Abstract
The role of interleukin-10 (IL-10) in malaria remains poorly characterized. The aims of this study were to investigate (i) whether genetic variants of the IL-10 gene influence IL-10 production and (ii) whether IL-10 production as well as the genotypes and haplotypes of the IL-10 gene in young children and their mothers are associated with the incidence of clinical malaria in young children. We genotyped three IL-10 single nucleotide polymorphisms in 240 children and their mothers from a longitudinal prospective cohort and assessed the IL-10 production by maternal peripheral blood mononuclear cells (PBMCs) and cord blood mononuclear cells (CBMCs). Clinical episodes of Plasmodium falciparum malaria in the children were documented until the second year of life. The polymorphism IL-10 A-1082G (GCC haplotype of three SNPs in IL-10) in children was associated with IL-10 production levels by CBMC cultured with P. falciparum-infected erythrocytes (P = 0.043), with the G allele linked to low IL-10 production capacity. The G allele in children was also significantly associated with a decreased risk for clinical malaria infection in their second year of life (P = 0.016). Furthermore, IL-10 levels measured in maternal PBMCs cultured with infected erythrocytes were associated with increased risk of malaria infection in young children (P < 0.001). In conclusion, IL-10 polymorphisms and IL-10 production capacity were associated with clinical malaria infections in young children. High IL-10 production capacity inherited from parents may diminish immunological protection against P. falciparum infection, thereby being a risk for increased malaria morbidity.
INTRODUCTION
Interleukin-10 (IL-10) is a dominant cytokine in the regulation of immunity to infection, as it suppresses macrophages and dendritic cells (DCs), thereby limiting T helper type 1 (TH1) and T helper type 2 (TH2) effector responses (3). With the ability to downregulate inflammatory responses, IL-10 has been reported to be associated with decreased risk of cerebral malaria and severe malaria-associated anemia (12, 13, 21). However, higher IL-10 levels were associated with less effective clearance of Plasmodium falciparum parasites (7), thus facilitating malaria parasite infection in the human host. The exact role of IL-10 in malaria remains poorly characterized.
An individual's capacity to produce IL-10 is under genetic control. The IL-10 gene is located on chromosome 1q31-q32, with several variants in the promoter region associated with variable IL-10 production and infection phenotypes (8). One haplotype (GCC: -1082G/-819C/-592C) in the IL-10 gene that results in higher levels of IL-10 was reported to be associated with protection against severe malaria-related anemia in Kenyan children <3 years of age (22). In another study of Gambian children, in which eight IL-10 gene single nucleotide polymorphisms (SNPs) were investigated, a specific haplotype with a frequency of 11% was associated with a decreased risk for severe malaria, although the transmission disequilibrium test in families from the same population did not detect undertransmission of this haplotype (28). To our knowledge, no studies have been performed to investigate the association of IL-10 genetic variants with the incidence of clinical malaria in young children.
In the AgeMal collaborative study (ClinicalTrials.gov identifier NCT00231452), women from the Manhiça district of Southern Mozambique were recruited during pregnancy. Their children were followed until they were 2 years of age, with the aim of investigating age of exposure and immunity to malaria in young children (5). The AgeMal study provided a unique opportunity to longitudinally investigate the potential effects of IL-10 on clinical malaria in young children. IL-10 production in maternal peripheral blood mononuclear cells (PBMCs) and in cord blood mononuclear cells (CBMCs) was measured after stimulation with a lysate of uninfected or P. falciparum-infected erythrocytes, and episodes of clinical malaria were documented in the cohort of children followed up to 2 years of age. Three IL-10 SNPs were also genotyped in the young children and their mothers. We hypothesized that genetic variants in the IL-10 gene would influence the capacity of IL-10 production and that specific genotypes and haplotypes of the IL-10 gene in young children and their mothers would be associated with the incidence of clinical malaria during the first 2 years of life.
MATERIALS AND METHODS
Study population.
This study is part of the project entitled “Age of exposure and immunity to malaria in infants (AgeMal).” A three-arm randomized, double-blind, placebo-controlled trial was conducted in an area where malaria is endemic. First exposure to P. falciparum was selectively controlled at different periods during infancy (2 to 5 months, early exposure; 5 to 10 months, late exposure; or none, control) with monthly chemoprophylaxis with sulfadoxine-pyrimethamine and artesunate. A total of 298 HIV-negative pregnant women were recruited from the Manhiça district of Southern Mozambique. Transmission of P. falciparum is perennial, with marked seasonality and of moderate intensity, with an entomological inoculation rate of 38 infective bites/person/year (1). A total of 287 children were followed up to age 24 months. The study included 240 infants and their mothers, with cell culture and genotyping data in this cohort. For individual SNPs in infants and their mothers, there were missing values due to insufficient DNA or technical failures with genotyping. For specific IL-10 measurements, there were missing values due to low blood volumes and insufficient cell numbers. Written informed consent was obtained from all mothers. Analysis of the young children's cohort did not indicate significant effects of the controlled exposure on P. falciparum infection outcomes and immunity (5).
This study was approved by the National Mozambican Ethics Review Committee, the Hospital Clinic of Barcelona Ethics Review Committee, and the Princess Margaret Hospital for Children Ethics Committee (1473/EP) in Perth.
Surveillance for clinical malaria.
Young children were followed up by passive and active malaria case detection for the first 2 years of life to record the incidence of clinical malaria episodes. Passive case detection was done through the morbidity surveillance system at the Manhiça District Hospital, where standardized information on all pediatric outpatient visits and hospital admissions is collected. Active case detection consisted of weekly home visits by field workers to take axillary temperatures and record any history of fever from the child's caretaker from birth to approximately 10.5 months. From the age of 10.5 to 24 months, these visits were monthly. In the case of a documented fever (axillary temperature of >37.5°C) or history of fever in the previous 24 h, a blood sample was collected by finger prick and 2 blood smears were prepared for the measurement of P. falciparum parasitemia under light microscopy. Antimalarials were administered if the smear reading was positive for asexual P. falciparum parasites. As we performed interventions in the child's first year of life in our analysis, we used only the episodes of malaria infection during the second year of life. There were 216 clinical malaria episodes recorded, and the incidence rate of clinical malaria during the second year of life was 0.91 (95% confidence interval [CI], 0.70 to 1.12) episodes/person-year. Among the clinical malaria episodes, there were 23 (11%) episodes in which the child was admitted to the hospital.
Genotyping.
Genomic DNA was extracted from PBMCs that were kept frozen at −80° by an automated DNA extraction instrument (Autopure LS; Qiagen, Hilden, Germany). Three SNPs in the IL-10 gene were genotyped in young children and their mothers: IL-10 A-1082G (rs1800896), IL-10 A-592C (rs1800872), and IL-10 G919T (rs1518110). Two SNPs (IL-10 A-1082G and IL-10 A-592C) are located in the promoter of the IL-10 gene and have been reported to be associated with infection (18, 24, 26), and IL-10 G919T is a common SNP in Africans. Genotyping of the three SNPs was performed by the Australian Genome Research Facility (AGRF) using the iPLEX assay on the MassARRAY system (Sequenom, San Diego, CA) (4) according to the manufacturer's instructions (Sequenom).
P. falciparum-specific IL-10 cytokine production.
Production of IL-10 by maternal PBMCs and CBMCs stimulated with uninfected and infected erythrocytes was determined as described previously (2). Briefly, cells were isolated using a Lymphoprep gradient and resuspended in complete RPMI medium. A total of 0.4 million fresh PBMCs and CBMCs were stimulated with 20 μl of a P. falciparum schizont extract corresponding to lysate from 2 million freeze-thawed synchronized infected erythrocytes (3D7 strain, tested to exclude endotoxin and Mycoplasma contamination) or uninfected erythrocytes. The supernatant was collected following incubation for 24 h and then frozen at −80°C. IL-10 was quantified with the Bender MedSystems Human Th1/Th2 11plex FlowCytomix multiplex kit and analyzed on a FACS Canto II (Becton Dickinson) (limit of detection, 1.9 pg/ml) (2).
Statistical analysis.
The primary case definition of a clinical malaria episode was measured axillary temperature of ≥37.5°C or history of fever within the prior 24 h plus the presence of P. falciparum asexual stage parasites of any density. For the three IL-10 SNPs, Hardy-Weinberg equilibrium (HWE) was examined separately in children and mothers using the online tool (https://regepi.bwh.harvard.edu/IIPGA2/). PHASE was employed to construct haplotypes of the three SNPs (23). Haploview version 4.2 was utilized to investigate linkage disequilibrium for the three SNPs in the IL-10 gene. The levels of IL-10 produced by stimulated PBMCs and CBMCs were log transformed to have an approximately normal distribution. The associations between levels of IL-10 cytokine with genotypes and haplotypes of the three IL-10 SNPs were investigated using analysis of variance (ANOVA) and independent sample t test. Linear regression was also employed to investigate these associations after adjusting for confounders such as mother's age, placental malaria infection, and child's gender. Principal component analysis (PCA), which can find a linear combination (a component) of variables that accounts for as much variation in the original variables as possible, was employed for the analysis of IL-10 levels in PBMCs and CBMCs cultured with uninfected/infected erythrocytes to extract the first component score that was examined for the association with IL-10 genotypes.
Poisson regression was used to estimate the risk of the incidence of clinical malaria in the second year of life and its association with the capacity of IL-10 production and genotypes of IL-10. In the regression analyses, confounders, including the variable of intervention group (early and late exposure and control), were fitted in the model for an adjustment.
A P value of <0.05 was used for significance, and all analyses were conducted using SPSS (PASW Statistics 18) and Stata (StataIC 11) programs.
RESULTS
In this study, we included 240 women and their children, excluding approximately 20% for a variety of reasons such as stillbirth, parental withdrawal, and insufficient blood collection.
General information on the children and their mothers included in the study is presented in Table 1. The mean (±standard deviation [SD]) age of mothers at recruitment was 24.3 ± 6.7 years. There were 52 (21.7%) pregnant women in whom placental malaria infection was detected, including 45 past infections, 5 acute infections, and 2 chronic infections. Within the group of young children there were 119 females, and the ratio of females to males was approximately 1.
Table 1.
Characteristics of the study participants
| Characteristica | Mean | SD | No. of participants | % of participants |
|---|---|---|---|---|
| Mothers | ||||
| Age (yr) | 24.3 | 6.7 | ||
| Use of ITNs | 26 | 10.8 | ||
| Use of IRS | 109 | 45.4 | ||
| Placental infection | 52 | 21.7 | ||
| Parity | ||||
| Primipara | 64 | 26.7 | ||
| Multiparous | 153 | 63.8 | ||
| Unknown | 23 | 9.6 | ||
| Infants | ||||
| Female | 119 | 49.6 | ||
| Birth wt (kg) | 3.0 | 0.4 | ||
| Birth length (cm) | 47.9 | 3.1 | ||
| Intervention | ||||
| Control | 84 | 35.0 | ||
| Late exposure | 74 | 30.8 | ||
| Early exposure | 82 | 34.2 |
ITN, insecticide-treated mosquito nets; IRS, indoor residual spraying.
Genotypes and haplotypes.
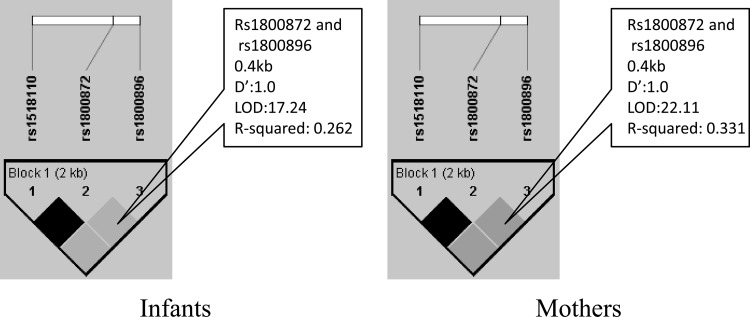
The genotype and haplotype frequencies of the three SNPs in young children and their mothers are shown in Table 2. The three SNPs were in HWE. Complete linkage disequilibrium was detected between the three SNPs (Fig. 1), with D′ (D/Dmax; a statistic that measures linkage disequilibrium) equal to 1 in both young children and mothers. The r2 between G919T and A-592C was 1, i.e., the T allele of G919T was always linked with the A allele of A-592C, and G with C. Due to the complete dependency of these two SNPs, we investigated only the genotype and phenotype relationships for SNP IL-10 A-592C. The correlation between A-592C and A-1082G was 0.26 (r2) in young children and 0.33 in their mothers, separately. Three haplotypes were inferred, namely, TAA, GCA, and GCG, with frequencies that varied from 26% to 39% in both groups. Due to complete linkage disequilibrium (D′ = 1) between the two SNPs, variations of haplotypes TAA and GCG in the population are equal to those in SNPs A-592C and A-1082G, respectively. For example, individuals with 0, 1, or 2 copies of GCG are the same individuals with 0, 1, or 2 copies of the G of A-1082G, respectively. Therefore, in the following haplotype and phenotype analyses, we investigated only the effect of haplotype GCA.
Table 2.
Genotypes and haplotypes in infants and mothersa
| Genotype or haplotype | Infants |
Mothers |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | MAF | HWE, exact P value | No. | % | MAF | HWE, exact P value | |
| Genotypes | ||||||||
| rs1518110(G919T) | ||||||||
| GG | 74 | 36.5 | 38.9 | 0.61 | 80 | 37.7 | 37.3 | 0.31 |
| GT | 100 | 49.3 | 106 | 50.0 | ||||
| TT | 29 | 14.3 | 26 | 12.3 | ||||
| rs1800872(A-592C) | ||||||||
| AA | 29 | 14.3 | 39.4 | 0.46 | 24 | 11.4 | 36.4 | 0.25 |
| CA | 102 | 50.2 | 105 | 50.0 | ||||
| CC | 72 | 35.5 | 81 | 38.6 | ||||
| rs1800896(A-1082G) | ||||||||
| AA | 91 | 44.6 | 33.6 | 0.75 | 94 | 45.4 | 31.4 | 0.27 |
| AG | 89 | 43.6 | 96 | 46.4 | ||||
| GG | 24 | 11.8 | 17 | 8.2 | ||||
| Haplotypes | ||||||||
| TAA | 36.3 | 39.4 | ||||||
| GCA | 32.1 | 26.8 | ||||||
| GCG | 31.6 | 33.8 | ||||||
MAF, minor allele frequency; HWE, Hardy-Weinberg equilibrium.
Fig 1.
Linkage disequilibrium for the three single nucleotide polymorphisms (rs1518110 [G919T], rs1800872 [A-592C], and rs1800896 [A-1082G]) was examined using Haploview version 4.2 in infants and their mothers, separately.
IL-10 cytokine production.
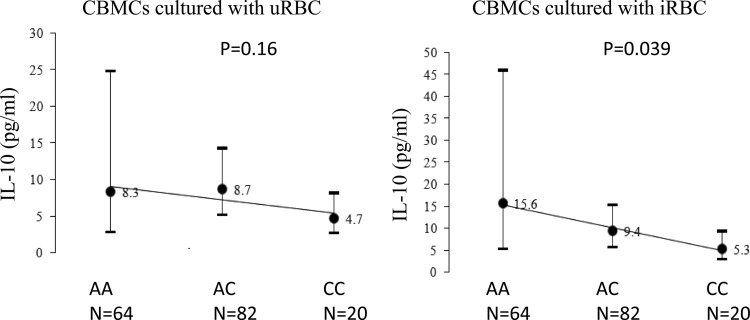
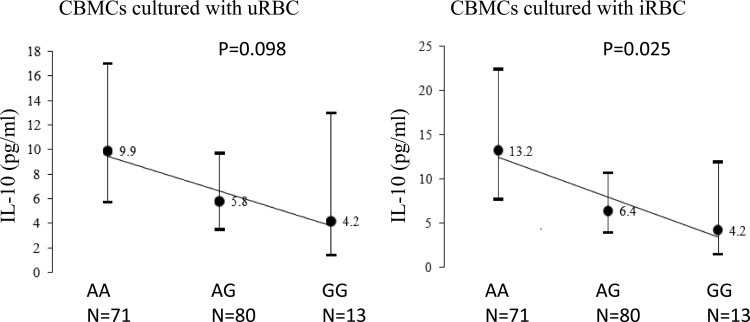
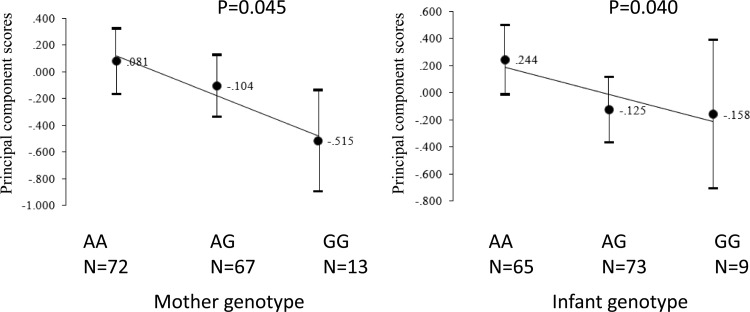
IL-10 production was strongly correlated in maternal PBMCs and CBMCs and in cells cultured with uninfected and infected erythrocytes. Figures 2 and 3 and Tables S1 and S2 in the supplemental material show the associations of IL-10 genotypes in children and mothers with IL-10 production capacity by PBMCs and CBMCs. In children, the A alleles of A-592C (P = 0.039) and A-1082G (P = 0.025) were associated with increased IL-10 production in CBMCs cultured with infected erythrocytes in an additive genetic model. The A allele of these two SNPs in children and mothers was consistently associated with higher IL-10 production by CBMCs and PBMCs. Acknowledging the strong correlation of IL-10 production levels by PBMCs and CBMCs, we utilized PCA to extract the first principal component that accounted for 67% of the variance for IL-10 levels in four cultures: PBMC cultured with uninfected/infected erythrocytes and CBMCs cultured with uninfected/infected erythrocytes (Fig. 4). The first principal component score was associated with A-1082G in young children (P = 0.04) and A-1082G in mothers (P = 0.045).
Fig 2.
IL-10 levels (pg/ml) in cord blood mononuclear cells (CBMCs) cultured with uninfected/infected erythrocytes (uRBC/iRBC), stratified by children's single nucleotide polymorphism IL-10 A-592C. Geometric means and 95% confidence intervals are presented.
Fig 3.
IL-10 levels (pg/ml) in cord blood mononuclear cells (CBMCs) cultured with uninfected/infected erythrocytes (uRBC/iRBC), stratified by children's single nucleotide polymorphism IL-10 A-1082G. Geometric means and 95% confidence intervals are presented.
Fig 4.
Associations of IL-10 A-1082G in mothers and infants with the first principal component score of IL-10 production in maternal peripheral blood mononuclear cells (PBMCs) and cord blood mononuclear cells (CBMCs).
In order to further verify the significant association of the two investigated SNPs with IL-10 production, we employed linear regression analysis with adjustment for confounders, including mother's age, parity, infant gender, use of insecticide-treated mosquito nets (ITNs), use of indoor residual spraying (IRS), and placental malaria infection. After adjustment, the P values for the association of A-592C and A-1082G with IL-10 in CBMCs cultured with infected erythrocytes were 0.070 and 0.043, respectively, and the P value for the first principal component score of IL-10 production by maternal PBMCs and infant CBMCs with the SNP A-1082G in young children and mothers was 0.054 and 0.057, respectively.
Incidence of clinical malaria.
We investigated the associations of IL-10 genotypes and IL-10 production with clinical malaria incidence in the second year of life. Table 3 shows the incidence rate ratios (IRRs) estimated using Poisson regression models after adjusting for intervention, mother's age, parity, infant gender, use of ITNs, use of IRS, and placental malaria infection. Significant effects were seen in the IL-10 production by maternal PBMCs cultured with both uninfected erythrocytes (P = 0.033) and infected erythrocytes (P < 0.001). The A allele of A-592C was associated with an increased risk in malaria infection in young children, with a boundary significance of 0.099; and the G allele of A-1082G was significantly associated with decreased risk in clinical malaria infection in young children (P = 0.016).
Table 3.
IRRs of IL-10 production and IL-10 polymorphisms for malaria infection in infantsa
| Predictor | IRR | 95% CI |
P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| IL-10 production | ||||
| IL-10 in maternal PBMC with uRBC (log value) | 1.07 | 1.01 | 1.15 | 0.033 |
| IL-10 in maternal PBMC with iRBC (log value) | 1.19 | 1.11 | 1.28 | <0.001 |
| IL-10 in CBMC with uRBC (log value) | 1.04 | 0.97 | 1.11 | 0.25 |
| IL-10 in CBMC with iRBC (log value) | 1.02 | 0.96 | 1.09 | 0.52 |
| PCA score | 1.13 | 0.97 | 1.33 | 0.12 |
| IL-10 SNPs in infants | ||||
| A-592C (coding AA, 2; AC, 1; CC, 0) | 1.19 | 0.97 | 1.48 | 0.099 |
| A-1082G (coding AA, 0; AG, 1; GG, 2) | 0.75 | 0.59 | 0.95 | 0.016 |
| IL-10 SNPs in mothers | ||||
| A-592C in mothers (coding AA, 2; AC, 1; CC, 0) | 0.96 | 0.77 | 1.20 | 0.73 |
| A-1082G in mothers (coding AA, 0; AG, 1; GG, 2) | 0.87 | 0.69 | 1.10 | 0.24 |
PBMC, peripheral blood mononuclear cells; CBMC, cord blood mononuclear cells; uRBC, uninfected erythrocytes; iRBC, infected erythrocytes; 95% CI, 95% confidence intervals; PCA, principal component analysis. Incidence rate ratios (IRRs) were estimated using the Poisson regression model after adjusting for intervention, mother's age, parity, infant gender, use of insecticide-treated mosquito nets, use of indoor residual spraying, and placental malaria infection.
We further investigated whether IL-10 production and IL-10 polymorphisms were independently associated with clinical malaria in young children. As expected, the effects of IL-10 production and the IL-10 SNPs were not independent, and after adjusting for IL-10 production by maternal PBMCs, the association of IL-10 SNPs with clinical malaria disappeared. However, even after adjusting for the two SNPs in the IL-10 gene in mothers and young children, the IL-10 levels produced by maternal PBMCs after stimulation with infected erythrocytes were still significantly associated with an increased risk for clinical malaria in the second year of life.
We also investigated the association of the GCA haplotype with IL-10 production by PBMCs and CBMCs (Table S3 in the supplemental material) and clinical malaria in young children. No significant effects of this haplotype were found (data not shown).
DISCUSSION
In the longitudinal prospective cohort of children investigated, we analyzed the effects of SNPs in IL-10 on IL-10 production by CBMCs and maternal PBMCs and on the incidence of clinical malaria in children during the second year of life. The variants A-592C and A-1082G in the promoter region of IL-10 were associated with IL-10 levels produced by CBMCs stimulated with infected erythrocytes, and the genetic variants were associated with clinical malaria in the second year of life. While not statistically significant, our data also suggested a relationship between IL-10 variants and IL-10 levels produced by maternal PBMCs. The IL-10 A-1082G polymorphism in young children and their mothers was associated with the first PCA score that represented overall IL-10 levels produced by PBMCs and CBMCs. Furthermore, IL-10 levels produced by maternal PBMCs cultured with infected erythrocytes were associated with an increased risk for clinical malaria in young children.
Three genetic variants (rs1800896, rs1800871, and rs1800872) in the IL-10 gene have been extensively investigated in relation to infectious diseases (9, 27, 34) and allergic conditions (11, 20). We previously identified complete linkage between rs1800871 and rs1800872, and therefore in the present study we excluded rs1800871 and included an additional intronic IL-10 SNP rs1518110 (6). However, the SNP rs1518110 was still in complete linkage with SNP rs1800872. Due to the strong linkage disequilibrium between the three investigated SNPs, the genotype and phenotype investigations for SNP rs1800896 (A-1082G) were the same for the haplotype GCG of the three SNPs (G919T, A-592C, and A-1082G) and the SNP rs1800872 (A-592C) for the haplotype TAA. Therefore, the identified association of A-1082G with IL-10 production and clinical malaria possibly reflected the effect of the IL-10 haplotype GCG.
Several studies have reported associations of IL-10 haplotypes and genotypes with severe malaria (22, 28). To our knowledge, no studies have reported on the association of IL-10 polymorphisms and clinical malaria incidence in young children. In healthy volunteers, the A allele of A-1082G was reported to be associated with decreased IL-10 production in PBMCs stimulated with concanavalin A (25). Another study reported that the haplotype GCC of the three SNPs A-1082G, T-819C, and A-592C was associated with increased circulating concentrations of IL-10 in plasma (22). These findings are not consistent with ours, as the A alleles of A-1082G and A-592C appeared to be associated with increased IL-10 production by CBMCs stimulated with infected erythrocytes. We acknowledge that there are differences in the populations and methods of our study and others (plasma cytokines versus secreted cytokines upon antigen stimulation); however, coexistent environmental factors and population-specific attributes warrant further investigation (31–33).
IL-10 has been suggested to have a key role in immune responses to clinical malaria due to its ability to dampen the proinflammatory response and thus prevent the subsequent development of severe malaria anemia and cerebral malaria (14, 19). Interleukin-12 is critical in providing protective immunity against malaria, and it has been suggested that IL-10 has counterregulatory effects on IL-12 production, thereby diminishing protective immunity to malaria (7, 10). Our study suggested that IL-10 levels produced by maternal PBMCs were associated with an increased risk for clinical malaria in the second year of life. Our design was longitudinal rather than cross-sectional, and increased IL-10 levels produced by maternal PBMCs and CBMCs preceded the susceptibility to clinical malaria in young children. This suggested that the susceptibility to clinical malaria in young children was more likely to be attributed to an increase in IL-10 production than a consequence of this infection. However, this association may be reflecting a relationship between domestic P. falciparum exposure and individual IL-10 production. We adjusted for several confounders that could be related to parasite exposure, such as use of ITN or IRS, and these analyses did not alter the findings. We acknowledge that the latent (unknown) confounders that represent a high risk for P. falciparum exposure may have enhanced the relationship between maternal IL-10 production and the susceptibility to clinical malaria in children. More studies with detailed assessments for malaria exposure will help explain the relationship.
The A allele of A-1082G was associated with higher IL-10 levels in CBMCs and was a risk factor for clinical malaria in young children. The effects of IL-10 production and IL-10 genotypes on clinical malaria were consistent in our population, as both the high levels of IL-10 from maternal PBMCs and the A allele of A-1082G, with the potential to produce more IL-10, were significantly associated with increased risk for clinical malaria. We assumed that the IL-10 production capacity would be transmitted from mothers to young children by the inheritance of genetic and epigenetic factors, thus resulting in a risk for clinical malaria. However, the higher maternal IL-10 production may have represented an acquired maternal immune state that changed the womb environment, imposing a risk for clinical malaria in young children. Moreover, some environmental and other genetic factors have been reported to be associated with IL-10 production and immune responses to malaria, such as mineral deficiency (17), Toll-like receptor 4, and tumor necrosis factor (15, 16). These cofactors may interact synergistically or agonistically to influence malaria susceptibility in young children.
An animal study showed that susceptible BALB/c mice produced more plasmacytoid and less mature DCs and had higher levels of IL-10 and transforming growth factor-β1 than resistant mice in the early stages of Plasmodium yoelii 17XL infection (35). In humans, a cross-sectional study reported that plasma IL-10 levels were elevated in asymptomatic malaria infection compared with those in healthy controls (29). These studies indicated a possible association between increased IL-10 production and P. falciparum infection. Protective immunity facilitates parasite clearance, and higher IL-10 levels have been associated with less effective clearance of P. falciparum parasites (7), partly due to its suppressive effects on immunoprotection in humans. Therefore, we postulate that the associations of maternal IL-10 produced by PBMCs, and the A allele of A-1082G, with the increased risk for clinical malaria in young children, were attributable to the increased IL-10 production with the consequent potential suppressive effects on protective immunity to clinical malaria. Consistent with our findings, there is evidence that Treg proliferation might be causally associated with the suppression of TH1 responses during early malaria infection, thereby increasing parasitemia in BALB/c mice in an IL-10-dependent manner (30).
In conclusion, in this longitudinal prospective study, we identified that IL-10 polymorphisms and IL-10 production capacity were associated with the risk of clinical malaria in young children. High IL-10 production capacity inherited from parents may diminish immunoprotection against malaria infection, thereby being a risk for increased malaria morbidity. Our findings have significantly contributed to the understanding of the immunopathological mediators of malaria in children.
Supplementary Material
ACKNOWLEDGMENTS
We thank all children and their families for their participation in the study, the field workers, field supervisors, laboratory staff, and other staff at CISM for their work during the study, and Laura Puyol and Sònia Tomàs for their support to the study.
The study was funded by a EU Framework Program 6 STREP project (Malaria Age Exposure, project reference no. 18902), the Instituto de Salud Carlos III (Ayuda de incentivación a la participación en proyectos del Espacio Europeo de Investigación), the Spanish Ministerio de Educación y Ciencia (project reference no. A107190024), and NHMRC. G.Z. was supported by NHMRC and BrightSpark Foundation, C.D. was supported by a Ramón y Cajal grant from the Spanish Ministerio de Ciencia e Innovación (RYC-2008-02631), C.G. by a grant from the Spanish Ministry of Health (Contrato post-Formación Sanitaria Especializada, Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, reference no. CM04/00028), and A.M. by a Miguel Servet grant from the Instituto de Salud Carlos III (CP-04/00220). The Manhiça Health Research Centre receives core funding from the Spanish Agency for International Cooperation and Development (AECID).
Footnotes
Published ahead of print 7 May 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Alonso PL, et al. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411–1420 [DOI] [PubMed] [Google Scholar]
- 2. Berthoud TK, et al. 2011. Comparison of commercial kits to measure cytokine responses to Plasmodium falciparum by multiplex microsphere suspension array technology. Malar. J. 10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Couper KN, Blount DG, Riley EM. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 180:5771–5777 [DOI] [PubMed] [Google Scholar]
- 4. Gabriel S, Ziaugra L, Tabbaa D. 2009. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. 2:Unit 2.12 [DOI] [PubMed] [Google Scholar]
- 5. Guinovart C, et al. 2012. The role of age and exposure to Plasmodium falciparum in the rate of acquisition of naturally acquired immunity: a randomized controlled trial. PLoS One 7:e32362 doi:10.1371/journal.pone.0032362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayden CM, et al. 2011. Regulatory role of IL10 genetic variations in determining allergen-induced T(H)2 cytokine responses in children. J. Allergy Clin. Immunol. 128:237–239 [DOI] [PubMed] [Google Scholar]
- 7. Hugosson E, Montgomery SM, Premji Z, Troye-Blomberg M, Bjorkman A. 2004. Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Parasite Immunol. 26:111–117 [DOI] [PubMed] [Google Scholar]
- 8. Jin X, et al. 2012. Association of IL-10-1082 G/G genotype with lower mortality of acute respiratory distress syndrome in a Chinese population. Mol. Biol. Rep. 39:1–4 [DOI] [PubMed] [Google Scholar]
- 9. Karaoglan I, et al. 2009. TNF-alpha, TGF-beta, IL-10, IL-6 and IFN-gamma gene polymorphisms as risk factors for brucellosis. New Microbiol. 32:173–178 [PubMed] [Google Scholar]
- 10. Keller CC, et al. 2006. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect. Immun. 74:5249–5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SH, et al. 2009. Combined effect of IL-10 and TGF-beta1 promoter polymorphisms as a risk factor for aspirin-intolerant asthma and rhinosinusitis. Allergy 64:1221–1225 [DOI] [PubMed] [Google Scholar]
- 12. Kossodo S, et al. 1997. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91:536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurtzhals JA, et al. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768–1772 [DOI] [PubMed] [Google Scholar]
- 14. Li C, Corraliza I, Langhorne J. 1999. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect. Immun. 67:4435–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. May J, Lell B, Luty AJ, Meyer CG, Kremsner PG. 2000. Plasma interleukin-10:tumor necrosis factor (TNF)-alpha ratio is associated with TNF promoter variants and predicts malarial complications. J. Infect. Dis. 182:1570–1573 [DOI] [PubMed] [Google Scholar]
- 16. May L, et al. 2010. Polymorphisms in TLR4 and TLR2 genes, cytokine production and survival in rural Ghana. Eur. J. Hum. Genet. 18:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mbugi EV, et al. 2010. Alterations in early cytokine-mediated immune responses to Plasmodium falciparum infection in Tanzanian children with mineral element deficiencies: a cross-sectional survey. Malar. J. 9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Medina TS, et al. 2011. Increased interleukin-10 and interferon-gamma levels in Plasmodium vivax malaria suggest a reciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar. J. 10:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165–190 [DOI] [PubMed] [Google Scholar]
- 20. Movahedi M, et al. 2008. IL-10, TGF-beta, IL-2, IL-12, and IFN-gamma cytokine gene polymorphisms in asthma. J. Asthma. 45:790–794 [DOI] [PubMed] [Google Scholar]
- 21. Othoro C, et al. 1999. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179:279–282 [DOI] [PubMed] [Google Scholar]
- 22. Ouma C, et al. 2008. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum. Genet. 124:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheet P, Stephens M. 2006. A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am. J. Hum. Genet. 78:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swiatek BJ. 2012. Is interleukin-10 gene polymorphism a predictive marker in HCV infection? Cytokine Growth Factor Rev. 23:47–59 [DOI] [PubMed] [Google Scholar]
- 25. Turner DM, et al. 1997. An investigation of polymorphism in the interleukin-10 gene promoter. Eur. J. Immunogenet. 24:1–8 [DOI] [PubMed] [Google Scholar]
- 26. Wang C, et al. 2012. Relationships between tumour necrosis factor-alpha, interleukin-12B and interleukin-10 gene polymorphisms and hepatitis B in Chinese Han haemodialysis patients. Nephrology (Carlton) 17:167–174 [DOI] [PubMed] [Google Scholar]
- 27. Wang S, Huang D, Sun S, Ma W, Zhen Q. 2011. Interleukin-10 promoter polymorphism predicts initial response of chronic hepatitis B to interferon alfa. Virol. J. 8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson JN, et al. 2005. Analysis of IL10 haplotypic associations with severe malaria. Genes Immun. 6:462–466 [DOI] [PubMed] [Google Scholar]
- 29. Wilson NO, et al. 2010. Elevated levels of IL-10 and G-CSF associated with asymptomatic malaria in pregnant women. Infect. Dis. Obstet. Gynecol. doi:10.1155/2010/317430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y, et al. 2007. Plasmodium yoelii: distinct CD4(+)CD25(+) regulatory T cell responses during the early stages of infection in susceptible and resistant mice. Exp. Parasitol. 115:301–304 [DOI] [PubMed] [Google Scholar]
- 31. Zhang G, Goldblatt J, LeSouef P. 2009. The era of genome-wide association studies: opportunities and challenges for asthma genetics. J. Hum. Genet. 54:624–628 [DOI] [PubMed] [Google Scholar]
- 32. Zhang G, Goldblatt J, LeSouef PN. 2008. Does the relationship between IgE and the CD14 gene depend on ethnicity? Allergy 63:1411–1417 [DOI] [PubMed] [Google Scholar]
- 33. Zhang G, et al. 2009. Opposite gene by environment interactions in Karelia for CD14 and CC16 single nucleotide polymorphisms and allergy. Allergy 64:1333–1341 [DOI] [PubMed] [Google Scholar]
- 34. Zhang LZ, Zhang TC, Pan FM, Zhang ZH, Li X. 2010. Interleukin-10 gene polymorphisms in association with susceptibility to chronic hepatitis C virus infection: a meta-analysis study. Arch. Virol. 155:1839–1842 [DOI] [PubMed] [Google Scholar]
- 35. Zheng W, et al. 2010. Distinct host-related dendritic cell responses during the early stage of Plasmodium yoelii infection in susceptible and resistant mice. Parasite Immunol. 32:324–334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.