IL-10 induction is important for the virulence function of YopM in two ways. First, Y. pseudotuberculosis strain IP32953, which is a widely used model for the species due to the availability of its genomic sequence (8), was tested for the ability to induce IL-10 in a YopM-dependent manner in a mouse infection model. Second, the amount of IL-10 in mice infected with the wild-type or yopM mutant Y. pseudotuberculosis strain was experimentally controlled by supplementation with exogenous IL-10 or by the use of IL-10−/− animals.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A description of the Y. pseudotuberculosis and Escherichia coli strains used in this study is presented in Table 1. Strains 32777 and IP32953 are serogroup O1 strains. The complete genome sequence of IP32953 was reported previously (8), and the sequence of the yopM gene is conserved between IP32953 and 32777 (data not shown). The deletion of codons 85 to 446 of yopM in IP32953, resulting in the IP32953ΔyopM strain, was carried out by using allelic recombination and S17-1λpir harboring plasmid pSB890-yopMYPTB:npt (Table 1), as described previously for strain 32777 (29). The 32777yopM::kan strain was an intermediate in the construction of the 32777ΔyopM strain (29) (i.e., before the excision of the kanamycin resistance cassette [npt] using plasmid pFLP2 [Table 1]). Bacterial strains were routinely grown in Luria-Bertani (LB) medium or on LB agar plates at 28°C (Yersinia) or at 37°C (E. coli).
Table 1.
List of strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Reference |
|---|---|---|
| Strains | ||
| Y. pseudotuberculosis | ||
| 32777 | Serogroup O1 wild-type strain (originally called IP2777) | 45 |
| 32777yopM::kan | yopM codons 85 to 446 replaced by a kanamycin resistance cassette (npt) | 29 |
| 32777ΔyopM | yopM codons 85 to 446 deleted | 29 |
| IP32953 | Serogroup O1 wild-type strain | 8 |
| IP32953yopM::kan | yopM codons 85 to 446 replaced by a kanamycin resistance cassette (npt) | This study |
| IP32953ΔyopM | yopM codons 85 to 446 deleted | This study |
| E. coli S17-1λpir | Tpr Smr hsdR pro recA RP4-2-Tc::Mu-Km::Tn7; lysogenized with λpir | 12 |
| Plasmids | ||
| pSB890-yopMYPTB:npt | Suicide vector carrying yopM sequence with codons 85 to 446 replaced with an npt cassette flanked by FRT sites; Kanr Tetr | 29 |
| pFLP2 | Encodes Flp recombinase; sacB Tetr | 19 |
FRT, Flp recombination target.
Mouse infections.
All experimentation with mice was approved by the Stony Brook University IACUC. Six- to eight-week-old female C57BL/6J mice or B6.129P2-Il10tm1Cgn/J mice were purchased from Jackson Laboratory, Bar Harbor, ME. Y. pseudotuberculosis cultures were grown overnight in LB medium. The cultures were washed twice in sterile phosphate-buffered saline (PBS) before being diluted to the desired CFU/ml in PBS. An aliquot of 100 μl was delivered via tail vein injection using a 30-gauge needle.
For infection with IP32953, C57BL/6J mice were infected with 1.2 × 103 to 1.5 × 103 CFU of the wild type or the IP32953ΔyopM mutant as described above. Mice were monitored for up to 14 days following infection for signs of severe illness (hunched posture, ruffled fur, and immobility). Moribund mice were euthanized and scored as deceased. Surviving mice were euthanized on day 14. For organ burden assays, liver and spleen were harvested from euthanized mice at the time points indicated in the figure legends and homogenized in 5 ml PBS, and serial dilutions were spread onto LB agar plates to determine CFU values.
IL-10 supplementation experiments were performed under conditions similar to those previously described (20). C57BL/6J mice were infected with 32777 or 32777ΔyopM mutant cells as described above. Recombinant murine IL-10 (rmIL-10) was purchased from Peprotech. The lyophilized powder was resuspended to 2 mg/ml in PBS, and aliquots were stored at −80°C. The stock solution was diluted to 50 μg/ml, and 100 μl (5 μg) was delivered to each mouse via intraperitoneal (i.p.) injection on days 3, 4, and 5 postinfection. Control mice received injections of 100 μl of PBS alone. Organ burden assays were performed as described above.
For infections to determine the competitive index (CI), cultures of 32777 or the 32777yopM::kan mutant grown overnight were individually diluted to 1.2 × 104 to 1.5 × 104 CFU/ml. Equal volumes of each of these cultures were then mixed, and CFU were enumerated by plating serial dilutions onto LB agar or LB agar containing 50 μg/ml kanamycin (LB-Kan) to determine the input (in) CFU ratios. The mixture of 32777 and the 32777yopM::kan mutant was delivered via tail vein injection, as described above, into C57BL/6J mice or into B6.129P2-Il10tm1Cgn/J mice. Organ burden assays were performed as described above, with the exception that serial dilutions were plated onto LB or LB-Kan agar to determine output (out) CFU ratios. The CI was calculated by the following formula: CI = (number of CFU yopM::kanout/number of CFU 32777out)/(number of CFU yopM::kanin/number of CFU 32777in).
ELISA of mouse serum.
IL-10 and IFN-γ enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Biosystems (Minneapolis, MN). IL-18 ELISA kits were purchased from MBL (Nagoya, Japan). Blood was collected from infected mice by cardiac puncture after euthanization. Serum was prepared by the use of Sarstedt S1.1 serum gel tubes (Sarstedt, Nümbrecht, Germany) according to the manufacturer's instructions. All serum samples were diluted 1:10 in ELISA sample diluent and processed according to the manufacturer's instructions.
Statistical analysis.
Results of survival curve experiments were analyzed by using log-rank testing and the GraphPad Prism software suite (GraphPad, La Jolla, CA). The CFU, cytokine, and competitive index data were analyzed by the Mann-Whitney test of significance.
RESULTS
Requirement for YopM in virulence and IL-10 induction in mice is conserved in Y. pseudotuberculosis.
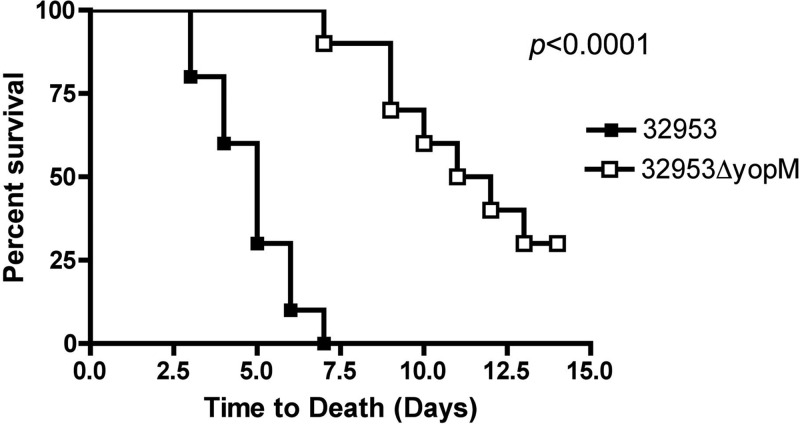
We previously demonstrated that the inactivation of yopM in Y. pseudotuberculosis strain 32777 resulted in a significant loss of virulence in mice by the orogastric or i.v. route of infection (29). In addition, unlike the wild type, the 32777ΔyopM mutant failed to induce high levels of IL-10 in the sera of i.v. infected mice (29). Moreover, this phenotype of the 32777ΔyopM mutant could be complemented by the expression of yopM+ in trans (29). In order to determine if this phenotype is specific to strain 32777 or a general feature of the Y. pseudotuberculosis species, we introduced the same inactivating mutation into yopM in the sequenced strain IP32953 (8) and carried out i.v. infection of C57BL/6J mice with the mutant (IP32953ΔyopM) or the parental strain. In the i.v. infection model, Y. pseudotuberculosis initially infects livers, spleens, and lungs of mice (27, 46). The bacteria grow in necrotic abscesses in the liver and spleen, and the animals begin to succumb to infection within several days (27, 46). As shown in Fig. 1, the IP32953ΔyopM mutant was significantly attenuated in C57BL/6J mice compared to the parental strain, as assessed by a time-to-death assay following i.v. infection with ∼103 CFU (P < 0.0001 by log rank test). IP32953 appeared to be more virulent than 32777 in this assay, as mice infected with this strain first became moribund by day 3 or 4, while mice similarly infected with 32777 first became moribund on day 6 or 7 (29).
Fig 1.
Survival curves of mice infected with Y. pseudotuberculosis IP32953 or the IP32953ΔyopM mutant. C57BL/6J mice were infected via tail vein injection with 1.2 × 103 to 1.5 × 103 CFU of Y. pseudotuberculosis IP32953 or the IP32953ΔyopM mutant grown at 26°C. The mice were monitored over 14 days for signs of illness and were euthanized if found to be moribund. Results are pooled from 2 independent experiments with five mice per group (n = 10 for both strains). Statistical significance was calculated by a log rank test.
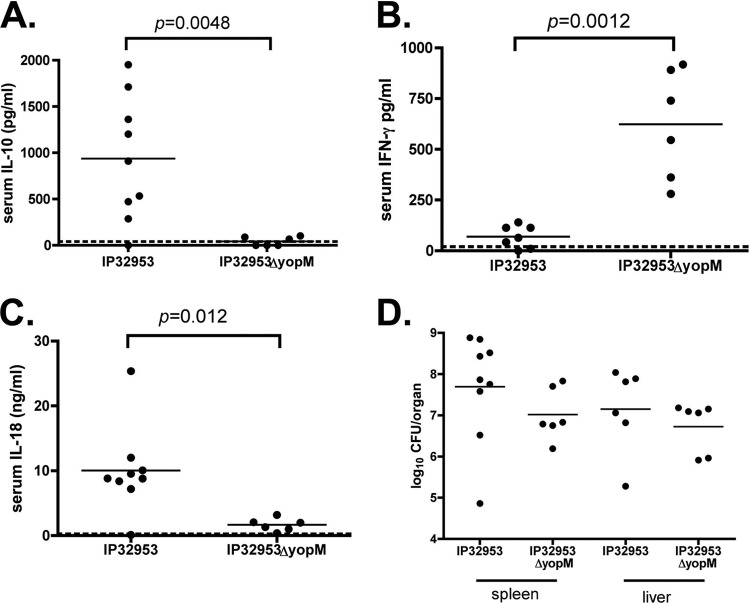
Groups of C57BL/6J mice infected with IP32953 or the isogenic yopM mutant, as described above, were euthanized on day 4 postinfection. Concentrations of cytokines in serum and bacterial CFU in liver and spleen were determined. The presence of an intact yopM allele in IP32953 was associated with a marked, statistically significant increase in IL-10 levels in sera of infected mice (Fig. 2A). In addition, there was a significant decrease in IFN-γ levels and an increase in IL-18 levels in sera of mice infected with yopM+ bacteria compared to levels in mice infected with yopM-deficient bacteria (Fig. 2B and C, respectively). We previously showed that there was a small but significant increase in the ability of wild-type strain 32777 to colonize the spleen compared to the 32777ΔyopM mutant at day 4 post-i.v. infection of C57BL/6J mice (29). There were, on average, higher CFU of IP32953 than of the yopM mutant in spleens and livers, but these differences were not significant (Fig. 2D). Taken together, as the above-described results with IP32953 are similar to what was previously observed for experiments with 32777 (29), we conclude that the requirement for YopM in virulence and IL-10 induction in mice is conserved in Y. pseudotuberculosis.
Fig 2.
Cytokine levels in sera and organ burdens in spleen and liver of C57BL/6J mice infected with IP32953 or the IP32953ΔyopM mutant. Mice were infected with IP32953 or the IP32953ΔyopM mutant as described in the legend to Fig. 1. Blood, spleens, and livers were collected from euthanized mice at day 4 postinfection. (A to C) IL-10 (A), IFN-γ (B), and IL-18 (C) concentrations in serum were determined by an ELISA. Each filled circle represents the concentration of cytokine present in the serum from a single mouse (pg/ml for IL-10 and IFN-γ and ng/ml for IL-18). (D) CFU assays were performed to determine numbers of bacteria per spleen and liver. Each point represents the log10 CFU per organ recovered from a single mouse. The horizontal lines represent the mean values for all mice in the group. Results are from 2 to 3 experiments with 3 to 4 mice per group (n = 9 for IP32953 IL-10, IL-18, and spleen CFU data; n = 7 for IP32953 IFN-γ data; and n = 6 for IP32953 liver CFU and IP32953ΔyopM mutant cytokine and CFU data). The limits of detection for the CFU assay were 500 CFU/organ. Cytokine values below the limit of detection for each ELISA were set at the limit of detection (dashed lines). Statistical significance was calculated via the Mann-Whitney test.
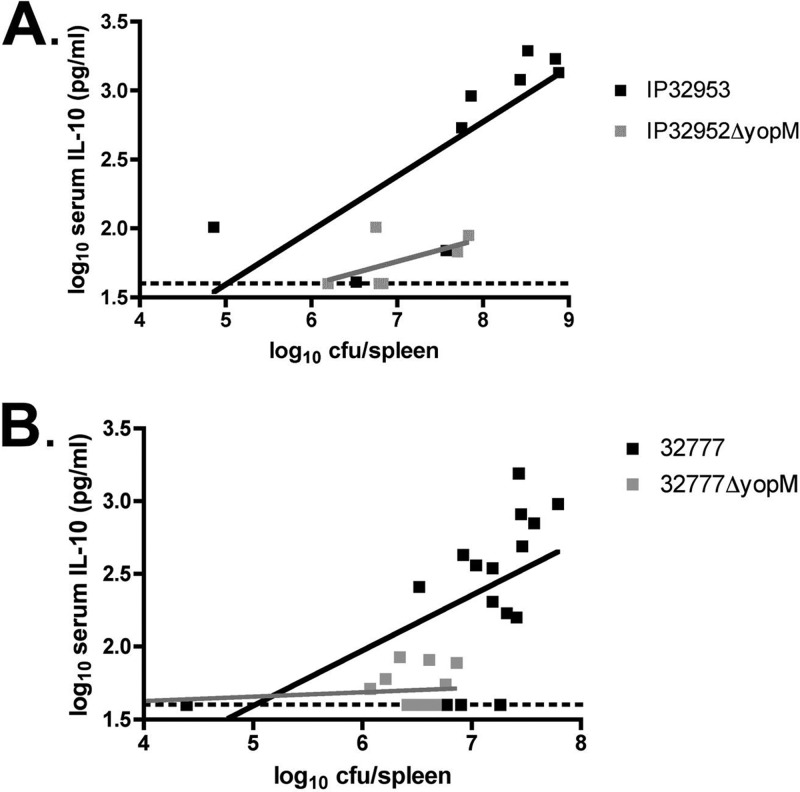
To characterize the relationship between serum IL-10 levels and organ burdens in mice infected with the yopM+ or yopM mutant strain, the log10 serum IL-10 concentration was plotted versus the log10 spleen CFU (59). This analysis was done with the results obtained here with IP32953 strains (Fig. 3A) as well as with previous data (29) resulting from infections with 32777 strains (Fig. 3B). In mice infected with the IP32953 or 32777 strain, there was a positive-sloped linear relationship between the colonization level and the serum IL-10 concentrations. In contrast, the data obtained with mice infected with yopM mutants formed slopes that did not deviate significantly from zero, as determined by a linear regression analysis. These results show that there is a direct correlation between bacterial burdens in spleens and concentrations of IL-10 in sera of mice infected with the yopM+ but not the yopM-deficient Y. pseudotuberculosis strain.
Fig 3.
Relationship between bacterial CFU values in spleens and IL-10 concentrations in sera of mice infected with Y. pseudotuberculosis. The log10 serum IL-10 concentrations and log10 CFU per spleen shown in Fig. 2A and D, respectively, for IP32953- or IP32953ΔyopM mutant-infected mice (A) or from data reported previously (29) for 32777- or 32777ΔyopM mutant-infected mice (B) are presented in a scatter plot format. Dashed lines represent the limit of detection of the IL-10 ELISA. By linear regression analysis, the R2 values were 0.603 for IP32953, 0.315 for the IP32953ΔyopM mutant, 0.309 for strain 32777, and 0.077 for the 32777ΔyopM mutant.
Supplementation of mice with IL-10 rescues the ability of a Y. pseudotuberculosis yopM mutant to colonize the liver.
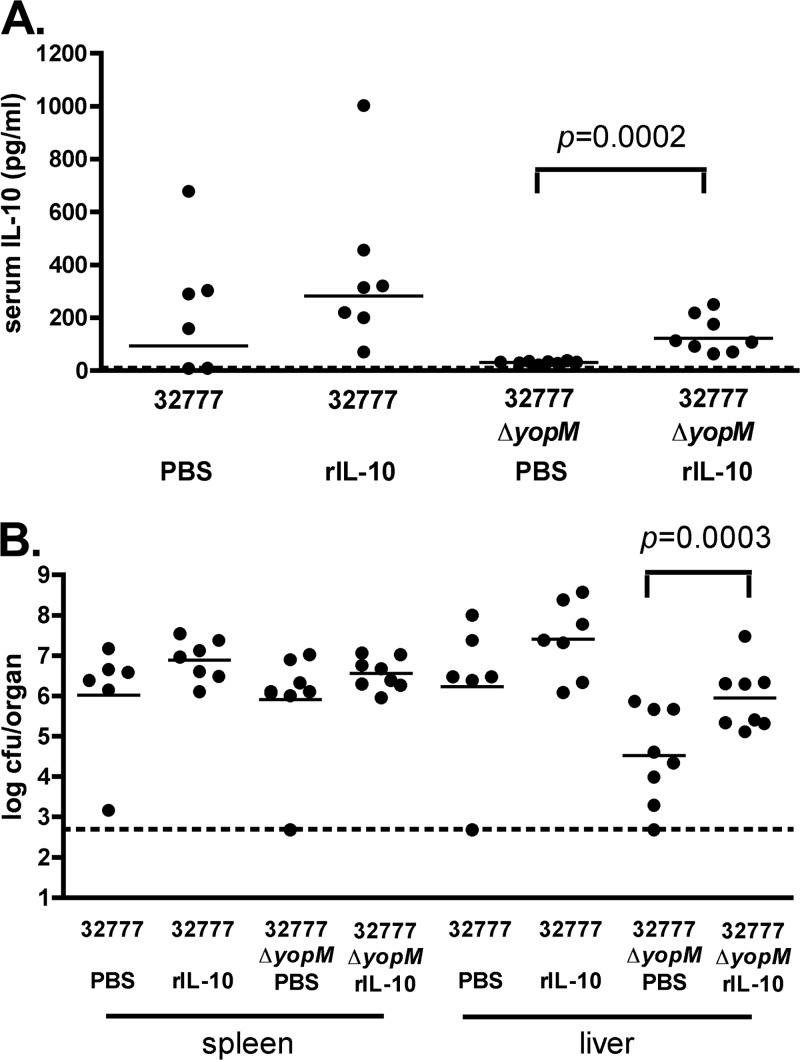
We reasoned that if a Y. pseudotuberculosis yopM mutant is defective for virulence in vivo due to a lack of IL-10 induction, the injection of exogenous IL-10 into mice should increase colonization by that mutant in the spleen and liver. To examine this possibility, groups of C57BL/6J mice were infected with ∼103 CFU of 32777 or the yopM mutant via tail vein injection, followed by the i.p. administration of recombinant murine IL-10. The 32777 background was used for this experiment because it is less virulent than IP32953 (Fig. 1) (29), allowing for a longer time course of infection and IL-10 treatment. Preliminary experiments suggested that the earliest time point at which increased serum IL-10 concentrations are observed for C57BL/6J mice infected with Y. pseudotuberculosis is between days 2 and 4 postinfection (data not shown). We therefore delivered 5 μg of IL-10 in PBS via i.p. injection on days 3, 4, and 5 postinfection. Control mice were injected with PBS alone on the same schedule. At 6 days postinfection, CFU assays were conducted on spleens and livers removed from euthanized mice. We also analyzed the serum levels of IL-10 from these mice and found significantly higher levels of IL-10 in the 32777ΔyopM mutant-infected, IL-10-supplemented mice than in mice that received PBS (P = 0.0003) (Fig. 4A). Similar to the results obtained for strain IP32953 in the spleen at day 4 (Fig. 2D), the yopM deficiency did not significantly reduce colonization by strain 32777 in spleens of control (PBS-treated) mice at day 6 (Fig. 4B). However, IL-10 supplementation caused a small (but not significant) increase in the colonization of spleens by 32777 and the 32777ΔyopM mutant (Fig. 4B). Significantly fewer CFU of yopM mutant bacteria than wild-type bacteria were present in livers of control mice at day 6 (Fig. 4B). These results suggest that the liver is a more restrictive environment for colonization by a Y. pseudotuberculosis yopM mutant than the spleen. Compared to the PBS control, average colonization levels of the yopM mutant were significantly higher in liver after IL-10 supplementation (P = 0.0003). Thus, the growth defect of the yopM mutant in liver could be partially rescued by the addition of exogenous IL-10.
Fig 4.
Effect of IL-10 supplementation on organ burdens of 32777 or the 32777ΔyopM mutant in spleens and livers of C57BL/6J mice. C57BL/6J mice were infected intravenously with 1.2 × 103 to 1.5 × 103 CFU of Y. pseudotuberculosis 32777 or the 32777ΔyopM mutant. At 3, 4, and 5 days postinfection, mice received 5 μg of recombinant murine IL-10 (rIL-10) in PBS or PBS alone via intraperitoneal injection. On day 6 postinfection, mice were euthanized, blood was obtained for analysis of serum IL-10 levels (A), and spleen and liver were collected and processed for a CFU assay (B). The experiment was repeated twice with four mice per group. Each filled circle represents the log10 CFU or the serum IL-10 concentration from an individual mouse. Mice that died before day 6 were excluded from the analysis, resulting in n values of <8 (n = 6 for strain 32777 plus PBS, n = 7 for strain 32777 plus IL-10, n = 8 for the 32777ΔyopM mutant plus PBS, and n = 8 for the 32777ΔyopM mutant plus IL-10). IL-10 and CFU values below the limit of detection were set at the limit of detection for each assay (dashed lines). Statistical significance was determined by the Mann-Whitney test.
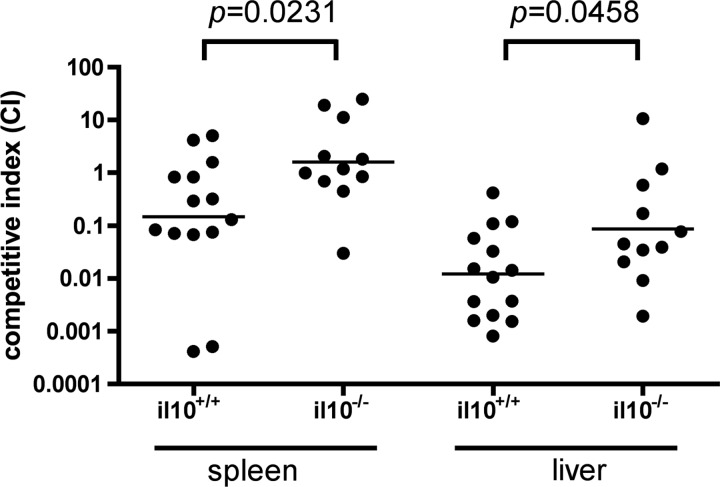
The competitive survival advantage of the Y. pseudotuberculosis yopM+ strain over the yopM mutant is decreased in IL-10−/− mice.
As an additional test to determine if the induction of IL-10 is important for the virulence function of YopM, a competitive infection experiment was carried out with IL-10+/+ or IL-10−/− C57BL/6J mice. The rationale for the test was that if the induction of IL-10 is important for the yopM+ Y. pseudotuberculosis strain to outcompete a yopM mutant for growth in C57BL/6J mice, then this advantage should be decreased in congenic IL-10−/− mice, which are unable to produce the cytokine. C57BL/6J (IL-10+/+) or B6.129P2-Il10tm1Cgn/J (IL-10−/−) mice were infected i.v. with a mixture of equal CFU of 32777 or the 32777yopM::kan strain (∼600 to 700 CFU of each bacterial strain). The mice were euthanized at 7 days postinfection, and CFU assays were conducted. Homogenates of the spleen and liver were plated onto medium with and without the addition of kanamycin to differentiate between colonies arising from the two strains and to allow the calculation of a competitive index (CI). As shown in Fig. 5, in IL-10+/+ mice, the mean CI was 0.23 for the spleen, showing that the yopM+ strain had a slight competitive advantage for splenic colonization over the yopM mutant. The yopM+ strain had a greater competitive advantage over the yopM mutant in the livers of IL-10+/+ mice, as the mean CI was 0.0125 (Fig. 5). Importantly, the competitive advantage of the yopM+ strain was absent in spleens (CI = 1.66) and was significantly reduced in livers (CI = 0.105) of IL-10−/− mice. In both spleens and livers, the change in CI associated with an IL-10 deficiency was significant (P = 0.0231 and P = 0.0458, respectively).
Fig 5.
Competitive index analysis of 32777 and 32777yopM::kan mutant infections in il10+/+ or il10−/− mice. C57BL/6J (il10+/+) or B6.129P2-Il10tm1Cgn/J (il10−/−) mice were infected intravenously with 1.2 × 103 to 1.5 × 103 CFU of an equal mixture of Y. pseudotuberculosis 32777 and 32777yopM::kan bacteria (600 to 750 CFU of each strain). The ratio of bacteria in the input was determined by the plating of serial dilutions followed by the enumeration of CFU. The infections were allowed to proceed for 7 days, the mice were euthanized, and the spleen and liver were processed for CFU analysis to determine output ratios. The competitive index (CI) was determined as described in Materials and Methods. The horizontal bar represents the geometric mean of the CI values. Results shown are from 5 (il10+/+) or 3 (il10−/−) independent experiments with 4 to 8 mice per group. Mice that died or had CFU values below the limit of detection were excluded from the analysis (n = 14 for il10+/+ mice and n = 11 for il10−/− mice). Statistical significance was determined by the Mann-Whitney test.
DISCUSSION
IL-10 is a potent anti-inflammatory cytokine that normally serves to counterregulate proinflammatory processes that can be detrimental in some circumstances (e.g., bacterial sepsis) (11, 44). It also plays a very important role in mediating immune tolerance in body sites that are routinely exposed to foreign antigens, like the lungs or the gastrointestinal tract. Indeed, mice lacking IL-10 have been successfully used as a model of inflammatory bowel disease, as these mice develop colitis in response to normal gut microbiota (22). Due to the immunosuppressive effects of this cytokine, it is perhaps unsurprising that a number of bacterial pathogens have evolved mechanisms to promote IL-10 production in the host, thereby dampening the normal host inflammatory response. For example, the causative agent of Lyme disease, Borrelia burgdorferi, induces IL-10 responses that allow it to persist within the host (15). Additionally, the induction of IL-10 has been demonstrated to occur via a T3SS effector, BopN, in mice infected with Bordetella bronchiseptica (32).
Brubaker and colleagues first proposed the concept that IL-10 production contributes to Yersinia pathogenesis (6). Nakajima et al. demonstrated that injecting mice with a recombinant protein A-LcrV fusion polypeptide resulted in the suppression of proinflammatory cytokines (33). This observation was later linked to upregulation of IL-10 (34). Other groups followed by demonstrating that recombinant LcrV was directly responsible for the induction of IL-10, via a Toll-like receptor 2 (TLR2)- and CD14-mediated mechanism (1, 6, 47, 49). However, different regions of Y. enterocolitica O8 LcrV (amino acids 31 to 49) (47) and Y. pestis LcrV (amino acids 271 to 300) (34, 36) were shown to be important for IL-10-inducing activity. In addition, the resistance of TLR2−/− mice to Y. enterocolitica infection (47, 48) turned out to be dependent upon the mouse strain background used (50), and TLR2−/− mice did not show enhanced resistance to infection with Y. pestis or Y. pseudotuberculosis (2, 13, 40, 42). More recent studies suggested that TLR6 as well as TLR2 and CD14 are important for the LcrV-dependent induction of IL-10 in mice infected with Y. pestis (13). LcrV is also required for the translocation of T3SS effectors, including YopM, into host cells infected with pathogenic Yersinia strains (24, 38). In this study, we have obtained evidence using genetic and immunological approaches that the induction of IL-10 is important for the virulence function of YopM in mice infected with Y. pseudotuberculosis. Thus, the ability of pathogenic Yersinia strains to induce IL-10 during infection may be multifactorial and species and/or strain dependent.
Consistent with results that we obtained previously with Y. pseudotuberculosis 32777 (29), we show here that YopM is important for virulence (Fig. 1) and for the induction of high levels of IL-10 in sera of mice infected with strain IP32953 (Fig. 2A). In addition, we demonstrate that a linear correlation exists between levels of IL-10 in sera and bacterial CFU in spleens of mice infected with IP32953 or 32777 but not the corresponding yopM mutants of these strains (Fig. 3). Although there were a few exceptions, in general, those mice infected with IP32953 or 32777 and with ≥107 CFU per spleen were also the mice with high serum IL-10 levels (more than 2 log10 pg/ml). However, two mice infected with the IP32953ΔyopM mutant had organ burdens of nearly 108 CFU but serum IL-10 levels of ≤2 log10 pg/ml (Fig. 3). These results suggest that the high level of IL-10 production in IP32953- or 32777-infected mice is not necessarily a consequence of higher organ burdens but rather reflects a differential cytokine response to these strains.
A recent report demonstrated that IL-10 was expressed in NK cells in blood and nonlymphoid organs (e.g., liver and lung) during systemic infection of mice with Y. pestis (37). This IL-10 expression in NK cells was also seen in response to systemic infections with other pathogens (Toxoplasma gondii and Listeria monocytogenes) or systemic challenge with cytosine-guanine oligodeoxynucleotides or LPS (37). Perona-Wright et al. showed previously that the IL-12 produced during the innate immune response to these challenges was necessary and sufficient for IL-10 expression in NK cells (37). Two observations suggest that the IL-10 that we detected in the sera of Y. pseudotuberculosis-infected mice is produced by a mechanism that is distinct from that observed previously by Perona-Wright et al. (37). First, IL-12-induced NK cell expression of IL-10 could be achieved by systemic challenge with Y. pestis lacking the virulence plasmid (and, hence, the T3SS and all effectors, including YopM) (37), while IL-10 induction in Y. pseudotuberculosis-infected mice required YopM (Fig. 3). Second, IL-12-induced NK cell expression of IL-10 was associated with the coproduction of IFN-γ (37), while IL-10 induction in Y. pseudotuberculosis-infected mice was associated with reduced levels of serum IFN-γ (Fig. 2B) (29). Nevertheless, it would be informative to determine if there is a YopM-dependent induction of IL-12 in mice infected with Y. pseudotuberculosis. Determining the cell types that are responsible for the YopM-dependent expression of IL-10 in Y. pseudotuberculosis-infected mice will also help elucidate the mechanism of this response. Recently, Bouabe et al. used transgenic mice with a transcriptional IL-10–β-lactamase reporter to identify cell types that express IL-10 following systemic infection with Y. enterocolitica (5). They obtained evidence that CD11b+ Ly6G+ PMNs were the predominant IL-10-expressing cell type in the spleen under these conditions (5).
Experiments were undertaken to determine if the virulence defect of the 32777ΔyopM strain could be overcome by providing mice with exogenous IL-10. The bacterial colonization of spleen and liver, as determined by a CFU assay, was used to measure the effect of IL-10 supplementation. CFU assays performed on organs of control (PBS-injected) mice infected for 6 days with wild-type 32777 or the yopM mutant showed that YopM was more important for bacterial colonization of the liver than the spleen by strain 32777 (Fig. 4B). Small but nonsignificant increases in CFU of 32777 and the 32777ΔyopM mutant were observed for the spleens of IL-10-treated mice. Similarly, IL-10 treatment caused a small but nonsignificant increase in the hepatic burden of 32777-infected mice. In contrast, IL-10 supplementation caused a significant (∼1.2-log) increase in CFU of the 32777ΔyopM mutant in livers of infected mice. Ye et al. (58) previously used a depletion approach to show that PMNs make a large contribution to the control of yopM-deficient Y. pestis in livers of mice. Additionally, those researchers showed that similar numbers of PMNs were present in spleens of mice infected with yopM+ or ΔyopM Y. pestis strains (58). Taken together with our results (Fig. 4), it is possible that IL-10 supplementation increases the colonization of the liver by the 32777ΔyopM mutant by reducing bactericidal activity in PMNs. The exposure of PMNs to IL-10 has been shown to reduce phagocytic activity and the expression of cytokines (3), and high levels of intrahepatic IL-10 cause the downregulation of Mac-1 on PMNs (30). Thus, IL-10 supplementation may decrease PMN function and maintenance at this site, thereby creating a permissive site for the replication of the 32777ΔyopM mutant in the liver. It is unknown why IL-10 supplementation did not restore the colonization of the 32777ΔyopM mutant to the level of wild-type bacteria in the livers of control mice. Although we demonstrate that supplementation with IL-10 causes sustained increased serum levels of IL-10 (Fig. 4A), it is still possible that insufficient levels of the exogenous cytokine were obtained in the microenvironment of the abscesses in the liver in which the bacteria grow. A second possibility is that YopM has an additional virulence function in the liver that is independent of IL-10 induction (discussed further below).
As a second test of the model that IL-10 induction is important for the virulence function of YopM, we compared the ability of wild-type bacteria to outcompete the ΔyopM strain in wild-type or IL-10−/− C57BL/6J mice. Competition infections were done for two reasons. First, it was reasoned that the subtle colonization defect of the ΔyopM mutant in the spleen would be easier to detect in competition with wild-type bacteria. Second, competition infections would avoid the known genetic background difference between wild-type and IL-10−/− C57BL/6J mice that can confound studies of the role of IL-10 in resistance to bacterial infection (54). Specifically, the commercially available IL-10-deficient mice in the C57BL/6J lineage (B6.129P2-Il10tm1Cgn/J [22]) have been shown to have a genetic background associated with resistance to Y. pestis infection (54). Turner et al. (54) demonstrated previously that B6.129P2-Il10tm1Cgn/J mice contain genomic DNA flanking the il10 allele derived from the original 129 line. The 129 DNA increases, in a nonrecessive manner, resistance to Y. pestis infection (54). Several 129 mouse substrains were previously shown to be resistant to conditionally virulent Y. pestis strains (9). Thus, studies in which single-strain infections of B6.129P2-Il10tm1Cgn/J mice or C57BL/6J mice were compared (13, 39, 48) likely overestimated the contribution of IL-10 to Yersinia pathogenesis (54), while competition infections would negate this issue.
We found that wild-type strain 32777 had a slight competitive advantage over the ΔyopM mutant in spleens of C57BL/6J mice (CI of 0.23), and this advantage was lost in IL-10−/− mice (CI of 1.66) (Fig. 5). Similar results were found for the liver, although the competitive advantage of the wild type over the ΔyopM mutant was greater in C57BL/6J mice (CI of 0.0125) and only partially lost in IL-10−/− mice (CI of 0.105). We interpret these results to indicate that the production of IL-10 is important for the virulence function of YopM in both the spleen and liver. As discussed above, PMNs (58) may be the target of YopM-induced IL-10 in the liver. In the spleen, inflammatory monocytes and inflammatory dendritic cells (iDCs) (also known as Tip-DCs) may be the most important cell types for controlling the growth of ΔyopM Yersinia strains (58). IL-10 is known to inhibit Tip-DC function. For example, during infection with Trypanosoma brucei, localized endogenous IL-10 attenuates the maturation of monocytes into Tip-DCs (17). Furthermore, the sustained expression of IL-10 under the control of a liver-specific promoter in an adenovirus vector can inhibit the differentiation of monocytes into Tip-DCs in mice chronically infected with T. brucei (17).
A requirement of the competition test was that the yopM+ Y. pseudotuberculosis strain would be unable to fully rescue in trans the growth defect of a ΔyopM mutant in wild-type C57BL/6J mice. Previously, Ye et al. showed that that a yopM+ Y. pestis strain was able to fully rescue in trans the growth defect of a ΔyopM mutant in spleens but not livers of mice (58). Our ability to detect a competitive advantage of wild-type bacteria over ΔyopM bacteria in spleens of C57BL/6J mice may have been due to the use of different Yersinia strains (Y. pseudotuberculosis versus Y. pestis) or different mouse strains (C57BL/6J versus C57BL/6N.HSD) from those used by Ye et al. The host immune responses to these two pathogens could differ because there are distinct forms of LPS (41) and different sets of proteins (56) localized to the outer membranes of Y. pseudotuberculosis and Y. pestis. The finding that the competitive advantage of the wild type over the ΔyopM mutant was only partially reduced in livers of IL-10−/− mice (Fig. 5) suggests that YopM has a virulence function in this organ that is independent of IL-10 induction, reinforcing the same conclusion reached with the IL-10 supplementation experiment (Fig. 4). Ye et al. (58) suggested that YopM subverts the function of PMNs in liver, possibly by interfering with their interaction with Kupffer cells (16). Thus, it is possible that YopM interferes with PMN function directly as well as indirectly by downregulating their bactericidal activity through the induction of IL-10.
ACKNOWLEDGMENTS
We thank Galina Romanov for excellent technical support and Ralph Isberg for providing strain IP32953.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (R56-AI043389 and P01-AI055621) and the Northeast Biodefense Center (U54-AI057158-Lipkin), awarded to J.B.B., and by contract DHHSN266200400057C, awarded to Myriad Genetics, Inc.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print 30 April 2012
REFERENCES
- 1. Abramov VM, et al. 2007. Attachment of LcrV from Yersinia pestis at dual binding sites to human TLR-2 and human IFN-gamma receptor. J. Proteome Res. 6:2222–2231 [DOI] [PubMed] [Google Scholar]
- 2. Auerbuch V, Isberg RR. 2007. Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect. Immun. 75:3561–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bazzoni F, Tamassia N, Rossato M, Cassatella MA. 2010. Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: lessons from neutrophils. Eur. J. Immunol. 40:2360–2368 [DOI] [PubMed] [Google Scholar]
- 4. Boland A, Havaux S, Cornelis GR. 1998. Heterogeneity of the Yersinia YopM protein. Microb. Pathog. 25:343–348 [DOI] [PubMed] [Google Scholar]
- 5. Bouabe H, Liu Y, Moser M, Bosl MR, Heesemann J. 2011. Novel highly sensitive IL-10-beta-lactamase reporter mouse reveals cells of the innate immune system as a substantial source of IL-10 in vivo. J. Immunol. 187:3165–3176 [DOI] [PubMed] [Google Scholar]
- 6. Brubaker RR. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bubeck SS, Cantwell AM, Dube PH. 2007. Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect. Immun. 75:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chain PS, et al. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. U. S. A. 101:13826–13831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Congleton YH, Wulff CR, Kerschen EJ, Straley SC. 2006. Mice naturally resistant to Yersinia pestis Δpgm strains commonly used in pathogenicity studies. Infect. Immun. 74:6501–6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cornelis GR, et al. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Couper KN, Blount DG, Riley EM. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 180:5771–5777 [DOI] [PubMed] [Google Scholar]
- 12. de Lorenzo V, Herrero M, Jakubzik U, Timmis KN. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568–6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Depaolo RW, et al. 2008. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe 4:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evdokimov AG, Anderson DE, Routzahn KM, Waugh DS. 2001. Unusual molecular architecture of the Yersinia pestis cytotoxin YopM: a leucine-rich repeat protein with the shortest repeating unit. J. Mol. Biol. 312:807–821 [DOI] [PubMed] [Google Scholar]
- 15. Giambartolomei GH, Dennis VA, Philipp MT. 1998. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 66:2691–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gregory SH, Wing EJ. 2002. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J. Leukoc. Biol. 72:239–248 [PubMed] [Google Scholar]
- 17. Guilliams M, et al. 2009. IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J. Immunol. 182:1107–1118 [DOI] [PubMed] [Google Scholar]
- 18. Hentschke M, et al. 2010. Yersinia virulence factor YopM induces sustained RSK activation by interfering with dephosphorylation. PLoS One 5(10):e13165 doi:10.1371/journal.pone.0013165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 20. Kato T, et al. 1995. Interleukin 10 reduces mortality from severe peritonitis in mice. Antimicrob. Agents Chemother. 39:1336–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kerschen EJ, Cohen DA, Kaplan AM, Straley SC. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72:4589–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274 [DOI] [PubMed] [Google Scholar]
- 23. Lathem WW, Crosby SD, Miller VL, Goldman WE. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. U. S. A. 102:17786–17791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee VT, Tam C, Schneewind O. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275:36869–36875 [DOI] [PubMed] [Google Scholar]
- 25. Leung KY, Reisner BS, Straley SC. 1990. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58:3262–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. 2005. Plague bacteria target immune cells during infection. Science 309:1739–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCoy MW, Marre ML, Lesser CF, Mecsas J. 2010. The C-terminal tail of Yersinia pseudotuberculosis YopM is critical for interacting with RSK1 and for virulence. Infect. Immun. 78:2584–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDonald C, Vacratsis PO, Bliska JB, Dixon JE. 2003. The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 278:18514–18523 [DOI] [PubMed] [Google Scholar]
- 29. McPhee JB, Mena P, Bliska JB. 2010. Delineation of regions of the Yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infect. Immun. 78:3529–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menezes GB, et al. 2009. Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10. J. Immunol. 183:7557–7568 [DOI] [PubMed] [Google Scholar]
- 31. Mueller CA, Broz P, Cornelis GR. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68:1085–1095 [DOI] [PubMed] [Google Scholar]
- 32. Nagamatsu K, et al. 2009. Bordetella evades the host immune system by inducing IL-10 through a type III effector, BopN. J. Exp. Med. 206:3073–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakajima R, Motin VL, Brubaker RR. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nedialkov YA, Motin VL, Brubaker RR. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nemeth J, Straley SC. 1997. Effect of Yersinia pestis YopM on experimental plague. Infect. Immun. 65:924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Overheim KA, et al. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 73:5152–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perona-Wright G, et al. 2009. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe 6:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pettersson J, et al. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961–976 [DOI] [PubMed] [Google Scholar]
- 39. Philipovskiy AV, et al. 2005. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect. Immun. 73:1532–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pouliot K, et al. 2007. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect. Immun. 75:3571–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rebeil R, Ernst RK, Gowen BB, Miller SI, Hinnebusch BJ. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 52:1363–1373 [DOI] [PubMed] [Google Scholar]
- 42. Reithmeier-Rost D, et al. 2007. The weak interaction of LcrV and TLR2 does not contribute to the virulence of Yersinia pestis. Microbes Infect. 9:997–1002 [DOI] [PubMed] [Google Scholar]
- 43. Ruter C, Buss C, Scharnert J, Heusipp G, Schmidt MA. 2010. A newly identified bacterial cell-penetrating peptide that reduces the transcription of pro-inflammatory cytokines. J. Cell Sci. 123:2190–2198 [DOI] [PubMed] [Google Scholar]
- 44. Saraiva M, O'Garra A. 2010. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10:170–181 [DOI] [PubMed] [Google Scholar]
- 45. Simonet M, Falkow S. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 60:4414–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simonet M, Richard S, Berche P. 1990. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sing A, et al. 2005. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. U. S. A. 102:16049–16054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sing A, Roggenkamp A, Geiger AM, Heesemann J. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315–1321 [DOI] [PubMed] [Google Scholar]
- 49. Sing A, et al. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sing A, et al. 2003. Contribution of Toll-like receptors 2 and 4 in an oral Yersinia enterocolitica mouse infection model. Int. J. Med. Microbiol. 293:341–348 [DOI] [PubMed] [Google Scholar]
- 51. Skrzypek E, Cowan C, Straley SC. 1998. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30:1051–1065 [DOI] [PubMed] [Google Scholar]
- 52. Trulzsch K, Sporleder T, Igwe EI, Russmann H, Heesemann J. 2004. Contribution of the major secreted Yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect. Immun. 72:5227–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Turner JK, McAllister MM, Xu JL, Tapping RI. 2008. The resistance of BALB/cJ mice to Yersinia pestis maps to the major histocompatibility complex of chromosome 17. Infect. Immun. 76:4092–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Turner JK, Xu JL, Tapping RI. 2009. Substrains of 129 mice are resistant to Yersinia pestis KIM5: implications for interleukin-10-deficient mice. Infect. Immun. 77:367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Viboud GI, Bliska JB. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69–89 [DOI] [PubMed] [Google Scholar]
- 56. Wren BW. 2003. The yersiniae—a model genus to study the rapid evolution of bacterial pathogens. Nat. Rev. Microbiol. 1:55–64 [DOI] [PubMed] [Google Scholar]
- 57. Ye Z, et al. 2009. Gr1+ cells control growth of YopM-negative Yersinia pestis during systemic plague. Infect. Immun. 77:3791–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ye Z, et al. 2011. Distinct CCR2(+) Gr1(+) cells control growth of the Yersinia pestis ΔyopM mutant in liver and spleen during systemic plague. Infect. Immun. 79:674–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Bliska JB. 2011. Mathematical relationship between cytokine concentrations and pathogen levels during infection. Cytokine 53:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]