Abstract
Summary: Invasive infections caused by Streptococcus pneumoniae continue to be a major cause of morbidity and mortality worldwide, especially in children under 5 years of age. In the United States, 90% of invasive pneumococcal infections in children are caused by 13 serotypes of S. pneumoniae. The licensure (in 2000) and subsequent widespread use of a heptavalent pneumococcal conjugate vaccine (PCV7) have had a significant impact on decreasing the incidence of serious invasive pneumococcal disease (IPD) in all age groups, especially in children under 2 years of age. However, the emergence of replacement non-PCV7 serotypes, especially serotype 19A, has resulted in an increase in the incidence of serious and invasive infections. In 2010, a 13-valent PCV was licensed in the United States. However, the impact that this vaccine will have on IPD remains to be seen. The objectives of this review are to discuss the epidemiology of serious and invasive pneumococcal infections in the United States in the PCV era and to review some of the pneumococcal vaccines that are in development.
INTRODUCTION
Invasive infections caused by Streptococcus pneumoniae continue to be a major cause of morbidity and mortality worldwide, especially in children under 5 years of age. In 2000, there were an estimated 14.5 million serious infections and over 825,000 deaths (11% of all-cause deaths) in this population (86). The organism is a major cause of serious invasive diseases, such as meningitis, bacteremia, and pneumonia, with young children in the first 2 years of life and adults aged ≥65 years being particularly susceptible (1). Of the 92 different pneumococcal serotypes (grouped into 46 serogroups based on immunologic similarities) that have been identified based on antigenic differences in their capsular polysaccharides, 10 serogroups account for most pediatric invasive pneumococcal infections worldwide, with serogroups 1, 3, 6, 14, 19, and 23 being the most common (45). The amounts of disease caused by these serotypes vary over time, with population age and ethnicity, and with geography. In the United States, 90% of invasive pneumococcal infections in children are caused by 13 serotypes of S. pneumoniae: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F (14, 21, 116). These 13 serotypes also account for 80% to 90% of antibiotic-resistant pneumococcal strains in most U.S. reports and in reports from other parts of the world (30, 50, 65). Prior to the licensure and recommendation of the heptavalent pneumococcal conjugate vaccine (PCV7) for all U.S. children aged 2 to 23 months and for older children at increased risk for invasive pneumococcal disease (IPD), approximately 65,000 cases of IPD occurred annually among all age groups; 25% of the IPD episodes occurred in children of <5 years of age (96). In the United States, the licensure (in 2000) and subsequent widespread use of PCV7 have had a significant impact on decreasing the incidence of serious IPD in all age groups, especially in children under 2 years of age. However, the emergence of replacement nonvaccine pneumococcal serotypes, especially serotype 19A, has resulted in an increase in the incidence of serious and invasive infections. The scope of disease caused by these emerging serotypes has not been defined fully. In 2010, a 13-valent pneumococcal conjugate vaccine (PCV13) was licensed in the United States. This vaccine contains the same seven serotypes found in PCV7, with an additional six serotypes (1, 3, 5, 6A, 7F, and 19A) that account for 63% of the IPD currently being seen in the pediatric population (32, 85). However, the impact that this vaccine will have on IPD remains to be seen. The objectives of this review are to discuss the epidemiology of serious and invasive pneumococcal infections in the United States in the PCV era and to review some of the pneumococcal vaccines that are in development.
PNEUMOCOCCAL ORGANISMS
Streptococcus pneumoniae organisms (pneumococci) are alpha-hemolytic, lancet-shaped, Gram-positive, catalase-negative diplococci. They are fastidious, growing best in 5% carbon dioxide, and require a source of catalase (e.g., blood) to grow on agar plates. In liquid media, S. pneumoniae replicates in chains; on solid media, pneumococci form alpha-hemolytic colonies, with a depression in the center of each colony. Pneumococci produce pneumolysin, which breaks down hemoglobin into a green pigment, causing the colonies to be surrounded by a green zone during growth on blood agar plates. On chocolate agar, pneumococcal colonies are surrounded by a greenish yellow pigment. They are susceptible to optochin, soluble in bile salts, and bile esculin negative. More than 90 pneumococcal serotypes have been identified on the basis of unique polysaccharide capsules, which are produced in various amounts and give the colonies a mucoid appearance. The organism also expresses several virulence factors which play a major role in its ability to cause disease. These have been the subject of multiple reviews and are not discussed further here (76, 77).
Capsular Switching
The capsule of the pneumococcal organism is one of the major determinants of organism invasiveness. It is also the target for serotype-specific disease prevention by vaccine, but the effectiveness of a vaccine may be impacted by the organism's ability to switch the serotype of its capsule. Capsular switching occurs when the genes encoding one capsular serotype are exchanged, via transformation and recombination, with the genes encoding a different capsular serotype. The most concerning of the capsular switches is the vaccine-to-nonvaccine serotype switch, during which a vaccine serotype capable of causing invasive disease acquires the capsule of a nonvaccine serotype; this contributes to serotype replacement disease through the development of “vaccine escape” serotypes. Studies have shown that the emergence of several of the clones of replacement serotype 19A developed through the process of capsular switching. The development of vaccine escape serotypes by genetic recombination at the capsular locus has the potential to significantly reduce the long-term effectiveness of pneumococcal conjugate vaccines (12, 23, 24, 87).
EPIDEMIOLOGY OF INVASIVE PNEUMOCOCCAL DISEASE
Invasive pneumococcal disease is defined as infection of any normally sterile body site. It occurs most commonly in children under the age of 5 years, especially those under 2 years of age (14, 21, 116). This age group is particularly susceptible to infections partly as the result of an immature immune response and frequent exposures to and colonizations by S. pneumoniae. During the first 2 to 3 months of life, full-term infants have some protection against pneumococcal infections through the passive transfer of maternal antibodies. Of the invasive forms of pneumococcal disease, meningitis is seen most frequently in children between the ages of 6 and 18 months, while bacteremia occurs most commonly between the ages of 6 and 36 months. The majority of pneumococcal bone and joint infections are seen between the ages of 3 and 34 months, while the majority of cases of pneumococcal pneumonia occur in children between 3 and 60 months of age (90, 116). Although pneumonia in itself is not usually thought of as an invasive infection, up to 25% of cases have an associated bacteremia (11, 67, 80, 113). Given that pneumonia is a potentially serious infection, it is discussed in this review.
Prior to the licensure of PCV7, a study conducted in Southern California found that the overall incidence of IPD was 12.5 cases per 100,000 persons per year, with higher incidence rates observed for both the very young and the very old (116). As noted in the study, the annual incidence of IPD in children of <2 years of age was 145 cases per 100,000 individuals, compared with 72 and 32 per 100,000 individuals for children of <5 years of age and adults of >65 years of age, respectively. In this study, over 95% of the invasive disease included bacteremia, while the incidence of meningitis (with or without bacteremia) was 0.8 case per 100,000 persons. Seventy-nine percent of the isolates were from children of 2 years of age or younger. The pneumococcal serotypes isolated most frequently from children of <2 years of age were 14, 23F, 19F, 6B, 6A, 9V, 18C, 4, and 19A (116).
The impact of widespread PCV7 use on IPD in all age groups has been substantial. A study using population-based data from the Active Bacterial Core Surveillance (ABCs) of the Centers for Disease Control and Prevention (CDC) that examined the burdens of invasive pneumococcal disease (defined by the isolation of S. pneumoniae from a normally sterile body fluid) pre- and post-PCV7 licensure demonstrated a substantial drop in the rate of IPD, from an average of 24.3 cases per 100,000 persons in the prevaccine years (1998 and 1999) to 17.3 cases per 100,000 persons after the vaccine's introduction in 2001. The largest decline was seen in children under 2 years of age, for whom the rate of disease decreased 69%, from 188 cases per 100,000 individuals to 59 cases per 100,000 individuals; the rates of disease caused by vaccine and vaccine-related serotypes declined by 78% and 50%, respectively. In this group, the magnitude of decline was larger for black children than for white children. Disease rates also fell in the adult population, with decreases of 32% for adults of 20 to 39 years of age (11.2 cases per 100,000 individuals to 7.6 cases per 100,000 individuals), 8% for those of 40 to 64 years of age (21.5 cases per 100,000 individuals to 19.7 cases per 100,000 individuals), and 18% for those of 65 years of age and older (60.1 cases per 100,000 individuals to 49.5 cases per 100,000 individuals). The rate of disease caused by penicillin-nonsusceptible isolates dropped by 35% compared to that in the prevaccine year 1999 (6.3 cases per 100,000 individuals to 4.1 cases per 100,000 individuals) (49, 113). Similar declines have been reported by other large surveillance networks (8, 54, 58). This decline was further reflected in a study from 2003 in which there was a significant decrease in the rate of hospital discharge for patients admitted with IPD, declining from a peak of 12.03 discharges per 100,000 individuals in 1999 to 5.6 discharges per 100,000 individuals (P < 0.001) (102).
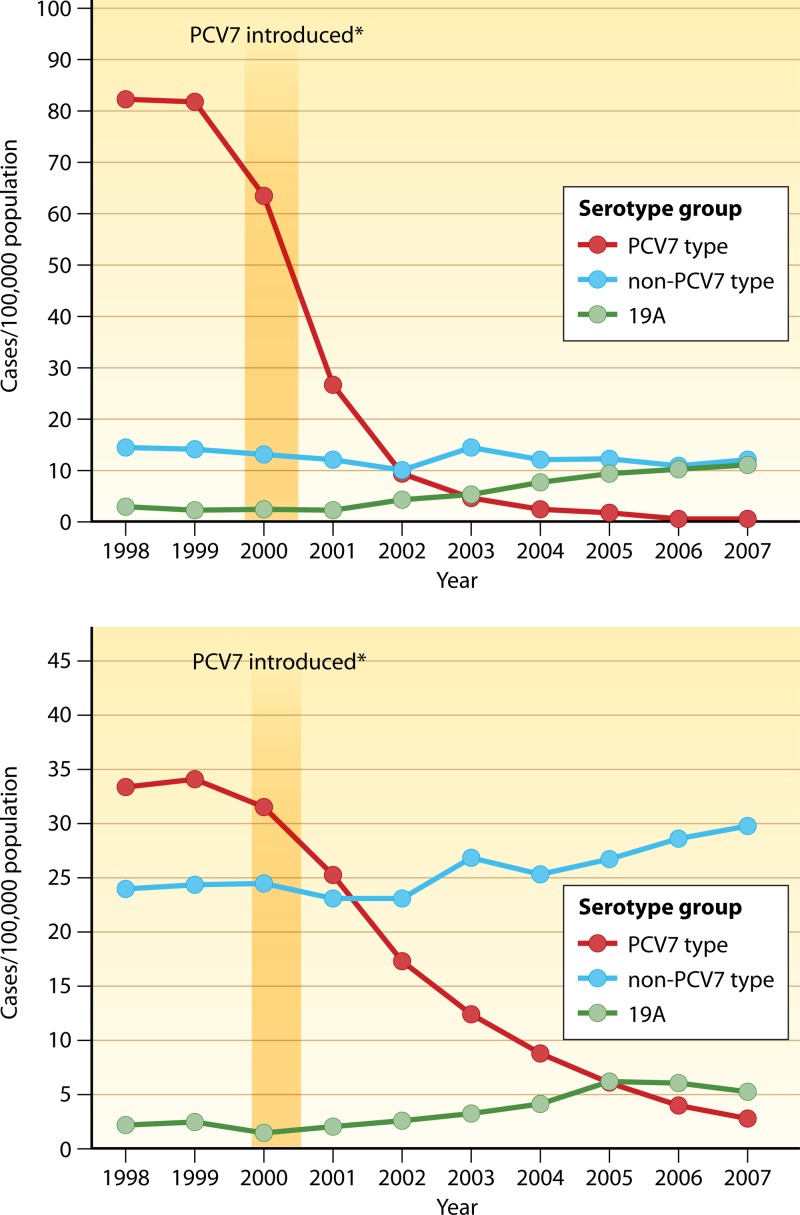
In 2004, further drops in the rates of IPD in all age groups were seen, and the overall rate of disease was noted to be 12.6 cases/100,000 individuals. The most significant decrease was again found to be among children younger than 2 years of age. In this population, there was a marked decrease in the rate of disease caused by vaccine serotypes; this rate decreased from an average of 78.9 cases/100,000 individuals pre-vaccine licensure (1998 to 1999) to 2.7 cases/100,000 individuals, a decrease of over 65%. Marked decreases in the rates of IPD were also observed in older children, adults, and the elderly. By 2007, the incidence of PCV7-type IPD had decreased by 99%, and the incidence of all IPD had decreased by 76% in children younger than 5 years of age. In adults of ≥65 years of age, IPD caused by PCV7 serotypes decreased 92%, and all-serotype invasive disease decreased by 37% compared to the rate pre-vaccine licensure (Fig. 1) (88). The reductions in IPD in these groups show the significant indirect benefits of PCV7 immunization through the interruption of transmission of pneumococci from children to adults (49, 66, 88). PCV7 use has also led to significant decreases in age-specific mortality rates across all age groups, further supporting the benefits of vaccine-induced herd immunity in the population (94).
Fig 1.
Changes in invasive pneumococcal disease incidence (1998 to 2007) by serotype among U.S. children aged <5 years (top) and adults aged ≥65 years (bottom). (Adapted from reference 88 by permission of the Infectious Diseases Society of America.)
However, over the same period, the annual rate of IPD due to nonvaccine serotypes increased from an average of 16.3 cases/100,000 individuals in the prevaccine years (1998 and 1999) to 19.9 cases/100,000 individuals in 2004 for children aged <5 years and from 27 cases/100,000 individuals during the prevaccine years to 29.8 cases/100,000 individuals in 2004 for adults aged ≥65 years. Disease caused by nonvaccine serotypes accounted for 17% of all IPD cases during the prevaccine years, compared to 88% of cases in 2004. Serotypes 3, 7F, 19A, 22F, and 33F and serogroup 15 were the predominant replacement serotypes that were seen in children under 5 years of age, with serotypes 3, 7F, and 19A accounting for 95% of IPD cases (21). The overall rate of IPD caused by vaccine serotypes among adults of 65 years of age and older decreased from 61.5 cases/100,000 individuals in 1998 and 1999 to 38 cases/100,000 individuals in 2004. However, the rate of disease caused by nonvaccine serotypes, which accounted for 44% of IPD cases in 1998 and 1999, increased to 78% in 2004 (49, 66, 88).
Serotype Replacement
Serotype replacement is defined as a decrease in the prevalence of PCV7 serotype pneumococci accompanied by a corresponding increase in non-PCV7 serotype pneumococci, which fill the ecological niche formerly occupied by the vaccine serotypes. Recent studies have shown that replacement serotypes 19A, 1, 5, 7F, and 33F and serogroup 15 have accounted for the most significant increases in IPD (21, 49, 66), with serotype 19A being recognized as the predominant replacement invasive pneumococcal serotype in the United States (37, 46, 59, 74, 79). The predominant replacement serotypes that are seen in children under 5 years of age are also the replacement serotypes seen in adults of 65 years of age and older.
There have been multiple reasons postulated, in addition to the impact of PCV7, to explain the dominance of serotype 19A replacement disease. Antibiotic usage patterns have been postulated to be one of the major contributing factors. In countries with little to no PCV7 use but high rates of antibiotic use, especially for antibiotics in the macrolide class, a significant increase in the amount of IPD caused by serotype 19A has been seen. This is further supported by findings from a dynamic compartmental transmission model of the pneumococcus which was developed to further study the reasons behind the increase in antibiotic-resistant serotype 19A disease and to estimate the impact of vaccines or changes in antibiotic use on future IPD incidence in those under 2 years of age in the United States. The model suggests that high rates of antibiotic usage play a major role in increasing IPD caused by antibiotic-resistant serotype 19A strains. Based on these findings, antibiotic usage patterns may have significantly impacted pneumococcal serotype distribution and contributed to the emergence of serotype 19A replacement disease (9, 81, 110). Other possible reasons include clonal expansion, with the emergence of a single predominant serotype 19A clone, recombinant capsular switching of other serotypes to 19A, development of significant antibiotic resistance by this serotype, and the lack of cross-protection by PCV7-induced antibodies for serotype 19F (79).
In contrast, among particular populations, the impact of PCV7 on IPD has not been as dramatic, and the emergence of serotype replacement disease by pneumococcal serotype 19A has not been observed. As an example, in the White Mountain Apache (WMA) population in Arizona, despite the introduction of PCV7, high rates of IPD in PCV7-vaccinated children under 5 years of age continue to be seen. This is attributed to the fact that the 7 serotypes covered in the vaccine account for only 56.2% of the IPD cases among WMA children, compared to about 80% to 90% of cases in the general population. It is interesting that serotype 19A replacement disease has not been observed in this population. In fact, the rate of serotype 19A IPD in the WMA population has been stable or has decreased since vaccine introduction. The reasons for these differences are not understood, but they demonstrate the wide variability in the coverage of PCV7 that has been observed worldwide and emphasize the geographic variability of pneumococcal serotype distribution (63).
Antibiotic Resistance among Replacement Serotypes
Even though the overall incidence of invasive disease caused by penicillin-nonsusceptible, erythromycin-resistant, and multidrug-resistant pneumococcal vaccine strains decreased significantly after the introduction of PCV7, there was a simultaneous marked increase in disease caused by antibiotic-resistant nonvaccine replacement serotypes of pneumococci (33, 62). Seven serotypes (6A, 6B, 9V, 14, 19A, 19F, and 23F) account for most of the clinically significant drug-resistant strains of Streptococcus pneumoniae. Five of these serotypes (6B, 9V, 14, 19F, and 23F) are included in PCV7, and all of these serotypes are included in PVC13. Only serotype 19A was unaffected by PCV7, and this has allowed the spread of drug-resistant and multidrug-resistant serotype 19A strains worldwide (26, 87). In the United States, the percentages of penicillin- and erythromycin-nonsusceptible serotype 19A isolates among children aged <5 years increased from 63% and 23%, respectively, in 1998 and 1999 to 74% and 46%, respectively, in 2004. Also, the percentage of strains resistant to levofloxacin has been increasing steadily (27, 49, 62, 74, 91). Over the same period, the rate of IPD due to penicillin-nonsusceptible serotype 19A strains increased from 2 to 8.3 cases/100,000 individuals in children under 2 years of age and from 1.3 to 2.2 cases/100,000 individuals in adults of ≥65 years of age (62).
Transmission and Carriage of S. pneumoniae
Streptococcus pneumoniae is part of the normal pharyngeal flora in healthy children and adults. The rates and duration of colonization are influenced by a number of factors, including age, race, exposure to cigarette smoke, and day care attendance (17, 34). Nasopharyngeal colonization usually is acquired around 6 months of age but can occur as early as the first week of life, and although the average length of carriage is 3 to 4 months, some individuals (especially young infants) may harbor pneumococci for over a year. At any given time, over 75% of infants and young children are colonized, with the highest rates occurring in children who live in institutions and in those who attend day care. Colonization rates decrease with age, dropping to 25% for teenagers and to 2% to 9% for adults who are not routinely exposed to young children (34). The differences in colonization rates between children and adults have been attributed to the gradual development of serotype-specific antibodies in response to multiple exposures to various pneumococcal serotypes that occurs with increasing age.
Nasopharyngeal colonization is a prerequisite for invasive disease, with the transition from asymptomatic carriage to invasive disease occurring through hematogenous spread or direct extension from the site of colonization. Infection occurs most often after acquisition of a new pneumococcal strain, and studies have shown that 15% of children who acquire a new strain become ill with acute otitis media (AOM) or other types of IPD within 1 month of the acquisition (38).
Immunization with PCV7 has had a beneficial indirect effect of decreasing nasopharyngeal colonization of vaccine serotypes in vaccinees and in adult and unvaccinated child household contacts. This in turn has led to a decrease in rates of IPD among these individuals. However, studies have shown that this has also led to an increase in nasopharyngeal carriage of nonvaccine serotypes, whose potential for IPD is not completely known and in which antibiotic resistance is increasing (42, 43, 52, 53, 75, 83). The effect of PCV13 on nasopharyngeal colonization is expected to be similar to that seen with PCV7.
CLINICAL MANIFESTATIONS OF INVASIVE PNEUMOCOCCAL DISEASE
Risk Factors Associated with Invasive Disease
Prior to the introduction of PCV7, the highest rates of IPD were seen in children under 2 years of age (96). Factors that increase the risk for pneumococcal disease include chronic medical conditions (e.g., congenital or acquired humoral immunodeficiency, human immunodeficiency virus infection, absent or deficient splenic function, abnormal innate immune responses, cochlear implants, or cerebrospinal fluid [CSF] leak), belonging to certain racial and ethnic groups (black, Native Alaskan, and American Indian populations), and attendance in group child care (2, 96). A case-control study performed by Pilishvili and colleagues to evaluate risk factors for IPD among children aged 3 to 59 months in the era of PCV7 found that children continued to be at increased risk for vaccine-type IPD when they had underlying illnesses, were male, or had no health care coverage, while vaccination with PCV7 reduced the risk for vaccine-type IPD that was associated with race and group child care attendance. For non-vaccine-type IPD, risk factors included the presence of underlying illnesses, attendance in group child care, male gender, low socioeconomic status, and asthma (89).
Pneumococcal Meningitis
Meningitis is the most serious manifestation of IPD and most often results from dissemination of the organism to the meninges via the bloodstream. Prior to PCV7, the incidence of meningitis in children under the age of 2 years was 6.6 cases per 100,000 individuals (101). Since the introduction of a Haemophilus influenzae type b conjugate vaccine, population-based studies of bacterial meningitis indicate that S. pneumoniae has emerged as the leading cause of bacterial meningitis in the United States (70, 101). Patients with pneumococcal meningitis may experience a wide range of disease findings, from a gradual onset of symptoms, progressing over a period of several days from nonspecific upper respiratory manifestations to the appearance of overt disease manifestations, to a fulminant disease course that can result in death less than 24 h after the onset of symptoms. Despite early diagnosis, appropriate antibiotic therapy, and intensive medical care, significant levels of morbidity and mortality continue to be associated with the disease. Neurologic sequelae have been detected in 25% to 56% of survivors, and death can be expected in 5% to 15% of cases, especially if disease is caused by specific serotypes which may be associated with more severe disease (5, 100).
After the introduction of PCV7, rates of pneumococcal meningitis and hospitalizations decreased substantially among children and adults (58, 109, 113). Findings from a recent study which examined trends in pneumococcal meningitis from 1998 through 2005, using active, population-based surveillance data from 8 sites in the United States, identified 1,379 cases of pneumococcal meningitis during the study period. The study found that the rate of pneumococcal meningitis declined significantly overall, from 1.13 cases to 0.79 case per 100,000 individuals for 1998 to 1999 and 2004 to 2005, respectively, representing a 30.1% decline (P < 0.001). The greatest decreases were seen among persons younger than 2 years of age and those of 65 years of age or older, for whom the incidence decreased by 64% and 54%, respectively (P < 0.001 for both). Overall, the rate of PCV7 serotype meningitis decreased from 0.66 case per 100,000 individuals to 0.18 case per 100,000 individuals among patients of all ages, i.e., a 73.3% decline (P < 0.001), with decreases ranging from 92.8% for children of <2 years of age to 61.6% among persons aged 40 to 64 years. The rate of PVC7 serotype-related disease for all age groups decreased by 32.1% (P = 0.08), from 0.14 case to 0.10 case per 100,000 individuals. However, in all age groups, the rate of non-PCV7 serotype-related disease increased significantly, from 0.32 to 0.51 case per 100,000 individuals for 1998 to 1999 and 2004 to 2005, respectively, representing an increase of 60.5% (P < 0.001). The most significant increases were seen in the percentages of cases caused by serotypes 19A, 22F, 11A, and 35B. Overall, the incidence of meningitis caused by isolates that were nonsusceptible to penicillin, meropenem, and cefotaxime decreased significantly between 1998 to 1999 and 2004 to 2005. All isolates were susceptible to vancomycin, and 99% were susceptible to rifampin and levofloxacin. As a whole, the proportion of penicillin-nonsusceptible isolates decreased between 1998 and 2003, from 32% to 19.4% (P = 0.01), but was noted to increase between 2003 and 2005, from 19.4% to 30.1% (P = 0.03), presumably due to an increase in cases caused by nonvaccine strains. Decreased susceptibility was most common among isolates of serotypes 15A (62.5%), 19A (60.7%), and 35B (69.6%). For the study period, even with the decline in the incidence of cases, the case fatality rate was 8.4% among children and 22.3% among adults (109). Information from another surveillance study showed that 20% of all meningitis cases were caused by nonvaccine serotypes 6A, 7F, 22, 15, and 33 (51).
Pneumococcal Bacteremia
For decades, high rates of pneumococcal bacteremia have been reported in very young children (28, 55, 64). Prior to PCV7, in the United States, the annual incidence of pneumococcal bacteremia in children of ≤4 years of age was estimated to be 242 cases per 100,000 individuals, with approximately 72% of these cases occurring in infants younger than 12 months of age. The incidence of pneumococcal bacteremia has also been shown to vary geographically and among certain ethnic groups, with rates as high as 1,195 cases per 100,000 individuals in Native Alaskan children under 2 years of age (28, 55).
Bacteremia may occur in association with meningitis, pneumonia, or septic arthritis; it may occur concurrently with localized disease, such as acute otitis media, or in the absence of any focal findings. Approximately 3% to 5% of febrile children between the ages of 3 and 36 months are at risk for asymptomatic or occult bacteremia, and 85% to 95% of these cases were caused by S. pneumoniae in the prevaccine era (57, 64). However, identifying these children is problematic, as symptoms may be nonspecific (e.g., decreased activity levels, reduced appetite, or irritability). Given that bacteremia is diagnosed on the basis of isolation of S. pneumoniae from blood cultures, diagnosis in these cases is generally made because blood cultures were obtained prior to initiation of antibiotic therapy. Although an increased peripheral white blood cell (WBC) count (≥15,000/mm3) or absolute neutrophil count (≥10,000/mm3) has been associated with the risk of asymptomatic pneumococcal bacteremia, it has limited value as a predictor of this disease because these signs may be indicative of any type of infection (61). Historically, the concern with missing the diagnosis of occult bacteremia was the fear of progression from bacteremia to focal or systemic infections, especially meningitis and sepsis.
The impact of PCV7 on pneumococcal bacteremia has been substantial. Studies of adults and children have shown that rates of bacteremia alone have decreased by at least 55%, especially in patients of ≥65 years of age (92, 102, 113). Herz et al. reported an 84% reduction of S. pneumoniae bacteremia following the implementation of routine vaccination with PCV7 in children aged 3 to 36 months who were seen in Northern California Kaiser Permanente outpatient clinics and emergency departments (48). Carstairs et al. reported that of 1,428 patients under 36 months of age presenting to an emergency room for evaluation of fever who had blood cultures obtained, 0% (0/833 patients) of those who had received a least one dose of PCV7 versus 2.4% (13/550 patients) of unimmunized patients had a positive blood culture for S. pneumoniae (18). However, the incidence of bacteremia caused by vaccine-related serotypes and nonvaccine serotypes has been reported to be increasing since the introduction of PCV7 (58, 107). Kaplan et al. reported that nonvaccine serotypes causing invasive disease in children younger than 24 months of age were observed to have increased 28% the year following introduction of PCV7 and 66% in the subsequent year (58), while Steenhoff et al. found that the percentage of episodes of vaccine-related pneumococcal bacteremia had increased from 6% of bacteremic episodes in the prevaccine era to 35% of these episodes following PCV7 introduction (107).
Pneumococcal Pneumonia
Pneumonia caused by S. pneumoniae is a serious worldwide health concern in infants and children, accounting for an estimated 1 million deaths among children of <5 years of age in developing countries (72, 86). Predisposing factors for pneumococcal pneumonia include immunodeficiency, crowded living conditions, chronic lung diseases, sickle cell disease, nephrotic syndrome, hematologic malignancies, chronic inhalation of smoke, and malnutrition. A higher incidence of disease is reported among certain racial groups, such as American Indians, Native Alaskans, Australian Aborigines, and blacks, than among Caucasians (72). Bacteremia may be associated with 20% to 40% of all cases of pneumococcal pneumonia (55, 80). The case fatality rate for pneumococcal pneumonia is lower for children than for adults (1% versus >35%, respectively) (70). The clinical presentation of pneumococcal pneumonia in children (especially infants and younger children) is broad, ranging from mild, nonspecific respiratory symptoms that can be managed on an outpatient basis to severe respiratory distress requiring mechanical ventilation. Pneumococci classically produce a lobar pneumonia that is characterized on physical examination by decreased breath sounds and/or rales over the affected lobe(s), with dullness to percussion. Chest radiograph demonstrates evidence of consolidation of one or more lung lobes. Bacteremic pneumococcal pneumonia may have a complicated course, with respiratory failure, pleural effusions, pleural empyema, and meningitis being the most common complications (72). Results from one study demonstrated that the risk of death in patients with bacteremic pneumococcal pneumonia varies depending on the infecting serotype. Among bacteremic pneumonia patients, patients with pneumonia caused by serotypes 3, 6A, 6B, 9N, 19A, 19F, and 23F were more likely to die than patients infected with serotype 14, while patients infected with serotypes 1, 4, 5, 7F, and 8 were less likely to die than patients infected with serotype 14 (112).
PCV7 has had a modest impact on the incidence of pneumococcal pneumonia. In a study performed by Northern California Kaiser Permanente, PCV7 was 32.2% effective at reducing episodes of pneumonia in infants in the first year of life and 23.4% effective in children during the first 2 years of life but only 9.1% effective in children of >2 years of age, presumably because of the wide range of serotypes that may be associated with pneumonia in the older age groups (7). A Cochrane review of 11 publications that examined the efficacy of PCV to prevent vaccine serotype IPD and X-ray-proven pneumonia found that the pooled vaccine efficacy for preventing X-ray-defined pneumonia was 27% and that for clinically diagnosed pneumonia was 6% for children under 2 years of age. From this information, it was concluded that PCV is effective at preventing X-ray-defined pneumonia and, to some extent, clinical pneumonia among children under 2 years of age (69). Studies have also shown that in the post-PCV7 era, there have been decreases in hospitalizations and ambulatory care visits for all-cause pneumonia (20, 39, 84, 117).
However, in contrast, there has been an increase in the incidence of childhood pneumonia complicated by empyema and an increase in empyema-related hospitalizations (40, 47, 68, 108). Analysis of Nationwide Inpatient Sample and Census data indicated that all-cause pneumonia complicated by empyema among children aged ≤2 years and those aged 2 to 4 years increased 2.01-fold, from 3.5 cases per 100,000 children in 1996 to 1998 to 7 cases per 100,000 children in 2005 to 2007, and 2.81-fold, from 3.7 cases per 100,000 children in 1996 to 1998 to 10.3 cases per 100,000 children in 2005 to 2007, respectively, with a 2.17-fold overall increase in the rate of pneumococcal pneumonia complicated by empyema (40). This is further supported by information from the Kids' Inpatient Database, which showed that among children of ≤18 years of age, the annual empyema-associated hospitalization rate increased from 2.2 cases per 100,000 children in 1997 to 3.7 cases per 100,000 children in 2006, an increase of almost 70% (68). Therefore, despite major decreases in the rates of IPD and pneumococcal pneumonia, PCV7 has not had an impact on the incidence of empyema. This may be explained in part by the fact that non-PCV7 serotypes 1, 3, and 19A now account for the majority of pneumonia cases being seen (59).
Pneumococcal Bone and Joint Infections
S. pneumoniae is the cause of up to 4% of all bacterial bone infections and up to 20% of all bacterial joint infections in children. Bone and joint infections represent approximately 3% to 6% of all IPD cases (10, 56, 97). The femur and humerus are the bones most commonly affected, and in some cases, the vertebrae may be involved; the most commonly involved joints include the knee and hip (10). Up to 50% of children with these infections will have septic arthritis associated with osteomyelitis, and nearly half of all children with bone and joint infections will also be bacteremic (10). Polyarticular disease and bacteremia are more common among adults with septic arthritis caused by S. pneumoniae than among adults with other causative organisms. One large study found that the mortality in patients with septic arthritis may be up to 19% among adults versus 0% among children, with pneumococcal bacteremia being the strongest predictor of mortality (97). Following the introduction of PCV7, bone and joint infections caused by pneumococci in children decreased by an estimated 50% compared to pre-PCV7 levels (58).
PNEUMOCOCCAL VACCINES
Table 1 shows the current pneumococcal vaccines that are licensed in the United States and/or other parts of the world and some of the vaccines that are in development.
Table 1.
Licensed pneumococcal vaccines and vaccines in development
| Status | Manufacturer | Type of vaccinea | Reference |
|---|---|---|---|
| Licensed | Pfizer | 7-valent conjugate (PCV7) | |
| Pfizer | 13-valent conjugate (PCV13) | 32 | |
| Licensed (not in United States) | GSK | 10-valent conjugate with nontypeable H. influenzae protein carrier | 6 |
| In development | Merck | 15-valent conjugate (adjuvanted and nonadjuvanted) | 106 |
| GSK | Pht proteins | 35 | |
| Sanofi-Pasteur | Recombinant PspA | 82 | |
| Intercell | Recombinant subunit vaccine (3 conserved proteins) | 35 | |
| Genocea | Protein-based vaccine | 77 | |
| Genocea | PspA-Lactococcus lactis vaccine delivery vehicle | 43 | |
| Genocea | Lactococcal GEM-based vaccine | 4 | |
| Genocea | Multicomponent adenovirus vector | 3 | |
| Genocea | Combination of PspA, PspC, and ClpP | 15 |
Pht, pneumococcal histidine triad surface protein; PspA, pneumococcal surface protein A; PspC, pneumococcal surface protein C; GEM, Gram-positive enhanced matrix; ClpP, caseinolytic protease.
Currently Licensed Vaccines
With the increasing prominence of pneumococcal infections caused by replacement serotypes and related concerns about multidrug antibiotic resistance, pneumococcal vaccination is the best strategy for decreasing the morbidity and mortality associated with pneumococcal disease in infants and children. Since 1983, a 23-valent pneumococcal polysaccharide vaccine, which contains the purified pneumococcal polysaccharide capsular antigens of the 23 most common disease-causing serotypes, has been licensed in the United States. This vaccine is effective at preventing IPD in adults, but this effectiveness wanes over time (19, 29, 103). Following immunization, there is a slow decline (>5 years) in antibody levels in healthy adults and a moderate decline (5 to 10 years) in adults aged 65 years or older and adults with diabetes mellitus or renal failure requiring dialysis. A rapid decline (within 3 to 5 years) is seen in children with sickle cell anemia and in individuals with functional or anatomic asplenia. The efficacy of the 23-valent pneumococcal vaccine has been reported to be between 56% and 86%; studies have documented an efficacy for preventing invasive disease in all patients of approximately 57% (13, 19, 103). The efficacy in persons of 65 years of age or older was 75% to 80% in these studies. The vaccine has been demonstrated to significantly reduce disease among individuals with diabetes mellitus, coronary artery disease, congestive heart failure, functional or anatomic asplenia, and chronic pulmonary disease (13, 103), but the vaccine fails to demonstrate protection against nonbacteremic pneumonia (71).
This vaccine has several major limitations that make it ineffective in the pediatric population. The most important of these limitations is that many of the 23 serotypes included in the vaccine are poorly immunogenic in infants and children under 2 years of age, which is the age group with the highest incidence of both local and invasive pneumococcal infections. This is especially true for the pneumococcal serotypes 6A, 6B, 14, 19A, 19F, and 23F, which most commonly cause drug-resistant disease in the pediatric population (41, 98, 99, 103). Because polysaccharide antigens are T lymphocyte independent, they do not induce immunologic memory and fail to prime for an anamnestic or booster response with subsequent reexposure. Also, the vaccine fails to reduce nasopharyngeal carriage of pneumococcal organisms and therefore does not provide any significant protection against mucosal infections (e.g., otitis media or sinusitis) or against the spread of resistant pneumococcal strains from one person to another (98).
To address the problem of decreased immunogenicity associated with the pneumococcal polysaccharide vaccine in infants and children, protein conjugate vaccines were developed in which the epidemiologically most important pneumococcal serotypes are coupled to various protein carriers. This approach involves covalent linking of the polysaccharide to a protein to enhance immunogenicity and increase serum antibody levels. These protein carriers are T-cell-dependent antigens that stimulate a T-helper cell response that primes the vaccinated individual for an anamnestic or booster response. The coupling of a polysaccharide antigen to a protein carrier converts the polysaccharide from a T-cell-independent to a T-cell-dependent antigen to which young children can respond immunologically (98).
In addition to PCV7, the 13-valent pneumococcal CRM197 conjugate vaccine (PCV13) was licensed in the United States in February 2010 and replaces PCV7 for the prevention of IPD and otitis media (22, 32). This vaccine contains serotypes 1, 3, 5, 6A, 7F, and 19A in addition to the serotypes found in PCV7; these serotypes account for 92% of the serotypes that cause IPD in children of <5 years of age in the United States. Results from phase 3 clinical trials in young infants demonstrated that the vaccine elicited similar antibody responses to those against the serotypes contained in PCV7 and induced very robust antibody responses to the 6 additional serotypes in the vaccine. The local and systemic side effects were noted to be similar to those seen with PCV7 (31, 32). This multivalent conjugate vaccine has the potential to have a significant impact on further decreasing the incidence of IPD.
A 10-valent pneumococcal conjugate vaccine (PHiD-CV) that uses a recombinant form of nontypeable Haemophilus influenzae (NTHI) protein D as a protein carrier for 8 of the 10 vaccine serotypes and diphtheria toxoid and tetanus toxoid as the carrier proteins for the other 2 serotypes was licensed in 2009 in over 60 countries, including European countries and Canada, as well as by the World Health Organization, for the prevention of invasive disease due to S. pneumoniae and otitis media due to S. pneumoniae and nontypeable H. influenzae. This vaccine contains serotypes 1, 5, and 7F in addition to the 7 serotypes contained in PCV7 and offers protection against both pneumococcal and NTHI diseases. The 10 pneumococcal serotypes contained in the vaccine account for about 90% of the cases of IPD in children under 5 years of age in Europe. There was a 33.6% reduction in clinical otitis media demonstrated in clinical trials of the vaccine. Trials to evaluate the immunogenicity of PHiD-CV coadministered with DTPw-HBV/Hib (diphtheria–tetanus–whole-cell pertussis—hepatitis B vaccine/Haemophilus influenzae type b vaccine) or DTPw-HBV/Hib plus IPV in young infants at 2, 4, and 6 months or 6, 10, and 14 weeks of age demonstrated that the vaccine was immunogenic against each of the 10 pneumococcal vaccine serotypes. The vaccine had no significant effect on reducing nasopharyngeal carriage of nontypeable H. influenzae but did reduce carriage of vaccine-type pneumococci after the primary series and a booster dose of vaccine at 12 to 15 months were given (6, 93). Further studies to assess booster vaccination outcomes and vaccine efficacy are currently ongoing. However, because these vaccines contain specific pneumococcal serotypes, there is real potential for the continued emergence of other pneumococcal serotypes not contained in the vaccines as a cause of IPD.
In an effort to decrease the morbidity and mortality caused by pneumococcal disease in developing countries, and based on the enormous success of pneumococcal conjugate vaccines in markedly decreasing IPD in resource-rich countries, the GAVI Alliance (in collaboration with the Bill & Melinda Gates Foundation) has begun to introduce pneumococcal conjugate vaccines into the national immunization programs of GAVI-eligible countries. To date, 15 countries in the developing world have begun the introduction of pneumococcal conjugate vaccines. The goal of GAVI and its alliance partners is to roll out pneumococcal conjugate vaccines to 58 countries by 2015; this has the potential to prevent over 650,000 deaths due to IPD in the pediatric population (www.gavialliance.org/support/nvs/pneumococcal).
Vaccines in Development
Several different manufacturers have created other multivalent pneumococcal conjugate vaccines (with 9 and 11 serotypes) conjugated to different protein carriers (e.g., the nontoxic diphtheria toxin mutant CRM197, the D protein of Haemophilus influenzae, or diphtheria toxoid). These vaccine candidates have been studied in infants as young as 2 months of age and appear to be well tolerated, with local reactions occurring less frequently than with concomitantly administered diphtheria, whole-cell pertussis, and tetanus (DPT) vaccines, but they are more reactogenic than DTaP and inactivated polio vaccine (60, 104).
The efficacy of a 9-valent PCV conjugated to CRM197 (PCV7 serotypes plus serotypes 1 and 5) was evaluated in a randomized double-blind study performed in Soweto, South Africa, in infants with or without HIV who received the vaccine at 6, 10, and 14 weeks of age. In children who were HIV negative, the vaccine reduced the incidence of IPD due to vaccine serotypes by 83% and that of radiologically confirmed pneumonia by 20%. In HIV-positive children, the reduction in the incidence of IPD was 65%. Invasive disease caused by penicillin-resistant strains was reduced by 67%, and that caused by trimethoprim-sulfamethoxazole-resistant strains was reduced by 56% (60). This vaccine has also been shown to be effective at decreasing nasopharyngeal carriage of vaccine serotypes in toddlers attending day care centers (25). Results of a trial to evaluate the safety and efficacy of a combination 9-valent pneumococcal conjugate and conjugate meningococcal C vaccine (9VPnC-MnCC) administered to young infants at 3, 4, and 5 months demonstrated that the vaccine was safe and induced significant antibody titers to all the components in the vaccine (60, 105). A study in the Philippines looking at the safety and immunogenicity of an 11-valent diphtheria toxoid- and tetanus protein-conjugated pneumococcal vaccine (11-PncTD) administered simultaneously with DTwP/PRP-T (diphtheria–tetanus–whole-cell pertussis/Haemophilus influenzae type B vaccine) and oral polio vaccine (OPV) to infants as a 3-dose series at 6, 10, and 14 weeks of age demonstrated good antibody responses to all of the pneumococcal serotypes, except for serotype 14, contained in the vaccine (16, 95, 115). However, despite initial promising results, further testing of the 9-valent and 11-valent pneumococcal conjugate vaccines has been slowed temporarily due to immunogenicity interference issues with coadministered vaccines.
Protein-based vaccines are the next generation of pneumococcal vaccines that are currently in development. These vaccines are based on pneumococcal surface proteins (e.g., PspA, PspC, IgA1p, PpmA, and SirA) and virulence factors (e.g., caseinolytic protease and pneumolysin) that are found in all pneumococcal organisms. The development of immunity to one or more of these agents has the potential to protect against all of the pneumococcal serotypes that cause disease in humans. Trials of these vaccines in animal models, with vaccines administered orally, intranasally, and by injection, have shown them to be immunogenic and to be protective against IPD (3, 4, 15, 36, 44, 73, 111, 114). One of the vaccines, IC47, which is a novel protein-based pneumococcal vaccine that contains 3 highly conserved proteins, has started phase I trials in 32 healthy adults. Two different antigen doses are being studied, with and without the addition of an aluminum hydroxide adjuvant. Preliminary results indicate that the vaccine is well tolerated, with a good safety profile.
Recently, it was reported that a vaccine designed to protect against pneumococcal nasopharyngeal colonization was tested in a mouse model and found to protect against infection with these bacteria. Proteomic screening was used to identify pneumococcal protein antigens that stimulate TH17 cells (CD4+ T cells) to secrete the cytokine interleukin-17A (IL-17A), which mediates resistance to mucosal colonization (78).
CONCLUDING REMARKS
Invasive infections due to S. pneumoniae remain a leading cause of morbidity and mortality worldwide, especially in children under the age of 2 years. Through the widespread use of PCV7 in the United States, there has been a significant decrease in the incidence of IPD and nasopharyngeal carriage of vaccine serotypes in all age groups. However, the emergence of replacement pneumococcal serotypes (e.g., 19A, 1, 5, 15, and 33) is now having a significant impact on the success of PCV7. These serotypes have become the most common causes of IPD in infants, children, and adults, with serotype 19A having emerged as the predominant replacement serotype associated with multidrug-resistant infections. The widespread use of PCV13 in the United States has the potential to effectively decrease the incidence of IPD that is currently being seen in infants and young children, with the added benefit of extension of protection to other age groups, especially the elderly, similar to what was seen with PCV7.
The rapid emergence of replacement pneumococcal serotypes has focused attention on the critical need for the continued development of other pneumococcal vaccines to prevent IPD and its associated morbidity and mortality. One possibility is serotype reformulation or expansion of protein conjugate vaccines on a scheduled basis every 5 to 10 years until the development of protein-based vaccines that provide immunity to all pneumococcal serotypes that cause disease in humans or of vaccines that prevent nasopharyngeal colonization. Until that time, the ongoing surveillance of nasopharyngeal and IPD isolates will be critical in identifying the emergence of additional replacement serotypes and directing vaccine reformulation and development.
ACKNOWLEDGMENTS
I am a member of the pneumococcal vaccine advisory board for Pfizer/Wyeth and have served as a consultant on Pfizer/Wyeth vaccines.
Biography

Tina Tan is a Professor of Pediatrics at the Feinberg School of Medicine, Northwestern University, and Pediatric Infectious Diseases Attending Codirector of the Pediatric Travel Medicine Clinic and Director of the International Adoptee Clinic at Children's Memorial Hospital, Chicago, IL. She is board certified in pediatrics and pediatric infectious diseases. She received her medical degree from Louisiana State University School of Medicine, New Orleans, LA. She completed her residency, chief residency, and pediatric infectious disease fellowship in the Department of Pediatrics, Baylor College of Medicine, Houston, TX. She is the Chicago area primary investigator for the U.S. Multicenter Pneumococcal Surveillance Study Group. Her research interests include pneumococcal disease epidemiology, antibiotic resistance, and vaccines; pertussis disease and vaccines; community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) infections; antibiotic resistance; and vaccines and vaccine-preventable diseases.
REFERENCES
- 1.Advisory Committee on Immunization Practices 2000. Preventing pneumococcal diseases among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 49(RR-9):1–35 [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases 2000. Recommendations for the prevention of pneumococcal infections, including use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 106:362–366 [DOI] [PubMed] [Google Scholar]
- 3. Arevalo MT, et al. 2009. Mucosal vaccination with a multicomponent adenovirus-vectored vaccine protects against Streptococcus pneumoniae infection in the lung. FEMS Immunol. Med. Microbiol. 55:346–351 [DOI] [PubMed] [Google Scholar]
- 4. Audouy SA, et al. 2007. Development of a lactococcal GEM-based pneumococcal vaccine. Vaccine 25:2497–2506 [DOI] [PubMed] [Google Scholar]
- 5. Baraff LJ, Lee SI, Schriger DL. 1993. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr. Infect. Dis. J. 12:389–394 [DOI] [PubMed] [Google Scholar]
- 6. Bermal N, et al. 2009. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr. Infect. Dis. J. 28:S89–S96 [DOI] [PubMed] [Google Scholar]
- 7. Black SB, et al. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810–815 [DOI] [PubMed] [Google Scholar]
- 8. Black S, et al. 2004. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr. Infect. Dis. J. 23:485–489 [DOI] [PubMed] [Google Scholar]
- 9. Black S. 2008. Changing epidemiology of invasive pneumococcal disease: a complicated story. Clin. Infect. Dis. 47:485–486 [DOI] [PubMed] [Google Scholar]
- 10. Bradley JS, et al. 1998. Pediatric pneumococcal bone and joint infections. Pediatrics 102:1376–1382 [DOI] [PubMed] [Google Scholar]
- 11. Brandenburg JA, Marine TJ, Colez CM. 2000. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J. Gen. Intern. Med. 15:638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brueggmann AB, Pai R, Crook DW, Beall B. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 3:e168 doi:10.1371/journal.ppat.0030168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butler JC, et al. 1993. Pneumococcal polysaccharide vaccine efficacy. JAMA 270:1826–1831 [PubMed] [Google Scholar]
- 14. Butler JC, Breiman RF, Lipman HB, Hofmann J, Facklam RR. 1995. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978–1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171:885–889 [DOI] [PubMed] [Google Scholar]
- 15. Cao J, et al. 2007. Enhanced protection against pneumococcal infection elicited by immunization with the combination PspA, PspC and ClpP vaccine. Vaccine 25:4996–5005 [DOI] [PubMed] [Google Scholar]
- 16. Capeding MZ, et al. 2003. Safety and immunogenicity of three doses of an eleven-valent diphtheria toxoid and tetanus protein conjugated pneumococcal vaccine in Filipino infants. BMC Infect. Dis. 3:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cardozo DM, et al. 2008. Prevalence and risk factors for nasopharyngeal carriage of Streptococcus pneumoniae among adolescents. J. Med. Microbiol. 57:185–189 [DOI] [PubMed] [Google Scholar]
- 18. Carstairs KL, Tanen DA, Johnson AS, Kailes SB, Riffenburgh RH. 2007. Pneumococcal bacteremia in febrile infants presenting to the Emergency Department before and after the introduction of the heptavalent pneumococcal vaccine. Ann. Emerg. Med. 49:772–777 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention 1997. Prevention of pneumococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 46(RR-8):1–24 [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention 2009. Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine—United States, 1997–2006. MMWR Morb. Mortal. Wkly. Rep. 58:1–4 [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) 2010. Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine—United States, 2007. MMWR Morb. Mortal. Wkly. Rep. 59:253–257 [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention 2010. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children. MMWR Morb. Mortal. Wkly. Rep. 59:258–261 [PubMed] [Google Scholar]
- 23. Coffey TJ, et al. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73–83 [DOI] [PubMed] [Google Scholar]
- 24. Coffey TJ, Daniels M, Enright MC, Spratt BG. 1999. Serotype 14 variants of the Spanish penicillin-resistant serotype 9V clone of Streptococcus pneumoniae arose by large recombinational replacements of the cpsA-pbp1a region. Microbiology 145:2023–2031 [DOI] [PubMed] [Google Scholar]
- 25. Dagan R, et al. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending daycare centers. J. Infect. Dis. 185:927–936 [DOI] [PubMed] [Google Scholar]
- 26. Dagan R, Klugman KP. 2008. Impact of conjugate pneumococcal vaccine on antibiotic resistance. Lancet Infect. Dis. 8:785–795 [DOI] [PubMed] [Google Scholar]
- 27. Dagan R. 2009. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin. Microbiol. Infect. 15(Suppl 3):16–20 [DOI] [PubMed] [Google Scholar]
- 28. Davidson M, et al. 1989. Invasive pneumococcal disease in an Alaska native population, 1980 through 1986. JAMA 261:715–718 [PubMed] [Google Scholar]
- 29. Dear K, Holden J, Andrews R, Tatham D. 2003. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2003:CD000422 doi:10.1002/14651858.CD000422 [DOI] [PubMed] [Google Scholar]
- 30. De Cunto Brandileone MC, et al. 1997. Prevalence of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated from Brazilian children with invasive infections. Microb. Drug Resist. 3:141–146 [DOI] [PubMed] [Google Scholar]
- 31. Dinleyici EC, Yargic ZA. 2009. Current knowledge regarding investigational 13-valent pneumococcal conjugate vaccine. Expert Rev. Vaccines 8:977–986 [DOI] [PubMed] [Google Scholar]
- 32. Duggan ST. 2010. Pneumococcal polysaccharide conjugate vaccine (13-valent, absorbed) [Prevnar 13]. Drugs 70:1973–1986 [DOI] [PubMed] [Google Scholar]
- 33. Farrell DJ, Klugman KP, Pichichero M. 2007. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr. Infect. Dis. J. 26:123–128 [DOI] [PubMed] [Google Scholar]
- 34. Ghaffar F, Friedland IR, McCracken GH., Jr 1999. Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 18:638–646 [DOI] [PubMed] [Google Scholar]
- 35. Giefing C, et al. 2008. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 205:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. 2011. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect. Immun. 79:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gonzalez BE, et al. 2006. Streptococcus pneumoniae serogroups 15 and 33: an increasing cause of pneumococcal infections in children in the United States after the introduction of the pneumococcal 7-valent conjugate vaccine. Pediatr. Infect. Dis. J. 25:301–305 [DOI] [PubMed] [Google Scholar]
- 38. Gray BM, Converse GM, Dillon HC. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923–933 [DOI] [PubMed] [Google Scholar]
- 39. Grijalva CG, et al. 2007. Decline in pneumonia admissions after routine childhood immunization with pneumococcal conjugate vaccine in the U. S. A.: a time series analysis. Lancet 369:1179–1186 [DOI] [PubMed] [Google Scholar]
- 40. Grijalva CG, Nuorti P, Zhu Y, Griffin MR. 2010. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin. Infect. Dis. 50:805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grijalva CG, Pelton SI. 2011. A second generation pneumococcal conjugate vaccine for prevention of pneumococcal diseases in children. Curr. Opin. Pediatr. 23:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hammitt LL, et al. 2006. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J. Infect. Dis. 193:1487–1494 [DOI] [PubMed] [Google Scholar]
- 43. Hanage WP, et al. 2007. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J. Infect. Dis. 195:347–352 [DOI] [PubMed] [Google Scholar]
- 44. Hanniffy SB, Carter AT, Hitchin E, Wells JM. 2007. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J. Infect. Dis. 195:185–193 [DOI] [PubMed] [Google Scholar]
- 45. Hausdorff WP, Feikin DR, Klugman KP. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83–93 [DOI] [PubMed] [Google Scholar]
- 46. Hausdorff W. 2007. The role of pneumococcal serotypes 1 and 5 in paediatric invasive disease. Vaccine 25:2406–2412 [DOI] [PubMed] [Google Scholar]
- 47. Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. 2008. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 27:1030–1032 [DOI] [PubMed] [Google Scholar]
- 48. Herz AM, et al. 2006. Changing epidemiology of outpatient bacteremia in 3- to 36-month-old children after the introduction of the heptavalent-conjugated pneumococcal vaccine. Pediatr. Infect. Dis. J. 25:293–300 [DOI] [PubMed] [Google Scholar]
- 49. Hicks LA, et al. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J. Infect. Dis. 196:1346–1354 [DOI] [PubMed] [Google Scholar]
- 50. Hofmann J, et al. 1995. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N. Engl. J. Med. 333:481–486 [DOI] [PubMed] [Google Scholar]
- 51. Hsu HE, et al. 2009. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 360:244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang SS, et al. 2005. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001–2004. Pediatrics 116:e408–e413 [DOI] [PubMed] [Google Scholar]
- 53. Huang SS, et al. 2009. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124:e1–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Isaacman DJ, Fletcher MA, Fritzell B, Ciuryla V, Schranz J. 2007. Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7; Prevnar). Vaccine 25:2420–2427 [DOI] [PubMed] [Google Scholar]
- 55. Jacobs NM, Lerdkachornsuk S, Metzger WI. 1979. Pneumococcal bacteremia in infants and children: a ten-year experience at the Cook County Hospital with special reference to the pneumococcal serotypes isolated. Pediatrics 64:296–300 [PubMed] [Google Scholar]
- 56. Jacobs NM. 1991. Pneumococcal osteomyelitis and arthritis in children. Am. J. Dis. Child. 145:70–74 [DOI] [PubMed] [Google Scholar]
- 57. Joffe MD, Alpern ER. 2010. Occult pneumococcal bacteremia—a review. Pediatr. Emerg. Care 26:448–457 [DOI] [PubMed] [Google Scholar]
- 58. Kaplan SL, et al. 2004. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 113:443–449 [DOI] [PubMed] [Google Scholar]
- 59. Kaplan SL, et al. 2010. Serotype 19A is the most common serotype causing invasive pneumococcal infections in children. Pediatrics 125:429–436 [DOI] [PubMed] [Google Scholar]
- 60. Klugman KP, Madhi SA, Huebner RE. 2003. A trial of a 9 valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341–1348 [DOI] [PubMed] [Google Scholar]
- 61. Kuppermann N, Fleisher GR, Jaffe DM. 1998. Predictors of occult pneumococcal bacteremia in young febrile children. Ann. Emerg. Med. 31:679–687 [DOI] [PubMed] [Google Scholar]
- 62. Kyaw MH, et al. 2006. Effect of the introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455–1463 [DOI] [PubMed] [Google Scholar]
- 63. Lacapa R, et al. 2008. Changing epidemiology of invasive pneumococcal disease among white Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin. Infect. Dis. 47:476–484 [DOI] [PubMed] [Google Scholar]
- 64. Lee GM, Harper MB. 1998. Risk of bacteremia for febrile young children in the post Haemophilus influenzae type b era. Arch. Pediatr. Adolesc. Med. 152:624–628 [DOI] [PubMed] [Google Scholar]
- 65. Levine MM, et al. 1998. Epidemiology of invasive pneumococcal infections in infants and young children in Metropolitan Santiago, Chile, a newly industrializing country. Pediatr. Infect. Dis. J. 17:287–293 [DOI] [PubMed] [Google Scholar]
- 66. Lexau CA, et al. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043–2051 [DOI] [PubMed] [Google Scholar]
- 67. Lexau CA. 2008. The changing epidemiology of pneumococcal pulmonary disease in era of the heptavalent vaccine. Curr. Infect. Dis. Rep. 10:229–235 [DOI] [PubMed] [Google Scholar]
- 68. Li ST, Tancredi DJ. 2010. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics 125:26–33 [DOI] [PubMed] [Google Scholar]
- 69. Lucero MG, et al. 2009. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst. Rev. 2009:CD004977 doi:10.1002/14651858.CD004977.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Malley R, Ambrosino D. 1998. Pneumococcal diseases in children: morbidity, mortality, and resistance. Univ. Chicago Child. Hosp. Rep. Curr. Concepts Use Pediatr. Vaccines 1:1–8 [Google Scholar]
- 71. Mangtani P, Cutts F, Hall AJ. 2003. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect. Dis. 3:71–78 [DOI] [PubMed] [Google Scholar]
- 72. Marrie TJ. 1999. Pneumococcal pneumonia: epidemiology and clinical features. Semin. Respir. Infect. 14:227–236 [PubMed] [Google Scholar]
- 73. Meng C, et al. 2009. Development of 5-valent conjugate pneumococcal protein A-capsular polysaccharide pneumococcal vaccine against invasive pneumococcal disease. Microb. Pathog. 47:151–156 [DOI] [PubMed] [Google Scholar]
- 74. Messina AF, et al. 2007. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr. Infect. Dis. J. 26:461–467 [DOI] [PubMed] [Google Scholar]
- 75. Millar EV, et al. 2008. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin. Infect. Dis. 47:989–996 [DOI] [PubMed] [Google Scholar]
- 76. Mitchell AM, Mitchell TJ. 2010. Streptococcus pneumoniae: virulence factors and variation. Clin. Microbiol. Infect. 16:411–418 [DOI] [PubMed] [Google Scholar]
- 77. Mitchell TJ. 2000. Virulence factors and pathogenesis of disease caused by Streptococcus pneumoniae. Res. Microbiol. 151:413–419 [DOI] [PubMed] [Google Scholar]
- 78. Moffitt KL, et al. 2011. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 9:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moore MR, et al. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016–1027 [DOI] [PubMed] [Google Scholar]
- 80. Mufson MA. 1981. Pneumococcal infections. JAMA 246:1942–1948 [PubMed] [Google Scholar]
- 81. Munoz-Almagro C, et al. 2008. Emergence of invasive pneumococcal disease caused by non-vaccine serotypes in the era of 7-valent conjugate vaccine. Clin. Infect. Dis. 46:174–182 [DOI] [PubMed] [Google Scholar]
- 82. Nabors GS, et al. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743–1754 [DOI] [PubMed] [Google Scholar]
- 83. Nahm MH, Lin J, Finkelstein JA, Pelton SI. 2009. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J. Infect. Dis. 199:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nelson JC, et al. 2008. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 26:4947–4954 [DOI] [PubMed] [Google Scholar]
- 85. Nuoti JP, et al. 2010. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 59(RR-11):1–18 [PubMed] [Google Scholar]
- 86. O'Brien KL, et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 87. Pai R, et al. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 192:1988–1995 [DOI] [PubMed] [Google Scholar]
- 88. Pilishvili T, et al. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 89. Pilishvili T, et al. 2010. Risk factors for invasive pneumococcal disease in children in the era of conjugate vaccine use. Pediatrics 126:e9–e17 [DOI] [PubMed] [Google Scholar]
- 90. Pineda SV, Perez BA, Domingo PM. 2002. Bacteremic pneumococcal pneumonia. An. Esp. Pediatr. 57:408–413 [PubMed] [Google Scholar]
- 91. Pletz MW, et al. 2004. Levofloxacin-resistant invasive Streptococcus pneumoniae in the United States: evidence for clonal spread and the impact of conjugate pneumococcal vaccine. Antimicrob. Agents Chemother. 48:3491–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Poehling KA, et al. 2006. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 295:1668–1674 [DOI] [PubMed] [Google Scholar]
- 93. Prymula R, et al. 2011. Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage. Vaccine 29:1959–1967 [DOI] [PubMed] [Google Scholar]
- 94. Pulido M, Sorvillo F. 2010. Declining invasive pneumococcal disease mortality in the United States, 1990–2005. Vaccine 28:889–892 [DOI] [PubMed] [Google Scholar]
- 95. Puumalainen T, et al. 2002. Antibody response to an eleven valent diphtheria- and tetanus-conjugated pneumococcal conjugate vaccine in Filipino infants. Pediatr. Infect. Dis. J. 21:309–316 [DOI] [PubMed] [Google Scholar]
- 96. Robinson K, et al. 2001. Epidemiology of Streptococcus pneumoniae infections in the U.S., 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA 285:1729–1735 [DOI] [PubMed] [Google Scholar]
- 97. Ross JJ, Saltzman CL, Carling P, Shapiro DS. 2003. Pneumococcal septic arthritis: review of 190 cases. Clin. Infect. Dis. 36:319–327 [DOI] [PubMed] [Google Scholar]
- 98. Rubin LG. 2000. Pneumococcal vaccine. Pediatr. Clin. North Am. 47:269–285 [DOI] [PubMed] [Google Scholar]
- 99. Rubin LG, et al. 2010. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine 28:7634–7643 [DOI] [PubMed] [Google Scholar]
- 100. Ruckinger S, von Kries R, Siedler A, van der Linden M. 2009. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr. Infect. Dis. J. 28:118–122 [DOI] [PubMed] [Google Scholar]
- 101. Schuchat A, et al. 1997. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970–976 [DOI] [PubMed] [Google Scholar]
- 102. Shah SS, Ratner AJ. 2006. Trends in invasive pneumococcal disease-associated hospitalizations. Clin. Infect. Dis. 42:e1–e5 [DOI] [PubMed] [Google Scholar]
- 103. Shapiro ED, et al. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453–1460 [DOI] [PubMed] [Google Scholar]
- 104. Shinefield HR, et al. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757–763 [DOI] [PubMed] [Google Scholar]
- 105. Sigurdardottir ST, et al. 2008. Safety and immunogenicity of CRM197 conjugated pneumococcal-meningococcal C combination vaccine (PVPnC-MnCC) whether given in two or three primary doses. Vaccine 26:4178–4186 [DOI] [PubMed] [Google Scholar]
- 106. Skinner JM, et al. 2011. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 29:8870–8876 [DOI] [PubMed] [Google Scholar]
- 107. Steenhoff AP, Shah SS, Ratner AJ, Patil SM, McGowan KL. 2006. Emergence of vaccine-related pneumococcal serotypes as a cause of bacteremia. Clin. Infect. Dis. 42:907–914 [DOI] [PubMed] [Google Scholar]
- 108. Tan TQ, et al. 2002. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics 110:1–6 [DOI] [PubMed] [Google Scholar]
- 109. Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. 2008. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin. Infect. Dis. 46:1664–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Van Effelterre T, et al. 2010. A dynamic model of pneumococcal infection in the United States: implications for prevention through vaccination. Vaccine 28:3650–3666 [DOI] [PubMed] [Google Scholar]
- 111. Villena J, Medina M, Raya R, Alvarez S. 2008. Oral immunization with recombinant Lactococcus lactis confers protection against respiratory pneumococcal infection. Can. J. Microbiol. 54:845–853 [DOI] [PubMed] [Google Scholar]
- 112. Weinberger DM, et al. 2010. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin. Infect. Dis. 51:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Whitney CG, et al. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737–1746 [DOI] [PubMed] [Google Scholar]
- 114. Wu K, et al. 2010. Immunization with a combination of three pneumococcal proteins confers additive and broad protection against Streptococcus pneumoniae infections in mice. Infect. Immun. 78:1276–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wuorimaa T, et al. 2001. Tolerability and immunogenicity of an eleven-valent pneumococcal conjugate vaccine in healthy toddlers. Pediatr. Infect. Dis. J. 120:272–277 [DOI] [PubMed] [Google Scholar]
- 116. Zangwill KM, et al. 1996. Epidemiology of invasive pneumococcal disease in southern California: implications for the design and conduct of a pneumococcal conjugate vaccine efficacy trial. J. Infect. Dis. 174:752–759 [DOI] [PubMed] [Google Scholar]
- 117. Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. 2007. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch. Pediatr. Adolesc. Med. 161:1162–1168 [DOI] [PubMed] [Google Scholar]