Abstract
Filarial lymphatic pathology is of multifactorial origin, with inflammation, lymphangiogenesis, and innate immune responses all playing important roles. The role of Toll-like receptors (TLRs) in the development of filarial pathology is well characterized. Similarly, the association of pathology with elevated levels of plasma angiogenic factors has also been documented. To examine the association between TLR function and the development of lymphangiogenesis in filarial infections, we examined TLR- and filarial antigen-induced expression and production of various angiogenic growth factors. We demonstrate that TLR ligands (specifically TLR2, -3, and -5 ligands) induce significantly increased expression/production of vascular endothelial growth factor A (VEGF-A) and angiopoietin-1 (Ang-1) in the peripheral blood mononuclear cells of individuals with lymphatic pathology (CP individuals) compared to that in cells of asymptomatic infected (INF) individuals. Similarly, filarial antigens induce significantly enhanced production of VEGF-C in CP compared with INF individuals. TLR2-mediated enhancement of angiogenic growth factor production in CP individuals was shown to be dependent on mitogen-activated protein kinase (MAPK) and NF-κB signaling, as pharmacologic inhibition of either extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK, or NF-κB signaling resulted in significantly diminished production of VEGF-A and Ang-1. Our data therefore strongly suggest an important association between TLR signaling and lymphangiogenesis in the development of pathology in human lymphatic filariasis.
INTRODUCTION
Filarial lymphatic pathology, in the form of hydrocele, lymphedema, and elephantiasis, occurs in a subset of filaria-infected individuals (18). Filarial infections are caused by the lymph-dwelling filariae—Wuchereria bancrofti, Brugia malayi, and Brugia timori—and the occurrence of disease is often a reflection of the in situ host-parasite interaction within or around the lymphatics. While the majority of filaria-infected individuals develop parasite antigen-specific CD4+ T cell hyporesponsiveness and interleukin-10 (IL-10)-dominated regulatory responses (14), individuals with pathology exhibit strong proinflammatory immune responses (18). This proinflammatory milieu is thought to promote the development of physiologic abnormalities of the lymphatic vasculature that result in lymphangiectasia, lymph vessel hyperplasia, and lymphangiogenesis (5, 10, 21). Lymphatic dilatation, in contrast, is likely an early event related to the parasite (and its secreted products) and may be independent of the host adaptive immune responses (5).
Toll-like receptors (TLRs) are important mediators of inflammatory processes in a variety of conditions, including infectious diseases (15). Indeed, TLR-mediated responses are considered important factors in induction and establishment of filarial lymphatic pathology (21, 26). Studies with animal models of filarial infection (12, 22) and in vitro studies of humans (6, 11, 25) have suggested that TLR-mediated responses in filarial infections are key inducers of proinflammatory cytokines, which contribute either directly or indirectly to development of lymphatic pathology. Downregulation of TLR on antigen-presenting cells and T cells has been shown to be a possible mechanism by which deleterious pathology in clinically asymptomatic filarial infections can be circumvented (23).
Angiogenic growth factors are vascular factors that are important mediators of angiogenesis and lymphangiogenesis (17). The two main families responsible for these angiogenic effects are those belonging to the vascular endothelial growth factor (VEGF) family and the angiopoietins. The VEGF family comprises five members, including VEGF-A, a potent regulator of angiogenesis, and VEGF-C, a key mediator of lymphangiogenesis (17). The angiopoietin family includes four ligands (Ang-1, Ang-2, Ang-3, and Ang-4). The best characterized are Ang-1—shown to induce lymphatic vessel enlargement, sprouting, and proliferation—and Ang-2—which can function either agonistically or antagonistically, depending on its dose and context (27). One consequence of stimulation of the innate response is production of VEGFs, possibly by macrophages and endothelial cells. Indeed, these factors have been shown to be elevated following filarial infections (3, 8, 9). Recent studies have implicated VEGF-mediated responses in the development of lymphedema and hydrocele (8, 9).
Because VEGFs have been shown to be upregulated by proinflammatory cytokines, and because TLR- and/or filarial antigen-mediated proinflammatory cytokine induction is a characteristic feature of chronic filarial lymphedema, we examined the expression and production of the major angiogenic growth factors (VEGF-A, -B, -C, and -D and Ang-1 and -2) in response to TLR or filarial antigen stimulation both in patients with filarial lymphedema and in those with subclinical (or asymptomatic) infection. Our findings reveal an important association between TLR- and filarial antigen-mediated production of VEGF-A, VEGF-C, and Ang-1 and the presence of overt filarial lymphatic pathology.
MATERIALS AND METHODS
Study population.
All individuals were examined as part of a clinical protocol to study the natural history of lymphatic filariasis and associated immune responses, approved by the Institutional Review Boards of both the National Institute of Allergy and Infectious Diseases and the Tuberculosis Research Center and registered at http://www.clinicaltrials.gov (trial NCT00001230). Informed written consent was obtained from all participants.
We studied a group of 34 unrelated patients with filarial lymphedema (CP patients) and 34 asymptomatic infected (INF) patients in an area in Tamil Nadu, South India, where lymphatic filariasis is endemic (Table 1). The first 42 subjects (equally divided between the CP and INF groups) were studied prospectively for responses to TLR agonists. For 10/21 CP subjects, there were sufficient numbers of cells to perform studies on pharmacological inhibition of cell signaling. The remaining 26 subjects (equally divided between the INF and CP groups) were then studied for their responses to filarial antigen.
Table 1.
Characteristics of the study population
| Characteristic | Value or descriptiona |
|
|---|---|---|
| CP individuals (n = 34) | INF individuals (n = 34) | |
| Median age (range) | 48 (20–60) | 44 (15–73) |
| Gender (no. of males/no. of females) | 18/16 | 21/13 |
| Lymphedema/elephantiasis | Yes | No |
| ICT card test | Negative | Positive |
| W. bancrofti circulating antigen level (U/ml) (GM [range]) | <32b | 2,814 (132–32,768) |
| IgG level (μg/ml) (GM [range]) | 44.32 (10.6–393) | 58.12 (5.1–3134) |
| IgG4 level (ng/ml) (GM [range]) | 165.2 (2.1–654.8) | 5,238 (98–36,836) |
CP individuals, patients with filarial pathology; INF individuals, patients with asymptomatic filarial infection.
Below the limit of detection.
CP patients were circulating filarial antigen negative by both the ICT filarial antigen test (Binax, Portland, ME) and the TropBio Og4C3 enzyme-linked immunosorbent assay (ELISA) (TropBio Pty. Ltd., Townsville, Queensland, Australia), indicating a lack of current active infection. They had previously undergone treatment with repeated doses of diethylcarbamazine. INF patients tested positive by both the ICT filarial antigen test and the TropBio Og4C3 ELISA, as well as by Brugia malayi adult worm extract (BmA)-specific IgG4 and IgG ELISAs. INF patients were not examined for the presence of microfilaremia and had not undergone any antifilarial treatment. BmA-specific IgG4 and IgG ELISAs were performed exactly as described previously (16). The two groups did not differ significantly in baseline hematologic and immunologic parameters, including T, B, and NK cell counts.
Reagents.
The TLR ligands (Invivogen, San Diego, CA) used were as follows: TLR2 ligands, Pam3CysSerLys4 (Pam3Cys) (0.5 μg/ml) and heat-killed Listeria monocytogenes (HKLM) (108 cells/ml); TLR3 ligand, poly(IC); TLR4 ligand, ultrapure lipopolysaccharide (LPS) (0.5 μg/ml); TLR5 ligand, Salmonella enterica serovar Typhimurium flagellin; and TLR9 ligand, CpG ODN 2006 (0.5 μM).
Parasite antigens.
Saline extracts of BmA and microfilariae (Mf) were used as the parasite antigens. The final concentration was 10 μg/ml for both BmA and Mf. The endotoxin levels of the final soluble BmA and Mf preparations were <0.1 endotoxin unit (EU)/ml as determined using a QCL-1000 chromogenic LAL test kit (BioWhittaker, Walkersville, MD).
Isolation of PBMCs and in vitro culture.
Peripheral blood mononuclear cells (PBMCs) were isolated as described previously (2). PBMCs were cultured in 24-well tissue culture plates (Corning Incorporated, Corning, NY) at a concentration of 5 × 106/well for 24 h.
ELISA.
Levels of VEGF-A and -C and Ang-1 and -2 in the culture supernatants were measured according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). Net levels were calculated by subtracting growth factor levels in the medium control for each stimulated condition.
RNA preparation.
RNA was isolated from PBMCs following culture with TLR ligands for 24 h. PBMCs were lysed using the reagents of a commercial kit (QIAshredder; Qiagen, Valencia, CA). Total RNA was extracted according to the manufacturer's protocol (RNeasy minikit; Qiagen), and RNA was dissolved in 50 ml of RNase-free water.
cDNA synthesis.
RNA (1 μg) was used to generate cDNA by use of TaqMan reverse transcription (RT) reagents according to the manufacturer's protocol (Applied Biosystems, Fullerton, CA). Briefly, random hexamers were used to prime RNA samples for reverse transcription using MultiScribe reverse transcriptase.
Real-time RT-PCR.
Real-time quantitative RT-PCR was performed in an ABI 7500 sequence detection system (Applied Biosystems), using TaqMan Assays-on-Demand reagents for VEGF-A, -B, -C, and -D and an endogenous 18S rRNA control. Relative transcript levels were determined according to the manufacturer's protocol.
Pharmacologic inhibition of signaling pathways.
The pharmacologic inhibitors used were U0126 (extracellular signal-regulated kinase 1/2 [ERK1/2] inhibitor), SB203580 (p38 mitogen-activated protein kinase [MAPK] inhibitor), and Bay 11-7082 (NF-κB inhibitor), all from CalBiochem Biosciences (San Diego, CA). They were reconstituted in sterile, cell culture-grade dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). For inhibition experiments, PBMCs from CP patients were first pretreated with 20 μM U0126, 30 μM SB203580, or 20 μM Bay 11-7082 or with DMSO alone for 60 min before culture with TLR ligands for 24 h, and supernatants were collected. The efficacy of the inhibitors in terms of inhibition of phosphorylation or activation of a specific molecule was tested independently.
Statistical analysis.
Geometric means (GM) were used as the measure of central tendency. Comparisons were made using the Mann-Whitney U test followed by Holm correction for multiple comparisons or the Wilcoxon signed rank test. All statistics were performed using GraphPad Prism, version 5, for Windows (GraphPad Software, Inc., San Diego, CA).
RESULTS
Baseline expression of angiogenic growth factors is not significantly altered in filarial pathology.
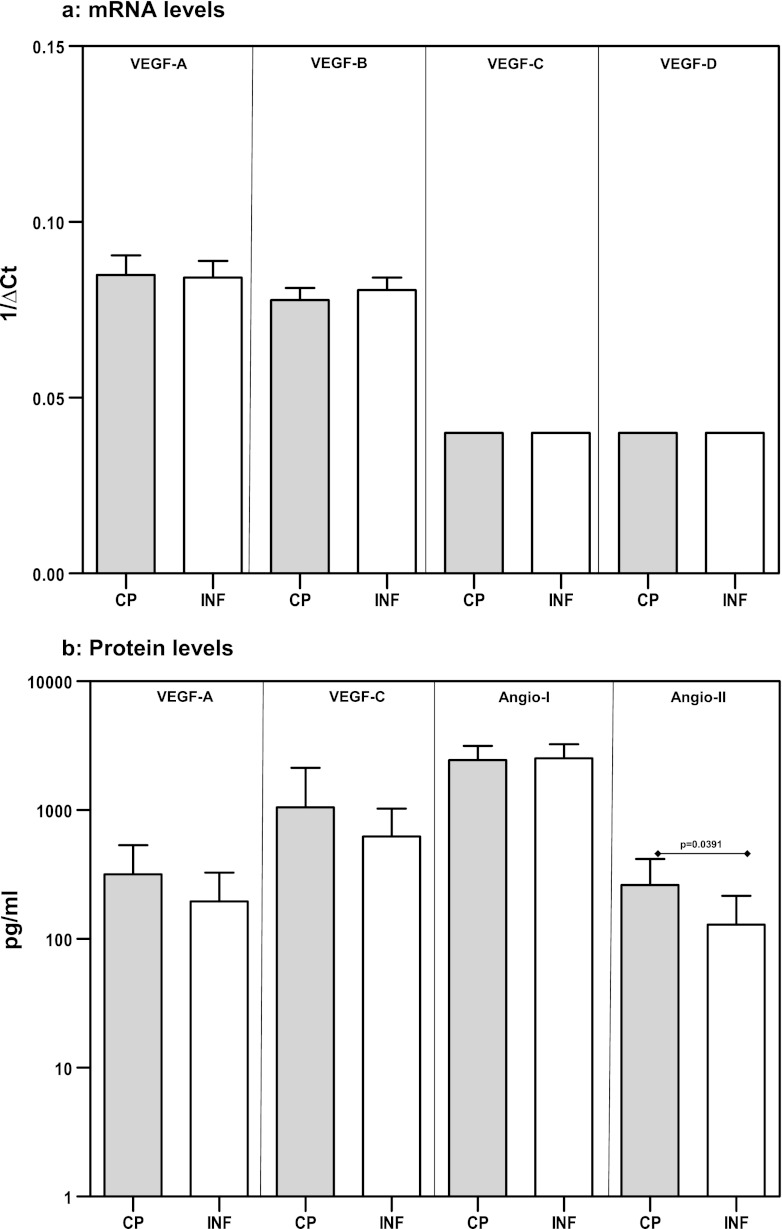
Because expression of VEGF by PBMCs is not well defined, we first examined the expression patterns of VEGF-A, -B, -C, and -D in PBMCs by real-time RT-PCR. We found that PBMCs expressed detectable levels of all of the VEGF genes examined. Next, we compared the baseline expression of VEGF-A, -B, -C, and -D in PBMCs from CP individuals and INF individuals. As shown in Fig. 1a, we did not detect any significant difference in the mRNA levels of these genes between the two groups. To further characterize the role of VEGF proteins, we measured the levels of VEGF-A, -C, and -D at baseline in CP and INF individuals. While VEGF-D was undetectable, we did not find any significant differences in the levels of VEGF-A and -C between the two groups (Fig. 1b). Finally, we also examined the baseline production of Ang-1 and Ang-2 in CP and INF individuals and found that only Ang-2 was detected at significantly higher levels in CP individuals than in INF individuals (GM of 261.9 pg/ml in CP individuals versus 129.4 pg/ml in INF individuals; P = 0.0391). Thus, baseline expression and/or production of angiogenic growth factors (with the exception of Ang-2) is not significantly elevated in CP individuals compared with INF individuals.
Fig 1.
Baseline levels of VEGF mRNA and VEGF and angiopoietin proteins in filarial pathology. PBMCs from individuals with chronic pathology (CP) and from infected (INF) individuals (n = 21 each) were cultured with medium alone for 24 h, and mRNA expression of VEGF-A, -B, -C, and -D (by RT-PCR) (a) and protein expression of VEGF-A and -C and Ang-1 and −2 (by ELISA) (b) were measured. Data are shown as relative transcript levels (a) or baseline protein levels (b). Data are shown in the form of bar graphs depicting the geometric means + 95% confidence intervals.
TLR ligand stimulation induces increased expression of VEGF-A mRNA in filarial pathology.
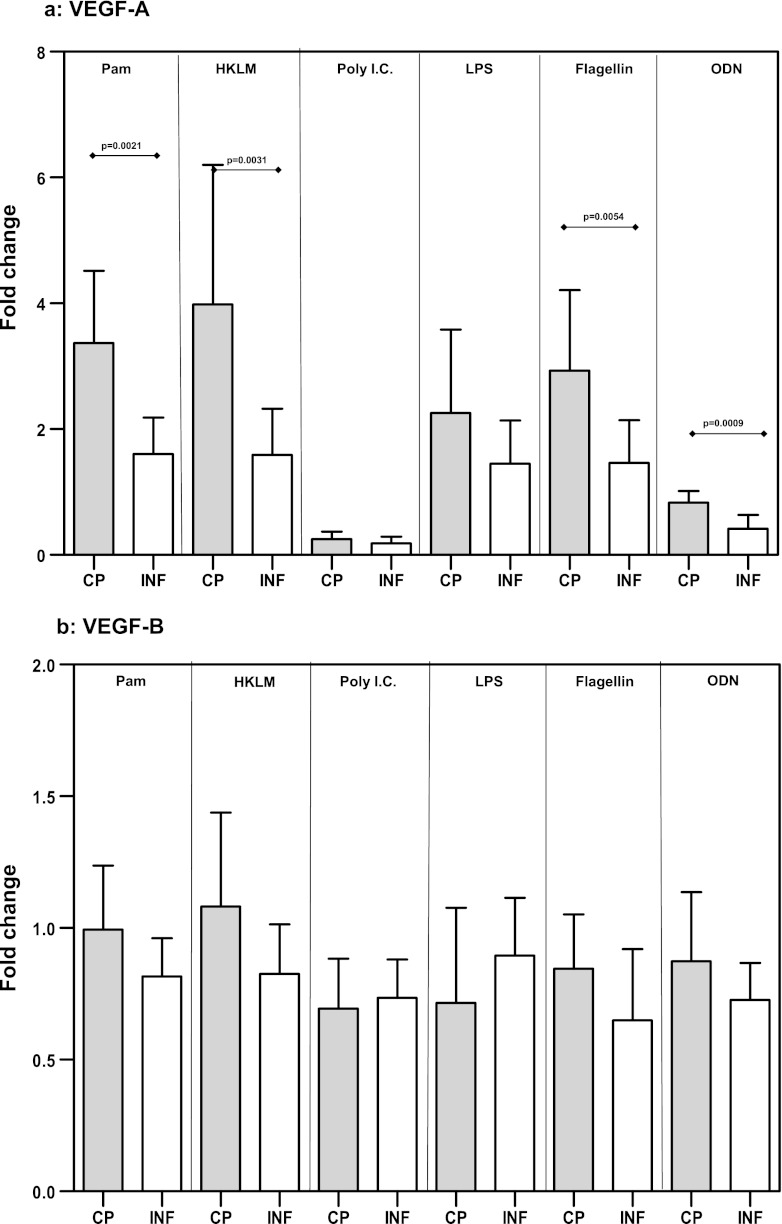
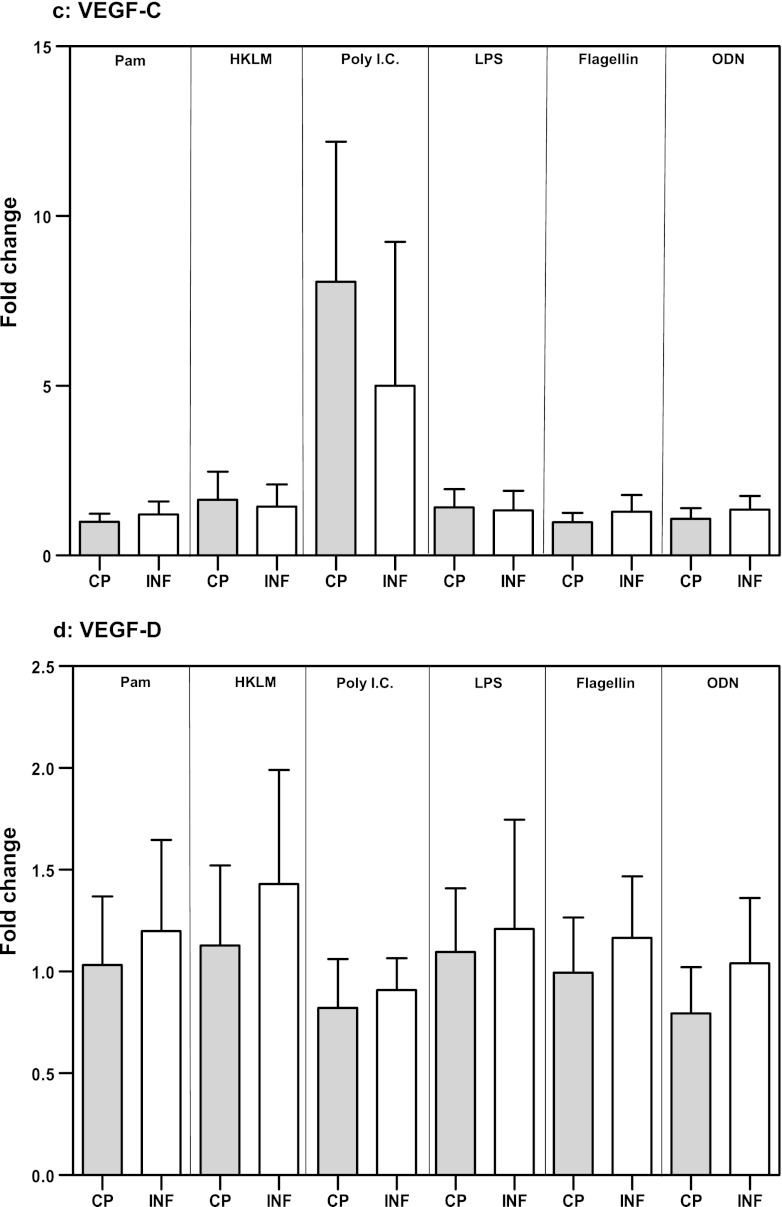
Because the effect of TLR stimulation on VEGF gene expression in PBMCs is not known, we examined the mRNA levels of VEGF-A, -B, -C, and -D in response to TLR2, -3, -4, -5, and -9 agonists. While the expression levels of VEGF-B (Fig. 2b), VEGF-C (Fig. 2c), and VEGF-D (Fig. 2d) were not significantly different between CP and INF individuals, we detected significantly increased expression of VEGF-A mRNA in response to the TLR2 ligands Pam3Cys (GM change of 3.4-fold in CP individuals versus 1.6-fold in INF individuals; P = 0.0021) and HKLM (GM change of 3.9-fold versus 1.6-fold; P = 0.0031), the TLR5 ligand flagellin (GM change of 2.9-fold versus 1.5-fold; P = 0.0054), and the TLR9 ligand CpG ODN 2006 (GM change of 0.83-fold versus 0.42-fold; P = 0.0009) in CP individuals compared with INF individuals (Fig. 2a). Our data therefore demonstrate that altered expression of VEGF-A mRNA is associated with filarial lymphatic pathology.
Fig 2.
TLR ligand stimulation induces increased expression of VEGF-A mRNA in filarial pathology. PBMCs from individuals with chronic pathology (CP) and from infected (INF) individuals (n = 21 each) were stimulated with Pam3Cys, HKLM, poly(IC), LPS, flagellin, or CpG ODN 2006 for 24 h, and the mRNA levels of VEGF-A (a), VEGF-B (b), VEGF-C (c), and VEGF-D (d) were measured by RT-PCR. Data are expressed as fold changes over the medium control level. Data are shown in the form of bar graphs depicting the geometric means + 95% confidence intervals.
TLR ligand stimulation induces increased production of VEGF-A and Ang-1 in filarial pathology.
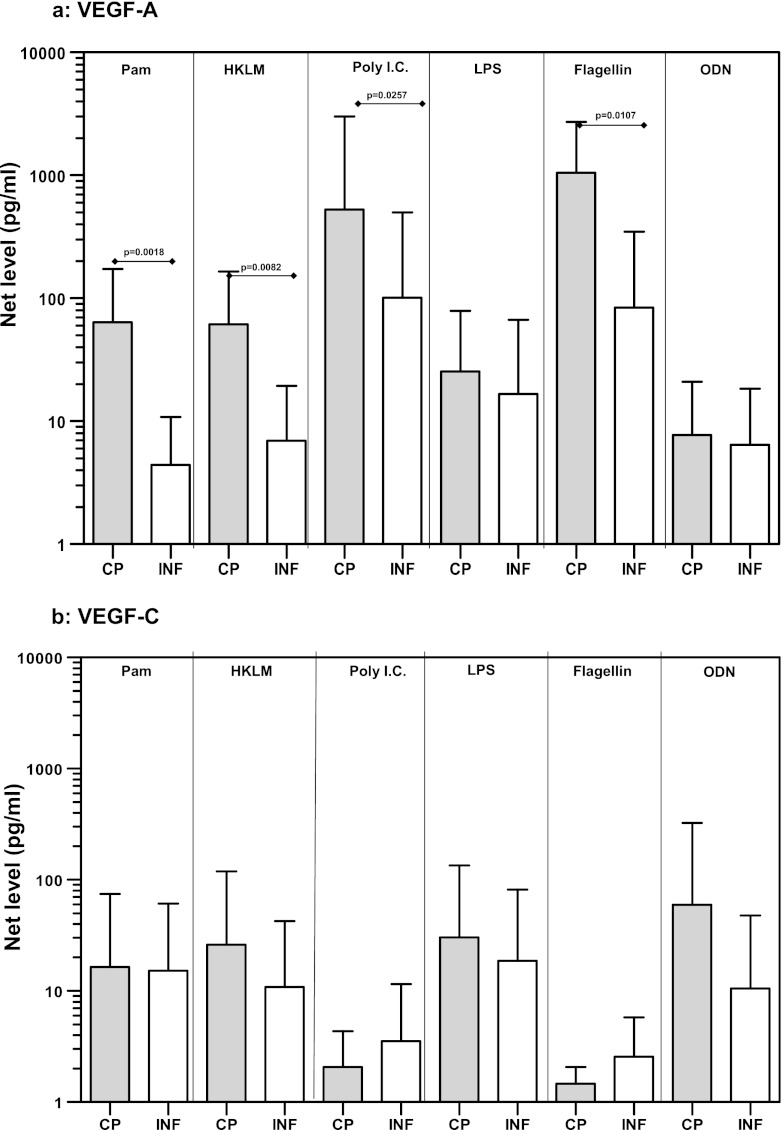
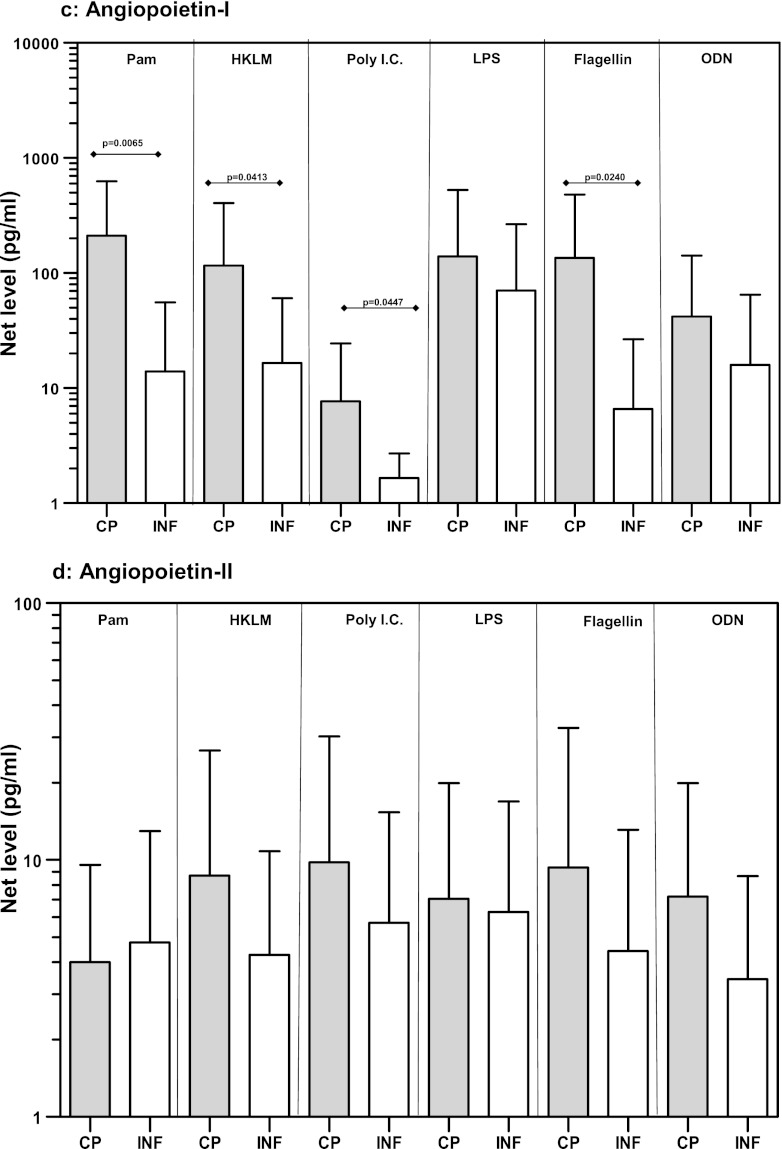
Because TLR stimulation resulted in increased expression of VEGF gene expression, and because baseline production of Ang-2 was elevated in CP individuals, we next examined the protein levels of VEGF-A and -C as well as Ang-1 and Ang-2 in response to TLR2, -3, -4, -5, and -9 ligands. While the expression levels of VEGF-C (Fig. 3b) and Ang-2 (Fig. 3d) were not significantly different between CP and INF individuals, we detected significantly increased production of VEGF-A in response to the TLR2 ligands Pam3Cys (GM of 64 pg/ml in CP individuals versus 4.4 pg/ml in INF individuals; P = 0.0018) and HKLM (GM of 61.4 versus 6.9 pg/ml; P = 0.0082), the TLR3 ligand poly(IC) (GM of 528.3 versus 101.5 pg/ml; P = 0.0257), and the TLR5 ligand flagellin (GM of 1,051 versus 84 pg/ml; P = 0.0107) in CP individuals compared with INF individuals (Fig. 3a). Similarly, we noted significantly increased production of Ang-1 in response to the TLR2 ligands Pam3Cys (GM of 211.2 pg/ml in CP individuals versus 13.9 pg/ml in INF individuals; P = 0.0065) and HKLM (GM of 116 versus 16.5 pg/ml; P = 0.0413), the TLR3 ligand poly(IC) (GM of 7.7 versus 1.7 pg/ml; P = 0.0447), and the TLR5 ligand flagellin (GM of 135 versus 6.6 pg/ml; P = 0.0240) in CP individuals compared to INF individuals (Fig. 3c). Our data therefore demonstrate that altered protein levels of VEGF-A and Ang-1 are associated with filarial lymphatic pathology.
Fig 3.
TLR ligand stimulation induces increased production of VEGF-A and Ang-1 in filarial pathology. PBMCs from individuals with chronic pathology (CP) and from infected (INF) individuals (n = 21 each) were stimulated with Pam3Cys, HKLM, poly(IC), LPS, flagellin, or CpG ODN 2006 for 24 h, and the protein levels of VEGF-A (a), VEGF-C (b), Ang-1 (c), and Ang-2 (d) were measured by ELISA. Data are expressed as net levels, with medium control levels subtracted. Data are shown in the form of bar graphs depicting the geometric means + 95% confidence intervals.
Filarial antigen stimulation induces increased production of VEGF-A and -C during filarial pathology.
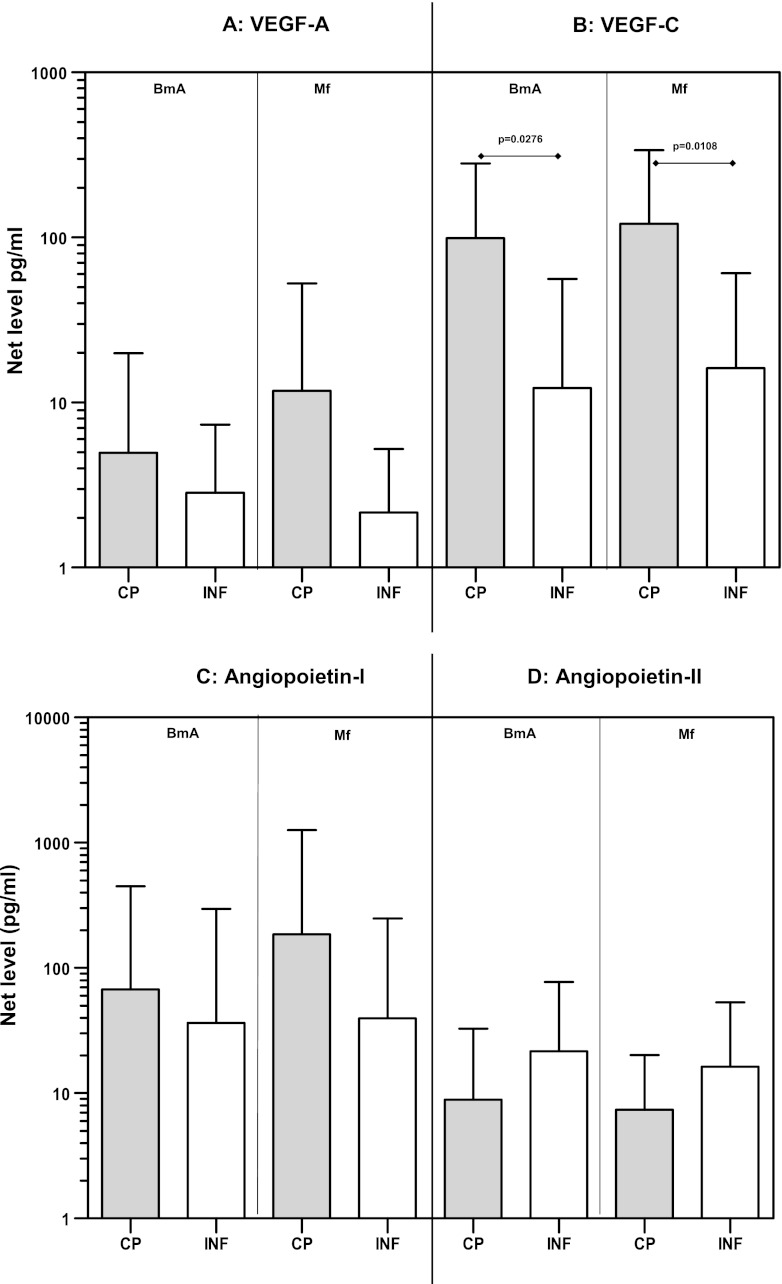
Because TLR stimulation resulted in increased production of VEGF-A and Ang-1, we wanted to examine whether filarial antigens could also induce production of angiogenic growth factors and whether such production was associated with the outcome of filarial infection. While the levels of VEGF-A (Fig. 4A), Ang-1 (Fig. 4C), and Ang-2 (Fig. 4D) were not significantly different between CP and INF individuals upon stimulation with either BmA or Mf antigen, we detected significantly increased production of VEGF-C in response to both BmA (GM of 99.1 versus 12.3 pg/ml; P = 0.0276) and Mf (GM of 121.6 versus 16.2 pg/ml; P = 0.0108) antigens in CP individuals compared with INF individuals (Fig. 4B). Our data therefore suggest that production of angiogenic growth factors associated with filarial lymphatic pathology could also be induced directly by filarial antigens.
Fig 4.
Filarial antigen stimulation induces increased production of VEGF-A and -C in filarial pathology. PBMCs from individuals with chronic pathology (CP) and from infected (INF) individuals (n = 13 each) were stimulated with BmA and Mf antigens for 24 h, and the protein levels of VEGF-A (A), VEGF-C (B), Ang-1 (C), and Ang-2 (D) were measured by ELISA. Data are expressed as net levels, with medium control levels subtracted. Data are shown in the form of bar graphs depicting the geometric means + 95% confidence intervals.
TLR2 ligand-induced VEGF-A and Ang-1 production is dependent on phosphorylation of p38 MAPK and ERK1/2 and on NF-κB activation.
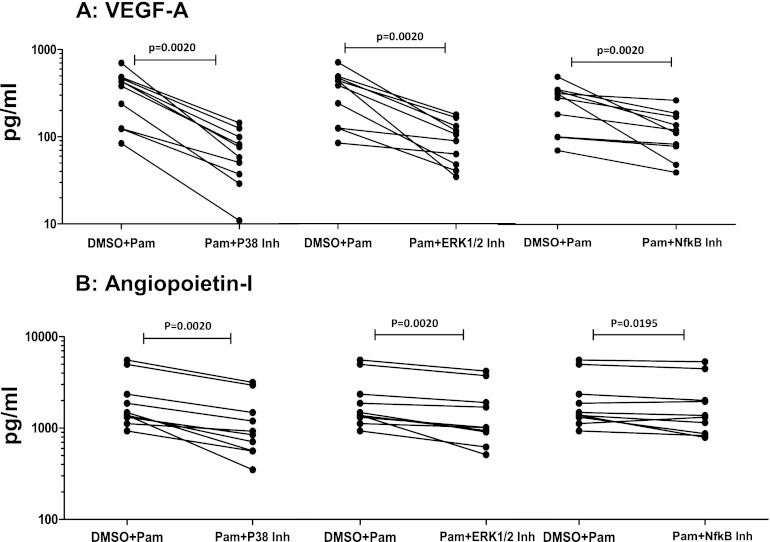
Because TLR stimulation has been shown to signal by phosphorylation of p38 MAPK and ERK1/2 and through activation of NF-κB (20), we sought to determine whether the angiogenic growth factor production observed in response to TLR2 agonists was dependent on these signaling pathways. We therefore utilized pharmacologic inhibitors of p38 MAPK, ERK1/2, and NF-κB activation to inhibit these signaling molecules before stimulating PBMCs from CP individuals with Pam3Cys. As shown in Fig. 5A, inhibition of the p38 MAPK, ERK1/2, and NF-κB pathways resulted in significantly decreased production of VEGF-A in response to Pam3Cys (P = 0.0020 for all). Similarly, as shown in Fig. 5B, inhibition of the above pathways resulted in significantly decreased production of Ang-1 in response to Pam3Cys (P = 0.0020 for p38 and ERK inhibition; P = 0.0195 for NF-κB inhibition). Thus, the p38 MAPK and ERK1/2 pathways, as well as NF-κB activation, play an important role in mediating the angiogenic growth factor response to TLR ligands in CP individuals.
Fig 5.
Decreased angiogenic growth factor production in filarial pathology by inhibition of p38 MAPK, ERK, and NF-κB signaling pathways. PBMCs from individuals with chronic pathology (CP) (n = 10) were cultured with inhibitors (Inh) of the p38 (SB203580), ERK (U0126), and NF-κB (Bay 11-7082) signaling pathways or with DMSO alone for 1 h prior to stimulation with a Pam3Cys control for 24 h, and the production of VEGF-A (A) and Ang-1 (B) was measured by ELISA. Data are shown as net levels in the presence of inhibitor or the DMSO control, with each line representing a single individual.
DISCUSSION
Among the 120 million people worldwide who are infected with lymphatic filarial parasites, a subset exhibit demonstrable clinical pathology, characterized most notably by lymphedema, hydrocele, and elephantiasis (18). The events that lead to development of lymphatic pathology in filariasis are not fully understood, although host-parasite interactions, parasite products, and opportunistic secondary infections are all believed to play significant roles in determining the development of pathology (21). TLRs are important initiators of immune responses through their ability to recognize a variety of microbial products (24), but if they are unregulated, TLR-dependent proinflammatory cascades can also escalate to cause severe pathology (19). It has been shown previously that TLR-dependent proinflammatory cytokine production—most notably of Th1 and Th17 cytokines—is strongly associated with chronic pathology in lymphatic filariasis (1).
A potential mechanism by which proinflammatory cytokines could mediate lymphatic damage is through their ability to induce production of various angiogenic and lymphangiogenic factors. This, in turn, could lead to perturbations in maintenance and function of the lymphatic endothelial system, resulting in a variety of complications, including lymphatic dilatation and lymphedema (5, 21). Indeed, the presence of elevated levels of lymphangiogenic factors has been shown to be associated with the severity of lymphatic pathology (3, 8, 9); however, no data are available on whether there is a direct association between TLR stimulation and angiogenic growth factor production in filarial pathology. In addition, filarial antigens themselves have been shown to induce proliferation and differentiation of human lymphatic endothelial cells in vitro (4). The VEGFs and angiopoietins are important regulatory growth factors for vascular and lymphatic endothelial function (17). While the VEGF family of proteins can promote both angiogenesis and lymphangiogenesis by interacting with cognate receptors (VEGFR-1, -2, and -3) on endothelial cells, Ang-1 and Ang-2 can interact with Tie-2 receptors and contribute to maturation and stabilization of the vasculature (17, 27). Although Ang-1 is implicated primarily in vascular stabilization, it has been shown to cause endothelial cell proliferation and differentiation (27). There is a dearth of literature on the different cell populations that can produce angiogenic growth factors per se, with even more limited data on the nature of this response to TLR stimulation. We therefore first established that PBMCs do have the capacity to produce these growth factors, although the exact cell population(s) that is responsible for the production of these factors is under investigation. Next, our examination of TLR stimulation demonstrated clearly delineated responses in individuals with overt clinical pathology compared with asymptomatic infected individuals. We have previously shown that expression as well as function (in the form of activation and cytokine production) of TLR2 is significantly enhanced in CP individuals compared with INF individuals (1). Here we demonstrated that TLR2 stimulation also leads to significantly enhanced VEGF-A and Ang-1 production, further confirming the importance of TLR2 in development of clinical filarial pathology. In addition, we also implicate a role for TLR3 and TLR5 in the activation of the angiogenic vascular network that might underlie the development of lymphatic complications in lymphedema. We can envision a couple of mechanisms (not mutually exclusive) by which TLR stimulation could influence angiogenic growth factor production in PBMCs: (i) a direct effect on VEGF and angiopoietin production and/or (ii) an indirect effect due to TLR-induced proinflammatory cytokine production. Proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and IL-17, are known inducers of VEGF proteins (13), and hence an indirect effect of TLR stimulation on angiogenic growth factor production cannot be excluded.
TLR signaling involves activation of the MAPK family of molecules, specifically ERK1/2, Jun N-terminal kinase (JNK), and p38 (24). Enhanced p38 MAPK and ERK1/2 phosphorylation, as well as NF-κB activation, has been associated with increased TLR-mediated cytokine responses (20). Therefore, we examined the roles of these MAP kinases as well as NF-κB activation in the observed immune response to TLR stimulation in CP individuals. Our data obtained using pharmacologic inhibition of the above-mentioned pathways clearly indicate that the elevated angiogenic growth factor production in CP patients is dependent on the ERK1/2 and MAPK pathways as well as on NF-κB activation. This provides a mechanistic framework in which TLR (and perhaps filarial antigen) stimulation could promote lymphangiogenesis that ultimately results in lymphatic pathology. While the use of TLR ligands in this study serves as a useful model for studying the effects of filarial products and bystander antigens on the innate immune response, it does not recapitulate the exact effects of parasite stimulation. Hence, we also examined whether filarial antigens of the relevant life cycle stages (adult worms and Mf) in humans have any effect on angiogenic growth factor production. We demonstrated that filarial antigens induce differential production of VEGF-C, illustrating certain similarities and (perhaps) differences between filarial antigen and TLR stimulation. The fact that filarial antigens induce significantly elevated levels of VEGF-C also strengthens previous data suggesting that this important angiogenic/lymphangiogenic factor plays a crucial role in lymphatic pathology. In addition, preliminary data indicate that TLR- and filarial antigen-induced VEGF production is also significantly higher in CP individuals than in uninfected individuals in areas where the disease is endemic (data not shown), reinforcing the importance of these factors in pathogenesis.
Inhibition of the VEGF-VEGFR and Ang-Tie systems is gaining importance in the fields of tumor immunology, vascular diseases, and other inflammatory disorders in terms of maximizing the efficacy of anti- and proangiogenic therapies (7). Our data suggest that targeting of these pathways, as well as new treatment regimens involving TLR modulation, could potentially provide new avenues in ameliorating pathology in chronic lymphatic filariasis. In addition, our data also reveal a new link between innate immune responses (in the form of TLR activation) and angiogenic/lymphangiogenic pathways (in the form of elevated growth factors), thereby providing additional insight into the pathways connecting the immune system and normal physiologic processes such as lymphatic circulation.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank NIAID intramural editor Brenda Rae Marshall for assistance.
Because S.B. and T.B.N. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside the United States subject to a government use license.
Footnotes
Published ahead of print 16 April 2012
REFERENCES
- 1. Babu S, et al. 2009. Filarial lymphedema is characterized by antigen-specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl. Trop. Dis. 3:e420 doi:10.1371/journal.pntd.0000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. 2006. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 176:3248–3256 [DOI] [PubMed] [Google Scholar]
- 3. Bennuru S, Maldarelli G, Kumaraswami V, Klion AD, Nutman TB. Elevated levels of plasma angiogenic factors are associated with human lymphatic filarial infections. Am. J. Trop. Med. Hyg. 83:–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennuru S, Nutman TB. 2009. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLoS Pathog. 5:e1000688 doi:10.1371/journal.ppat.1000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennuru S, Nutman TB. 2009. Lymphatics in human lymphatic filariasis: in vitro models of parasite-induced lymphatic remodeling. Lymphat. Res. Biol. 7:215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brattig NW, et al. 2004. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 173:437–445 [DOI] [PubMed] [Google Scholar]
- 7. Carmeliet P, Jain RK. 2011. Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Debrah AY, et al. 2009. Reduction in levels of plasma vascular endothelial growth factor-A and improvement in hydrocele patients by targeting endosymbiotic Wolbachia sp. in Wuchereria bancrofti with doxycycline. Am. J. Trop. Med. Hyg. 80:956–963 [PubMed] [Google Scholar]
- 9. Debrah AY, et al. 2006. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2:e92 doi:10.1371/journal.ppat.0020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Figueredo-Silva J, Noroes J, Cedenho A, Dreyer G. 2002. The histopathology of bancroftian filariasis revisited: the role of the adult worm in the lymphatic-vessel disease. Ann. Trop. Med. Parasitol. 96:531–541 [DOI] [PubMed] [Google Scholar]
- 11. Hartgers FC, et al. 2008. Enhanced Toll-like receptor responsiveness associated with mitogen-activated protein kinase activation in Plasmodium falciparum-infected children. Infect. Immun. 76:5149–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hise AG, et al. 2007. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J. Immunol. 178:1068–1076 [DOI] [PubMed] [Google Scholar]
- 13. Honorati MC, Cattini L, Facchini A. 2004. IL-17, IL-1β and TNF-α stimulate VEGF production by dedifferentiated chondrocytes. Osteoarthritis Cartilage 12:683–691 [DOI] [PubMed] [Google Scholar]
- 14. King CL, Nutman TB. 1991. Regulation of the immune response in lymphatic filariasis and onchocerciasis. Immunol. Today 12:A54–A58 [DOI] [PubMed] [Google Scholar]
- 15. Kopp E, Medzhitov R. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15:396–401 [DOI] [PubMed] [Google Scholar]
- 16. Lal RB, Ottesen EA. 1988. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J. Infect. Dis. 158:1034–1037 [DOI] [PubMed] [Google Scholar]
- 17. Lohela M, Bry M, Tammela T, Alitalo K. 2009. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 21:154–165 [DOI] [PubMed] [Google Scholar]
- 18. Nutman TB, Kumaraswami V. 2001. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 23:389–399 [DOI] [PubMed] [Google Scholar]
- 19. O'Neill LA. 2008. When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity 29:12–20 [DOI] [PubMed] [Google Scholar]
- 20. O'Neill LA, Bowie AG. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7:353–364 [DOI] [PubMed] [Google Scholar]
- 21. Pfarr KM, Debrah AY, Specht S, Hoerauf A. 2009. Filariasis and lymphoedema. Parasite Immunol. 31:664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saint Andre A, et al. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892–1895 [DOI] [PubMed] [Google Scholar]
- 23. Semnani RT, Nutman TB. 2004. Toward an understanding of the interaction between filarial parasites and host antigen-presenting cells. Immunol. Rev. 201:127–138 [DOI] [PubMed] [Google Scholar]
- 24. Takeda K, Kaisho T, Akira S. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376 [DOI] [PubMed] [Google Scholar]
- 25. Turner JD, et al. 2009. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J. Biol. Chem. 284:22364–22378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venugopal PG, Nutman TB, Semnani RT. 2009. Activation and regulation of Toll-like receptors (TLRs) by helminth parasites. Immunol. Res. 43:252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X, Liu N. 2010. The role of Ang/Tie signaling in lymphangiogenesis. Lymphology 43:59–72 [PubMed] [Google Scholar]