Abstract
The Enterococcus faecalis prg and pcf genes of plasmid pCF10 encode a type IV secretion system (T4SS) required for conjugative transfer. PrgJ is a member of the VirB4 family of ATPases that are universally associated with T4SSs. Here, we report that purified PrgJ dimers displayed ATP binding and hydrolysis activities. A PrgJ nucleoside triphosphate (NTP) binding site mutation (K471E) slightly diminished ATP binding but abolished ATP hydrolysis in vitro and blocked pCF10 transfer in vivo. As shown with affinity pulldown assays, PrgJ and the K471E mutant protein interacted with the substrate receptor PcfC and with relaxase PcfG and accessory factor PcfF, which together form the relaxosome at the oriT sequence to initiate plasmid processing. The purified PrgJ and K471E proteins also bound single- and double-stranded DNA substrates without sequence specificity in vitro, and both PrgJ derivatives bound pCF10 in vivo by a mechanism dependent on an intact oriT sequence and cosynthesis of PcfC, PcfF, and PcfG, as shown by a formaldehyde-cross-linking assay. Our findings support a model in which the PcfC receptor coordinates with the PrgJ ATPase to drive early steps of pCF10 processing/transfer: (i) PcfC first binds the pCF10 relaxosome through contacts with PcfF, PcfG, and DNA; (ii) PcfC delivers the plasmid substrate to PrgJ; and (iii) PrgJ catalyzes substrate transfer to the membrane translocase. Substrate engagement with a VirB4-like subunit has not been previously described; consequently, our studies point to a novel function for these signature T4SS ATPases in mediating early steps of type IV secretion.
INTRODUCTION
Enteroccocus faecalis donor cells transfer pheromone-responsive plasmids at high frequencies by conjugation upon perception of octapeptide pheromones synthesized by neighboring E. faecalis recipient cells. The regulatory networks controlling pheromone sensing and transfer (tra) gene expression are increasingly well understood for the pheromone-responsive plasmids pCF10 and pAD1 (13, 18, 20). However, the mechanism by which these and other mobile elements are delivered across the envelopes of Gram-positive cells remains poorly characterized (2).
The tra region of pCF10 carries three functionally distinct subsets of genes encoding (i) the Dtr (DNA transfer and replication) functions required for processing of the plasmid for transfer, (ii) the Mpf (mating-pair formation) proteins composing the mating or translocation channel, and (iii) 3 cell-wall-anchored proteins implicated in formation of the donor-recipient cell junction (2, 8, 9, 12, 21, 37, 43). The two Dtr proteins, the relaxase PcfG and the accessory factor PcfF, assemble at the plasmid's origin of transfer (oriT) sequence as the relaxosome to cleave the DNA strand destined for transfer (here termed the T strand) (8, 43, 44). PcfF initiates processing by binding the double-stranded (ds) oriT sequence, and oriT-bound PcfF then recruits the PcfG relaxase for strand-specific cleavage at the nic site. PcfG remains covalently bound to the 5′ end of the T strand, forming the PcfG–T-strand intermediate (here termed the T complex) (8). PcfG is postulated to pilot the T strand through the translocation channel, reminiscent of relaxase functions described in Gram-negative bacterial systems (2). PcfG also catalyzes the rejoining of cleaved nic sites in vitro (8), a biochemical activity that is thought to promote T-strand recircularization, second-strand synthesis, and plasmid stabilization in the recipient cell.
The estimated 11 Mpf proteins form the translocation channel, also termed the type IV secretion system (T4SS) (2, 27). Most of the Mpf proteins are likely constituents of the channel that extends across the cytoplasmic membrane and peptidoglycan layer to the cell surface. Two putative ATPases, PcfC and PrgJ, are thought to energize early steps of substrate transfer at the cytoplasmic entrance to the channel. PcfC is a member of a large family of T4SS ATPases that are structurally related to the SpoIIIE and FtsK DNA translocases (24, 25, 34). These subunits function as receptors for T4SS secretion substrates and are also called type IV coupling proteins (T4CPs) because they link DNA, as well as protein substrates, with cognate secretion channels (9, 26). Characterized receptor ATPases include TraD, TrwB, and TraG, encoded by the Gram-negative bacterial plasmids F, R388, and RP4, respectively, and VirD4, encoded by the Agrobacterium tumefaciens VirB/VirD4 system (here, plasmid-encoded proteins are designated TraDF, VirD4pTiA6, etc.) (5, 24–26, 41, 42, 46). Recent studies of T4SSs in Gram-positive species have defined the biochemical properties of E. faecalis PcfCpCF10 (9), Streptococcus agalactiae Orf10pIP501 (1), and Clostridium perfringens TcpApCW3 (45). In the pCF10 system, PcfC binds the Dtr factors PcfF and PcfG independently of each other, and all three subunits form punctate foci at the peripheries of pheromone-induced E. faecalis cells. PcfC also binds single-stranded (ss) and dsDNA substrates in vitro and the pCF10 plasmid in vivo, as shown by a formaldehyde (FA)-cross-linking assay termed transfer DNA immunoprecipitation (TrIP) (7, 9). PcfC cross-linking to pCF10 in vivo requires an intact oriT sequence and cosynthesis of PcfF and PcfG, suggesting that relaxosome assembly at oriT is necessary for plasmid engagement with the substrate receptor (9).
PrgJ, the subject of the present study, is a member of the VirB4-like ATPase superfamily; these subunits are associated with all T4SSs described to date (2). These signature ATPases function in assembly of the T4SS channel and biogenesis of the extracellular pili in Gram-negative systems, and they are also required for translocation of secretion substrates (2, 4, 7, 29). In the A. tumefaciens VirB/VirD4 system, the VirB4 ATPase coordinates its activity with two other ATPases, the VirD4 substrate receptor and VirB11, to mediate transfer of the oncogenic T-DNA across the inner membrane. Interestingly, VirD4 and VirB11, but not VirB4, formed FA-cross-linkable complexes with the translocating DNA substrate, as shown with the TrIP assay (4, 7). Consequently, we have postulated that VirD4 and VirB11 interact directly with the substrate to promote its delivery to the secretion channel, whereas VirB4 contributes indirectly through complex formation with the other ATPases to mediate the early steps of transfer (2).
We were intrigued by the fact that the E. faecalis pCF10 Prg/Pcf system, like all other described T4SSs of Gram-positive bacteria and a few in Gram-negative species, possesses the VirD4-like PcfC and VirB4-like PrgJ ATPases but lacks a VirB11-like ATPase (2). We biochemically characterized PrgJ, and here, we report that a purified, soluble form of PrgJ is dimeric and catalytically active. PrgJ dimers interact with the PcfC receptor, PcfF, and PcfG and with DNA substrates in vitro, and PrgJ also forms an FA-cross-linkable complex with pCF10 in vivo, as shown with the TrIP assay. Our data support a model in which the PcfC and PrgJ ATPases act sequentially and through direct interactions with the plasmid substrate to catalyze its transfer to the Prg/Pcf mating channel.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains, plasmids, and oligonucleotides are listed in Table 1. Escherichia coli BL21(DE3) (Novagen) was used for protein production, and strains DH5α and EC1000 served as hosts for the generation and maintenance of plasmid constructs. E. coli strains were cultured in Luria broth (LB) at 37°C with shaking. E. faecalis strains were cultured in brain heart infusion (BHI) broth (Difco Laboratories) at 37°C without shaking. Antibiotics (from Sigma Co.) were added to E. faecalis cultures at the following final concentrations: erythromycin, 100 μg ml−1 for plasmid markers and 10 μg ml−1 for chromosomal markers; fusidic acid, 25 μg ml−1; rifampin, 200 μg ml−1; chloramphenicol, 10 μg ml−1; spectinomycin, 1,000 μg ml−1 for plasmid markers and 250 μg ml−1 for chromosomal markers; streptomycin, 1,000 μg ml−1; and tetracycline, 10 μg ml−1. The following antibiotics were added to E. coli: carbenicillin (50 μg ml−1), chloramphenicol (20 μg ml−1), erythromycin (100 μg ml−1), kanamycin (50 μg ml−1), and spectinomycin (50 μg ml−1).
Table 1.
Bacterial strains, plasmids, and oligonucleotides used in the study
| Strain, plasmid, or oligonucleotide | Relevant features | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Gibco-BRL |
| BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm (DE3) | Novagen |
| EC1000 | E. coli cloning host; provides RepA in trans | 32 |
| E. faecalis | ||
| CK104 | OG1RFΔupp2 | 31 |
| OG1ES | Ermr Strr | 43 |
| OG1SSp | Strr Spcr | 19 |
| Plasmids | ||
| Expression plasmids/vectors | ||
| pET-28 b(+) | Kanr; expression vector for His tagging | Novagen |
| pAP1 | Kanr; pET-28a+ derivative | 6 |
| pGEX6P-1 | Crbr; expression vector for GST tagging | Amersham |
| pGEM-T Easy | Crbr; TA cloning vector | Promega |
| pDL278p23 | Spcr; pDL278 with Lactococcus lactis constitutive promoter P23 | 8 |
| pCJK47 | Carries oriTpCF10, lacZ, and P-pheS* cassette | 30 |
| pCJK21 | Ermr Spcr; shuttle vector; inducible promoter Pnis | 31 |
| pCF10 derivatives | ||
| pCF10 | Pheromone-inducible conjugative plasmid | 17 |
| pCF10ΔprgJ | pCF10 with prgJ deleted | This study |
| pCF10ΔprgH | pCF10 with prgH deleted | This study |
| pCF10ΔpcfC | pCF10 with pcfC deleted | 9 |
| pCF10ΔpcfF | pCF10 with pcfF deleted | 8 |
| pCF10ΔpcfG | pCF10 with pcfG inactivated by Ll.ltrBΔORF-Kan insertion | 43 |
| pCF10ΔoriT | pCF10 with oriT deleted | 43 |
| prgJ expression plasmids | ||
| pCY51 | pCJK21 expressing Pnis-prgJ-short | This study |
| pCM62 | pDL278p23 expressing P23-prgJ-short | This study |
| pCY81 | pCJK47 with prgJ flanking sequences for construction of pCF10ΔprgJ | This study |
| pCM58 | pGEM-T Easy containing prgJ.K471E for cloning into pCJK21 | This study |
| pCM59 | pGEM-T Easy containing prgJ.K471E for cloning into pET-28b(+) | This study |
| pCM64 | pCJK21 expressing Pnis-prgJ | This study |
| pCM69 | pCJK21 expressing Pnis-prgJ.K471E | This study |
| pINY1807 | pWM401 containing an ∼4.2-kb EcoRI fragment of pCF10 that includes prgIΔN5, full-length prgJ, and prgKΔC379 | 10 |
| pCY50 | pAP1 expressing prgJ-short-His6 | This study |
| pCM67 | pET28b(+) expressing prgJ-His6 | This study |
| pCM70 | pET28b(+) expressing prgJ.K471E-His6 | This study |
| pcfC, pcfF, pcfG, and pcfH expression plasmids | ||
| pCY26 | pGEX2T expressing Ptac-GST-pcfCΔN103 | 9 |
| pCY34 | pGEX6p-1 expressing GST-pcfF | 9 |
| pCY52 | pGEX6p-1 expressing GST-pcfG | This study |
| pCY82 | pCJK47 with prgH flanking sequences for construction of pCF10ΔprgH | This study |
| Oligonucleotides | ||
| Oligonucleotides for cloning | ||
| prgJ5F | 5′ GCTCTAGACATGGCCTATGCGCTTCAAAG 3′ | |
| prgJ5R | 5′ TCCCCCCGGGATCCTCTACAATGTGCATGAG 3′ | |
| prgJ3F | 5′ TCCCCCCGGGATTATGAACACCGACGCTTAG 3′ | |
| prgJ3R | 5′ ACATGCATGCCAAGCCAATACCTTGTTC 3′ | |
| PrgJ-pET5 | 5′ CATCCATGGCGCACATTGTAGAGGATGAGTTT 3′ | |
| PrgJ-pET3 | 5′ CCGCTCGAGGGTACCTTAGTGGTGGTGGTGGTGGTGCCTAGGAGCGTCGGTGTTCATAATTTCAAA 3′ | |
| PrgJ-XbaI | 5′ GCTCTAGACTAAGCGTCGGTGTTCAT 3′ | |
| PrgJ_rev_Xho | 5′ AATACTCGAGCTAAGCGTCGGTGTTCAT 3′ | |
| PrgJ_for_Nde | 5′ AATACATATGCACATTGTAGAGGAT 3′ | |
| KE_for | 5′ TCTGGTTCTGGTGAGGGAATGGCGACCAAAT 3′ | |
| KE_rev | 5′ ATTTGGTCGCCATTCCCTCACCAGAACCAGA 3′ | |
| PrgJ-pET6 | 5′ TAACCATGGGCAATTTGTTCAAAAAGAAACAATC 3′ | |
| pcfG-NdeI | 5′ GGAATTCCATATGGTGTATACAAAACATTTTGTT 3′ | |
| pcfG-XhoI | 5′ CCGCTCGAGTTATAGTTTGGGCTTAATGTC 3′ | |
| prgH5F | 5′ GCTCTAGAGATGTCTATATTCAGGTTGAA 3′ | |
| prgH5R | 5′ TCCCCCCGGGTCCTTTTGGATTTCTCATTGC 3′ | |
| prgH3F | 5′ TCCCCCCGGGGCCAAACGATTAGTAGGAGCG 3′ | |
| prgH3R | 5′ ACATGCATGCCGCTCGTCTTGTTCATACTGC 3′ | |
| Oligonucleotides for TrIP | ||
| TrIP-ChromF | 5′ TTACCAGTTTTGCAGTAGGG 3′ | |
| TrIP-ChromR | 5′ TTCAGCCACTGTCATAGCTTG 3′ | |
| TrIP-pcfE-F | 5′ ATGGACCAATTAACGGACG 3′ | |
| TrIP-pcfE-R | 5′ CCCATCGCTACACTTTGACAT 3′ | |
| Oligonucleotides for EMSA | ||
| pCF10oriTF3 | 5′ GGTAAGTCGAAACGTCAAT 3′ | |
| pCF10oriTR1 | 5′ CTCCTTAGTTTCGACAATTG 3′ | |
| 50-mer oriTS | 5′ ATATCGCAACATGCTAGCATGTTGCTCCGCTTGCAAAAAGAAAGCCTACC 3′ | |
| 50-mer oriTAS | 5′ GGTAGGCTTTCTTTTTGCAAGCGGAGCAACATGCTAGCATGTTGCGATAT 3′ | |
| 50-mer non-oriT | 5′ ATGCAGACGTACAAAAAAGAACTGAAACCGTATCTCTACCTTGGTGTTTT 3′ |
Plasmid construction.
Plasmid pCY81 was constructed for deletion of prgJ from pCF10. A 752-bp sequence upstream of prgJ (based on the annotated prgJ start site; accession no. AAW51315) was amplified by primers prgJ5F and prgJ5R (the primers used in this study were from Genosys Inc.). A 728-bp sequence downstream of prgJ was amplified by primers prgJ3F and prgJ3R. The amplified upstream fragment was digested with XbaI and XmaI, and the downstream fragment was digested with XmaI and SphI. The digested fragments were then cloned sequentially into pCJK47 to generate pCY81. The final construct contains the first 6 and the last 7 codons of prgJ separated by the XmaI site. A similar strategy was used to generate a ΔprgH mutation. A 754-bp DNA sequence upstream of prgH was amplified by primers prgH5F and prgH5R. A 766-bp DNA sequence downstream of prgH was amplified by primers prgH3F and prgH3R. The amplified upstream fragment was digested with XbaI and XmaI enzymes, and the downstream fragment was digested with XmaI and SphI enzymes. The digested fragments were then cloned sequentially into pCJK47 to generate pCY82. The final construct contains the first 6 and the last 9 codons of prgH separated by the XmaI site. pCY81 and pCY82 were used to delete prgJ and prgH, respectively, from pCF10 by marker exchange, as described previously (30).
prgJ and pcfG expression plasmids.
Plasmid pCY50 carries prgJ (designated prgJ-short) beginning with the Met start codon as annotated in the published pCF10 sequence (accession no. AAW51315). pCY50 was constructed by amplification of prgJ with primers PrgJ-pET5 and PrgJ-pET3, digestion of the PCR product with NcoI and XhoI, and introduction of the resulting fragment into the similarly digested vector pAP1. Plasmid pCM67 carries prgJ beginning with a Met codon located 51 codons upstream of the annotated start site. Primers PrgJ-pET6 and PrgJ-pET3 were used to amplify prgJ with flanking NcoI and XhoI sites for introduction into pET28b. In pCY50 and pCM67, prgJ is joined at its 3′ end to a 6-His tag, which was included in the reverse primer PrgJ-pET3. prgJ-short and prgJ were amplified by primers PrgJ-pET5 or PrgJ-pET6 and PrgJ-XbaI, the PCR products were digested with NcoI and XbaI, and the resulting DNA fragments were ligated to similarly digested pCJK21 for nisin-inducible expression. The resulting plasmids, pCY51 and pCM64, express prgJ-short and prgJ, respectively. prgJ-short was also placed under the control of the constitutive promoter P23 by amplification with primers prgJ_for_Nde and prgJ_rev_Xho, digestion with NdeI and XhoI, and introduction of the resulting fragment into similarly digested pCY31 (9) to generate pCM62. A Glu codon substitution for the invariant Lys471 residue within the Walker A motif was introduced into prgJ by two-step PCR using pINY1807 as the template and mutagenic primers (KE_for and KE_rev), together with the corresponding upstream primer (prgJ-pET6) and the downstream primer (prgJ-pET3). The resulting full-length PCR product was introduced into the pGEMT-Easy vector to yield pCM59. The mutated gene was introduced as an Nco/XhoI fragment into pET28b, resulting in pCM70, which expresses His-tagged prgJ.K471E. The same strategy was employed to express prgJ.K471E from a nisin-inducible promoter in E. faecalis, with the exception that the downstream primer prgJ_Xba was used for amplification instead of prgJ-pET3. Full-length prgJ.K471E was first cloned into pGEMT-Easy, resulting in pCM58, and then isolated as an NcoI-XbaI fragment for introduction into similarly digested pCJK21, resulting in pCM69. To synthesize an N-terminally glutathione S-transferase (GST)-tagged version of PcfG in E. coli, pcfG was amplified by PCR using primers pcfG-NdeI and pcfG-XhoI, which introduce NdeI and XhoI sites, respectively, into the ends of pcfG. The PCR fragment was digested with NdeI and XhoI and introduced into similarly digested pCY34, replacing pcfF with pcfG in the resulting plasmid, pCY52.
Conjugation assays.
Conjugative transfer of pCF10 plasmid was performed by liquid matings, as described previously (8). Briefly, overnight cultures of E. faecalis donor and recipient strains were washed with PBS and diluted 10 times in BHI medium without antibiotics. Cultures were grown for 1.5 h at 37°C without agitation and mixed at a donor/recipient cell ratio of 1:10. The mating mixtures were incubated for 1 h at 37°C, and serial dilutions were plated onto selective medium for determination of the numbers of transconjugants and donors. The efficiency of conjugation was expressed as the number of transconjugants per donor. Experiments were performed in triplicate.
Cellular fractionation.
Exponential-phase cultures (500 ml) of E. faecalis strains of interest were induced with 20 ng/ml of cCF10 peptide for 1.5 h and collected by centrifugation at 5,000 × g for 10 min at 4°C. The cell pellet was frozen at −20°C, thawed to facilitate cell breakage, and then resuspended in 5 ml of lysis buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 20% sucrose, 10 mg/ml lysozyme, 10 μg/ml mutanolysin, 1× complete protease inhibitor cocktail [Roche]) and incubated for 20 min at 37°C. The suspension was sonicated (Branson Sonifier 250) and centrifuged twice at 15,700 × g for 30 min to remove unbroken cells and cell debris. The soluble material was centrifuged at 165,000 × g for 2 h at 4°C to separate the cytosolic (supernatant) and membrane (pellet) fractions. The membrane pellets were resuspended by brief sonication in 1 ml of lysis buffer, and another round of ultracentrifugation was performed to remove residual cytosolic proteins. The membrane fractions were resuspended in 1 ml of lysis buffer containing 1% Triton X-100. The membrane and cytosolic fractions were loaded on a per-cell equivalent basis onto SDS-polyacrylamide gels, and proteins of interest were detected by SDS-PAGE and immunostaining.
Protein production and purification.
PrgJ-His6 and the Walker A mutant PrgJ.K471E-His6 were enriched by growth of E. coli strains BL21(DE3, pCM67) and BL21(DE3, pCM70) at 37°C in 500 ml LB medium and Kan (50-μg/ml final concentration) to an optical density at 600 nm (OD600) of 0.4, followed by addition of IPTG (isopropyl-β-d-thiogalactopyranoside) (0.1 mM final concentration) and incubation at room temperature (RT) for 3 h. Cells were harvested by centrifugation at 6,000 × g for 10 min, resuspended in 5 ml buffer A (20 mM Tris-HCl, pH 8.0, 0.15 M NaCl) supplemented with lysozyme (0.5 mg/ml) and protease inhibitors (Roche), and incubated for 10 min at 37°C. The cells were sonicated, and the lysate was centrifuged at 40,000 × g at 4°C for 30 min. The supernatant was mixed with 1 ml of Ni2+-nitrilotriacetic acid (NTA) (Qiagen) or Co2+ (Talon; Clontech) affinity resin, washed extensively with buffer A containing 10 mM imidazole, and incubated for 12 h at 4°C. The bound proteins were eluted with buffer A containing 200 mM imidazole. Alternatively, following sonication, the clarified supernatant was mixed with Ni2+-NTA agarose for 30 min, and the protein-resin complexes were packed onto a column and washed with buffer A containing 20 mM imidazole. Bound protein was eluted with buffer A containing 200 mM imidazole. Fractions with PrgJ were diluted five times with buffer B (20 mM Tris-HCl, pH 8.0), loaded onto a HiTrap-Q column (GE Healthcare), and eluted with a linear gradient of 50 mM to 1 M NaCl in buffer B by fast protein liquid chromatography (FPLC) (Akta; GE Healthcare). The peak fractions were combined and further purified using a HiPrep 16/60 Sephacryl S-200 HR gel filtration column or a Tricorn high-performance gel filtration column (Superose 6 10/300 GL; GE Healthcare) equilibrated with 20 mM Tris-HCl, pH 8.0, and 150 mM NaCl. The gel filtration columns were calibrated by using catalase, aldolase, and albumin (GE Healthcare) as standards.
GST and GST-tagged PcfCΔN103, PcfF, or PcfG were isolated by incubation of clarified lysates of E. coli cells prepared as described above with 1 ml glutathione-Sepharose beads (BioWorld) and gentle shaking overnight at 4°C. The beads were washed 5 times with buffer A and eluted with buffer A supplemented with 10 mM glutathione (9).
Purified PrgJ-His6 was sent to Cocalico Biologicals, Inc. (Reamstown, PA), for antibody production in New Zealand White rabbits, as previously described (28).
Protein-protein interaction assays.
PrgJ was assayed for interaction with PcfCΔN103, PcfF, or PcfG as follows. For GST affinity chromatography, soluble fractions of E. coli cells producing GST-tagged proteins or GST alone were mixed with 50 μl of glutathione-Sepharose beads for 1 h at RT. The Sepharose beads were pelleted by centrifugation at 500 × g for 5 min and washed three times with 1× phosphate-buffered saline (PBS). The beads were resuspended in 500 μl of binding buffer (20 mM Tris-HCl [pH 8.0], 0.1 M NaCl, 10 mg bovine serum albumin [BSA]). Purified PrgJ-His6 or PrgJ.K471E-His6 (20 μg) prepared as described above was incubated with the glutathione beads prebound with GST or the GST-tagged proteins for 3 h at RT. The beads were pelleted and washed 5 times with 1 ml of binding buffer containing 0.1% Triton X-100. The extensively washed Sepharose beads were eluted twice with elution buffer (20 mM Tris-HCl, pH 8, 0.1 M NaCl, 10 mM glutathione).
For Co2+ affinity (Talon) chromatography, PrgJ-His6 and PrgJ.K471E-His6 purified as described above were bound to the Talon resin by incubation for 3 h at 4°C, followed by extensive washes with buffer A containing 10 mM imidazole. Clarified extracts from E. coli cells producing GST or GST-tagged proteins were incubated with the Talon resin prebound with PrgJ-His6 for 3 h at RT. The beads were washed 4 times in buffer A containing 0.05% Triton X-100 and 10 mM imidazole, and bound proteins were eluted with buffer A containing 200 mM imidazole.
Proteins eluted from the glutathione or Talon affinity resins were identified by SDS-PAGE and immunostaining of Western blots with anti-His or anti-GST monoclonal antibodies.
TrIP assay.
The TrIP assay was carried out as previously described (7, 9). Following immunoprecipitation with anti-PrgJ antibodies, formaldehyde cross-links were broken by resuspension of the immunoprecipitates in 150 μl of elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS) and incubation overnight at 65°C. The supernatants were treated at 37°C for 2 h with 150 μl of 60 mM Tris-HCl, pH 6.8, 5% glycerol, and proteinase K (100 μg/ml). The DNA was purified by phenol-chloroform extraction, precipitated with isopropanol, and washed with 70% ethanol. Immunoprecipitated DNA was resuspended in 25 μl of Tris-EDTA (TE) and subjected to PCR amplification as previously described (9). For DNA detection, primers were designed to amplify a 315-bp region of pCF10 and a 485-bp region of the chromosome (Table 1).
EMSA.
The following single-stranded and double-stranded DNA substrates were used in electrophoretic mobility shift assays (EMSAs): (i) a 186-bp fragment encompassing the oriT sequence and upstream inverted repeat of pCF10, amplified with the primer pair pCF10oriTF3/pCF10oriTR1 and with pCF10 as the template and purified with a QIAquick PCR Purification Kit (Qiagen); (ii) a 39-bp nonspecific dsDNA sequence supplied with the DIG Gel Shift kit; (iii) a 50-mer oriT-S oligonucleotide containing the sense strand sequence encompassing the pCF10 oriT; (iv) a 50-mer oriT-AS oligonucleotide containing the antisense strand sequence encompassing the pCF10 oriT; and (v) a 50-mer non-oriT oligonucleotide corresponding to a pcfC fragment. Oligonucleotides were synthesized and purified by high-performance liquid chromatography (HPLC) and PAGE, and ssDNA and dsDNA substrates were labeled at the 3′ ends with digoxigenin (DIG)-11–ddUTP with the DIG Gel Shift kit (Roche) according to the manufacturer's instructions. EMSAs were carried out in 20-μl reaction mixtures composed of recombinant proteins, DIG-labeled ssDNA or dsDNA, 0.2 μg of poly(dA-dT), 0.1 μg of poly-l-lysine, 1× reaction buffer (20 mM Tris-HCl, pH 8.0, 0.1 M KCl, 10% glycerol, 5 mM EDTA). The reaction mixtures were incubated at RT for 15 min, loaded onto 8% polyacrylamide gels in 0.5× TBE buffer (Tris, borate, EDTA, pH 7.9), and then subjected to electrophoresis at 100 V for 1.5 h and electrotransferred to nylon membranes (Roche) at 6 V for 2 h with the Genie electrophoretic blotter (Idea Scientific). DIG-labeled DNA was visualized by an enzyme immunoassay (DIG Gel Shift Kit; Roche) following the manufacturer's instructions. In competitive EMSAs, a molar excess (10- or 100-fold) of cold competitor DNA was added to the preformed DNA substrate-protein complexes.
TNP-ATP binding assay.
ATP binding was assessed by measuring the fluorescence emission of a fluorescent ATP analog, 2′,3′-O-(2,4,6-trinitrophenyl) (TNP)-ATP (Molecular Probes, Inc.), with a fluorescence microplate reader (BioTek). Reactions were performed in black flat-bottom 96-well plates by mixing recombinant PrgJ (15 μg) with various concentrations of TNP-ATP in a reaction buffer of 20 mM Tris-HCl, pH 8.0, 50 mM NaCl at 37°C. The binding of the substrate was monitored by continuously measuring the increase of fluorescence intensity at an excitation wavelength of 410 nm and scanning wavelengths from 470 to 650 nm. Data analysis was carried out using Sigmaplot version 10.0 software, and binding curves were obtained by nonlinear regression fitting of the ligand equation (9, 33).
ATP hydrolysis assays.
ATPase assays were measured using a continuous coupled spectrophotometry assay (3). The reaction mixture (300 μl) containing 50 mM Tris-HCl, pH 6.8, 20 mM magnesium acetate, 1 mM dithiothreitol (DTT), 0.5 mg/ml BSA, 0.2 mM β-NADH, 2 mM phosphoenolpyruvate, 1.75 U pyruvate kinase, 2.5 U lactate dehydrogenase, and 0.9 to 12 mM ATP was preincubated at room temperature for 5 min. The reaction was initiated by the addition of 69 μg native or Walker A mutant PrgJ (2.67 μM), and the initial rates were measured by monitoring the decrease of absorbance at 340 nm for 1 min. One unit was defined as the amount of enzyme that produced 1 nmol of ADP per minute under standard assay conditions.
RESULTS
PrgJ is essential for pCF10 transfer.
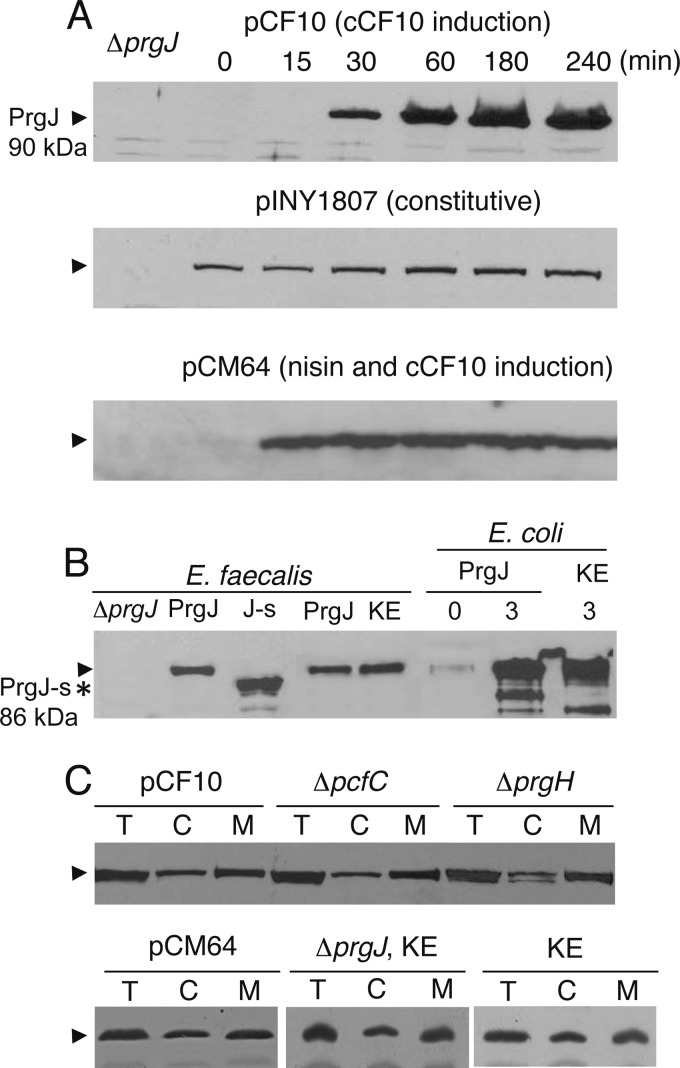
E. faecalis cells transfer pCF10 at high frequencies within 30 min of induction with the peptide pheromone cCF10 and then enter a decline phase marked by a reduction in transfer frequencies to preinduction levels (27). PrgJ accumulated within 30 min of cCF10 induction of CK104(pCF10), but levels remained high through the next 4 h, suggesting that PrgJ is not rate limiting for plasmid transfer over a prolonged postinduction period (Fig. 1A). Complementation tests confirmed the importance of PrgJ for plasmid transfer; CK104(pCF10ΔprgJ) lacks detectable PrgJ (Fig. 1A) and failed to transfer the plasmid (Table 2), whereas CK104(pCF10ΔprgJ, pINY1807) expressing prgJ from a cloned ∼4.2-kb EcoRI fragment accumulated detectable levels of PrgJ and transferred pCF10 at near wild-type levels (Table 2). PrgJ appears to be constitutively synthesized from pINY1807, likely from a promoter on the plasmid vector, although the protein accumulated at lower levels over a 4-h period compared with production levels in pCF10-carrying cells (Fig. 1A).
Fig 1.
PrgJ synthesis and subcellular localization. In all panels, the position of the native-size (∼90-kDa) functional form of PrgJ is marked by an arrowhead on the left. (A) Steady-state accumulation of PrgJ as a function of induction time with cCF10 or cCF10 and nisin. Strains (top to bottom): CK104(pCF10) producing PrgJ from its native promoter, CK104(pCF10ΔprgJ, pINY1807) producing PrgJ from a cryptic promoter on the pWM401 vector, and CK104(pCF10ΔprgJ, pCM64) producing PrgJ from a nisin-inducible promoter. Lanes 1, CK104(pCF10ΔprgJ) lacking PrgJ. Protein loading was normalized on a per-cell equivalent basis for comparisons of PrgJ steady-state levels. (B) PrgJ production in E. faecalis CK104 with the following plasmids: ΔprgJ, pCF10ΔprgJ; PrgJ, pCF10; J-s, pCF10ΔprgJ, pCY51 producing the PrgJ short form (∼86-kDa, marked by an asterisk on the left); PrgJ, pCF10ΔprgJ, pCM64 producing PrgJ native size; KE, pCF10ΔprgJ, pCM69 producing PrgJ.K471E. Also shown is PrgJ production in E. coli BL21(DE3) with the following plasmids: PrgJ, pCM67, PrgJ-His6 accumulation assessed at 0 and 3 h postinduction with IPTG; KE, pCM70, PrgJ.K471E-His6 accumulation assessed at 3 h postinduction. (C) Subcellular localization of PrgJ. (Top) cCF10-induced CK104 strains with pCF10; ΔpcfC, pCF10ΔpcfC; and ΔprgH, pCF10ΔprgH. (Bottom) Nisin-induced CK104 strains with PrgJ and pCM64 in the absence of other pCF10-encoded proteins; ΔprgJ, KE, pCF10ΔprgJ, pCM69, and PrgJ.K471E produced in the presence of other pCF10-encoded proteins; and KE, pCM69 in the absence of other pCF10-encoded proteins. Abundances of PrgJ are shown in total cell (T), cytoplasm (C), and membrane (M) fractions.
Table 2.
Transfer frequencies of E. faecalis strains
| Plasmida | Relevant genotype | Transfer frequency (transconjugants/donor)b |
|---|---|---|
| pCF10 | WTc | 3.2 × 10−2 |
| pCF10ΔprgJ | ΔprgJ | <10−8 |
| pCF10ΔprgJ, pSM3545 | ΔprgJ + vector plasmid | <10−8 |
| pCF10ΔprgJ, pINY1807 | EcoRI C fragment carrying prgJ | 2.1 × 10−2 |
| pCF10ΔprgJ, pCY51 + nisin | ΔprgJ + prgJ-short | <10−8 |
| pCF10ΔprgJ, pCM64 − nisin | ΔprgJ + prgJ | 1.7 × 10−5 |
| pCF10ΔprgJ, pCM64 + nisin | ΔprgJ + prgJ | 1.5 × 10−2 |
| pCF10ΔprgJ, pCM69 + nisin | ΔprgJ + prgJ.K471E | <10−8 |
| pCF10ΔprgH | ΔprgH | <10−8 |
Donor strain, CK104: recipient strain, OG1SSP or OG1ES.
Experiments were repeated three times in triplicate, and a representative experimental result is reported.
WT, wild type.
We expressed prgJ, beginning at the annotated prgJ start codon (bp 21359) in the pCF10 sequence (accession no. AAW51315), from a nisin-inducible promoter on pCY51. However, PrgJ synthesized from this construct had an ∼5-kDa-smaller molecular mass than native PrgJ produced from pCF10 or pINY1807, as shown by immunostaining of Western blots (Fig. 1B). prgJ expressed from pCY51 also failed to complement the ΔprgJ mutation (Table 2). We identified an in-frame Met codon located 153 bp upstream of the annotated prgJ start site within prgI, and when this longer prgJ gene was expressed from the nisin-inducible promoter, it both complemented the ΔprgJ mutation (Table 2) and yielded a protein migrating in gels at the same position as native PrgJ (Fig. 1B). In CK104(pCF10ΔprgJ, pCM64) cells expressing prgJ from the nisin-inducible promoter, PrgJ accumulated within 15 min of induction, and levels remained constant through the next 4 h of growth (Fig. 1A). These findings indicate that prgJ begins at the Met codon located 12 codons upstream from the 3′ end of prgI at bp 21206 of pCF10.
PrgJ lacks transmembrane domains (TMDs), as predicted by hydropathy algorithms, e.g., TMHMM, TopPred, and TmPred. PrgJ partitioned predominantly with the membrane fraction of pheromone-induced CK104(pCF10) cells, although a significant amount of the protein was also cytosolic (Fig. 1C). PrgJ partitioned similarly in subcellular fractions obtained from mutant strains lacking the PcfC receptor or the polytopic VirB6-like channel subunit PrgH (Fig. 1C). PrgJ had a similar distribution profile upon fractionation of OG1RF(pCM64), which lacks pCF10 and expresses prgJ from a nisin-inducible promoter (Fig. 1C).
PrgJ carries conserved Walker A and B nucleoside triphosphate (NTP)-binding motifs and therefore likely binds and hydrolyzes nucleoside triphosphates. We mutated the Walker A motif and, as expected, the prgJ.K471E allele failed to complement the ΔprgJ mutation despite accumulation of the K471E mutant protein at high levels (Fig. 1B and Table 2). The K471E mutant also copartitioned with membrane and cytosolic fractions in both the presence and absence of other pCF10-encoded proteins (Fig. 1C). Taken together with evidence that this mutant lacks ATPase activity (see below), these findings suggest that PrgJ's catalytic activity does not significantly influence its subcellular localization.
Soluble PrgJ is dimeric and binds and hydrolyzes ATP.
In E. coli BL21(DE3, pCM67) cells, the longer form of PrgJ fused to a His6 tag had the same apparent molecular mass as the native protein produced in E. faecalis cells (Fig. 1B). We purified PrgJ-His6 from E. coli cell lysates by Ni2+-NTA affinity chromatography followed by sequential anion-exchange and gel filtration chromatography. The purified protein migrated close to its predicted theoretical mass (90,849 Da) in SDS-polyacrylamide gels (Fig. 2A). PrgJ comigrated with the aldolase size marker (∼160 kDa), as assessed by gel filtration chromatography (Fig. 2A). The soluble form of PrgJ is therefore most likely dimeric, although the predicted mass is slightly less than expected of a dimer (∼180 kDa). We were unable to detect even small amounts of monomeric or higher-order species, further suggesting that soluble PrgJ is exclusively dimeric (Fig. 2A). The purified PrgJ.K471E mutant similarly migrated as an ∼90-kDa species in SDS-polyacrylamide gels and an ∼160-kDa species by size exclusion chromatography. Addition of ATP throughout purification and size exclusion chromatography did not alter the fractionation profiles of the native protein or the PrgJ.K471E mutant, suggesting that ATP binding/hydrolysis does not affect PrgJ's oligomeric state (F. Li, data not shown).
Fig 2.
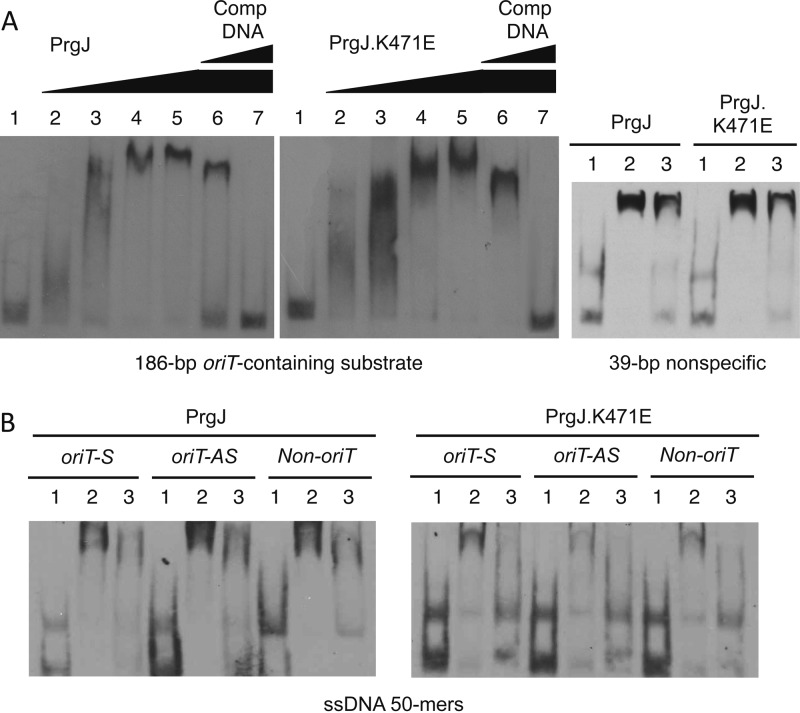
PrgJ purification, oligomeric state, and ATP binding/hydrolysis activities. (A) (Top) PrgJ-His6 enriched by Ni2+-NTA affinity chromatography was subjected to anion-exchange (not shown) and gel filtration (Sephacryl S-200 HR) column chromatography. Elution fractions of PrgJ (19 to 21) and peak positions of protein standards (1, aldolase, 158 kDa; 2, albumin, 67 kDa) are indicated. (Bottom) Coomassie-stained gel (10% polyacrylamide) showing relative amounts of PrgJ-His6 protein in the fractions listed. (B) ATP binding activities of PrgJ-His6 and PrgJ.K471E-His6 (15 μg) upon incubation with the fluorescent ATP analog TNP-ATP at the concentrations indicated. Fluorescence was measured with an excitation wavelength of 410 nm and scanning wavelengths from 470 to 650 nm. Reaction rates were calculated as described in Materials and Methods and were further plotted as a function of the TNP-ATP concentration. (C) ATP hydrolysis activities of PrgJ-His6 and PrgJ.K471E-His6 upon incubation with ATP at the concentrations indicated. ATP hydrolysis activities were monitored, and kinetic parameters were determined by fitting initial rate data to the Michaelis-Menten equation. The error bars indicate standard deviations.
Purified PrgJ bound the ATP fluorescence analog TNP-ATP, displaying a protein concentration-dependent increase in fluorescence intensity (Fig. 2B). A similar concentration-dependent increase in fluorescence was observed upon TNP-ATP incubation with the PrgJ.K471E mutant, suggesting that the Walker A mutant retains the capacity to bind ATP (Fig. 2B). In vitro ATPase activity was assessed by the coupled-enzyme method. Native PrgJ hydrolyzed ATP with a Vmax of 73.9 nmol/min/mg protein and an apparent Km of 2.9 mM, as determined by fitting initial rate data to the Michaelis-Menten equation. We consistently observed the highest ATP hydrolysis activity in a buffer containing Tris-HCl, pH 6.8, and Mg2+, whereas low reaction rates were observed in the presence of glycerol (∼2%) and HEPES buffer. The PrgJ.K471E mutant displayed only residual ATPase activity, with a high concentration of ATP over a prolonged incubation period (Fig. 2C).
PrgJ interacts with the PcfC substrate receptor.
To further define the contribution of PrgJ to pCF10 transfer through the T4SS, we assayed for its possible interaction with the PcfC substrate receptor. We prebound GST-PcfCΔN103, a soluble form of PcfC lacking its N-terminal TMD, to a glutathione resin and then incubated the washed bead-protein complexes with purified PrgJ-His6. As shown in Fig. 3, both GST-PcfCΔN103 and PrgJ-His6 were detected in material eluted from the beads with glutathione. In parallel experiments in which PrgJ-His6 was coincubated with glutathione beads prebound with GST only, GST, but not PrgJ-His6, was detected in the eluted fractions (Fig. 3A). Conversely, when GST-PcfCΔN103 was incubated with PrgJ-His6 prebound to a Co2+ affinity resin, a presumptive complex of PrgJ-His6 and GST-PcfCΔN103 was coeluted from the Co2+ beads when exposed to imidazole, whereas unconjugated GST showed no affinity for the PrgJ-His6-bead complexes (Fig. 3B).
Fig 3.

Interactions between PrgJ and Pcf proteins. (A and C) GST affinity pulldowns. GST, GST-PcfC, GST-PcfF, or GST-PcfG produced in the E. coli strains listed in Table 1 were bound to glutathione beads and then mixed with PrgJ-His6 (A) or PrgJ.K471E-His6 (C) purified as described in the legend to Fig. 2. Following extensive washes, bound proteins were eluted from the beads with glutathione; proteins in E. coli cell lysates and eluates were identified by immunostaining with anti-GST and anti-His antibodies (for PrgJ-His6). (B and D) Co2+ affinity pulldowns. Purified PrgJ-His6 (B) or PrgJ.K471E-His6 (D) was bound to Co2+ beads, and the protein-bead complexes were mixed with cell lysates containing the GST-tagged proteins shown. Following extensive washes, bound proteins were eluted from the beads with imidazole, and the proteins recovered in the eluates were identified by immunostaining with anti-GST and anti-His antibodies (for PrgJ-His6).
Pulldown experiments with PrgJ.K471E-His6 yielded similar results. The Walker A mutant protein and GST-PcfCΔN103 coeluted from glutathione resin prebound with the GST-tagged protein and Co2+ resin prebound with the His-tagged protein (Fig. 3C and D). PrgJ, therefore, interacts with the PcfC substrate receptor independently of its ATP hydrolysis activity.
PrgJ interacts with the relaxase PcfG and the accessory processing factor PcfF.
We next assayed for binding of purified PrgJ-His6 and PrgJ.K471E-His6 with GST-tagged forms of the Dtr factors PcfF and PcfG. Glutathione resins prebound with GST-PcfF or GST-PcfG, but not GST alone, retained both His-tagged versions of PrgJ (Fig. 3A and C). In reciprocal experiments, GST-PcfG and GST-PcfF, but not GST alone, coeluted from the Talon resin prebound with PrgJ-His6 or PrgJ.K471E-His6 (Fig. 3B and D). PrgJ, therefore, binds not only the PcfC receptor, but also the PcfF and PcfG processing factors. Our data further suggest that PrgJ binds each of these Dtr factors independently of the others and that formation of both PrgJ-PcfF and PrgJ-PcfG complexes occurs independently of PrgJ's catalytic activity.
PrgJ interacts with DNA in vitro and in vivo.
In view of evidence for interactions with components of the pCF10 relaxosome, we tested whether PrgJ also binds DNA substrates in vitro and in vivo. Purified PrgJ bound single- and double-stranded DNA substrates without sequence specificity, as shown by EMSAs (Fig. 4). A 186-bp dsDNA substrate containing the pCF10 oriT sequence exhibited a pronounced gel shift when incubated with PrgJ. Incubation with increasing amounts of PrgJ correlated with more pronounced mobility shifts, suggestive of noncooperative binding of the protein at multiple sites along the DNA substrate. DNA binding was partially inhibited by incubation with a 10-fold excess of unlabeled substrate as a competitor and completely inhibited by incubation with a 100-fold excess of the competitor DNA. PrgJ also bound a shorter heterologous dsDNA substrate provided in the DIG kit (39 bp nonspecific). Binding was less effectively inhibited with a 100-fold excess of unlabeled competitor DNA, possibly because of higher-affinity binding to this short dsDNA substrate.
Fig 4.
PrgJ DNA binding in vitro. Purified PrgJ-His6 and PrgJ.K471E-His6 (see the text) were analyzed by EMSAs for binding to the 3≪-end DIG-labeled double-stranded (A) and single-stranded (B) DNA substrates listed below the gels. (A) (Left) PrgJ binding to a 186-bp sequence encompassing the pCF10 oriT sequence. Lane 1, DNA probe only. Lanes 2 to 7, DNA probe plus the PrgJ proteins shown at the following concentrations: lane 2, 76 nM; lane 3, 152 nM; lane 4, 458 nM; lanes 5 to 7, 610 nM. Lanes 6 and 7, protein was preincubated with unlabeled 186-bp dsDNA competitor DNA at concentrations 10- and 100-fold higher than that of the DIG-labeled substrate. (Right) Binding of PrgJ proteins to a 39-bp nonspecific dsDNA substrate. Lanes: 1, DNA probe only; 2, DNA probe plus PrgJ (458 nM); 3, same as lane 2, except PrgJ was preincubated with unlabeled competitor DNA. (B) PrgJ (left) and PrgJ.K471E (right) binding to the DIG-labeled ssDNA 50-mers listed. The lanes are the same as in panel A, right; in all cases, competitor DNA was the unlabeled form of the test substrate at a 100-fold-higher concentration.
PrgJ also induced mobility shifts of all tested ssDNA substrates, including oligonucleotides corresponding to nicked (oriT-S) and unnicked (oriT-AS) DNA strands encompassing the pCF10 oriT sequence and a fragment of pcfC (Fig. 4). ssDNA binding was partially inhibited by incubation with a 100-fold excess of unlabeled competitor DNA.
The purified K471E Walker A mutant protein also bound dsDNA and ssDNA substrates, yielding band shifts similar to those observed with the native protein (Fig. 4A and B). The addition of NTPs to the EMSA reaction mixtures did not alter gel shift patterns generated with the native and mutant proteins (data not shown). The results of these in vitro studies suggest that PrgJ binds ssDNA and dsDNA substrates without sequence specificity or a requirement for ATP binding/hydrolysis.
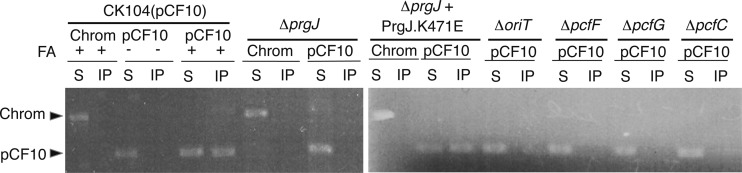
Our evidence for PrgJ interactions with the Dtr processing factors and DNA substrates in vitro prompted studies testing whether PrgJ binds the pCF10 substrate in vivo. By use of the TrIP assay, we found that anti-PrgJ antibodies precipitated pCF10 DNA from extracts of FA-treated CK104(pCF10) cells, but not detectable amounts of (i) chromosomal DNA from extracts of FA-treated CK104(pCF10) cells, (ii) pCF10 from extracts of non-FA-treated cells, or (iii) chromosomal or plasmid DNA from extracts of the FA-treated ΔprgJ mutant (Fig. 5). Of further interest, the anti-PrgJ antibodies did not precipitate pCF10 DNA from strains carrying mutant plasmids with the oriT sequence, pcfF, or pcfG deleted (Fig. 5). These findings suggest PrgJ interacts with pCF10 in vivo and that this interaction requires assembly of the relaxosome, e.g., PcfF and PcfG, bound at the plasmid's oriT sequence. Mutation of the Walker A motif (K471E) did not abrogate PrgJ-pCF10 complex formation (Fig. 5), further suggesting that PrgJ ATPase activity is not required for substrate binding in vivo. Finally, the PrgJ antibodies did not precipitate detectable levels of plasmid DNA from extracts of the FA-treated CK104(pCF10ΔpcfC) strain, suggesting that synthesis of the PcfC receptor is necessary for formation of cross-linkable PrgJ-pCF10 complexes.
Fig 5.
Detection of PrgJ-pCF10 close contacts in vivo by TrIP. (Left) Detection of pCF10 or chromosomal DNA in immunoprecipitates recovered with anti-PrgJ antibodies from FA-treated (+) or untreated (−) CK104(pCF10) or the FA-treated isogenic strains shown. PCF amplification products were generated with primers against a pCF10 gene fragment (shown as pCF10 at top) or a chromosomal fragment (shown as Chrom at the top). PCR products were generated using supernatant (nonprecipitated) (S) or immunoprecipitated (IP) material. (Right) Plasmid or chromosomal DNA precipitated from extracts of the ΔprgJ mutant producing the PrgJ.K471E Walker A mutant and other pCF10 deletion mutants producing native PrgJ.
DISCUSSION
VirB4 ATPases are associated with all known T4SSs, but to date, detailed structure-function studies have been restricted to subunits of Gram-negative systems (3, 14, 15, 22, 23, 29, 38, 47). In these systems, the VirB4 ATPases participate in biogenesis of the translocation channel and conjugative pilus (29, 47), and they contribute in an unspecified way to early substrate transfer reactions (4, 7). Here, we characterized PrgJ, a VirB4-like subunit of the E. faecalis pCF10 transfer system. Its biochemical properties generally resemble those of other VirB4 family members, although important differences also exist. Most importantly, whereas homologs in Gram-negative systems have not been shown to bind secretion substrates, purified PrgJ interacted with the Dtr factors PcfF and PcfG and DNA in vitro and also bound pCF10 in vivo. VirB4-like PrgJ of the E. faecalis pCF10 T4SS thus appears to catalyze early conjugative processing/translocation reactions through direct substrate interactions.
Our initial complementation studies established that PrgJ is required for pCF10 transfer and also supplied evidence that prgJ begins 14 codons upstream of the termination codon of prgI, whose product is related to the VirB3 subunits of Gram-negative T4SSs. In the Gram-negative systems, virB3- and virB4-like genes are highly conserved and invariably genetically linked, often overlapping and sometimes even fused to yield a single VirB3/VirB4 polypeptide (2, 11). The VirB3/PrgI subunits are typically small integral membrane proteins with two predicted transmembrane domains, and evidence has been presented for complex formation between VirB3/PrgI- and VirB4/PrgJ-like subunits in the A. tumefaciens VirB/VirD4 and S. agalactiae pIP501 transfer systems (1, 36, 47). prgI and prgJ thus are likely translationally coupled to facilitate formation of a PrgI/PrgJ subcomplex, although the precise contribution of PrgI to PrgJ function in mediating early steps of pCF10 transfer remains to be defined.
There is accumulating evidence from studies of VirB4 ATPases from Gram-negative T4SSs, such as TraCF (40), VirB4pTiA6 (14, 15), TraBpKM101 (22, 23), TrbERP4 (38), and TrwKR388 (3, 38), that the VirB4 ATPases vary in their membrane topologies/affinities and oligomeric states. Here, we found that PrgJ lacks a predicted transmembrane domain, yet it copartitioned with the membrane and cytosolic fractions. This distribution pattern was unaltered when PrgJ was produced in a pCF10-minus strain, suggesting that PrgJ's membrane affinity is not mediated through contacts with another T4SS channel subunit (Fig. 1). The K471E Walker A mutant also displayed a wild-type fractionation profile, excluding a role for ATP hydrolysis in mediating membrane binding (Fig. 1C). In the Gram-negative systems, TraBpKM101 and TrwKR388 similarly copartition with the membrane and cytoplasmic fractions (3, 22), whereas VirB4pTiA6 and TrbERP4 are exclusively membrane bound via N-terminal or internal TMDs (14, 38).
The soluble, cytosolic form of PrgJ most likely is dimeric, as assessed by gel filtration chromatography (Fig. 2A). VirB4pTiA6 also assembles minimally as a dimer (15), as does the soluble form of TraBpKM101 (22), whose low-resolution structure was recently solved by small-angle X-ray scattering (23). In contrast, soluble forms of Legionella pneumophila LvhB4 and E. coli TrwKR388 fractionate primarily as monomers (3, 23). By use of in silico modeling, it was reported that the C-terminal halves of VirB4pTiA6 and TraBpKM101 are related in tertiary structure to the TrwB/VirD4 ATPases (22, 35). This finding, coupled with evidence that the nucleotide-binding domain of TrwB crystallizes as a hexamer (24), led to a proposal that the VirB4 ATPases might function as higher-order hexamers (22, 35).
At this time, however, the only experimental support for this proposal is observations that small amounts (<5%) of TrwKR388 adopt ring-shaped structures that might correspond to hexamers (3), and higher-order TraBpKM101 species that also might correspond to hexamers were recovered during purification with an Na-acetate buffer system (22). Interestingly, the hexameric form of TraBpKM101 is soluble and catalyzes ATP hydrolysis, whereas the dimeric form is membrane bound and catalytically inactive (22). We have not detected PrgJ hexamers with any buffer systems, yet modeling studies with the Swiss-model workspace of the Swiss pdb viewer (http://swissmodel.expasy.org) showed that the closest structural homologs of PrgJ's C-terminal domain (residues 363 to 722) are the hexameric ATPases TrwBR388 (Protein Data Bank [PDB] ID, 1e9s) and FtsK (PDB ID, 2ius) (24, 34; M. Sarkar and P.J. Christie, unpublished data). At this time, we cannot exclude the possibility that PrgJ exists in different oligomeric states, possibly transitioning between dimeric and hexameric or other higher-order forms, as was also suggested for TraBpKM101 (22). It is noteworthy, however, that in contrast to TraBpKM101, the soluble dimeric form of PrgJ is catalytically active (Fig. 2), binds DNA (Fig. 4), and interacts with the PcfC receptor and the Dtr processing factors (Fig. 3). Thus, if PrgJ does transition between different oligomeric states, such a transition likely modulates rather than activates PrgJ's substrate binding activity. Conceivably, PrgJ undergoes a change in oligomeric state upon binding of substrate that is important for driving a subsequent reaction in the transfer pathway.
We found that the ATP binding properties of PrgJ, e.g., fluorescence spectra and affinity, were similar to the characteristics of other VirB4 subunits. The apparent dissociation constants for TNP-ATP (KdTNP-ATP) were 8.0 ± 0.99 μM and 10.0 ± 1.99 μM for native PrgJ and the PrgJ.K471E mutant, respectively. These values are similar to the KdTNP-ATP (6.91 ± 0.79 μM) of the full-length TraBpKM101 (22). In addition, the ATP hydrolysis activity of purified PrgJ (Vmax = 73.9 nmol/min/mg protein) was comparable to values reported for other recently characterized VirB4 homologs, including TrwKR388 (48.4 nmol/min/mg protein), TraBpKM101 (126 nmol/min/mg), and Aeromonas veronii TraEpAC3249A (53.75 nmol/min/mg protein) from T4SSs of Gram-negative species (3, 22, 39). However, purified PrgJ showed Mg2+-dependent ATPase activity with an apparent Km of 2.9 mM, which is approximately 2- to 5-fold higher than Km values reported for VirB4 homologs (3, 22, 39). Whether VirB4 homologs associated with other Gram-positive T4SSs also possess enhanced enzymatic activity over their Gram-negative counterparts remains an intriguing question for future studies.
The results of our protein-protein interaction studies supplied evidence for complex formation among the two ATPases, PcfC and PrgJ (Fig. 3). Such an interaction was expected on the basis of previous work in our laboratory showing that the A. tumefaciens VirD4, VirB4, and VirB11 ATPases form a ternary complex required for T-DNA transfer across the inner membrane (4, 7). However, our further evidence that PrgJ interacts with the Dtr processing factors and DNA substrates in vitro (Fig. 3 and 4) and pCF10 in vivo (Fig. 5) was unexpected and suggests a novel role for the signature VirB4-like ATPases. Importantly, we detected FA cross-linking of PrgJ only with oriT-containing pCF10 and only in strains coproducing PcfC, PcfF, and PcfG (Fig. 5). These findings suggest, first, that PrgJ does not directly recruit pCF10 to the transfer channel but engages the substrate only once it has docked with the PcfC receptor. Second, PrgJ likely binds either the PcfF-PcfG-oriT relaxosomal complex or a more fully processed form of the substrate composed of the Dtr factors bound to the free T strand.
Together with previous work on this system (8, 9), our findings build on a mechanistic understanding of early steps in the pCF10 processing and transfer pathway: (i) PcfF initiates processing by binding oriT and recruiting the PcfG relaxase to form the relaxosome, (ii) the PcfC receptor recruits pCF10 through binding of the relaxosome, and (iii) PcfC delivers the relaxosome or processed transfer intermediate to PrgJ. Our data further suggest that these early reactions proceed independently of the ATP hydrolysis activities of both PcfC and PrgJ (reference 9 and this study). The role of ATP energy in driving the early transfer reactions remains to be elucidated; for example, one or both ATPases might catalyze dissociation of the relaxosome, release of accessory factor PcfF from the T complex, or unfolding of the relaxase component of the T complex prior to translocation through the T4SS channel. It is also possible that PrgJ possesses a SecA ATPase motor-like activity (16) to initiate translocation of bound substrate through the Prg/Pcf channel.
Finally, we speculate that PrgJ is representative of a clade of VirB4 ATPases associated with T4SSs of all Gram-positive bacteria and some Gram-negative species that lack a VirB11 ATPase (2). These VirB4 subunits might have acquired a novel function(s) requiring direct substrate engagement to compensate for loss of the VirB11 subunit at some point during evolution. However, it also remains possible that substrate binding is a more widespread property of the signature VirB4 ATPases than we previously envisioned. Although we were unable to detect a VirB4pTiA6–T-DNA cross-link with our TrIP assays (4, 7), a recent report by Durand et al. (22) presented evidence that the VirB4 homolog TraBpKM101 binds DNA in vitro. This finding, together with the results of our present study, underscores the importance of further mechanistic work exploring how the signature VirB4 ATPases contribute to substrate transfer through phylogenetically diverse T4SSs.
ACKNOWLEDGMENTS
We thank members of the Christie laboratory for helpful discussions.
Studies in the Christie laboratory were supported by NIH GM48476. Work in the Yeo laboratory was supported in part by grant E-1616 from the Welch Foundation. C.A.-M. was supported in part by a Brazilian postdoctoral fellowship from CAPES-Brazil.
Footnotes
Published ahead of print 25 May 2012
REFERENCES
- 1. Abajy MY, et al. 2007. A type IV-secretion-like system is required for conjugative DNA transport of broad-host-range plasmid pIP501 in gram-positive bacteria. J. Bacteriol. 189:2487–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez-Martinez CE, Christie PJ. 2009. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73:775–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arechaga I, et al. 2008. ATPase activity and oligomeric state of TrwK, the VirB4 homologue of the plasmid R388 type IV secretion system. J. Bacteriol. 190:5472–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atmakuri K, Cascales E, Christie PJ. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54:1199–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atmakuri K, Ding Z, Christie PJ. 2003. VirE2, a type IV secretion substrate, interacts with the VirD4 transfer protein at cell poles of Agrobacterium tumefaciens. Mol. Microbiol. 49:1699–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burgess RR, Arthur TM, Pietz BC. 2000. Mapping protein-protein interaction domains using ordered fragment ladder far-western analysis of hexahistidine-tagged fusion proteins. Methods Enzymol. 328:141–157 [DOI] [PubMed] [Google Scholar]
- 7. Cascales E, Christie PJ. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Staddon JH, Dunny GM. 2007. Specificity determinants of conjugative DNA processing in the Enterococcus faecalis plasmid pCF10 and the Lactococcus lactis plasmid pRS01. Mol. Microbiol. 63:1549–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, et al. 2008. Enterococcus faecalis PcfC, a spatially localized substrate receptor for type IV secretion of the pCF10 transfer intermediate. J. Bacteriol. 190:3632–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christie PJ. 1987. Characterization of a pheromone-inducible plasmid transfer system and a conjugative transposon associated with the Streptococcus faecalis R-plasmid pCF10. Ph.D. thesis Cornell University, Ithaca, NY [Google Scholar]
- 11. Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:451–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuang ON, et al. 2009. Multiple functional domains of Enterococcus faecalis aggregation substance Asc10 contribute to endocarditis virulence. Infect. Immun. 77:539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clewell DB. 2007. Properties of Enterococcus faecalis plasmid pAD1, a member of a widely disseminated family of pheromone-responding, conjugative, virulence elements encoding cytolysin. Plasmid 58:205–227 [DOI] [PubMed] [Google Scholar]
- 14. Dang TA, Christie PJ. 1997. The VirB4 ATPase of Agrobacterium tumefaciens is a cytoplasmic membrane protein exposed at the periplasmic surface. J. Bacteriol. 179:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dang TA, Zhou XR, Graf B, Christie PJ. 1999. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter. Mol. Microbiol. 32:1239–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Driessen AJ, Nouwen N. 2008. Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 77:643–667 [DOI] [PubMed] [Google Scholar]
- 17. Dunny G, Funk C, Adsit J. 1981. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid 6:270–278 [DOI] [PubMed] [Google Scholar]
- 18. Dunny GM. 2007. The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell-cell signalling, gene transfer, complexity and evolution. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 362:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunny GM, Brown BL, Clewell DB. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. U. S. A. 75:3479–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dunny GM, Johnson CM. 2011. Regulatory circuits controlling enterococcal conjugation: lessons for functional genomics. Curr. Opin. Microbiol. 14:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dunny GM, Zimmerman DL, Tortorello ML. 1985. Induction of surface exclusion (entry exclusion) by Streptococcus faecalis sex pheromones: use of monoclonal antibodies to identify an inducible surface antigen involved in the exclusion process. Proc. Natl. Acad. Sci. U. S. A. 82:8582–8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Durand E, Oomen C, Waksman G. 2010. Biochemical dissection of the ATPase TraB, the VirB4 homologue of the Escherichia coli pKM101 conjugation machinery. J. Bacteriol. 192:2315–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durand E, Waksman G, Receveur-Brechot V. 2011. Structural insights into the membrane-extracted dimeric form of the ATPase TraB from the Escherichia coli pKM101 conjugation system. BMC Struct. Biol. 11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomis-Ruth FX, et al. 2001. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature 409:637–641 [DOI] [PubMed] [Google Scholar]
- 25. Gomis-Ruth FX, Sola M, de la Cruz F, Coll M. 2004. Coupling factors in macromolecular type-IV secretion machineries. Curr. Pharm. Des. 10:1551–1565 [DOI] [PubMed] [Google Scholar]
- 26. Hamilton CM, et al. 2000. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J. Bacteriol. 182:1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirt H, et al. 2005. Characterization of the pheromone response of the Enterococcus faecalis conjugative plasmid pCF10: complete sequence and comparative analysis of the transcriptional and phenotypic responses of pCF10-containing cells to pheromone induction. J. Bacteriol. 187:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jakubowski SJ, Cascales E, Krishnamoorthy V, Christie PJ. 2005. Agrobacterium tumefaciens VirB9, an outer-membrane-associated component of a type IV secretion system, regulates substrate selection and T-pilus biogenesis. J. Bacteriol. 187:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerr JE, Christie PJ. 2010. Evidence for VirB4-mediated dislocation of membrane-integrated VirB2 pilin during biogenesis of the Agrobacterium VirB/VirD4 type IV secretion system. J. Bacteriol. 192:4923–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kristich CJ, Chandler JR, Dunny GM. 2007. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid 57:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kristich CJ, Manias DA, Dunny GM. 2005. Development of a method for markerless genetic exchange in Enterococcus faecalis and its use in construction of a srtA mutant. Appl. Environ. Microbiol. 71:5837–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leenhouts K, et al. 1996. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253:217–224 [DOI] [PubMed] [Google Scholar]
- 33. Li F, Terzyan S, Tang J. 2011. Subsite specificity of anthrax lethal factor and its implications for inhibitor development. Biochem. Biophys. Res. Commun. 407:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Massey TH, Mercogliano CP, Yates J, Sherratt DJ, Lowe J. 2006. Double-stranded DNA translocation: structure and mechanism of hexameric FtsK. Mol. Cell 23:457–469 [DOI] [PubMed] [Google Scholar]
- 35. Middleton R, Sjolander K, Krishamurthy N, Foley J, Zambryski P. 2005. Predicted hexameric structure of the Agrobacterium VirB4 C-terminus suggests VirB4 acts as a docking site during type IV secretion. Proc. Natl. Acad. Sci. U. S. A. 102:1685–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mossey P, Hudacek A, Das A. 2010. Agrobacterium tumefaciens type IV secretion protein VirB3 is an inner membrane protein and requires VirB4, VirB7, and VirB8 for stabilization. J. Bacteriol. 192:2830–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olmsted SB, Erlandsen SL, Dunny GM, Wells CL. 1993. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encoded by the pheromone-inducible conjugative plasmid pCF10. J. Bacteriol. 175:6229–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rabel C, Grahn AM, Lurz R, Lanka E. 2003. The VirB4 family of proposed traffic nucleoside triphosphatases: common motifs in plasmid RP4 TrbE are essential for conjugation and phage adsorption. J. Bacteriol. 185:1045–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rangrez AY, Abajy MY, Keller W, Shouche Y, Grohmann E. 2010. Biochemical characterization of three putative ATPases from a new type IV secretion system of Aeromonas veronii plasmid pAC3249A. BMC Biochem. 11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schandel KA, Muller MM, Webster RE. 1992. Localization of TraC, a protein involved in assembly of the F conjugative pilus. J. Bacteriol. 174:3800–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schroder G, et al. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schroder G, Lanka E. 2003. TraG-like proteins of type IV secretion systems: functional dissection of the multiple activities of TraG (RP4) and TrwB (R388). J. Bacteriol. 185:4371–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Staddon JH, Bryan EM, Manias DA, Chen Y, Dunny GM. 2006. Genetic characterization of the conjugative DNA processing system of enterococcal plasmid pCF10. Plasmid 56:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Staddon JH, Bryan EM, Manias DA, Dunny GM. 2004. Conserved target for group II intron insertion in relaxase genes of conjugative elements of gram-positive bacteria. J. Bacteriol. 186:2393–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steen JA, Bannam TL, Teng WL, Devenish RJ, Rood JI. 2009. The putative coupling protein TcpA interacts with other pCW3-encoded proteins to form an essential part of the conjugation complex. J. Bacteriol. 191:2926–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szpirer CY, Faelen M, Couturier M. 2000. Interaction between the RP4 coupling protein TraG and the pBHR1 mobilization protein Mob. Mol. Microbiol. 37:1283–1292 [DOI] [PubMed] [Google Scholar]
- 47. Yuan Q, et al. 2005. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J. Biol. Chem. 280:26349–26359 [DOI] [PubMed] [Google Scholar]