Abstract
Light is one crucial environmental signal which can determine whether a fungus reproduces asexually or initiates sexual development. Mating in the ascomycete Hypocrea jecorina (anamorph Trichoderma reesei) occurs preferentially in light. We therefore investigated the relevance of the light response machinery for sexual development in H. jecorina. We found that the photoreceptors BLR1 and BLR2 and the light-regulatory protein ENV1 have no effect on male fertility, while ENV1 is essential for female fertility. BLR1 and BLR2 were found to impact fruiting body formation although they are not essential for mating. Quantitative reverse transcription-PCR (qRT-PCR) analyses revealed that BLR1, BLR2, and ENV1 negatively regulate transcript levels of both pheromone receptors as well as peptide pheromone precursors in light but not in darkness and in a mating type-dependent manner. The effect of BLR1 and BLR2 on regulation of pheromone precursor and receptor genes is less severe than that of ENV1 as strains lacking env1 show 100-fold (for ppg1) to more than 100,000-fold (for hpp1) increased transcript levels of pheromone precursor genes as well as more than 20-fold increased levels of hpr1, the pheromone receptor receiving the HPP1 signal in a MAT1-1 strain. ENV1 likely integrates additional signals besides light, and our results indicate that its function is partially mediated via regulation of mat1-2-1. We conclude that ENV1 is essential for balancing the levels of genes regulated in a mating-type-dependent manner, which contributes to determination of sexual identity and fruiting body formation.
INTRODUCTION
Sexual development is one of the most important evolutionary achievements in nature. “Nothing in biology makes sense unless it is in the light of reproduction” (14). This quotation briefly summarizes the relevance of sexual development for almost all living organisms and, hence, for its application in research and industry. Accordingly, a complex regulatory network of signaling and metabolic pathways is responsible for timing and efficiency of mating in fungi (2, 11). Peptide pheromones and their cognate receptors, which have been characterized in many fungi, are of crucial importance for communication of mating partners and subsequent sexual development (3, 11). In heterothallic fungi, only partners of different mating types can mate with each other. Thereby, the mating type is defined by a specific genomic locus, which contains one of two different sequences (often called idiomorphs) occupying the same chromosomal locus in the genome (12, 44). Fungi are usually hermaphroditic and can form both male and female reproductive structures, which are strongly dependent on environmental factors. At the molecular level, in most cases pheromone receptors are crucial for female fertility (33), while peptide pheromone precursors are reported to be essential for male fertility (9, 34, 54).
Sexual development remained undiscovered for decades in Trichoderma reesei, albeit Hypocrea jecorina could be identified as its teleomorph (36). Detection of this process in the industrial workhorse H. jecorina represents a major improvement in research with this organism. Since successful mating has been achieved (57), the mechanism and determinants of the underlying process have been subjected to elaborate analysis aiming at a more detailed understanding of physiology of H. jecorina as well as strategies for industrial strain improvement. The female sterile phenotype of heterothallic QM6a, however, is a drawback in this respect. At the same time the availability of a female sterile strain offers the possibility to investigate determinants of female fertility in mutants of sexually competent wild-type isolates. Sexual development in H. jecorina is favored in light and upon growth on rich medium (57), which is in contrast to many other fungi (11). Another peculiarity in H. jecorina is the composition of its pheromone system. H. jecorina has the usual types of pheromone receptors (50): one Ste3-type receptor termed HPR1 and one Ste2-type receptor (HPR2) as well as a normal alpha-type peptide pheromone precursor, PPG1. However, the peptide pheromone precursor HPP1 is the first member of the new class of h-type (hybrid type) peptide pheromone precursors, which assumed a-type function in H. jecorina. No classical a-type peptide pheromone precursor was found in the genome (54).
Light is a crucial environmental factor for fungi (10, 24) and also for the developmental decision whether to reproduce sexually or asexually. The perception of light and thus the physiological adaptation to light are evolutionarily old mechanisms found in zygomycetes (27), ascomycetes (26), basidiomycetes (60), and several other lineages (28). In many fungi, the light-induced changes in gene expression impact growth, the direction of growth, asexual and sexual reproduction, pigment formation, carbon metabolism, and circadian rhythms (63). In Cryptococcus neoformans an interesting connection between blue light perception, virulence, and mating inhibition was found: the deletion of the photoreceptor gene bwc1 or bwc2 results in reduced virulence and causes release of mating inhibition by light (26). Further connections between mating and the photoreceptors LreA and LreB were characterized in Aspergillus nidulans. Both proteins positively affect cleistothecium formation and secondary metabolism in A. nidulans (48).
The conserved Neurospora crassa blue light photoreceptor complex (White Collar complex [WCC]), consisting of White Collar 1 (WC-1) and WC-2, represents a central means of light perception and regulation of light-responsive genes (49).
Upon illumination, the WCC acts as a transcriptional activator of a third photoreceptor, VVD, which acts as a universal brake of light responses upon prolonged illumination and is essential for gating of light responses (7, 23). VVD hence also acts as negative regulator by inactivating the WCC and consequently its own expression (18, 23). This function in photoadaptation is achieved by physical interaction of VVD with the WCC (6, 25), and lack of VVD consequently causes increased effects of WCC.
The H. jecorina photoreceptor homologues of WC-1 and WC-2, denominated BLR1 (for blue light receptor 1) and BLR2, contain the characteristic PAS domains as well as zinc fingers, reflecting their function as transcription factors (5, 51). The PAS domain protein ENVOY (ENV1), representing the VVD homologue, is essential for light tolerance of H. jecorina (52). ENV1 represents an important node in the network connecting the light response pathway, heterotrimeric G-protein signaling, and the cyclic AMP (cAMP) pathway (61) and is induced by BLR1 and BLR2 (5). In particular, ENV1 acts negatively on transcription of the G-protein alpha subunit gene gna3 (53) and interferes with the positive feedback of gna1 upon activation of its encoded G-alpha subunit (61). In accordance with its function in regulation of heterotrimeric G-protein signaling, ENV1 also impacts light responsiveness of the class I phosducin-like protein-encoding gene phlp1. Due to the fact that PhLP1 is assumed to act as a cochaperone for G-protein beta and gamma subunit folding, this finding further supports the interrelationship of ENV1 with G-protein signaling (62). Since the heterotrimeric G-protein pathway is assumed to predominantly transmit nutrient signals besides the pheromone signals (39), ENV1 is likely to integrate these signals with the light response pathway.
In this study, we provide insights into the role of the blue light photoreceptors BLR1 and BLR2 and the regulatory protein ENVOY in regulation of sexual development, all of which contribute to the respective adjustment of signal transmission and reception. We further show that env1 is essential for balanced regulation of pheromone precursor genes and receptor genes. Consequently, ENV1 is important for successful mating, and its regulatory function was found to be at least in part achieved by a negative effect on the mating type gene mat1-2-1 in a MAT1-2 strain.
MATERIALS AND METHODS
Microbial strains and culture conditions.
H. jecorina (T. reesei) wild-type strain QM6a, its derivative QM9414 (ATCC 26921), and H. jecorina CBS999.97 MAT1-1 and MAT1-2 strains (40, 57) as well as H. jecorina (T. reesei) QM9414 strains lacking blr1, blr2, and env1 (5) were used throughout this study (Table 1). Propagation of strains occurred on 2% (wt/vol) malt extract-agar plates. If not indicated otherwise, sexual development was analyzed at room temperature and under daylight conditions (cycles 12 h of light and 12 h of darkness [12:12]). For the analysis of fruiting body formation, strains of opposite mating types were inoculated on opposing sides of a petri dish and monitored over a period of 14 days (57). For the additional evaluation of mating success, spore solution (0.1% Triton X-100, mixture of equal amounts of conidia from strains of opposite mating type; 2 × 104 conidia per strain were applied) was inoculated at the center of a petri dish and monitored over 14 days at room temperature and under daylight conditions. For determination of stroma biomass, strains were inoculated accordingly. Per strain, stromata from five equally treated plates were harvested, cleaned from agar residues, dried overnight at 80°C, and weighed subsequently. For transcript analysis, malt extract-agar plates were covered with cellophane to facilitate harvesting of mycelia. Mycelia of at least three equally treated plates were harvested and pooled. To avoid a possible influence of circadian rhythms, harvesting occurred at noon for each respective day. Mycelia were harvested 3, 4, and 6 days after inoculation, corresponding, respectively, to stages of precontact, contact, and the beginning of fruiting body formation of sexually developing cultures.
Table 1.
Strains used in this study
| Strain | Relevant genotype | Source and/or reference |
|---|---|---|
| QM6a | Wild-type MAT1-2 | 43 |
| QM9414 | QM6a derivative, MAT1-2 | ATCC 26921; 64 |
| QM9414 Δenv1 | Δenv1::hph+ MAT1-2 | 5 |
| QM9414 Δblr1 | Δblr1::hph+ MAT1-2 | 5 |
| QM9414 Δblr2 | Δblr2::hph+ MAT1-2 | 5 |
| CBS999.97 MAT1-1 | Wild-type MAT1-1 | 57 |
| CBS999.97 MAT1-2 | Wild-type MAT1-2 | 57 |
| CBS999.97 MAT1-1 Δenv1 | Δenv1::hph+ MAT1-1 | This study |
| CBS999.97 MAT1-2 Δenv1 | Δenv1::hph+ MAT1-2 | This study |
| CBS999.97 MAT1-1 Δblr1 | Δblr1::hph+ MAT1-1 | This study |
| CBS999.97 MAT1-2 Δblr1 | Δblr1::hph+ MAT1-2 | This study |
| CBS999.97 MAT1-1 Δblr2 | Δblr2::hph+ MAT1-1 | This study |
| CBS999.97 MAT1-2 Δblr2 | Δblr2::hph+ MAT1-2 | This study |
| CBS999.97 MAT1-1 Δblr1 Δblr2 | Δblr1blr2::hph+ MAT1-1 | This study |
| CBS999.97 MAT1-2 Δblr1 Δblr2 | Δblr1blr2::hph+ MAT1-2 | This study |
Escherichia coli JM109 (65) was used for DNA manipulations.
Nucleic acid isolation and transcript analysis.
For DNA isolation, strains were grown on malt extract-agar, and chromosomal DNA was isolated as described previously (8, 41).
RNA was isolated as described by Tisch et al. (61). For reverse transcription and quantitative PCR (RT-qPCR), 1 μg of total RNA was treated with DNase I (Fermentas, Vilnius, Lithuania) for 30 min. Termination of the DNase digest was accomplished by adding EDTA to a final concentration of 2.5 mM and incubation at 65°C for 10 min. A RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific/Fermentas, St. Leon-Rot, Germany) was used for first-strand synthesis according to the manufacturer's protocol. For qPCR cDNA was diluted 1:5, aliquoted, and stored at −80°C. For the quantification of hpr1 (GenBank accession number for QM6a, JN787117; CBS999.97, JN684208), hpr2 (GenBank accession number for QM6a, JN787118; CBS999.97, JN678730), hpp1, ppg1, and mat1-2-1 transcript levels, the primers shown in Table 2 were used. As a reference gene, rpl6e encoding the ribosomal protein RPL6e was used. The expression of rpl6e was shown to be unaffected by light or light regulators (61). The quantitative PCRs were performed in an IQ5 Real-Time PCR Detection System (Bio-Rad Laboratories GmbH, Munich, Germany) using the primer pairs hpp1F and hpp1R, ppg1F and ppg1R, hpr1F and hpr1R, and hpr2F and hpr2R. Melting curve analysis was performed after the PCR to confirm that the signal was the result of single-product amplification and not due to primer dimers or arbitrary amplification. Cycle threshold (CT) values were determined for a minimum of three biological replicates and three technical replicates. Analysis of qPCR data was performed using REST software (46).
Table 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequencea |
|---|---|
| BLR1DEL3F | 5′-ATGAATTCCTTGCTCATTTGATGCGAG-3′ |
| BLR1DEL3R | 5′-ATGGATCCAGCGTCCGATCGTATTCC-3′ |
| BLR1DEL5F | 5′-ATGGTACCACCAATTGTCCTCGTGAG-3′ |
| BLR1DEL5R | 5′-ATCTCGAGAAAGAATGAGGGAGAGGC-3′ |
| BLR1c1F | 5′-TGTGCCTTTGTCGTTTGTG-3′ |
| BLR1c1R | 5′-CAATCTCAGCCAGTCCGA-3′ |
| HPHin1NF | 5′-CGTTATGTTTATCGGCACTTTG-3′ |
| BLR1ex5F | 5′-ATTCTAGACTCGAGTTGCCAGGGACTAGGAAG-3′ |
| BLR1ex3R | 5′-AATTAACCCTCACTAAAGGG-3′ |
| BLR1c3R | 5′-ATACTAGTCCTTTGCCTCACCTCAACC-3′ |
| BLR2DEL5F | 5′-ATTCTAGACAGATACAAAGCCGAGGACC-3′ |
| BLR2DEL5R | 5′-ATAAGCTTGGTCGCGGTAGTATTGCTATAC-3′ |
| BLR2DEL3F | 5′-ATGTCGACATGGCGGAGAAAGAAAG-3′ |
| BLR2DEL3R | 5′-ATGGTACCTCCGATCCTGCACGATC-3′ |
| BLR2cDF | 5′-CATCGCATTTGCCTTCCAG-3′ |
| BLR2cDR | 5′-AAAGAATCGACAGCAACAATGG-3′ |
| ENVDEL5F2 | 5′-ATGGTACCTACGATTATTGGCATTGC-3′ |
| ENVDEL5R2 | 5′-ATCTCGAGTAAAGAAGAGGTCACAGCC-3′ |
| ENVDEL3F | 5′-AACCCGGGATAGATGCTAGGCGTACC-3′ |
| ENVDEL3R | 5′-ACGGATCCGAGAAGATTGCATTCATTAC-3′ |
| ENV1F | 5′-TCCCTGGATCTGGATACG-3′ |
| ENV2R | 5′-CTGGCGTGGTATTTCTCTGAC-3′ |
| MATa1-F | 5′-GCGCACCACGGTATTTCATTG-3′ |
| MATa1-R | 5′-ATTTGCGCGGCTTGTATTGG-3′ |
| hpp1F | 5′-ACAATCACCGTGGGACATCTG-3′ |
| hpp1R | 5′-TCCCTGCTGTTCCGCTGATG-3′ |
| ppg1F | 5′-TGGAGACGAAGGAGAAGACTG-3′ |
| ppg1R | 5′-GCGATGTGTGGTGATGGAG-3′ |
| hpr1F | 5′-TTGGCACCTTGATTGGCTG-3′ |
| hpr1R | 5′-CGGCGGGAGAATCACAAAG-3′ |
| hpr2F | 5′-TGGCACCACTTCATCAACTTC-3′ |
| hpr2R | 5′-GGAGTAGGAGGAGGATGTGTTG-3′ |
Introduced restriction sites are underlined.
Preparation of deletion strains.
To obtain deletion mutants of blr1, blr2, and env1 (Table 1), strain CBS999.97 was transformed with plasmids pDELBLR1-C, pDELBLR2-C, and pDELENV1-C, respectively. In these plasmids the sequence spanning the mRNA of blue light regulator genes, as predicted in the Joint Genome Institute (JGI) T. reesei genome database, version 2.0, is replaced by the E. coli hph gene under H. jecorina expression signals (42). Primer sequences mentioned in the following are shown in Table 2. The vector pDELBLR1-C was constructed using H. jecorina CBS999.97 genomic DNA as the template for PCR amplifications. A 1,542-bp fragment of the 3′ flanking region of blr1 was amplified by PCR using primers BLR1DEL3F and BLR1DEL3R. The amplicons were digested with EcoRI-BamHI (all restriction enzymes were from Thermo Fisher Scientific/Fermentas) and cloned into the EcoRI-BamHI sites of pBluescript (pBS) SK+. Thereafter, a 1,530-bp fragment of the 5′ flanking sequence of blr1 was amplified by PCR using primers BLR1DEL5F and BLR1DEL5R. The fragment was digested using SalI (cleaving 22 bp from the 5′ flanking region) and the XhoI site, which was introduced by the primer BLR1DEL5R and cloned into the XhoI digested vector. After dephosphorylation of the resulting plasmid pBS::3′-blr1, the hygromycin resistance cassette excised from pRLMex30 (42) using XhoI and HindIII was filled in using Klenow polymerase to create blunt ends and integrated into EcoRV-digested and dephosphorylated pBS::3′,5′-blr1.
The resulting transformation cassette now carried in pDELBLR1-C was amplified by PCR using the primers BLR1ex5F and BLR1ex3R and used for transformation (20) of H. jecorina CBS999.97. Transformants were selected on plates containing 50 μg/ml hygromycin B (Calbiochem, Merck KGaA, Darmstadt, Germany). Fungal DNA was isolated (see above) and subjected to PCR analysis to verify replacement of the gene. To determine the presence of the wild-type fragment, PCR verification was performed using the primers BLR1c1F and BLR1c1R. The wild-type fragment yielded a band at 2,098 bp. The presence of the deletion construct was verified using the primers HPHin1NF and BLR1c3R, which bind within the hph marker and outside the cassette. The deletion construct yielded a band at 2,912 bp. Transformants underwent three rounds of single-spore isolation until no wild-type PCR product was detectable. Two deletion strains of CBS999.97 (MAT1-1) (Δblr115b1β and -66a1α) were used and displayed clear and similar phenotypes.
The vector pDELBLR2-C was constructed with H. jecorina CBS999.97 DNA as the template for PCR amplifications. A 1,222-bp fragment of the 3′ flanking region of blr2 was amplified by PCR using primers BLR2DEL3F and BLR2DEL3R, digested with SalI-Acc65I and cloned into the XhoI-Acc65I sites of pBS SK+. Thereafter, a 1,300-bp fragment of the 5′ flanking sequence of blr2 was amplified by PCR using primers BLR2DEL5F and BLR2DEL5R. The amplicons were cleaved with HindIII and XbaI and cloned into the HindIII-XbaI sites of pBS::3′-blr2.
The hygromycin resistance cassette was digested with XhoI/HindIII and integrated in SalI and HindIII sites of pBS::3′-5′-blr2. The resulting pDELBLR2-C was cleaved with Acc65I and NotI, and the excised fragment obtained was used for transformation of H. jecorina CBS999.97. PCR verification was performed using the primers BLR2cDF and BLR2cDR. The presence of wild-type blr2 was indicated by a band at 2,345 bp, and the deletion construct yielded a band at 3,649 bp (data not shown). Positive transformants underwent at least three rounds of single-spore isolation until no wild-type background was detectable by PCR.
The vector pDELENV-C was constructed as follows. A 1,094-bp fragment of the 5′ flanking region of env1 was amplified by PCR using primers ENVDEL5F2 and ENVDEL5R2, digested with XhoI-Acc65I, and cloned into the XhoI-Acc65I sites of pBSXH (52), which contains the pki1p::hph::cbh2t cassette from pRLMex30. Thereafter, a 986-bp fragment of the 3′ flanking sequence of env1 was amplified by PCR using primers ENVDEL3F and ENVDEL3R; the amplicons were cleaved with XmaI and BamHI and cloned into the respective sites of pBSXH::5′-env1.
The resulting pDELENV-C was cleaved with Acc65I and NotI, and the excised fragment thus obtained was used for transformation of H. jecorina CBS999.97. PCR verification was performed using the primers ENV1F and ENV2R. The presence of wild-type env1 was indicated by a band at 1,099 bp; the deletion construct yielded a band at 3,067 bp. Positive transformants underwent at least three rounds of single-spore isolation until no wild-type background was detectable by PCR.
In order to obtain deletion strains in both H. jecorina CBS999.97 mating types, primary transformants were crossed with the H. jecorina CBS999.97 wild-type strain. Resulting ascospores were cultivated on plates containing 50 μg/ml hygromycin B (Calbiochem, Merck KGaA, Darmstadt, Germany). Hygromycin-resistant progeny were PCR verified for deletion of blr1, blr2, and env1. In order to determine the mating type of ascospore clones, primer pairs binding within the mat1-1 (57) or mat1-2 (Table 1, MATa1F and MATa1R) mating type locus were chosen. Double mutants in blr1 and blr2 were constructed and verified accordingly.
RESULTS
Sexual development and the H. jecorina QM9414 light response machinery.
Sexual development in H. jecorina preferentially occurs in light (57). In order to achieve a first insight into the role of the light response machinery in sexual development, we used the available H. jecorina deletion strains QM9414 Δblr1 and QM9414 Δblr2 (MAT1-2) (5) for crosses with the wild-type strain CBS999.97 (MAT1-1). Deletion of env1 is known to enhance pheromone gene expression and sexual development with this strain (54). Accordingly, mating was successful using Δenv1, Δblr1, and Δblr2 strains, and ascospore discharge was not abolished (see Fig. S1 in the supplemental material). However, since these strains are derivatives of the wild-type isolate QM6a, which is female sterile (57), any influence on female fertility would not be testable using this approach. Additionally, defects in gene regulation of pheromone precursors or receptors due to female sterility could not be excluded. A defect in male fertility, however, would abolish mating in these crosses because this strain would then be both male and female sterile. Therefore, it can be concluded that deletion of blr1, blr2, and env1 does not cause major defects in male fertility.
Photoreceptors BLR1, BLR2, and ENV1 in H. jecorina CBS999.97.
In order to investigate an effect of the blue light regulators on sexual reproduction in a fully (male and female) fertile wild-type background, we used H. jecorina CBS999.97 (57). Sequence analysis of blr1, blr2, and env1 in CBS999.97 revealed only minor differences to QM6a at the DNA (identities in blr1, 99%; blr2, 99%; env1, 96%) and protein (identities in BLR1, 99%; BLR2, 100%; ENV1, 99%) levels. blr1, blr2, and env1 were thus deleted in H. jecorina CBS999.97 (MAT1-1). The same deletions in the respective MAT1-2 strain as well as Δblr1 Δblr2 double mutants in both mating types were constructed by crossing. Strains lacking blr1 or blr2 did not show a significant growth defect. In contrast, growth of the Δenv1 strain was severely perturbed in light (Fig. 1A), hence confirming the phenotype reported for H. jecorina QM9414 Δenv1 (52). As in the wild-type, conidiation is enhanced by light in all mutant strains. Lack of blr1 or blr2 caused a less distinct phenotype in light and darkness than observed for the wild type (Fig. 1B). Conidiation of the Δenv1 strain was unaltered in darkness but dense in light, which contrasts with earlier data and may be due to slightly altered regulation of asexual development in QM6a and its derivatives in comparison to CBS999.97.
Fig 1.
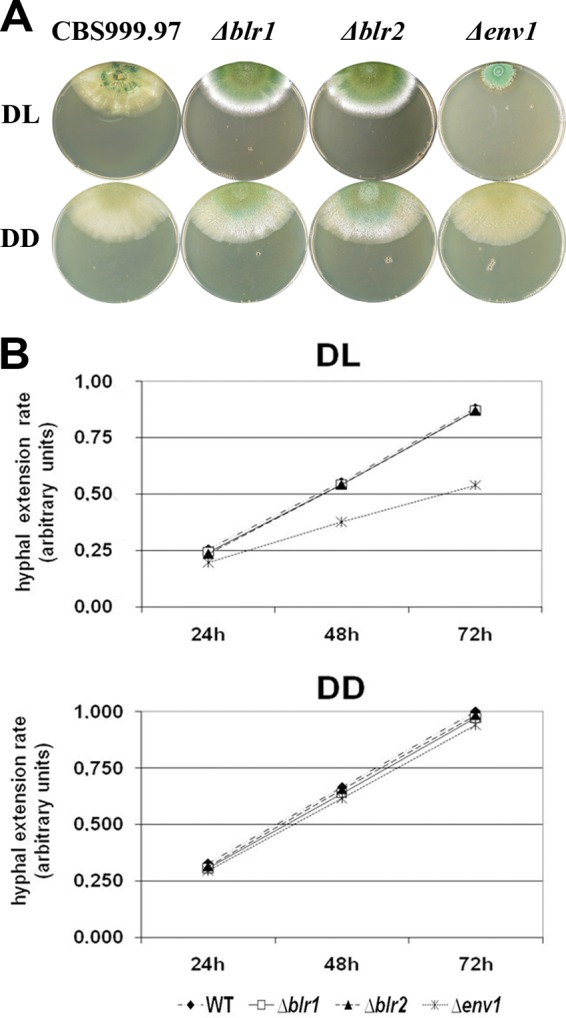
Phenotypes of CBS999.97 (MAT1-1) Δblr1, Δblr2, and Δenv1 deletion strains. (A) Plates show growth characteristics and sporulation of the wild type (WT) and Δblr1, Δblr2, and Δenv1 mutant strains in daylight (DL; 12:12) and constant darkness (DD) on malt extract-agar plates. One representative plate out of at least three replicates is shown. (B) Hyphal extension rates upon growth on malt extract-agar plates in daylight (DL; 12:12) and constant darkness (DD).
Blue light regulators are involved in sexual development of H. jecorina CBS999.97.
Deletion mutants of blr1, blr2, and env1 in the CBS999.97 background now allow for assessment of the influence of these genes also on female fertility. The relevance of the light response machinery for sexual development in the sexually competent H. jecorina CBS999.97 was evaluated by crosses of Δb1r1, Δb1r2, and Δenv1 mutant strains of both mating types with the respective wild-type mating partner. As conditions for this assay, we chose daylight (12:12 cycles) and room temperature, which is favorable for sexual development in H. jecorina. Crosses of strains bearing similar mutations in blr1, blr2, or both genes clearly showed altered fruiting body formation compared to the wild type with fewer, but larger, fruiting bodies (Fig. 2A). This characteristic is also reflected by only a slight decrease in total dry mass of stromata (Fig. 2B). When Δenv1 (MAT1-1) and Δenv1 (MAT1-2) strains were used as partners, no fruiting body formation at all was apparent (Fig. 2A), thereby indicating a defect in sexual development caused by the lack of ENV1. Altered fruiting body formation was detected for crosses of mutant strains with a wild-type strain of the opposite mating type. However, the phenotypes were less severe than if both mating partners lacked components of the light signaling machinery (see Fig. S2 in the supplemental material). In crosses of Δenv1 (MAT1-1 or MAT1-2) strains with the wild-type as partners, fruiting body formation was altered but not abolished.
Fig 2.
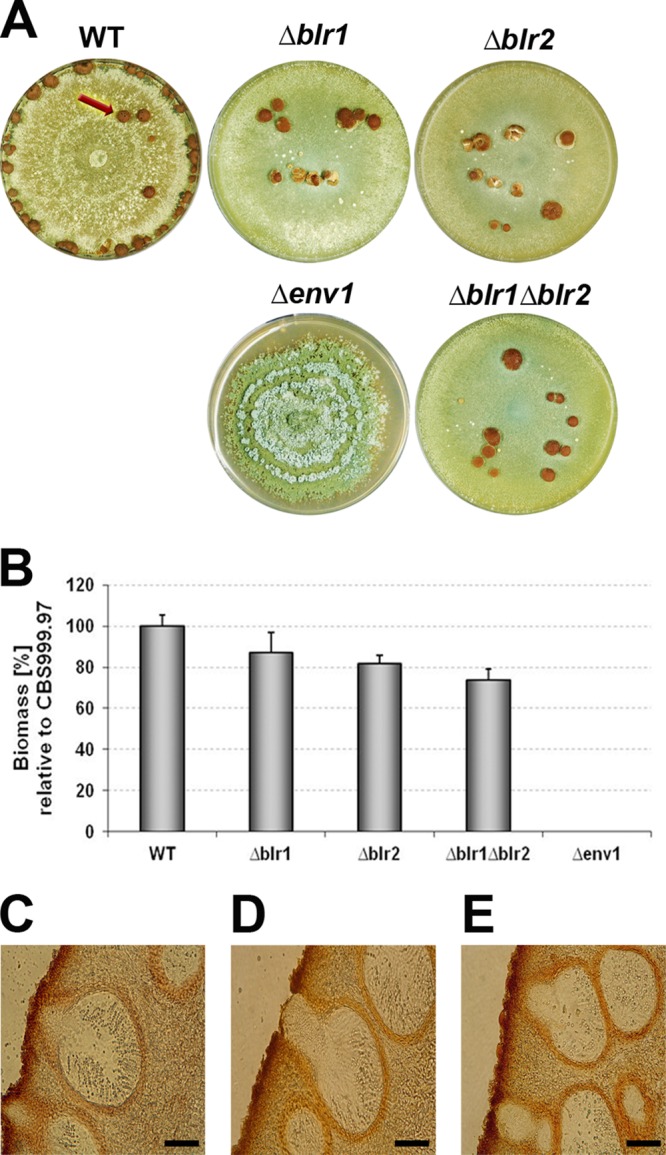
Effect of deletion of blr1, blr2, and env1 on stroma formation. (A) Sexual development of H. jecorina CBS999.97 wild type (WT) and Δblr1, Δblr2, Δblr1 Δblr2, and Δenv1 mutant strains. Equal amounts of conidia from strains of opposite mating types (deletions in both mating partners) were inoculated in the center of a petri dish and cultivated under mating conditions (DL; 22°C on malt extract-agar plates). Crosses were performed between strains carrying the same wild-type or mutant allele (WT × WT, Δblr1 × Δblr1, Δblr2 × Δblr2, Δblr1 Δblr2 × Δblr1 Δblr2, Δenv1 × Δenv1). For the wild type, sexual structures are indicated by a red arrow. (B) Average dry weight of stromata (fruiting bodies) from wild-type or mutant crosses. At least five biological replicates were considered. (C to E) Microscopic analysis of stromata from representative wild type (C), Δblr1 Δblr2 double mutant (D), and wild-type × Δenv1 (E) strains. Cross-sections of perithecia are shown for strains grown in light-dark cycles (DL; 22°C on malt-agar plates). Scale bar, 25 μm.
Except for the crosses of two stains lacking env1, where no fruiting bodies are formed, mating of strains lacking blr1, blr2, or env1 resulted in discharge of viable ascospores. Perithecium formation of these crosses did not reveal obvious defects (Fig. 2C to E).
In addition to analysis of mating efficiency of these strains, the female sterile strain QM6a and its derivative QM9414 enabled us to test whether BLR1, BLR2, or ENV1 would affect female fertility in the H. jecorina wild-type strain CBS999.97. We therefore mated the newly constructed Δenv1, Δblr1, and Δblr2 strains with the CBS999.97 background of mating type MAT1-1 with the female sterile QM6a. In case of a defect in female fertility, in these strains sexual development would be abolished because of a lack of a female partner. While fruiting body formation still occurred with the Δblr1 or Δblr2 strain as a mating partner, sexual development was not observed with the Δenv1 strain (Fig. 3). Together with the defect in fruiting body formation observed in a cross of two strains both lacking env1 as described above, this indicates that ENV1 is essential for female fertility in H. jecorina CBS999.97.
Fig 3.
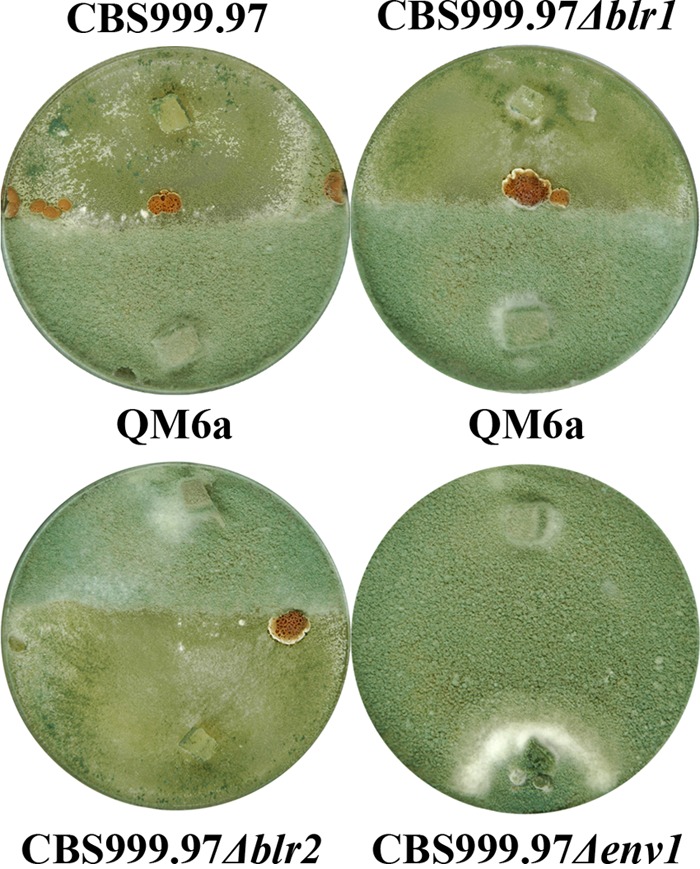
Effect of blr1, blr2, and env1 deletion on female fertility. H. jecorina CBS999.97 (MAT1-1) wild type and Δblr1, Δblr2, and Δenv1 mutant strains in confrontation with female sterile H. jecorina QM6a (MAT1-2). The cross between CBS999.97 Δenv1 × QM6a did not result in fruiting body formation. Strains were grown under daylight conditions and at room temperature.
Regulatory effects of the light signaling machinery.
Peptide pheromone precursor genes and the heptahelical G-protein-coupled receptors of their matured gene product are major determinants of sexual development. Due to the clear effects of BLR1, BLR2, and ENV1 on sexual development, we investigated these factors as possible regulatory targets of the light response machinery on the molecular level. For ENV1, previous data indicate a function in regulation of pheromone levels (54).
We analyzed transcript patterns of peptide pheromone precursor genes (hpp1 and ppg1) as well as pheromone receptor genes (hpr1 and hpr2) in mutant strains of both mating types. Strains were therefore harvested at time points when parallel mating control cultures were in the stage of precontact (3 days), contact of hyphae (4 days), or at the onset of fruiting body formation (6 days).
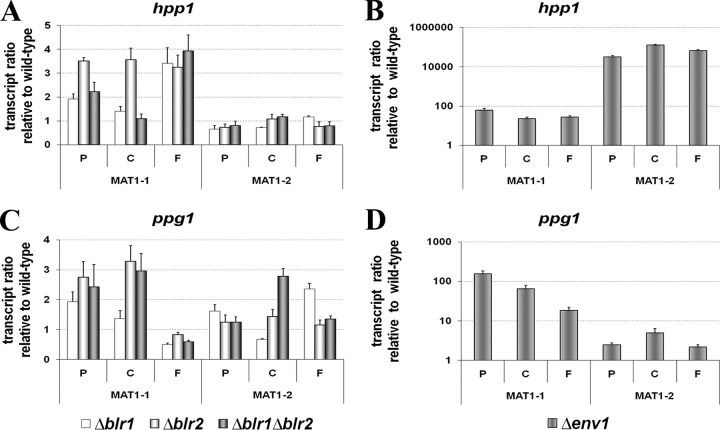
Deletion of blr1, blr2, or env1 consistently enhanced transcription of pheromone genes, albeit to different extents (Fig. 4). With respect to the h-type peptide pheromone precursor gene hpp1, the effect was clearly mating type dependent in Δblr1, Δblr2 (Fig. 4A), and Δenv1 strains (Fig. 4B). The effect of the blue light photoreceptors BLR1 and BLR2 on hpp1 occurred only in a MAT1-1 strain, while hardly any difference relative to the wild type was observed in the MAT1-2 strain. In the Δenv1 strain, transcription of hpp1 was strongly upregulated, even in a MAT1-1 background, reaching several thousand-fold in a MAT1-2 strain. For the alpha-type peptide pheromone precursor gene ppg1, the mating type dependence of gene regulation was less intense for blr1 and blr2, but again the effect of their deletion was clearly positive (Fig. 4C). Lack of env1, however, again caused a strong and clearly mating-type-dependent upregulation of ppg1, albeit to a lesser extent than seen for hpp1 (Fig. 4D).
Fig 4.
Transcription analysis of peptide pheromone precursor genes, as indicated, in H. jecorina CBS999.97 in Δblr1, Δblr2, Δblr1 Δblr2, and Δenv1 strains compared to the wild type in both mating types. Strains were cultivated in daylight at 22°C on malt extract-agar plates, i.e., conditions corresponding to mating assays. At indicated time points of 3, 4, and 6 days, confronted wild-type strains of opposite mating types undergo stages of precontact (P), initial contact between the colonies (C), and the macroscopically visible start of fruiting body formation (F), respectively. RNA was isolated at these time points after inoculation of the indicated strain from mycelia harvested from the growth front of the hyphae.
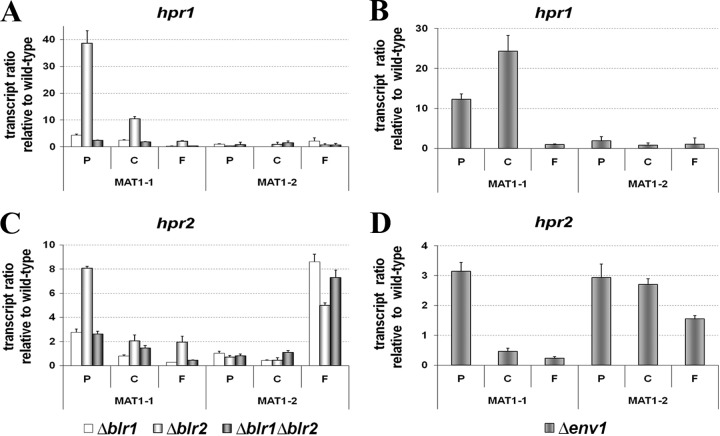
In case of the ste3-type pheromone receptor hpr1, deletion of blr2 has a clearly positive effect on transcript levels in a MAT1-1 strain (Fig. 5A). In contrast, the effect of blr1 is considerably weaker, and transcript levels of hpr1 fall below wild-type levels upon prolonged cultivation (6 days). Also, lack of ENV1 caused considerably increased levels of hpr1 in the MAT1-1 strain (Fig. 5B). In the MAT1-2 strain, however, no effect of ENV1 or BLR1 or BLR2 on hpr1 transcription was detected.
Fig 5.
Transcription analysis of pheromone receptor genes hpr1 and hpr2 in Δblr1, Δblr2, Δblr1 Δblr2 and Δenv1 strains compared to the wild type. Strains were cultivated in daylight at 22°C on malt-agar plates, i.e., conditions corresponding to mating assays. At indicated time points of 3, 4, and 6 days, confronted wild-type strains of opposite mating types undergo stages of precontact (P), initial contact between the colonies (C), and the macroscopically visible start of fruiting body formation (F), respectively. RNA was isolated at these time points after inoculation of the indicated strain from mycelia harvested from the growth front of the hyphae.
As already observed for the alpha-type peptide pheromone precursor gene ppg1, the putative cognate receptor gene hpr2 also shows less strict mating-type-dependent regulation by BLR1, BLR2, and ENV1. The regulation of hpr2 by BLR1 and BLR2 strongly resembles the situation of hpr1, with BLR2 having the strongest effect and levels of hpr2 falling below wild-type levels upon prolonged cultivation in the case of a lack of blr1 in a MAT1-1 strain (Fig. 5C). In a Δenv1 strain, hpr2 was clearly upregulated in a MAT1-2 strain, while in a MAT1-1 strain initial upregulation was followed subsequently by levels lower than those of the wild type (Fig. 5D). Again, as observed for ppg1, the regulatory effect of ENV1 on hpr2 is moderate compared to the strong effect on hpr1 at early time points in the MAT1-1 background.
In general, the effect of BLR2 on transcript levels of pheromones and their receptors appears stronger, but since the effect seen in the double mutant in almost all cases resembles that of BLR1, we conclude that regulation by BLR1 is pivotal in this mechanism.
In order to evaluate if these regulatory patterns also occur on contact with a mating partner, we analyzed transcription of peptide pheromone precursor genes in wild-type and mutant strains of mating type MAT1-1, which showed clear regulatory effects of BLR1, BLR2, and ENV1, in the presence of a wild-type MAT1-2 mating partner. Since to some extent blending of mating partners has to be expected upon fruiting body formation, this time point, corresponding to 6 days, has to be treated with caution. Indeed, comparison of transcript levels of mutant crosses with those of wild-type crosses in the respective same mating type showed that the negative effect of BLR1, BLR2, and ENV1 was also observed under conditions of sexual development for both ppg1 and hpp1 strains (see Fig. S3A and B in the supplemental material). Additionally, the wild-type mating partner (CBS999.97 MAT1-2) showed an enhanced response upon encountering the mutants, especially in case of ppg1 strains (see Fig. 3B).
Light- and mating-type-specific effects of the light response machinery.
Our studies of regulation of sexual development by the light signaling machinery were performed in daylight. We were thus interested whether this effect would be specific for light or if a regulatory function of ENV1, BLR1, or BLR2 would also be detected in darkness, which would indicate a function of these components independent of light.
We therefore analyzed regulation of hpp1, ppg1, hpr1, and hpr2 in the same strains and under the same conditions as described above except that we cultivated the strains in constant darkness. The “contact” time point was used as a test case. Transcript analysis under these conditions did not show the regulatory and mating-type-dependent effects as seen in light (see Fig. S4 and S5 in the supplemental material). In contrast, we found that in darkness gene transcription of hpp1, ppg1, hpr1, and hpr2 is more or less similar to that of the wild type (P values of >0.1). Only for a Δenv1 strain did we detect a minor regulation of hpr1 and ppg1 under these conditions (about 1.5-fold). These data indicate that the function of ENV1, BLR1, and BLR2 in regulation of sexual development is specific to light.
As described above, we did not observe the strong regulation of pheromone precursor and receptor genes by ENV1 in darkness. If this strong regulation, indeed, interferes with sexual development and abolishes female fertility, this defect should be restricted to light conditions, and sexual development should be possible in darkness. In order to evaluate this hypothesis, we first analyzed whether sexual development is possible with these strains in darkness. Indeed, fruiting body formation eventually occurs in crossings with all strains in darkness (albeit with considerable delay compared to strains under light conditions), and if both blr1 and blr2 are absent, the strains behave as in light (see Fig. S6 in the supplemental material).
We also found that fruiting body formation was delayed in darkness both in the wild-type and in strains lacking env1. In a Δenv1 MAT1-1 strain, which has strongly increased levels of both pheromone precursor and receptor genes, fruiting body formation started earlier and more vigorously than in the wild type (Fig. 6A). Fruiting body formation eventually also occurs in the Δenv1 MAT1-2 strain, but the extremely strong upregulation of hpp1 in this strain (>100,000-fold) may here interfere with proper regulation of this process—even upon crossing with the wild type. Crossing of strains lacking env1 in both mating partners did not lead to earlier fruiting body formation in darkness (Fig. 6A). However, in accordance with our hypothesis, sexual development was possible in darkness between two strains lacking env1 (Fig. 6B). Accordingly, transcript levels of both pheromone precursor genes and pheromone receptor genes are at wild-type levels under these conditions.
Fig 6.
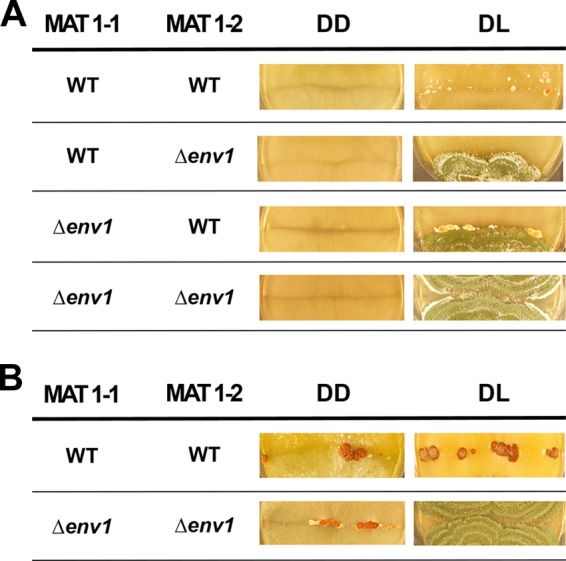
Fruiting body formation in wild-type (WT) and mutant crosses in daylight and darkness. (A) After 8 days of incubation, no fruiting body development is visible in darkness in all strains. (B) After 11 days of incubation in darkness in the wild-type as well as in the Δenv1 mutant strains, fruiting bodies could be detected. Strains were cultivated either in constant darkness (DD) at 22°C, or they were kept under daylight conditions (12:12 cycles; DL).
ENVOY impacts transcription of the mating type gene mat1-2-1.
The transcript analysis described above showed a mating-type-dependent effect for both BLR1/BLR2 and especially for ENV1 biased toward a MAT1-2 strain. Consequently, it is reasonable to assume that the regulatory output of BLR1, BLR2, and ENV1 is at least in part due to regulation of mating type genes. We used mat1-2-1 in MAT1-2 strains as a test case for mating-type-specific regulation by the light response machinery. Indeed, we detected high transcript levels of mat1-2-1 in strains lacking blr1, blr2, or both genes. Similar to the findings for pheromone and receptor genes, the effect was much stronger in the Δenv1 strain (Fig. 7). As a consistently negative effect of BLR1, BLR2, and ENV1 was found, the effect on mat1-2-1 is likely to be exerted by the photoreceptors via regulation of ENV1. The divergent transcript patterns for hpp1, ppg1, hpr1, and hpr2 indicate that the mating-type-dependent involvement of BLR1, BLR2, and ENV1 in regulation of sexual development is not solely exerted via regulation of mating type genes but additionally uses other pathways.
Fig 7.
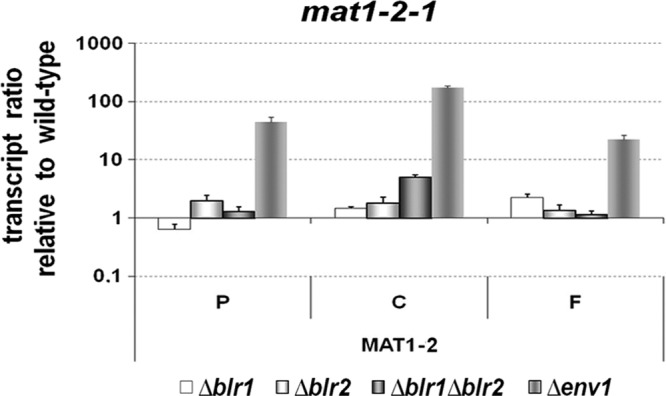
Transcript analysis of the mating type gene mat1-2-1 in Δblr1, Δblr2, Δblr1 Δblr2, and Δenv1 strains compared to the wild type. Strains were cultivated in daylight at 22°C on malt-agar plates, i.e., conditions corresponding to mating assays. At indicated time points of 3, 4, and 6 days, confronted wild-type strains of opposite mating types undergo stages of precontact (P), initial contact between the colonies (C), and the macroscopically visible start of fruiting body formation (F), respectively. RNA was isolated at these time points after inoculation of the indicated strain from mycelia harvested from the growth front of the hyphae.
Since the growth phenotype of H. jecorina QM9414 Δenv1 (5, 52) could not be rescued by deletion of hpp1 in this strain (data not shown), it was concluded that pheromone-induced growth arrest potentially caused by the abnormally high levels of hpp1 in the Δenv1 strain (54) is not the reason for the strongly retarded growth of QM9414 Δenv1 in light. Strong upregulation of mating type genes was, however, shown to result in suppression of vegetative growth and stimulation of sexual development in A. nidulans (45).
DISCUSSION
In several fungi blue light spectra modulate crucial developmental processes by the cooperative action of White Collar (4, 26, 37, 38, 58) and Vivid-like blue light regulators (52, 56). Recently, the effects of BLR1, BLR2, and ENV1 on conidiation and cellulase expression were described to be restricted to blue light in H. jecorina (5).
Both light induction of protoperithecium formation and phototropism of perithecia are subject to blue light induction and are impaired in N. crassa White Collar mutant strains (21, 29). Since neither phototropism nor protoperithecia formation has been observed so far in H. jecorina (54, 57), we could not evaluate if there is an influence on these phenomena.
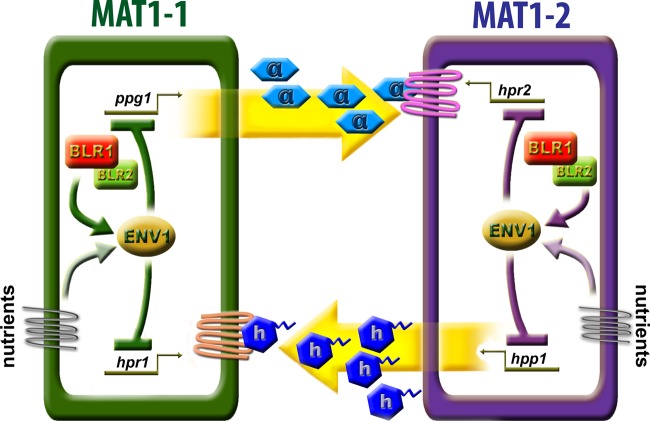
We found altered sexual development in H. jecorina mutants lacking either BLR1, BLR2, or both photoreceptors in daylight. For C. neoformans, regulation of one pheromone gene and one homeodomain gene by BWC1 and BWC2 was observed (26). In N. crassa, an influence of circadian rhythms, which are subject to regulation by WC-1 and WC-2, on the pheromone precursor genes ccg-4 and mfa-1 is in accordance with these results (1). Together with the regulation of hpp1 by ENV1 (54), these findings suggest an involvement of the light response machinery in regulation of genes important for mating (Fig. 8).
Fig 8.
Schematic representation of the regulatory role of the light signaling machinery in MAT1-1 and MAT1-2 strains. Both pheromone precursors and receptors are influenced by the photoreceptors BLR1 and BLR2, likely via their impact on ENV1. The impact of the light response machinery is to a certain extent mating type dependent. In the MAT1-1 strain, the light signaling machinery predominantly acts on ppg1 transcription and on transcript levels of the pheromone receptor presumably receiving the HPP1 (MAT1-2 mating type) signal, hpr1. In contrast, BLR1, BLR2, and ENV1 in the MAT1-2 background more strongly act on transcript levels of hpp1 and the cognate receptor of the MAT1-1 pheromone precursor PPG1, hpr2. This strong effect on hpp1 in this mating type is also reflected in earlier and more vigorous fruiting body formation of the Δenv1 strain with the wild-type strain. The strong effect of ENV1 indicates that this factor may act as a node integrating mating signals with the nutrient signaling pathway. The pale arrows indicate this hypothesis, which remains to be proven.
In this study, we show an involvement of BLR1, BLR2, and ENV1 in fruiting body formation and regulation of pheromone precursor and receptor genes as well as the mating type gene mat1-2-1. Interestingly, the effect of BLR1, BLR2, and ENV1 is mating type dependent. In N. crassa or Sordaria macrospora, regulation of pheromone receptor genes is mating type dependent and influenced by mating type genes (31, 35, 47). This regulation is considerably less distinct in H. jecorina, where both pheromone precursor genes were detected in both mating types (54).
The transcript profiles of hpp1, ppg1, hpr1, hpr2, and mat1-2-1 in Δblr1 and Δblr2 strains suggest that BLR1 and BLR2 predominantly act as a complex and show a consistently negative effect on transcription of these genes. Lack of ENV1 was found to have a considerably stronger effect than that of BLR1 or BLR2. This finding suggests that the effect of BLR1 and BLR2 is mediated by ENV1. Hence, ENV—as a node between pheromone and nutrient signals—may be responsible for cross talk between the respective pathways. The expression patterns in env1 deletion strains, which are partially diverging from a blr1 or blr2 mutant, moreover, indicate that additional signals are integrated at this stage. We conclude that the signal introduced by light via photoreceptors is only one determinant for sexual development as regulated by this cascade. The involvement of ENV1 in the regulatory network of heterotrimeric G-protein signaling (53, 61, 62), which also transmits nutritional signals (39), supports this hypothesis.
Although BLR1 and BLR2 can be expected to act as a complex, our data indicate that they also have individual functions. The effect in mutants lacking blr2 is in most cases stronger than that in mutants lacking blr1. In this respect a constitutive expression of BLR2 and thus higher abundance than BLR1, as suggested for their homologues in N. crassa, could be assumed. Together with the predicted importance of WC-2 for the interaction of WC-1 with FRQ (13), this could explain the more severe effect of deletion of blr2 for regulation of sexual development. In N. crassa, neither the pheromone receptor genes nor the pheromone precursor genes or mating type genes are targets of the White Collar complex (59). Moreover, these genes also do not seem to be light induced in N. crassa (7). Consequently, we assume that in H. jecorina the homologues of these genes are likely to be indirect targets of BLR1 and BLR2. A flat hierarchical network (59) of transcription factors, as suggested for N. crassa, could be targeted by BLR1 and BLR2 in H. jecorina. However, since photoreceptors were shown to have a role in regulating carbon sensing and utilization (17), it cannot be excluded that altered cultivation conditions would also show binding to promoters of genes involved in sexual development.
While some evidence for an involvement of photoreceptor genes in regulation of sexual development in fungi was available, the possible relevance of genes homologous to env1 for this process was suggested only in one previous report on H. jecorina (54). Our data suggest that the strongly negative effect of ENV1 on pheromone precursor and receptor genes and the mating type gene mat1-2-1 are likely to be essential for proper regulation of sexual development in H. jecorina. The considerable deregulation of genes crucial for sexual development in a Δenv1 strain can obviously be compensated by a wild-type mating partner or at least one that does not lack env1 (such as a Δblr1 or Δblr2 strain). In this case the overexpression of pheromone and receptor genes results in earlier and enhanced sexual development (54; also this study). Such an effect can also be observed for Δblr1 and Δblr2 strains, which show certain upregulation of these genes but do not reach levels as high as in a Δenv1 strain. Upon combination of equal amounts of spores, fruiting body formation seems to be initiated earlier and more vigorously than in the wild type as they are formed closer to the center of the petri dish (Fig. 2). In a Δenv1 strain, however, signals specific to both mating types likely exceed saturation levels and consequently inhibit appropriate coordination of developmental programs, which abolishes fruiting body formation.
Since ENV1 is not a transcription factor, its effect is exerted by a downstream signal transduction cascade. This cascade has been shown to involve the heterotrimeric G-protein pathway as well as cAMP signaling in H. jecorina (53, 55, 61, 62). Considering an effect of env1 on G-protein signaling, mate recognition, cell fusion and postfusion processes like ascospore or fruiting body development could be impaired, as shown for N. crassa G-alpha subunits (30, 32). In Saccharomyces cerevisiae or Ustilago maydis, cell fusion in mating is a process that requires pheromone signaling for the polarization of the cytoskeleton, polarisome, and Spitzenkörper (15, 19). Additionally, the pheromone-regulated plasma membrane merger protein PRM1 is required for cell fusion in sexual development of S. cerevisiae and N. crassa (16, 22). Hence, the mating defect of strains lacking env1 may involve perturbed cell fusion.
In summary, we show that the light response machinery with its major constituents BLR1, BLR2, and ENV1 plays an important role in sexual development of H. jecorina. BLR1, BLR2, and ENV1 negatively influence expression of pheromone precursor and receptor genes. Alleviation of the strong repression exerted by ENV1 even disables fruiting body formation, likely due to signal strengths exceeding saturation levels and/or defects in cell fusion. The mating-type-dependent effects of these factors suggest a contribution to determination of sexual identity signaling in this heterothallic, hermaphroditic organism.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Freitag and Kyle Pomraning for providing sequence information of H. jecorina CBS999.97 prior to publication of the genome. Sequencing of CBS999.97 was done at the U.S. Joint Genome Institute, Department of Energy.
This work was supported by the Austrian Research Fund (FWF), projects P20004 and V152-B20, to M.S.
Footnotes
Published ahead of print 11 May 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Bobrowicz P, Pawlak R, Correa A, Bell-Pedersen D, Ebbole DJ. 2002. The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol. Microbiol. 45:795–804 [DOI] [PubMed] [Google Scholar]
- 2. Bölker M. 1998. Sex and crime: heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 25:143–156 [DOI] [PubMed] [Google Scholar]
- 3. Bölker M, Kahmann R. 1993. Sexual pheromones and mating responses in fungi. Plant Cell 5:1461–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casas-Flores S, Rios-Momberg M, Bibbins M, Ponce-Noyola P, Herrera-Estrella A. 2004. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 150:3561–3569 [DOI] [PubMed] [Google Scholar]
- 5. Castellanos F, et al. 2010. Crucial factors of the light perception machinery and their impact on growth and cellulase gene transcription in Trichoderma reesei. Fungal Genet. Biol. 47:468–476 [DOI] [PubMed] [Google Scholar]
- 6. Chen CH, DeMay BS, Gladfelter AS, Dunlap JC, Loros JJ. 2010. Physical interaction between VIVID and white collar complex regulates photoadaptation in Neurospora. Proc. Natl. Acad. Sci. U. S. A. 107:16715–16720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen CH, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. 2009. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 28:1029–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159 [DOI] [PubMed] [Google Scholar]
- 9. Coppin E, de Renty C, Debuchy R. 2005. The function of the coding sequences for the putative pheromone precursors in Podospora anserina is restricted to fertilization. Eukaryot. Cell 4:407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corrochano LM. 2007. Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochem. Photobiol. Sci. 6:725–736 [DOI] [PubMed] [Google Scholar]
- 11. Debuchy R, Berteaux-Lecellier V, Silar P. 2010. Mating systems and sexual morphogenesis in ascomycetes, p 501–535 In Borkovich KA, Ebbole DJ. (ed), Cellular and molecular biology of filamentous fungi. ASM Press, Washington, DC [Google Scholar]
- 12. Debuchy R, Turgeon BG. 2006. Mating-type structure, evolution and function in euascomycetes, p 293–323 In Kues U, Fischer R. (ed), The Mycota I. Springer-Verlag, Berlin, Germany [Google Scholar]
- 13. Denault DL, Loros JJ, Dunlap JC. 2001. WC-2 mediates WC-1-FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J. 20:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dobzhansky T. 1973. Nothing in biology makes sense except in the light of evolution. Am. Biol. Teacher 35:125–129 [Google Scholar]
- 15. Etienne-Manneville S. 2004. Cdc42-the centre of polarity. J. Cell Sci. 117:1291–1300 [DOI] [PubMed] [Google Scholar]
- 16. Fleissner A, Diamond S, Glass NL. 2009. The Saccharomyces cerevisiae PRM1 homolog in Neurospora crassa is involved in vegetative and sexual cell fusion events but also has postfertilization functions. Genetics 181:497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedl MA, Kubicek CP, Druzhinina IS. 2008. Carbon source dependence and photostimulation of conidiation in Hypocrea atroviridis. Appl. Environ. Microbiol. 74:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Froehlich AC, Loros JJ, Dunlap JC. 2003. Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc. Natl. Acad. Sci. U. S. A. 100:5914–5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuchs U, Hause G, Schuchardt I, Steinberg G. 2006. Endocytosis is essential for pathogenic development in the corn smut fungus Ustilago maydis. Plant Cell 18:2066–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gruber F, Visser J, Kubicek CP, de Graaff LH. 1990. The development of a heterologous transformation system for the cellulolytic fungus Trichoderma reesei based on a pyrG-negative mutant strain. Curr. Genet. 18:71–76 [DOI] [PubMed] [Google Scholar]
- 21. Harding RW, Melles S. 1983. Genetic analysis of phototropism of Neurospora crassa perithecial beaks using White Collar and Albino mutants. Plant Physiol. 72:996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heiman MG, Walter P. 2000. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J. Cell Biol. 151:719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heintzen C, Loros JJ, Dunlap JC. 2001. The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell 104:453–464 [DOI] [PubMed] [Google Scholar]
- 24. Herrera-Estrella A, Horwitz BA. 2007. Looking through the eyes of fungi: molecular genetics of photoreception. Mol. Microbiol. 64:5–15 [DOI] [PubMed] [Google Scholar]
- 25. Hunt SM, Thompson S, Elvin M, Heintzen C. 2010. VIVID interacts with the WHITE COLLAR complex and FREQUENCY-interacting RNA helicase to alter light and clock responses in Neurospora. Proc. Natl. Acad. Sci. U. S. A. 107:16709–16714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Idnurm A, Heitman J. 2005. Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol. 3:e95 doi: 10.1371/journal.pbio.0030095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Idnurm A, et al. 2006. The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc. Natl. Acad. Sci. U. S. A. 103:4546–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Idnurm A, Verma S, Corrochano LM. 2010. A glimpse into the basis of vision in the kingdom Mycota. Fungal Genet. Biol. 47:881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Innocenti FD, Pohl U, Russo VE. 1983. Photoinduction of protoperithecia in Neurospora crassa by blue light. Photochem. Photobiol. 37:49–51 [DOI] [PubMed] [Google Scholar]
- 30. Ivey FD, Hodge PN, Turner GE, Borkovich KA. 1996. The G alpha i homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 7:1283–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karlsson M, Nygren K, Johannesson H. 2008. The evolution of the pheromonal signal system and its potential role for reproductive isolation in heterothallic Neurospora. Mol. Biol. Evol. 25:168–178 [DOI] [PubMed] [Google Scholar]
- 32. Kays AM, Rowley PS, Baasiri RA, Borkovich KA. 2000. Regulation of conidiation and adenylyl cyclase levels by the Gα protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20:7693–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim H, Borkovich KA. 2004. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 52:1781–1798 [DOI] [PubMed] [Google Scholar]
- 34. Kim H, Borkovich KA. 2006. Pheromones are essential for male fertility and sufficient to direct chemotropic polarized growth of trichogynes during mating in Neurospora crassa. Eukaryot. Cell 5:544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klix V, et al. 2010. Functional characterization of MAT1-1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and nonessential sexual regulators. Eukaryot. Cell 9:894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuhls K, et al. 1996. Molecular evidence that the asexual industrial fungus Trichoderma reesei is a clonal derivative of the ascomycete Hypocrea jecorina. Proc. Natl. Acad. Sci. U. S. A. 93:7755–7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lauter FR, Russo VE. 1991. Blue light induction of conidiation-specific genes in Neurospora crassa. Nucleic Acids Res. 19:6883–6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee K, et al. 2006. Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae. Fungal Genet. Biol. 43:694–706 [DOI] [PubMed] [Google Scholar]
- 39. Li L, Wright SJ, Krystofova S, Park G, Borkovich KA. 2007. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 61:423–452 [DOI] [PubMed] [Google Scholar]
- 40. Lieckfeldt E, Kullnig CM, Samuels GJ, Kubicek CP. 2000. Sexually competent, sucrose- and nitrate-assimilating strains of Hypocrea jecorina (Trichoderma reesei) from South American soils. Mycologia 92:374–380 [Google Scholar]
- 41. Liu D, Coloe S, Baird R, Pederson J. 2000. Rapid mini-preparation of fungal DNA for PCR. J. Clin. Microbiol. 38:471 (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mach RL, Schindler M, Kubicek CP. 1994. Transformation of Trichoderma reesei based on hygromycin B resistance using homologous expression signals. Curr. Genet. 25:567–570 [DOI] [PubMed] [Google Scholar]
- 43. Martinez D, et al. 2008. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat. Biotechnol. 26:553–560 [DOI] [PubMed] [Google Scholar]
- 44. Metzenberg RL, Glass NL. 1990. Mating type and mating strategies in Neurospora. Bioessays 12:53–59 [DOI] [PubMed] [Google Scholar]
- 45. Paoletti M, et al. 2007. Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr. Biol. 17:1384–1389 [DOI] [PubMed] [Google Scholar]
- 46. Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36 doi: 10.1093/nar/30.9.e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poggeler S. 2001. Mating-type genes for classical strain improvements of ascomycetes. Appl. Microbiol. Biotechnol. 56:589–601 [DOI] [PubMed] [Google Scholar]
- 48. Purschwitz J, et al. 2008. Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr. Biol. 18:255–259 [DOI] [PubMed] [Google Scholar]
- 49. Schafmeier T, Diernfellner AC. 2011. Light input and processing in the circadian clock of Neurospora. FEBS Lett. 585:1467–1473 [DOI] [PubMed] [Google Scholar]
- 50. Schmoll M. 2008. The information highways of a biotechnological workhorse—signal transduction in Hypocrea jecorina. BMC Genomics 9:430 doi: 10.1186/1471-2164-9-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmoll M, Esquivel-Naranjo EU, Herrera-Estrella A. 2010. Trichoderma in the light of day—physiology and development. Fungal Genet. Biol. 47:909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmoll M, Franchi L, Kubicek CP. 2005. Envoy, a PAS/LOV domain protein of Hypocrea jecorina (anamorph Trichoderma reesei), modulates cellulase gene transcription in response to light. Eukaryot. Cell 4:1998–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmoll M, Schuster A, Rdo Nascimento Silva, Kubicek CP. 2009. The G-alpha protein GNA3 of Hypocrea jecorina (anamorph Trichoderma reesei) regulates cellulase gene expression in the presence of light. Eukaryot. Cell 8:410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schmoll M, Seibel C, Tisch D, Dorrer M, Kubicek CP. 2010. A novel class of peptide pheromone precursors in ascomycetous fungi. Mol. Microbiol. 77:1483–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schuster A, Tisch D, Seidl-Seiboth V, Kubicek CP, Schmoll M. 2012. Roles of protein kinase A and adenylate cyclase in light-modulated cellulase regulation in Trichoderma reesei. Appl. Environ. Microbiol. 78:2168–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwerdtfeger C, Linden H. 2003. VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation. EMBO J. 22:4846–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seidl V, Seibel C, Kubicek CP, Schmoll M. 2009. Sexual development in the industrial workhorse Trichoderma reesei. Proc. Natl. Acad. Sci. U. S. A. 106:13909–13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Silva F, Torres-Martinez S, Garre V. 2006. Distinct white collar-1 genes control specific light responses in Mucor circinelloides. Mol. Microbiol. 61:1023–1037 [DOI] [PubMed] [Google Scholar]
- 59. Smith KM, et al. 2010. Transcription factors in light and circadian clock signaling networks revealed by genome wide mapping of direct targets for Neurospora white collar complex. Eukaryot. Cell 9:1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Terashima K, Yuki K, Muraguchi H, Akiyama M, Kamada T. 2005. The dst1 gene involved in mushroom photomorphogenesis of Coprinus cinereus encodes a putative photoreceptor for blue light. Genetics 171:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tisch D, Kubicek CP, Schmoll M. 2011. New insights into the mechanism of light modulated signaling by heterotrimeric G-proteins: ENVOY acts on gna1 and gna3 and adjusts cAMP levels in Trichoderma reesei (Hypocrea jecorina). Fungal Genet. Biol. 48:631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tisch D, Kubicek CP, Schmoll M. 2011. The phosducin-like protein PhLP1 impacts regulation of glycoside hydrolases and light response in Trichoderma reesei. BMC Genomics 12:613 doi: 10.1186/1471-2164-12-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tisch D, Schmoll M. 2010. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 85:1259–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vitikainen M, et al. 2010. Array comparative genomic hybridization analysis of Trichoderma reesei strains with enhanced cellulase production properties. BMC Genomics 11:441 doi: 10.1186/1471-2164-11-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.