Abstract
Acinetobacter baumannii has recently emerged as a highly troublesome nosocomial pathogen, especially in patients in intensive care units and in those undergoing mechanical ventilation. We have identified a surface protein adhesin of A. baumannii, designated the Acinetobacter trimeric autotransporter (Ata), that contains all of the typical features of trimeric autotransporters (TA), including a long signal peptide followed by an N-terminal, surface-exposed passenger domain and a C-terminal domain encoding 4 β-strands. To demonstrate that Ata encoded a TA, we created a fusion protein in which we replaced the entire passenger domain of Ata with the epitope tag V5, which can be tracked with specific monoclonal antibodies, and demonstrated that the C-terminal 101 amino acids of Ata were capable of exporting the heterologous V5 tag to the surface of A. baumannii in a trimeric form. We found that Ata played a role in biofilm formation and bound to various extracellular matrix/basal membrane (ECM/BM) components, including collagen types I, III, IV, and V and laminin. Moreover, Ata mediated the adhesion of whole A. baumannii cells to immobilized collagen type IV and played a role in the survival of A. baumannii in a lethal model of systemic infection in immunocompetent mice. Taken together, these results reveal that Ata is a TA of A. baumannii involved in virulence, including biofilm formation, binding to ECM/BM proteins, mediating the adhesion of A. baumannii cells to collagen type IV, and contributing to the survival of A. baumannii in a mouse model of lethal infection.
INTRODUCTION
Acinetobacter baumannii is a Gram-negative opportunistic pathogen that has recently emerged as a significant cause of nosocomial infections worldwide (54). Multidrug-resistant (MDR) A. baumannii infections tend to occur in debilitated patients, especially those in intensive care units (ICUs) and/or in the context of serious underlying disease, in patients subjected to invasive procedures, such as mechanical ventilation, or those undergoing long hospitalizations or being treated with broad-spectrum antibiotics (25, 39). The most common clinical manifestations of A. baumannii infections in the ICUs are ventilator-associated pneumonia (VAP) and bacteremia, which are associated with morbidity and mortality rates as high as 52% (12, 64). Other hospital-acquired A. baumannii infections include urinary tract infections, wound infections, and meningitis (55). In addition, A. baumannii infections have been a recurrent problem during wars and natural disasters (53, 76), and recently MDR A. baumannii has become a major pathogen found in combat-associated wounds in military personnel deployed to Iraq or Afghanistan (18, 29). Problematically, 89% of Acinetobacter strains isolated from patients injured in Iraq and Afghanistan were resistant to at least two major classes of antibiotics (72). The lack of new antibiotics to treat MDR A. baumannii infections has led the Infectious Disease Society of America (IDSA) to describe A. baumannii as “an emblematic case of the mismatch between unmet medical needs and the current antimicrobial research and development pipeline” (48).
Although A. baumannii is a pathogen of considerable health care interest, surprisingly little is known about this organism's virulence determinants, bacterial regulatory networks, and host defense mechanisms. Recent DNA genome sequencing revealed that this organism harbors an extraordinary number of putative virulence-associated genes and elements homologous to the Legionella/Coxiella type IV secretion apparatus (66). Several virulence determinants involved in biofilm formation (24, 41), iron acquisition (79), lipopolysaccharide (LPS) synthesis (62), resistance to the bactericidal activity of human serum (33), adherence, host cell invasion (11, 42), and death (9, 10, 35) have been reported in previous studies. While these presumably encompass just a minor fraction of elements involved in A. baumannii virulence, new approaches are needed to expand our understanding of the basic features of this organism which will ultimately be essential to control the spread of A. baumannii infections and to develop effective means to prevent and/or treat this harmful pathogen.
To gain greater insight into A. baumannii virulence factors, we identified an ORF in A. baumannii ATCC 17978, A1S_1032, that codes for a protein belonging to the trimeric autotransporter (TA) family, which was termed the Acinetobacter trimeric autotransporter, or Ata. TAs encompass a large family of proteins produced by many Gram-negative bacteria that contain a C-terminal domain that is believed to form the trimeric β-barrel that allows for the transport of the N-terminal passenger domain to the bacterial cell surface. These proteins form lollipop-shaped surface projections on the bacterial surface and have been extensively studied as vaccine candidates against multiple pathogens (52). Two relevant examples of TAs as vaccine components include the Neisseria meningitidis NadA autotransporter (AT) which is undergoing phase III clinical evaluation against serogroup B meningococcal disease (65), and the conventional AT pertactin, produced by Bordetella pertussis strains (30), that is a component of four out of the five pertussis vaccines currently licensed for use in the United States (1).
In this report, we conducted an in silico structural analysis of the Ata protein and investigated its role in biofilm formation, binding to extracellular matrix/basal membrane (ECM/BM) proteins, and adhesion of whole A. baumannii cells to collagen type IV, as well as in virulence in a mouse model of lethal infection in immunocompetent mice.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All strains were routinely grown in lysogeny broth (LB) with the exception of Escherichia coli LMG194, which was grown in M9 minimal medium supplemented with 0.25% Casamino Acids. Carbenicillin and kanamycin were added to the growth medium at 50 μg/ml each.
Table 1.
Bacterial strains and plasmids used in this work
| Strains and plasmids | Description | Source or reference |
|---|---|---|
| A. baumannii strains | ||
| ATCC 17978 | Reference sequenced strain, susceptible to antibiotics | ATCC |
| ATCC 17978 Δata | ATCC 17978 derivative with an in-frame deletion of ata | This work |
| ATCC 17978 Δata-c | ATCC 17978 Δata complemented with pBAD-Ata | This work |
| ATCC 17978 Δata-pLVB-Ata | ATCC 17978 Δata complemented with pLVB-Ata | This work |
| ATCC 17978 Δata-pBAD18kan-Ori | ATCC 17978 Δata complemented with pBAD18kan-Ori | This work |
| E. coli strains | ||
| DH5α λpir | λpir lysogen of DH5α | Laboratory strain |
| LMG194 | F− ΔlacX74 galE thi rpsL ΔphoA (PvuII) Δara714 leu::Tn10 | Invitrogen |
| Top10 | Used as host for gene cloning | Invitrogen |
| Plasmids | ||
| pSSK10 | Derived from pDS132 (R6K ori mobRP4 cat sacB), Kmr | 66 |
| pCR-XL-TOPO | PCR cloning plasmid, Kmr | Invitrogen |
| pΔata | pSSK10 carrying an ∼3.4-kb fragment of the 5′ and 3′ flanking regions of ata | This work |
| pBAD18kan-Ori | E. coli-Acinetobacter shuttle vector | 8 |
| pBAD-Ata | A. baumannii ATCC 17978 promoterless ata gene (5,682 bp) cloned in pBAD18Kan-Ori | This work |
| pBAD-TOPO-TA | Protein expression vector carrying C-terminal V5-6×His tag; Apr | This work |
| pBAD-SP | Promoter and signal peptide of ata cloned in pBAD-TOPO-TA | This work |
| pBAD-TD | Translocator domain of ata cloned in pBAD18Kan-Ori | This work |
| pAta-V5-6×His | SP-V5-6×His fragment cloned in pBAD-TD | This work |
| pAta | pBAD-TOPO derivative carrying 5,274-bp coding sequence coding for the passenger domain of ata | This work |
| pTOPO-XL-Ata | A. baumannii ATCC 17978 ata gene with its own promoter (5,789 bp) cloned in pCR-XL-TOPO | This work |
| pLVB-Ata | ata from pTOPO-XL-Ata cloned in pBAD18kan-Ori | This work |
Bioinformatics.
The signal peptide and secondary structure features of Ata were predicted using the SignalP 3.0 program, available at http://www.cbs.dtu.dk/services/SignalP/ (2), and the PSIPRED software (38), respectively.
A model of the three-dimensional (3D) structure of the C-terminal 101 Ata residues was built by the combined use of homology modeling and molecular mechanics. A homology model of the Ata membrane anchor was built in CPH-models (51) using the crystal structure of the membrane anchor of Haemophilus influenzae Hia protein as a template. DeepView software (27) was used for construction of the homology model and for manipulation of torsion angles.
Construction of strains.
We constructed a vector to generate an in-frame deletion of ata in A. baumannii ATCC 17978 in the following manner. An approximately 3.4-kb fragment of the 5′ and 3′ regions of ata were amplified by PCR using the primer pairs NdeI-Ata-3.4-F/MluI-Ata-R and MluI-Ata-F/XhoI-Ata-3.4-R, respectively (Table 2). The fragments were then digested with NdeI/MluI and MluI/XhoI and ligated between the NdeI and XhoI sites of pSSK10 (68). The resulting plasmid, pΔata, contained approximately 3.4 kb of DNA flanking regions on both sides of ata. ATCC 17978 Δata, harboring an in-frame deletion of ata, was made by first transforming pΔata into A. baumannii strain ATCC 17978 by electroporation. Clones in which the plasmid was integrated into the chromosome were selected on LB agar plates supplemented with kanamycin. A. baumannii ATCC 17978 merodiploids were grown for 2 days in LB without antibiotic selection, plated on LB agar supplemented with 10% sucrose, and grown at 25°C for 48 h to select for cells that had lost the plasmid after homologous recombination. Kanamycin-sensitive, sucrose-resistant colonies were screened by PCR using primers Ata-F-out/Ata-R-out to confirm deletion of ata. In-frame deletion of the ata gene was confirmed by sequencing using primers Ata-F-out/Ata-R-out. The resulting strain contains the first 13 and last 3 amino acids (aa) of the ata product in frame.
Table 2.
Oligonucleotide primers used in this study
| Primer name | Sequence (5′ to 3′)a | Purpose |
|---|---|---|
| NdeI-Ata-3.4-F | GGGCCCCATATGGGGCATAAAAAACGCAGTCCAAAAAACGGTG | Clone 5′ upstream region of ata |
| MluI-Ata-R | GGGCCCACGCGTAATCGAAGCATTCCAAATGACCTTGTAAAC | Clone 5′ upstream region of ata |
| MluI-Ata-F | GGGCCCACGCGTATTAATTAAGAAACTGGTTGGGAGGGCAAT | Clone 3′ downstream region of ata |
| XhoI-Ata-3.4-R | GGGCCCCTCGAGAGAGCTTCGGCTGATTGAACAAACTTTTAGG | Clone 3′ downstream region of ata |
| Ata-F-out | ATTTATTCAATTAGGATGCCGCCTCTTTTTTTGG | Confirm in-frame deletion of ata |
| Ata-R-out | CTAAGACTAAGCTTTGGATTGTTTTGTTCATCTC | Confirm in-frame deletion of ata |
| XbaI-Ata-C-F | GGGCCCTCTAGATTGGTCGTTGAGTTCG | Complementation of ata |
| SphI-Ata-C-R | GGGCCCGCATGCTTAATTAATCACACCACTAATACCA | Complementation of ata |
| Ata-SP-F | TATTTGTCTGAGAAGTTTTATGAATAAAGTTTACAAGG | Amplification of promoter and signal peptide of ata |
| Ata-SP-R | AGCAAAAGCATTTGGAGCAAAACAAATTACACCC | Amplification of promoter and signal peptide of ata |
| KpnI-TD-F | GGGCCCGGTACCAACAAAATTACCAATCTGGGTGATCAGTTACAA | Amplification of ata translocator domain |
| SphI-TD-R | GGGCCCGCATGCTTAATTAATCACACCACTAATACCAACGCGGAC | Amplification of ata translocator domain |
| SacI-SP-V5-6×His-F | GGGCCCGAGCTCTATTTGTCTGAGAAGTTTTATGAATAAAGTTTACAAG | Amplification of ata promoter-signal peptide-V5-6×His |
| KpnI-SP-V5-6×His-R | GGGCCCGGTACCATGGTGATGGTGATGATGACCGGTAC | Amplification of ata promoter-signal peptide-V5-6×His |
| NdeI-Ata-PD-F | GGGCCCCATATGGGGACAAATACCGAAGGGGGAATAG | Cloning passenger domain of ata |
| XhoI-Ata-PD-R | GGGCCCCTCGAGTTCTAAGGCCATGGCAGCG | Cloning passenger domain of ata |
| Ata-C2-F | ATTTATTCAATTAGGATGCCGCCTCTTTTTTTGG | Complementation of ata with its native promoter |
| Ata-C2-R | CTAAGACTAAGCTTTGGATTGTTTTGTTCATCTC | Complementation of ata with its native promoter |
| pBAD-R | CCGCCAGGCAAATTCTGTTTTATCAG | Confirm orientation of ata in pLVB-Ata vector |
Restriction sites are underlined.
Complementation plasmid pBAD-Ata was constructed as follows. The ata gene was amplified by PCR with the KAPA HiFi DNA polymerase (Kapa Biosystems) from the chromosomal DNA of A. baumannii ATCC 17978 using primers XbaI-Ata-C-F and SphI-Ata-C-R. The resulting 5,682-bp DNA fragment was then digested with XbaI/SphI ligated between the XbaI and SphI sites of pBAD18kan-Ori to produce the plasmid pBAD-Ata, which was then transformed into A. baumannii ATCC 17978 Δata by electroporation, generating strain ATCC 17978 Δata-c.
To construct the complementation plasmid pLVB-Ata containing ata with its own promoter, the ata gene was amplified by PCR with the KAPA HiFi DNA polymerase (Kapa Biosystems) from the chromosomal DNA of A. baumannii ATCC 17978 using primers Ata-C2-F and Ata-C2-R. Following amplification, the resulting 5,789-bp PCR product was mixed with 2.5 U of Taq DNA polymerase (Invitrogen) and 2 mM dATP in 1× Taq buffer and incubated at 72°C for 10 min to allow addition of 3′ A-overhangs before ata was subcloned into the pCR-XL-TOPO vector to generate pTOPO-XL-Ata. Ligation of DNA into pCR-XL-TOPO and subsequent transformation into E. coli TOP10 cells were performed according to the manufacturer's protocol. The ata gene was then isolated from pTOPO-XL-Ata after digestion with EcoRI and cloned into the EcoRI-digested shuttle vector pBAD18kan-Ori to generate the complementation vector pLVB-Ata. Finally, the pLVB-Ata plasmid was introduced in E. coli TOP10 cells by electroporation. Primers pBAD-R and Ata-C2-R were then used to deliberately select a clone where the orientation of ata in pBAD18kan-Ori was in the opposite direction of the pBAD promoter and therefore where the expression of ata was controlled by its own native promoter rather than by the pBAD promoter. pBAD18kan-Ori was chosen to construct the complementation plasmid pLVB-Ata mainly for its ability to replicate in E. coli-A. baumannii and its stability in vivo rather than for its arabinose-inducible pBAD promoter. The pLVB-Ata plasmid was finally electroporated into A. baumannii Δata cells to generate the complemented strain A. baumannii Δata-pLVB-Ata.
The control strain A. baumannii Δata-pBAD18kan-Ori was prepared by electroporating the empty vector pBAD18kan-Ori into the mutant strain A. baumannii ATCC 17978 Δata.
Replacement of the Ata passenger domain with the V5-6×His epitope tags.
The V5-6×His peptide was fused to the C-terminal region of Ata according to the following steps. First, a fragment containing the promoter and signal sequence of ata was amplified by PCR, with primers Ata-SP-F and Ata-SP-R, and ligated upstream of the V5-6×His sequence contained in the plasmid pBAD-TOPO-TA to generate the plasmid pBAD-SP. A second PCR amplicon containing the predicted translocator domain of ata was amplified with primers SphI-TD-R/KpnI-TD-F digested with SphI and KpnI and cloned into SphI/KpnI digested pBAD18Kan-Ori to generate pBAD-TD. The entire ata promoter-SP-V5-6×His fragment then was amplified from pBAD-SP with primers SacI-SP-V5-6×His-F and KpnI-SP-V5-6×His-R, cloned in the SacI/KpnI-digested pPBAD-TD to generate fusion plasmid pAta-V5-6×His, and introduced into E. coli LMG194 by electroporation. All constructs were examined by nucleotide sequencing to ensure that the inserts were in frame and that all PCR products were free of mutations.
Immunoblotting.
E. coli LMG194 pAta-V5-6×His cells were grown in M9 plus 0.25% Casamino Acids and induced with either 0.2% arabinose or 0.2% glucose for 4 h. Outer membrane fractions of whole-cell bacterial sonicates were prepared on the basis of Sarkosyl insolubility as described previously (19). The samples were resuspended in SDS-PAGE loading buffer, boiled for 5 min, and separated on a 10% Bis-Tris gel (Invitrogen) using morpholineethanesulfonic acid (MES) running buffer. Proteins were transferred to Invitrolon polyvinylidene difluoride (PVDF) membranes (Invitrogen) and blocked with phosphate-buffered saline (PBS)–5% nonfat dry milk for 1 h at room temperature. Blocked membranes were incubated for 1 h with mouse IgG1 anti-V5 monoclonal antibody (MAb) (Santa Cruz Biotechnology) diluted 1:1,000 in PBS plus 0.05% Tween 20 (PBST)–2% nonfat dry milk. V5 epitopes were detected with goat anti-mouse IgG (Southern Biotech) (1:1,000 dilution in PBST–2% nonfat dry milk) by enhanced chemiluminescence (Western blotting reagent; Santa Cruz Biotechnology). Some arabinose-induced pAta-V5-6×His outer membrane protein (OMP) samples were also subjected to depolymerization by overnight treatment with 70% formic acid at room temperature as described elsewhere (69) and then analyzed by Western blotting as described before.
To investigate the levels of Ata expression among clinical isolates by Western blotting, cultures of eight A. baumannii clinical strains (ATCC 17978, S19, I30, N10, I42, I25, I31, and I28) as well as the ata-negative strain ATCC 17978 Δata were grown in LB to an optical density at 650 nm (OD650) of 0.025, and OMPs were extracted and processed as described above for the strain E. coli LMG194 pAta-V5-6×His. Purified OMPs (2.5 μg) were resolved on a 4 to 12% Bis-Tris gel using morpholinepropanesulfonic acid (MOPS) running buffer, transferred to PVDF membranes, and analyzed by Western blotting as before but using rabbit sera raised to Ata (1:1,000 dilution) as the primary antibody and a secondary goat anti-rabbit IgG (Southern Biotech) diluted 1:1,000.
Purification of recombinant Ata.
The passenger domain of Ata was PCR amplified from the genomic DNA of A. baumannii strain ATCC 17978 with primers NdeI-Ata-PD-F/XhoI-Ata-PD-R, digested with NdeI and XhoI, and cloned into NdeI/XhoI-digested pBAD-TOPO-TA (Invitrogen) to generate pAta. Recombinant protein expression from pAta was induced in E. coli TOP10 cultured to an OD650 of 0.4 in LB containing carbenicillin by addition of 2% l-arabinose and further incubation for 4 h. Soluble His-tagged Ata was purified by affinity chromatography on a nickel column according to the protocols of the manufacturer (Novagen). The purity was checked by SDS-PAGE stained with Coomassie blue, and protein content was quantified with the Bradford assay (3).
Biofilm formation.
A. baumannii overnight cultures were diluted 1:100 in 2 ml of either fresh LB (ATCC 17978 and Δata strains) or LB plus 2% arabinose or 0.2% glucose (Δata-c strain), and biofilms were allowed to form in polystyrene plastic tubes under static conditions for 24 h. At this point, the cultures were monitored for planktonic growth by measuring the OD650. Once the medium and planktonic cells were removed, biofilms were gently washed with PBS to remove loosely attached cells, dried, and stained with a 0.2% crystal violet solution. After biofilms were rinsed with deionized water, the biomass was quantified by adding 2 ml of 100% ethanol and vortexing until the stained biomass was completely removed from the tube surface, and the OD595 was determined (8). Biofilm formation was expressed as a normalized value: OD595 divided by the planktonic cell density (OD650) of each tube.
Flow-cytometric analysis of bacterial production of Ata.
Flow cytometry was used to examine the production of Ata by A. baumannii ATCC 17978 at various phases of growth, from early exponential to stationary phase, to optimize the induction/repression conditions from the complementation vector pBAD-Ata as well as to compare Ata production in wild-type A. baumannii ATCC 17978 to that in Δata, Δata-pLVB-Ata, and Δata-pBAD18kan-Ori strains and among A. baumannii clinical isolates.
To investigate the production of Ata during all phases of growth, wild-type A. baumannii ATCC 17978 was grown to an OD650 of 0.025, 0.1, 0.4, 0.8, and 1.2, whereas for the optimizing of Ata production from pBAD-Ata, Δata-c cells were grown to an OD650 of 0.4 and induced for 2 h with arabinose or glucose at concentrations ranging from 0.2 to 2%. After cells were washed with PBS, the OD650 of all samples was adjusted to 0.4, and then they were incubated for 1 h at room temperature with rabbit sera raised to recombinant Ata (1:1,000 dilution in PBS) that had been previously absorbed with the A. baumannii ATCC 17978 Δata strain to remove any reactivity to antigens other than Ata. Following three washes with PBST, cells were incubated for 30 min with Alexa 488-conjugated goat antibody to rabbit IgG (Invitrogen) diluted 1:250 in PBS. Bacterial cells were washed three times with PBST and then analyzed by a FACSCalibur flow cytometer (Becton, Dickinson). We found that the induction of A. baumannii ATCC 17978 Δata-c with 2% arabinose for 2 h resulted in high levels of surface Ata, comparable to those seen in the wild-type strain grown to an OD650 of 0.025, whereas growth with 0.2% glucose was optimal for maximum repression of ata expression from the recombinant plasmid. Therefore, these concentrations of arabinose and glucose were used as our standard induction/repression conditions for the complementation vector pBAD-Ata throughout this study (data not shown).
Finally, for testing the levels of Ata in A. baumannii ATCC 17978, Δata, Δata-pLVB-Ata, and Δata-pBAD18kan-Ori and among A. baumannii clinical isolates, bacteria were grown to an OD650 of 0.025 and washed with PBS. After the OD650 was adjusted to 0.4, samples were processed as described above for analysis by fluorescence-activated cell sorting (FACS).
Quantitative real-time PCR (qRT-PCR).
A. baumannii ATCC 17978 cells grown to early log (OD650 of 0.1) and mid-log (OD650 of 0.4) phase were harvested with RNA Protect (Qiagen), and total RNA was isolated using the RNeasy kit (Qiagen) according to the manufacturer's recommendations. Genomic DNA was removed by incubating the samples twice with Turbo DNase at 37°C for 30 min according to the manufacturer's instructions (Ambion). One microgram of RNA was used to synthesize cDNA using the SuperScript III first-strand synthesis system (Invitrogen), and quantitative analysis of cDNAs was performed with a Light Cycler 480 system instrument using SYBR green I master mix (Applied Biosystems) to detect PCR products. Primers for PCR were designed to amplify a product of approximately 200 bp with the primer3 software. Reactions were set up according to the manufacturer's instructions, and three replicates for each sample were included. The amplification conditions were 95°C for 5 min (ramp rate of 4.5°C/s), followed by 45 cycles of 95°C for 10 s (ramp rate of 4.5°C/s), 55°C for 20 s (ramp rate of 2.5°C/s), and 72°C for 30 s (ramp rate of 4.5°C/s). The specificity of the reaction was confirmed by obtaining a melting curve from 95 to 55°C. The threshold cycle (CT) value was defined as the cycle in which the fluorescence value was above the background level. Relative gene expression was quantified using the 2−ΔΔCT method (44) with the 16S rRNA gene as a control for normalization.
CLSM.
Confocal laser-scanning microscopy (CLSM) was used to evaluate the surface localization of the Ata-V5-His fusion protein on E. coli pAta-V5-6×His as well as the production of Ata in A. baumannii ATCC 17978, Δata, and Δata-c strains. E. coli pAta-V5-6×His cells were grown to an OD650 of 0.4 and induced for 4 h with either 0.2% arabinose or 0.2% glucose. For A. baumannii strains, cells were grown to an OD650 of 0.025 (ATCC 17978 and Δata) or 0.4 and then induced for 2 h with either 2% arabinose or 0.2% glucose (ATCC 17978 Δata-c). Bacteria were washed with PBS, and the OD650 of all samples was adjusted to 0.4. Cells were then incubated for 15 min in blocking buffer (PBS–5% bovine serum albumin [BSA]) and then with a 1:100 dilution of anti-V5 MAb or 1:50 dilution of polyclonal rabbit antiserum raised against Ata. Finally, cells were incubated with a 1:200 dilution of a secondary antibody coupled to Alexa 488 along with 1 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI) in PBS. Following three washes with PBS, bacteria were analyzed by CLSM as described elsewhere (8).
Binding of Ata to ECM/BM proteins.
Microtiter plates were coated overnight at 4°C with 5 μg of fibronectin, laminin, heparan, collagen types I, II, III, IV, and V (all from Sigma), vitronectin (R&D Systems), or BSA (Sigma) per well in 100 mM phosphate buffer, pH 7. Wells were washed three times with PBST and then treated with blocking solution (PBST–2% nonfat dry milk) for 2 h at 37°C. After three washes with PBST, 50 μl of purified recombinant Ata (0.02 to 2.5 μg/ml) diluted in PBS plus 0.1% blocking solution was added to the plate wells and incubated at 37°C for 2 h. After three washes with PBST, wells were incubated at 37°C for 1 h with rabbit antiserum diluted 1:1,000 in 0.1% blocking solution. Following three washes in PBST, wells were incubated with a secondary anti-rabbit antibody (alkaline phosphatase conjugated and diluted 1:1,000) in 0.1% blocking solution at 37°C for 1 h. Once wells were washed three times with PBST, the binding of Ata was detected with p-nitrophenyl phosphate (pNPP) substrate by measuring the absorbance at 405 nm.
Binding of A. baumannii to collagen type IV.
Microtiter tissue culture plates were coated with collagen type IV, blocked, and washed as described before. A. baumannii ATCC 17978, Δata, and Δata-c strains induced with arabinose or glucose were grown as described above and resuspended in phosphate-buffered saline (PBS) to an OD650 of 0.4. One hundred μl containing 5 × 106 CFU was added to each well and incubated for 1 h at 37°C. Wells were rinsed four times with PBS and treated with trypsin-EDTA to release the bound bacteria. The number of adherent A. baumannii cells was determined by serial dilution and plating.
Rabbit antibodies to Ata and enzyme-linked immunoabsorbent assay (ELISA).
Antibodies to recombinant Ata were raised in rabbits after immunization with 10-μg doses of recombinant Ata by following protocols described elsewhere (47).
A. baumannii virulence studies.
The role of Ata in A. baumannii virulence was investigated in a recently developed lethal model of systemic infection (4). In this model, wild-type A. baumannii ATCC 17978, Δata, Δata-pLVB-Ata, or Δata-pBAD18kan-Ori were grown to an OD650 of 0.025, washed once with PBS, and used to infect groups of immunocompetent mice (C57BL/6; female; n = 8; 3 to 5 weeks of age) intraperitoneally (i.p.) with a dose of approximately 107 CFU per animal. Mortality was scored for a 5-day period.
Statistical analysis.
All statistical analyses were performed using Prism 4.0 (GraphPad Software). Results from comparisons of biofilm formation and binding to collagen IV for A. baumannii strains were analyzed by one-way analysis of variance (ANOVA) with Tukey's correction. Survival data for the different mouse groups were analyzed by using Kaplan-Meier survival curves and the log-rank test. A P value of <0.05 was considered significant
Accession numbers.
The complete genome sequence and annotation of A. baumannii ATCC 17978 has been deposited in the GenBank/EMBL/DDBJ database under accession no. CP000521. The nucleotide and predicted amino acid sequence accession numbers of the ata gene and protein are CP000521 and ABO11464, respectively.
RESULTS
In silico predicted structure of Ata.
The 5,622-bp ata gene, ORF A1S_1032, from A. baumannii ATCC 17978 is predicted to encode a protein that shares many structural features with the other AT proteins, including YadA from Yersinia enterocolitica, NhhA from N. meningitidis, or Uspa1 from Moraxella catarrhalis TA proteins, among others.
Signal peptide and secondary structure prediction analysis of Ata revealed that this protein contains a long putative signal sequence with a signal peptidase cleavage site predicted to be between amino acids 53 and 54 that was followed by a large N-terminal passenger domain (amino acids 54 to 1772) containing four pentameric collagen binding consensus sequences (SVAIG) and one Arg-Gly-Asp (RGD) motif. The 101-amino-acid C-terminal sequence of Ata is predicted to contain a translocator domain that can be separated into an initial 39-amino-acid α-helix and a 9-amino-acid hairpin loop that connects the α-helix to the β-barrel domain. The β-barrel domain is predicted to contain four transmembrane antiparallel β-sheets (β1 to β4) of 9, 10, 10, and 11 amino acids connected by short turns (see Fig. S1A in the supplemental material). By analogy to other members of the TA family, the trimerization of this region in the outer membrane may form 12-stranded pore-forming β-barrels containing four strands from each of three subunits to mediate the exposure of the N-terminal part of the protein at the cell surface.
Based on results from secondary structure predictions and the strong amino acid sequence similarity of Ata to other TAs, we generated a computer model of the putative 3D structure of the last 101 amino acids of Ata with the Chimera computer modeling tool (16, 56) using the crystal structure of Hia (Protein Data Bank identification no. 2GR7) as a template. Figure S1B in the supplemental material represents an overview of the modeled structures of monomeric and trimeric Ata. The three individual α-domains for each Ata monomer are predicted to be exposed at the surface of the structure, looping out of the 12-stranded β-barrel pore formed with four antiparallel β-sheets from each of the monomers of Ata.
The C-terminal 101-amino-acid region of Ata contains functional translocator activity.
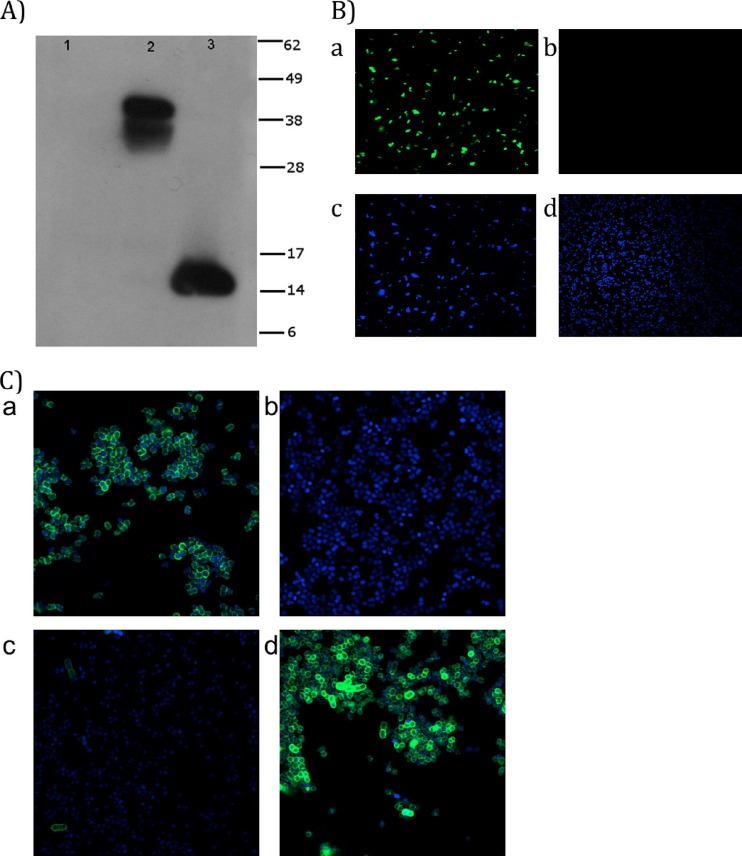
Results from the in silico analysis of Ata suggested that the C-terminal region of Ata contains the translocator activity of the protein. To test this hypothesis, we investigated if the C-terminal 101 amino acid residues of Ata, predicted to contain the translocator function, were capable of presenting a heterologous passenger domain on the bacterial surface using a fusion protein, Ata-V5-His. After induction of E. coli pAta-V5-6×His cells with either 2% arabinose or 0.2% glucose, extraction of the OMPs, and analysis by Western blotting with anti-V5 MAbs, we found that OMP extracts from arabinose-induced E. coli cells had an anti-V5 reactive band that ran between 38 and 49 kDa (Fig. 1A, lane 2). No bands were visible when E. coli was grown under the repressing conditions containing glucose (Fig. 1A, lane 1). As the predicted size of the mature monomeric Ata-V5-His fusion protein is approximately 13.8 kDa, it appears that the V5 band observed was a trimer of the Ata-V5-His fusion protein. Previous accounts indicated that C termini of other AT proteins, such as Hia and YadA, formed heat-resistant, sodium dodecyl sulfate-resistant trimers in the outer membrane, and that Hia requires formic acid denaturation for dissociation (61, 70). After treatment of our samples with 70% formic acid overnight at room temperature, we found that OMP samples from arabinose-grown E. coli pAta-V5-6× had an altered migration of the V5 reactive band that ran at approximately 14 kDa, a size consistent with a monomer of the Ata-V5-His protein (Fig. 1A, lane 3). Similar results were obtained when Western blottings were probed with anti-His MAbs (data not shown).
Fig 1.
(A) Immunoblot of Ata-V5-His fusion protein expressed in E. coli. Outer membrane proteins (OMPs) of E. coli LMG194 harboring pAta-V5-6×His were prepared after induction with either 0.2% glucose (lane 1) or 0.2% arabinose (lane 2) and then tested by Western blotting with a monoclonal antibody (MAb) directed against the V5 peptide. Samples from arabinose-induced OMPs were treated with 70% formic acid and detected as before (lane 3). The predicted monomeric mass of Ata-V5-His fusion proteins is 13.8 kDa. Migration distances of molecular mass markers (in kDa) are indicated on the right. (B) Detection of Ata-V5-His fusion protein in E. coli pAta-V5-6×His by CLSM. E. coli pAta-V5-6×His cultures were grown in M9 plus 0.25% Casamino Acids and induced with 0.2% arabinose or 0.2% glucose. The V5 epitope was labeled with a mouse anti-V5 MAb and a secondary goat anti-mouse Alexa 488 fluorescent antibody (green channel), and cell nucleic acids were stained with DAPI (blue channel). (a) E. coli pAta-V5-6×His induced with arabinose (green channel) or (b) glucose (green channel). Also shown are DAPI-stained nuclei of E. coli cells (blue channel) after induction with arabinose (c) or glucose (d). (C) CLSM detection of Ata in A. baumannii ATCC 17978, Δata, and Δata-c strains. A. baumannii ATCC 17978, Δata, and Δata-c induced with arabinose or glucose were labeled with rabbit anti-Ata antisera and a secondary goat anti-rabbit Alexa 488 fluorescent antibody. Nucleic acids were stained with DAPI. Merged images of Alexa 488- and DAPI-stained A. baumannii ATCC 17978 (a) and Δata (b), Δata-c induced with glucose (c), and Δata-c induced with arabinose (d) are shown.
We next used CLSM to confirm the surface location of the Ata-V5-His chimeric protein in arabinose- or glucose-induced E. coli pAta-V5-6×His cells. As shown in Fig. 1B, high levels of labeling were observed in arabinose-induced E. coli pAta-V5-6×His (Fig. 1B, panel a) but not when the E. coli cells were grown under repressing effects of glucose (Fig. 1B, panel b). Control DAPI-stained E. coli pAta-V5-6×His cells induced with arabinose or glucose are shown in panels c and d, respectively.
The surface localization of Ata in A. baumannii cells was further confirmed by CLSM studies using wild-type A. baumannii ATCC 17978, Δata, and Δata-c strains grown in the presence of arabinose or glucose. Results presented in Fig. 1C demonstrated high levels of surface Ata production in both wild-type A. baumannii ATCC 17978 (Fig. 1C, panel a) and arabinose-induced Δata-c (Fig. 1C, panel d) but not in the Δata strain (Fig. 1C, panel b) or the glucose-induced Δata-c strain (Fig. 1C, panel c).
All CLSM studies with E. coli pAta-V5-6×His and A. baumannii ATCC 17978, Δata, and Δata-c were carried out with intact, nonpermeabilized cells.
In summary, the Western blot analysis of OMP extracts from the Ata-V5-His fusion protein demonstrated that ORF A1S_1032 encodes a TA protein, and CLSM confirmed its surface localization.
Production of Ata is growth phase dependent.
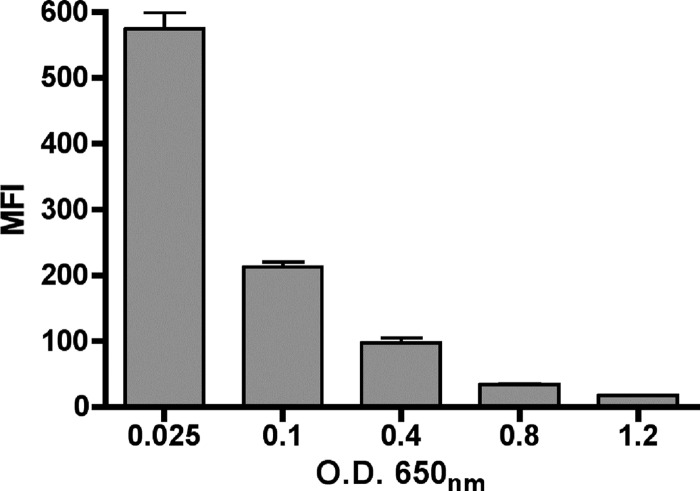
We investigated the production of Ata in A. baumannii ATCC 17978 at various growth phases in LB (OD650 of 0.025, 0.1, 0.4, 0.8, or 1.2) by measuring surface Ata production by flow cytometry. Results presented in Fig. 2 showed growth phase-dependent Ata production, with the highest levels found at very early exponential phase (mean fluorescence intensity [MFI], 574 at an OD650 of 0.025), followed by a continuous decline throughout the logarithmic and stationary phases. In contrast, the MFI levels of ata-negative strain ATCC 17978 Δata grown to an OD650 of 0.025, which was included as a control in these experiments, was only 7.6. Since A. baumannii ATCC 17978 and Δata differ only in the production of Ata, the differences in MFI levels observed (574 for A. baumannii ATCC 17978 and 7.6 for the ata mutant) validate the specificity of the primary antibodies used in these studies to the Ata protein.
Fig 2.
Quantification of Ata expression in A. baumannii ATCC 17978 by flow cytometry during various growth phases. A. baumannii was grown in LB to early exponential (OD650 of 0.025 or 0.1), mid-exponential (OD650 of 0.4), late exponential (OD650 of 0.8), or stationary phase (OD650 of 1.2), labeled with rabbit antibody to Ata and secondary goat antibody to rabbit IgG conjugated to Alexa 488 fluorescent dye, and analyzed by flow cytometry. Results represent the mean fluorescent intensity (MFI) of 500,000 cells, and bars indicate the averages from three independent experiments ± standard errors of the means (SEM).
In addition to FACS analysis, we investigated ata expression by quantitative real-time PCR (qRT-PCR) studies on A. baumannii ATCC 17978 cells grown to early log (OD650 of 0.1) and mid-log (OD650 of 0.4) phases in LB. Our results showed that the level of Ata mRNA was approximately 3-fold higher at an OD650 of 0.1 that at an OD650 of 0.4 (means ± standard deviations, 2.98 ± 0.1; n = 3 independent experiments). This pattern of ata transcription is consistent with our previous FACS results showing higher levels of Ata production in the early logarithmic phase and a continuous decline throughout the logarithmic and stationary phases.
Ata is critical for biofilm development of A. baumannii.
The ability of bacteria to form biofilms is a trait closely associated with bacterial persistence and virulence (15, 21). We therefore investigated the role of ata in biofilm formation by A. baumannii. Results presented in Fig. 3 show that A. baumannii ATCC 17978 formed biofilms of adherent cells, while biofilm production was significantly lower in the ATCC 17978 Δata cultures (P < 0.05 by one-way ANOVA). Complementation of the biofilm-negative ATCC 17978 Δata strain with the ata gene in trans restored the biofilm-positive phenotype of the A. baumannii ATCC 17978 strain only when cells were grown under arabinose-inducing conditions (for Δata versus Δata-c arabinose, P < 0.001 by one-way ANOVA) but not when they were incubated in the presence of the repressing effects of glucose (for Δata versus Δata-c glucose, not significant according to one-way ANOVA).
Fig 3.
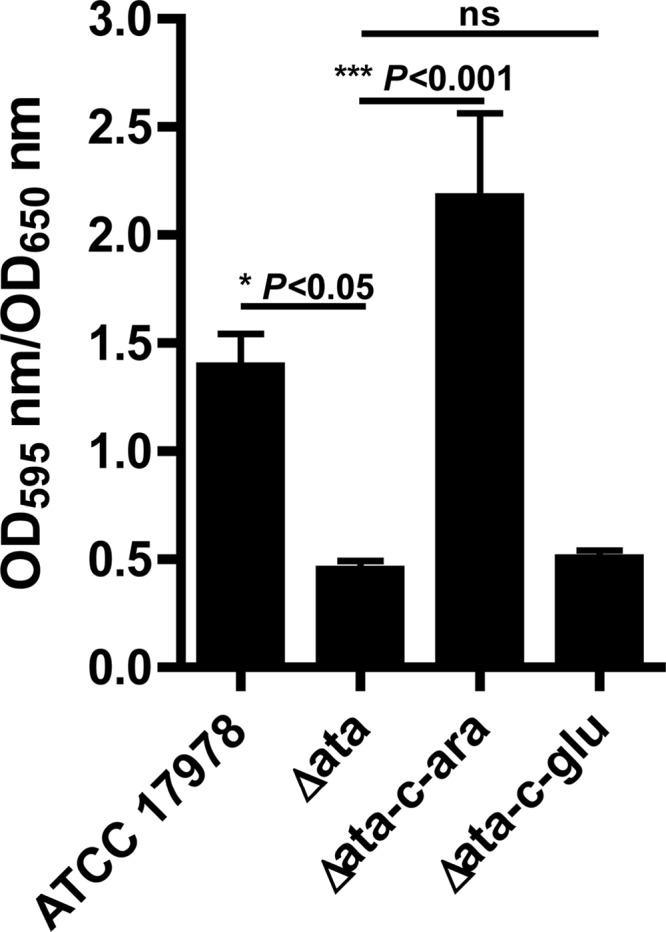
Quantitative biofilm formation by A. baumannii ATCC 17978, Δata, and Δata-c on polystyrene surfaces. A. baumannii 17978 and Δata were grown in LB and ATCC 17978 Δata-c in LB plus 2% arabinose or 0.2% glucose under static conditions for 24 h at 37°C. Total biofilm formation (OD595) was normalized by bacterial growth (OD650). The bars indicate the means of 15 tubes from five independent experiments ± SEM. P values were determined by one-way ANOVA with Tukey's post hoc analysis. ns, not significant.
Ata binds multiple extracellular/basal matrix proteins and mediates the binding of A. baumannii to collagen type IV.
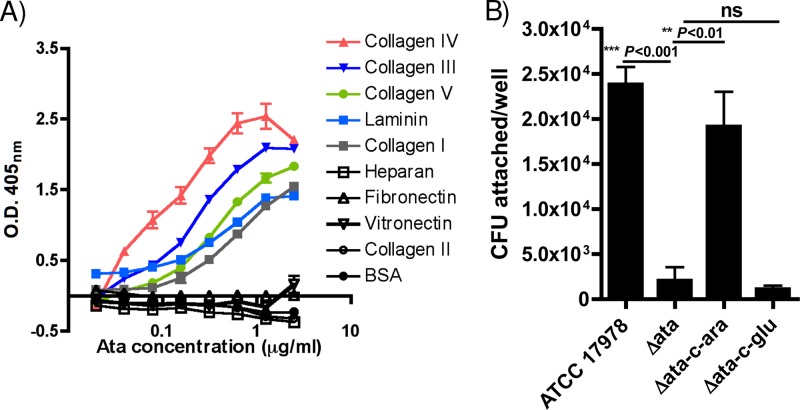
As one of the main roles of TAs is to act as adhesins, facilitating the binding of bacteria to host tissues (43), we evaluated the ability of purified recombinant Ata to bind various ECM/BM proteins, including purified collagen types I, II, III, IV, and V, laminin, heparan, fibronectin, and vitronectin. As presented in Fig. 4A, there was a clear dose-dependent binding of recombinant Ata to collagen types I, III, IV, and V and laminin. There was no binding of Ata to collagen type II, fibronectin, vitronectin, or heparan, which was comparable to binding to the negative control, BSA.
Fig 4.
Adhesive properties of Ata. (A) Dose-dependent binding of Ata to selected ECM/BM components. ECM/BM proteins, including collagen types I, II, III, IV, and V, heparan, fibronectin, vitronectin, and laminin, and BSA (as a control) were used at 5 μg/well, and binding of Ata was quantified by ELISA. Data points represent the means from four independent experiments ± SEM. (B) Binding of A. baumannii ATCC 17978, Δata, and Δata-c induced with 2% arabinose or 0.2% glucose to immobilized collagen type IV. The bars indicate the means from three independent experiments ± SEM. P values were determined by one-way ANOVA with Tukey's post hoc analysis. ns, not significant.
We then investigated if, in addition to binding multiple ECM/BMs, Ata could promote the adhesion of whole A. baumannii cells to ECM/BMs. For this, we compared the binding of the wild-type A. baumannii ATCC 17978, Δata, and Δata-c strains grown in arabinose or glucose to immobilized collagen type IV, which was used as a prototype ECM/BM protein. Results presented in Fig. 4B demonstrate that the deletion of ata results in a significant decrease in the binding of A. baumannii ATCC 17978 cells to collagen IV-coated plates (for A. baumannii ATCC 17978 versus Δata, P < 0.001 by one-way ANOVA), and that this binding was restored when the Δata-c strain was grown under arabinose-inducing conditions (for Δata versus Δata-c-arabinose, P < 0.01 by one-way ANOVA) but not in the presence of repressive effects of glucose (for Δata versus Δata-c-glucose, not significant according to one-way ANOVA).
In conclusion, our results demonstrate that Ata not only binds multiple ECM/BMs proteins but also mediates the adhesion of A. baumannii cells to immobilized collagen type IV.
Ata promotes the survival of A. baumannii in vivo.
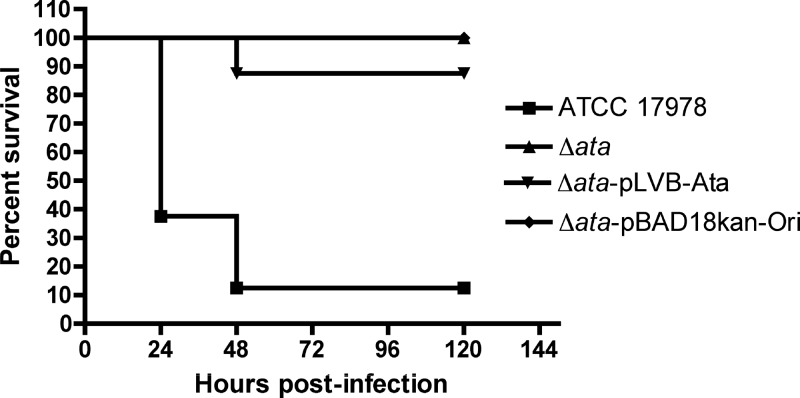
We investigated the contribution of Ata to the virulence of A. baumannii in vivo by comparing the survival of wild-type A. baumannii ATCC 17978, Δata, Δata-pLVB-Ata (ata complemented in trans and expressed from its own promoter), and Δata-pBAD18kan-Ori (complemented with the empty plasmid) strains in a lethal model of systemic infection based on that recently described by Breslow et al. (4).
In this study, groups of eight immunocompetent mice were infected i.p. with approximately 107 CFU of A. baumannii strains, and survival was monitored for a period of 5 days. As shown in Fig. 5, infection of mice with the wild-type A. baumannii ATCC 17978 strain expressing Ata resulted in high levels of mortality (87.5%) compared to mice infected with the ata-negative strains, Δata or Δata-pBAD18kan-Ori, complemented with the empty vector, which all survived until day 5 postinfection (0% mortality; for wild-type ATCC 17978 versus Δata and ATCC 17978 versus Δata-pBAD18kan-Ori, P = 0.0005 by log-rank test). Surprisingly, mice infected with A. baumannii Δata-pLVB-Ata showed little lethality (12.5%) in this animal model.
Fig 5.
Survival curves of mice (n = 8; C57BL/6) following intraperitoneal infection with A. baumannii ATCC 17978 (1.6 × 107 CFU/mouse), Δata (1.6 × 107 CFU/mouse), Δata-pLVB-Ata (ata gene with its native promoter cloned into pBAD18Kan-Ori; 1.4 × 107 CFU/mouse), and Δata-pBAD18Kan-Ori (empty vector pBAD18Kan-Ori; 1.2 × 107 CFU/mouse). P = 0.0005 by log-rank test in a Kaplan-Meier analysis of wild-type ATCC 17978 versus Δata and ATCC 17978 versus Δata-pBAD18kan-Ori.
To investigate if the reduced lethality of the trans-complemented strain A. baumannii Δata-pLVB-Ata was due to problems of stability of the complementation plasmid pLVB-Ata in vivo, we conducted a pilot study to assess the potential loss of pLVB-Ata from A. baumannii pLVB-Ata in our mouse model of lethal infection. C57BL/6 mice were infected i.p. with A. baumannii pLVB-Ata (2.5 × 107 CFU). Two to 3 days later animals were sacrificed, the peritoneum washed with 0.5 ml of PBS, the peritoneal lavage was plated in tryptic soy agar (TSA), and TSA plates were supplemented with kanamycin 50 μg/ml (TSA-kan). Our results revealed no significant differences in bacterial counts after growth in the presence or absence of kanamycin (480 CFU/ml TSA-kan/440 CFU/ml TSA by 48 h postinfection and 30 CFU/ml TSA-kan/10 CFU/ml TSA by 72 h postinfection), indicating no plasmid loss in vivo.
To determine if a marginal complementation of Ata production in the A. baumannii Δata-pLVB-Ata strain could explain the partial in vivo complementation of the virulence properties, FACS analysis of Ata production among A. baumannii ATCC 17978, Δata, Δata-pLVB-Ata, and Δata-pBAD18kan-Ori strains was carried out. As shown in Fig. S2 in the supplemental material, the levels of Ata produced by strain A. baumannii Δata-pLVB-Ata were 4.4-fold lower (MFI of 16.7) than those measured in the wild-type A. baumannii ATCC 17978 strain (MFI of 73.8). Conversely, the A. baumannii Δata and Δata-pBAD18kan-Ori (empty vector) strains that do not express ata had very low MFI values, 3.3 and 7.3, respectively. Thus, it appears that trans-complementation of ata from its own promoter does not fully restore protein synthesis.
Ata production among clinical isolates.
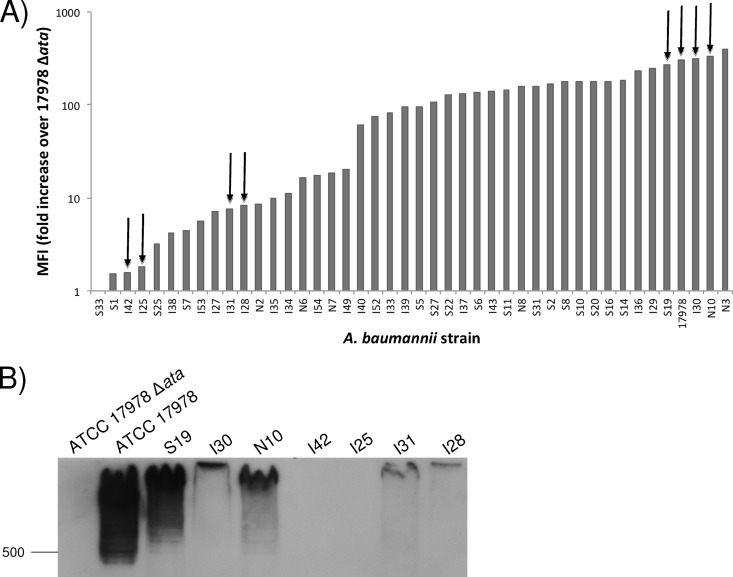
We determined the prevalence of the ata gene among A. baumannii strains from various geographic locations as well as clinical disease sources (lung, blood, and skin isolates). Our A. baumannii strain collection encompasses a total of 75 isolates obtained from American soldiers wounded in the Iraqi war (supplied by Murray Clinton), the Singapore General Hospital, (provided by Tse Hsien Koh), and serum-resistant isolates from various U.S. institutions, including the Centers for Disease Control and Prevention (CDC), the ATCC, and the University of Nebraska Medical Center (supplied to us by Paul M. Dunman). Our results showed that 44/75 (58.6%) of A. baumannii strains tested by PCR yielded an amplification product with an approximate size of 5.6 kb.
In addition to PCR analysis, all 44 A. baumannii clinical isolates positive for the ata gene by PCR were also tested for surface Ata production by FACS. Included in each assay as a specificity control was the ATCC 17978 Δata strain, and results were expressed as the ratio of the MFI of each strain to that of the ata-negative strain.
Results presented in Fig. 6A show that 43/44 A. baumannii strains that were positive for the ata gene by PCR also produced detectable surface Ata protein. The levels of Ata were high overall but varied among the A. baumannii clinical strains.
Fig 6.
Analysis of Ata expression among A. baumannii clinical isolates. (A) Flow-cytometric analysis of Ata production among A. baumannii clinical isolates positive for the ata gene by PCR. A. baumannii strains were grown in LB and probed with rabbit anti-Ata antibodies and a secondary goat anti-rabbit Alexa 488 fluorescent antibody. Approximately 500,000 cells were then analyzed by flow cytometry, and antibody binding was expressed as mean fluorescent intensity (MFI). Bars represent the fold ratio (in MFI) of each A. baumannii clinical isolate to the MFI of the ATCC 17978 Δata strain. (B) Western blot (WB) analysis of Ata levels in 4 strains producing high levels of Ata (ATCC 17978, S19, I30, and N10) and in 4 strains producing low levels of Ata (I42, I25, I31, and I28) as determined by FACS and the Ata-negative strain ATCC 17978 Δata, which was used as a control. Migration distances of molecular mass markers (in kDa) are indicated on the left. (Strains used for WB analysis are highlighted with arrows in panel A). Outer membrane proteins were extracted, resolved by SDS-PAGE, and transferred to a PVDF membrane, and Ata was detected by WB with anti-Ata rabbit antibodies and a secondary goat anti-rabbit IgG.
Although it was not surprising to find variability in the levels of Ata production among our A. baumannii clinical strains obtained from various geographical locations and types of infections, we decided to further investigate these findings by evaluating Ata production in outer membrane extracts of cells using Western blot analysis. Comparing levels of Ata production by FACS and Western blotting would help to rule out some explanations for the variability observed in the levels of surface Ata, including the nonspecific binding of anti-Ata antibodies to A. baumannii surface components other than Ata or differences in the levels of surface exposure of the Ata protein.
We evaluated eight clinical strains of A. baumannii that were selected based on their FACS profiles, including four strains producing high levels of Ata (ATCC 17978, S19, I30 and N10) and four strains producing low levels of Ata (I42, I25, I31, and I28), and we included A. baumannii ATCC 17978 Δata as a negative control. After OMPs were extracted and Western blots were probed with anti-Ata rabbit antibodies, we found that all four strains producing high levels of Ata also had a very-high-molecular-weight reactive band, which was consistent in size with a trimer of the Ata protein (Fig. 6B). On the other hand, the four strains producing low levels of Ata as determined by FACS had low to nondetectable levels of Ata by Western blotting, and no Ata was detected in the ata-negative strain Δata. In addition, these immunoblot studies suggested the presence of various Ata alleles among the A. baumannii strains, resulting in proteins with slightly different molecular weights.
DISCUSSION
In this study, we report the identification of Ata as a TA of A. baumannii. Ata is surface exposed on the A. baumannii outer membrane, plays a role in the virulence of A. baumannii in mice, mediates adherence to ECM/BM proteins, and contributes to biofilm formation. Ata has all the typical features of TAs, including a long signal peptide followed by a surface-exposed passenger domain and C-terminal translocator domain encoding 4 β-strands. With a size of 1,873 amino acids per monomer, the A. baumannii Ata is significantly larger than other well-described TAs, such as YadA (455 aa), UspA1 (863 aa), Nhha (590 aa), and NadA (364 aa). The passenger domain of Ata encodes an RGD domain and four SVAIG, collagen-binding domains. This SVAIG motif or its derivatives are widespread among other TAs, including, for example, YadA of Y. enterocolitica, EmaA of Aggregatibacter actinomycetemcomitans, BpaA of Burkholderia pseudomallei, variably expressed outer membrane proteins (Vomps) of Bartonella quintana, and BadA of Bartonella henselae (43, 71, 78), among others. While the presence of SVAIG motifs is consistent with the ability of recombinant Ata to bind various types of collagens, including types I, III, IV, and V, the direct involvement of these Ata motifs in binding to collagen has not been confirmed. The RGD motif, on the other hand, is found in many adhesive proteins, including various autotransporters (22, 23, 31), and is associated with attachment to mammalian cells via binding to integrin molecules on the plasma membrane (32). The significance of this motif in adhesion and pathogenesis of A. baumannii has yet to be determined.
Computer predictions indicate that the β-barrel translocator of Ata forms a trimeric 12-stranded β-barrel embedded into the outer membrane with three hairpin loops, one from each Ata monomer, passing through the pore and pointing toward the extracellular space, while the α-helix fragments protrude outside. These α-helices form a neck between the membrane-inserted anchor and the surface-exposed passenger domains. Similar models have been proposed for the YadA and NhhA membrane anchor regions (63, 77).
A. baumannii frequently causes biofilm infections associated with medical devices, such as vascular catheters, cerebrospinal fluid shunts, or Foley catheters (58, 59). A number of A. baumannii components have been identified to be important for biofilm formation on abiotic surfaces, including the pili synthesized by the csuA/BABCDE chaperone-usher secretion system (73), a biofilm-associated protein (Bap) (45), and the widely distributed surface polysaccharide, poly-N-acetylglucosamine (PNAG) (8). We have now identified Ata as another surface component that contributes to biofilm formation in A. baumannii, since the deletion of this protein from the wild-type ATCC 17978 strain significantly decreases biofilm formation in vitro. Moreover, complementation of Ata in the Δata-c strain restored biofilm formation only when cells were grown in arabinose-inducible conditions but not in the presence of the repressible effects of glucose.
Microbial pathogens use adhesins to bind to ECM/BM macromolecules and subsequently may use them as a stronghold for propagating and spreading to other parts of the body (28). We have shown that purified recombinant Ata binds to the ECM proteins, including collagen types I, III, IV, and V and the basement protein laminin. Furthermore, this study also demonstrated that in addition to binding ECM/BM proteins, Ata mediated the adhesion of A. baumannii cells to immobilized collagen type IV. Collagens are the most common ECM proteins in the body, with collagen type I being the most abundant type in the human lung, and it is also prominent in the peripheral lung tissue and pulmonary vascular tissue (13). Thus, it is thought that ECM/BM components serve as docking sites for microbial pathogen invasion. We speculate that in the case of A. baumannii infections, ECM/BM proteins become exposed in instances where there is tissue damage, and, following adherence to ECM/BM proteins, A. baumannii can grow as a biofilm at these sites, making the treatment of these infections very difficult.
When we investigated the prevalence of Ata among clinical isolates of A. baumannii, we found that 44 of the 75 strains tested by PCR were positive for the ata gene (58.6%), 43 of which synthesized surface-exposed Ata by flow cytometry (56.3%).
Moreover, Western blot analysis of eight A. baumannii clinical isolates (four strains producing high levels of Ata and four producing low levels of Ata) demonstrated an overall good correlation between levels of Ata measured by FACS and Western blot analysis and suggested the presence of various alleles of the Ata trimers that migrated at slightly different molecular weights. Allelic variation is not uncommon in the AT family. Some examples of ATs that display allelic diversity include Ag43 in E. coli (74), UspA in M. catarrhalis (5), NadA in N. meningitidis (14), and VacA (17), BabA, and BabB (57) in Helicobacter pylori, among others. Studies are now under way to investigate if the lack of amplification of ata in a subset of A. baumannii strains using a primer set designed based on sequenced strain ATCC 17978 is due to sequence divergence, gene localization in the chromosome, or the true absence of the ata gene.
This work also presents clear evidence of the surface exposure of Ata in A. baumannii, as the CLSM and FACS studies were carried out using intact, unpermeabilized bacterial cells. Moreover, the role of Ata in biofilm formation, adhesion to collagen type IV, and mouse virulence provide additional indications of its surface exposure.
Due to the overall low virulence of most A. baumannii strains in rodent models of infection, investigators have used various methods to enhance virulence, including mixing bacterial inocula with porcine mucin (49, 60) or the use of neutropenic mouse models of A. baumannii infections (36, 67, 75). However, neutropenia is a rare clinical risk factor for patients with A. baumannii infections (1a, 6, 7, 20, 26, 34, 40, 50), and therefore an alternative model of A. baumannii infection in immunocompetent animals is desirable to more accurately mimic the types of infections caused by this organism. For these reasons, we chose an immunocompetent mouse model of infection based on that recently developed by Breslow et al. (4) to evaluate the role of Ata in A. baumannii virulence. In this animal model, infections are induced via i.p. injection and A. baumannii virulence assessed by mortality during a 5-day period.
Using this lethal model of systemic infection, we could demonstrate the role of Ata in A. baumannii virulence. As shown, the deletion of ata from wild-type A. baumannii ATCC 17978 significantly decreased the virulence of this strain in the murine model of lethal infection. However, trans-complementation of Ata in the plasmid pLVB-Ata only marginally restored the virulence of the wild-type strain.
Considerable effort has been focused on the construction of a complementation plasmid suitable for in vivo studies aimed at investigating the role of Ata in A. baumannii virulence. After testing various vectors for complementation, we constructed the plasmid pLVB-Ata, carrying the ata gene, with its native promoter in the shuttle vector pBAD18kan-Ori. The pLVB-Ata plasmid was highly stable in vivo in the strain A. baumannii Δata-pLVB-Ata, even in the absence of antibiotic selection in our mouse model of lethal infection. Despite these encouraging results, FACS levels of Ata production in vitro in A. baumannii Δata-pLVB-Ata were 4.4-fold lower than those seen in the wild-type A. baumannii ATCC 17978 strain. Although the nature of this reduced complementation remains unclear, we speculate that an undetermined cis-regulatory element is required for the regulation of Ata, which would explain the limited trans-complementation of virulence seen in the A. baumannii Δata-pLVB-Ata strain, where ata is expressed from its native promoter in the stable plasmid pBAD18kan-Ori.
Therefore, our findings are consistent overall with the conclusion that Ata plays a role in A. baumannii virulence as determined in a murine lethal model of infection, promoting biofilm formation and ECM/BM protein binding and mediating the adhesion of A. baumannii cells to immobilized collagen type IV. These findings may help promote the development of novel therapeutic strategies to limit A. baumannii-associated morbidity and mortality.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the contributions to this work of Lai Ding (Optical Imaging Core Facility, Harvard Medical School) for his help with the CLSM studies and Valeria Risso for her assistance in generating a computer model of the 3D structure of the Ata translocator domain using the Chimera computer modeling tool.
This work was supported by NIH grant AI 046706.
Footnotes
Published ahead of print 18 May 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. American Association for Pediatrics 2006. Pertussis (whooping cough). Red Book Online 2006:498–520 [Google Scholar]
- 1a. Beavers SF, et al. 2009. Comparison of risk factors for recovery of Acinetobacter baumannii during outbreaks at two Kentucky hospitals, 2006. Public Health Rep. 124:868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bendtsen JD, Nielsen H, von Heijne G, Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 [DOI] [PubMed] [Google Scholar]
- 3. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 4. Breslow JM, et al. 2011. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect. Immun. 79:3317–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks MJ, et al. 2008. Modular arrangement of allelic variants explains the divergence in Moraxella catarrhalis UspA protein function. Infect. Immun. 76:5330–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caricato A, et al. 2009. Risk factors and outcome of Acinetobacter baumanii infection in severe trauma patients. Intensive Care Med. 35:1964–1969 [DOI] [PubMed] [Google Scholar]
- 7. Chiang D-H, et al. 2008. Risk factors for mortality in patients with Acinetobacter baumannii bloodstream infection with genotypic species identification. J. Microbiol. Immunol. Infect. 41:397–402 [PubMed] [Google Scholar]
- 8. Choi AHK, Slamti L, Avci FY, Pier GB, Maira-Litran T. 2009. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-β-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 191:5953–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi CH, et al. 2008. Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell Microbiol. 10:309–319 [DOI] [PubMed] [Google Scholar]
- 10. Choi CH, et al. 2005. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 7:1127–1138 [DOI] [PubMed] [Google Scholar]
- 11. Choi CH, Lee JS, Lee YC, Park TI, Lee JC. 2008. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 8:216 doi:10.1186/1471-2180-8-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cisneros JM, et al. 1996. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin. Infect. Dis. 22:1026–1032 [DOI] [PubMed] [Google Scholar]
- 13. Clark JG, Kuhn C, McDonald JA, Mecham RP. 1983. Lung connective tissue. Int. Rev. Connect. Tissue Res. 10:249–331 [DOI] [PubMed] [Google Scholar]
- 14. Comanducci M, et al. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 16. Cotter SE, Surana NK, St Geme JW. 2005. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 13:199–205 [DOI] [PubMed] [Google Scholar]
- 17. Cover TL, Tummuru MK, Cao P, Thompson SA, Blaser MJ. 1994. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 269:10566–10573 [PubMed] [Google Scholar]
- 18. Davis KA, Moran KA, McAllister CK, Gray PJ. 2005. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 11:1218–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 20. Dizbay M, Tunçcan OG, Sezer BE, Hizel K. 2010. Nosocomial imipenem-resistant Acinetobacter baumannii infections: epidemiology and risk factors. Scand. J. Infect. Dis. 42:741–746 [DOI] [PubMed] [Google Scholar]
- 21. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Souza SE, Ginsberg MH, Burke TA, Lam SC, Plow EF. 1988. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science 242:91–93 [DOI] [PubMed] [Google Scholar]
- 23. Finn TM, Stevens LA. 1995. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol. Microbiol. 16:625–634 [DOI] [PubMed] [Google Scholar]
- 24. Gaddy JA, Tomaras AP, Actis LA. 2009. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 77:3150–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia-Garmendia J, et al. 2001. Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically ill patients: a cohort study. Clin. Infect. Dis. 33:939–946 [DOI] [PubMed] [Google Scholar]
- 26. Gómez J, et al. 1999. Six-year prospective study of risk and prognostic factors in patients with nosocomial sepsis caused by Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 18:358–361 [DOI] [PubMed] [Google Scholar]
- 27. Guex N, Peitsch MC. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723 [DOI] [PubMed] [Google Scholar]
- 28. Hauck CR, Agerer F, Muenzner P, Schmitter T. 2006. Cellular adhesion molecules as targets for bacterial infection. Eur. J. Cell Biol. 85:235–242 [DOI] [PubMed] [Google Scholar]
- 29. Hawley JS, et al. 2007. Susceptibility of acinetobacter strains isolated from deployed U.S. military personnel. Antimicrob. Agents Chemother. 51:376–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hellwig SMM, Rodriguez ME, Berbers GAM, van de Winkel JGJ, Mooi FR. 2003. Crucial role of antibodies to pertactin in Bordetella pertussis immunity. J. Infect. Dis. 188:738–742 [DOI] [PubMed] [Google Scholar]
- 31. Henderson IR, Owen P. 1999. The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported protein and is regulated by a novel mechanism involving Dam and oxyR. J. Bacteriol. 181:2132–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Humphries J, Byron A. 2006. Integrin ligands at a glance. J. Cell Sci. 119:3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacobs AC, et al. 2010. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect. Immun. 78:1952–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jang TN, Lee SH, Huang CH, Lee CL, Chen WY. 2009. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J. Hosp. Infect. 73:143–150 [DOI] [PubMed] [Google Scholar]
- 35. Jin JS, et al. 2011. Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles. PLoS One 6:e17027 doi:10.1371/journal.pone.0017027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joly-Guillou ML, Wolff M, Pocidalo JJ, Walker F, Carbon C. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reference deleted. [Google Scholar]
- 38. Jones DT. 2007. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 23:538–544 [DOI] [PubMed] [Google Scholar]
- 39. Kapoor R. 2008. Acinetobacter infection. N. Engl. J. Med. 358:2845–2847 [DOI] [PubMed] [Google Scholar]
- 40. Lautenbach E, et al. 2009. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 30:1186–1192 [DOI] [PubMed] [Google Scholar]
- 41. Lee H-W, et al. 2008. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin. Microbiol. Infect. 14:49–54 [DOI] [PubMed] [Google Scholar]
- 42. Lee JC, et al. 2006. Adherence of Acinetobacter baumannii strains to human bronchial epithelial cells. Res. Microbiol. 157:360–366 [DOI] [PubMed] [Google Scholar]
- 43. Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VAJ. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 14:264–270 [DOI] [PubMed] [Google Scholar]
- 44. Livak K. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔ CT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 45. Loehfelm TW, Luke NR, Campagnari AA. 2008. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J. Bacteriol. 190:1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reference deleted. [Google Scholar]
- 47. Maira-Litran T, Kropec A, Goldmann DA, Pier GB. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect. Immun. 73:6752–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 46:1254–1263 [DOI] [PubMed] [Google Scholar]
- 49. McConnell MJ, et al. 2011. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect. Immun. 79:518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Metan G, Sariguzel F, Sumerkan B. 2009. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur. J. Intern. Med. 20:540–544 [DOI] [PubMed] [Google Scholar]
- 51. Nielsen M, Lundegaard C, Lund O, Petersen TN. 2010. CPHmodels-3.0–remote homology modeling using structure-guided sequence profiles. Nucleic Acids Res. 38:W576–W581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nishimura K, Tajima N, Yoon Y-H, Park S-Y, Tame JRH. 2010. Autotransporter passenger proteins: virulence factors with common structural themes. J. Mol. Med. 88:451–458 [DOI] [PubMed] [Google Scholar]
- 53. Oncül O, et al. 2002. Hospital-acquired infections following the 1999 Marmara earthquake. J. Hosp. Infect. 51:47–51 [DOI] [PubMed] [Google Scholar]
- 54. Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microb. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perez F, et al. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3471–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pettersen EF, et al. 2004. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605–1612 [DOI] [PubMed] [Google Scholar]
- 57. Pride DT, Meinersmann RJ, Blaser MJ. 2001. Allelic variation within Helicobacter pylori babA and babB. Infect. Immun. 69:1160–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Richet H, Fournier PE. 2006. Nosocomial infections caused by Acinetobacter baumannii: a major threat worldwide. Infect. Control Hosp. Epidemiol. 27:645–646 [DOI] [PubMed] [Google Scholar]
- 59. Rodríguez-Baño J, et al. 2004. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect. Control Hosp. Epidemiol. 25:819–824 [DOI] [PubMed] [Google Scholar]
- 60. Rodríguez-Hernández MJ, et al. 2000. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J. Antimicrob. Chemother. 45:493–501 [DOI] [PubMed] [Google Scholar]
- 61. Roggenkamp A, et al. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 185:3735–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Russo TA, et al. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 78:3993–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scarselli M, et al. 2006. Neisseria meningitidis NhhA is a multifunctional trimeric autotransporter adhesin. Mol. Microbiol. 61:631–644 [DOI] [PubMed] [Google Scholar]
- 64. Seifert H, Strate A, Pulverer G. 1995. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine 74:340–349 [DOI] [PubMed] [Google Scholar]
- 65. Serruto D, et al. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith MG, et al. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21:601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Song JY, Cheong HJ, Lee J, Sung AK, Kim WJ. 2009. Efficacy of monotherapy and combined antibiotic therapy for carbapenem-resistant Acinetobacter baumannii pneumonia in an immunosuppressed mouse model. Int. J. Antimicrob. Agents 33:33–39 [DOI] [PubMed] [Google Scholar]
- 68. Steyert SR, Pineiro SA. 2007. Development of a novel genetic system to create markerless deletion mutants of Bdellovibrio bacteriovorus. Appl. Environ. Microbiol. 73:4717–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. St Geme JW, Kumar VV, Cutter D, Barenkamp SJ. 1998. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect. Immun. 66:364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Surana NK, Cutter D, Barenkamp SJ, St Geme JW. 2004. The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J. Biol. Chem. 279:14679–14685 [DOI] [PubMed] [Google Scholar]
- 71. Tahir YE, Kuusela P, Skurnik M. 2000. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol. Microbiol. 37:192–206 [DOI] [PubMed] [Google Scholar]
- 72. Tien HC, et al. 2007. Multi-drug resistant Acinetobacter infections in critically injured Canadian forces soldiers. BMC Infect. Dis. 7:95 doi:10.1186/1471-2334-7-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. 2003. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484 [DOI] [PubMed] [Google Scholar]
- 74. van der Woude MW, Henderson IR. 2008. Regulation and function of Ag43 (Flu). Annu. Rev. Microbiol. 62:153–169 [DOI] [PubMed] [Google Scholar]
- 75. van Faassen H, et al. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect. Immun. 75:5597–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang Y, et al. 2010. Causes of Infection after earthquake, China, 2008. Emerging Infect. Dis. 16:974–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wollmann P, Zeth K, Lupas AN, Linke D. 2006. Purification of the YadA membrane anchor for secondary structure analysis and crystallization. Int. J. Biol. Macromol. 39:3–9 [DOI] [PubMed] [Google Scholar]
- 78. Zhang P, et al. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. U. S. A. 101:13630–13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zimbler DL, et al. 2009. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals 22:23–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.