Abstract
The competence-stimulating peptide (CSP) and the sigX-inducing peptide (XIP) are known to induce Streptococcus mutans competence for genetic transformation. For both pheromones, direct identification of the native peptides has not been accomplished. The fact that extracellular XIP activity was recently observed in a chemically defined medium devoid of peptides, as mentioned in an accompanying paper (K. Desai, L. Mashburn-Warren, M. J. Federle, and D. A. Morrison, J. Bacteriol. 194:3774–3780, 2012), provided ideal conditions for native XIP identification. To search for the XIP identity, culture supernatants were filtered to select for peptides of less than 3 kDa, followed by C18 extraction. One peptide, not detected in the supernatant of a comS deletion mutant, was identified by tandem mass spectrometry (MS/MS) fragmentation as identical to the ComS C-terminal sequence GLDWWSL. ComS processing did not require Eep, a peptidase involved in processing or import of bacterial small hydrophobic peptides, since eep deletion had no inhibitory effect on XIP production or on synthetic XIP response. We investigated whether extracellular CSP was also produced. A reporter assay for CSP activity detection, as well as MS analysis of supernatants, revealed that CSP was not present at detectable levels. In addition, a mutant with deletion of the CSP-encoding gene comC produced endogenous XIP levels similar to those of a nondeletion mutant. The results indicate that XIP pheromone production is a natural phenomenon that may occur in the absence of natural CSP pheromone activity and that the heptapeptide GLDWWSL is an extracellular processed form of ComS, possibly the active XIP pheromone. This is the first report of direct identification of a ComR/ComS pheromone.
INTRODUCTION
The ability of bacteria to modify their hereditary material by taking up and incorporating extracellular DNA into their genomes via natural transformation is a widespread phenomenon (17). In streptococci, the mechanisms involved in natural transformation have been described in the most detail in Streptococcus pneumoniae, but the phenomenon is also observed in at least 14 other streptococcal species (10, 17, 26).
The pheromones triggering competence for natural transformation in S. pneumoniae are the competence-stimulating peptides (CSPs). They are encoded by the comC gene of the comCDE operon and are thought to be produced as propeptides with a double-glycine leader sequence (11). Cleavage of the leader sequence at the double glycine is concomitant with the export of the mature peptide by the ComA ABC transporter and its accessory protein, ComB (15). Upon reaching a threshold concentration, the CSP mature peptide binds to the ComD histidine kinase. The binding probably leads to autophosphorylation of ComD, followed by the transfer of the phosphate group to the cognate ComE response regulator (12, 30). Phosphorylated ComE is then thought to recognize a direct repeat in the promoter sequences of the comAB and comCDE operons, as well as in the sigX promoter, activating their transcription (42). SigX or ComX is the alternative sigma factor that links the CSP autocatalytic system to competence by activating transcription of competence effector genes involved in DNA binding, uptake, and recombination (22, 35). Other mitis streptococci, as well as streptococci from the anginosus group are thought to use a similar CSP autocatalytic system to activate competence (13, 14, 32, 41).
In the salivarius group, the competence pheromone belongs to a new class of small hydrophobic peptides that most probably bind to the Rgg-like regulator ComR to activate transcription of sigX and the pheromone-encoding gene comS (10). The pheromones in the salivarius group belong to the type I class of ComR/ComS pheromone systems (26). Like most other pheromones, the ComR/ComS type I pheromones are predicted to be produced as propeptides that are processed to yield the mature pheromone by a mechanism that has not yet been elucidated.
The Streptococcus mutans competence-signaling system is unique in that competence can be triggered by two pheromones. One is the S. mutans CSP, which, like the CSP in S. pneumoniae, belongs to the class of double-glycine-type leader peptides (24, 31). The other is the recently identified small hydrophobic peptide XIP (for sigX-inducing peptide), which belongs to the type II class of ComR/ComS pheromone systems and is characterized by the presence of conserved aspartate and double-tryptophan residues (26).
The S. mutans CSP pheromone triggers early events associated with increased expression of the ComDE two-component system, such as upregulation of bacteriocin-related genes, including cipB (mutacin V; SMU.1914) and other mutacin loci (19, 20, 29, 39). Comparison of S. mutans ComDE with S. pneumoniae two-component systems reveals that ComDE is more closely related to the S. pneumoniae BlpHR system involved in bacteriocin production than to the S. pneumoniae ComDE system involved in competence (25). Increased expression of sigX and effector genes of the DNA binding and uptake machinery regulated by sigX, however, is also observed, but this is a delayed response, and in contrast to the system in S. pneumoniae, no ComE binding site is found in the S. mutans sigX promoter. Instead, the sigX promoter has a motif that is most probably recognized by ComR, which places the ComR/ComS system at the core of the S. mutans competence response (26). Supporting the core role of the ComR/ComS system are the facts that sigX expression and competence are abolished in comR or comS deletion mutants in complex or defined growth medium and that such phenotypes are restored to normal levels in the comS mutant in the presence of synthetic XIP, but not CSP (26). This is also supported by the fact that competence development, albeit at lower levels, is frequently observed in mutants with deletion of comDE (1, 5, 24, 26, 34) and that deletion of comC, at least in S. mutans UA159, may result in mutants that are not affected in competence (1).
ComS is most probably produced as a propeptide that is processed into the active XIP pheromone. XIP is imported via an oligopeptide permease system and, inside the cells, is thought to bind to ComR to activate transcription of comS and sigX (26). Eep metalloproteases of Enterococcus faecalis, and of some streptococci, have been implicated in the processing of pheromones (4, 9, 40). In streptococci, Eep also has a purported role in the processing of signal peptides directing oligopeptide permeases to the bacterial membrane (9). A single Eep homologue is found in the S. mutans genome (2), but whether it is involved in the possible processing of XIP or in the assembly of permeases, such as the Opp permease system associated with XIP import in S. mutans, remains to be determined.
ComR/ComS pheromones are found in at least eight other species of streptococci (9, 10, 26). In all of them, a predicted ComR/ComS binding site is found in the sigX promoter sequence, indicating that the system has evolved to regulate competence in a wide range of streptococcal species. In no case, however, has the native structure of the secreted peptides been identified.
Endogenous XIP activity was recently described in the culture filtrates of S. mutans grown in a chemically defined medium (CDM) (see the accompanying paper by Desai et al. [8]). The CDM is devoid of peptides, which, together with the high levels of extracellular XIP activity attained, creates ideal conditions for the identification of the native XIP and possibly other peptides. In this study, we used the growth conditions described in the accompanying paper (8) to identify the extracellular native XIP and to investigate the putative involvement of Eep in XIP production or response. The possible link between the accumulation of native extracellular XIP and CSP was also investigated.
MATERIALS AND METHODS
Bacteria and growth conditions.
S. mutans UA159 and the isogenic mutants used in this study are presented in Table 1. Cultures of S. mutans were grown in 5% CO2 at 37°C in Todd-Hewitt Broth (THB) (Becton, Dickinson and Company, Le Pont de Claix, France) or CDM and stored at −80°C in the same medium supplemented with 15% glycerol. CDM was prepared as previously described (26). The selective antibiotics for S. mutans were erythromycin, kanamycin, and spectinomycin at final concentrations of 20 μg ml−1, 500 μg ml−1, and 200 μg ml−1, respectively.
Table 1.
Strains used in this study
| S. mutans strain | Descriptiona | Source or reference |
|---|---|---|
| UA159 | Wild-type transformable strain; sequenced genome; Kans Erms Spcs | 2 |
| SM059 | UA159::Φ(PcipB-luc); Spcr | This study |
| SM065 | UA159 ΔcomS::spc; Spcr (from strain MW05 [26]) | This study |
| SM068 | UA159::Φ(PsigX-luc); Spcr | This study |
| SM074 | SM068 ΔcomC::kan Φ(PsigX-luc); Kanr Spcr (from strain SM004 [31]) | This study |
| SM089 | UA159 ΔcomS::erm; Ermr | This study |
| SM091 | SM068 ΔcomS::erm Φ(PsigX-luc); Ermr Spcr (from strain SM089) | This study |
| SM100 | SM068 Δeep::kan Φ(PsigX-luc); Kanr Spcr | This study |
| SM101 | SM091 Δeep::kan Φ(PsigX-luc); Kanr Ermr Spcr (from strain SM100) | This study |
Kan, kanamycin; Erm, erythromycin; Spc, spectinomycin.
Construction of mutants.
The PCR-ligation mutagenesis strategy (21) was used for the construction of deletion mutants, using the oligonucleotide primers listed in Table 2. Mutants with deletions of comS, comC, and eep were constructed. AscI or FseI restriction sites were incorporated into the 5′ ends of the oligonucleotide primers complementary to the gene target used to generate the flanking DNA fragments and into both 5′ ends of the resistance cassettes (Table 2). The erythromycin and kanamycin resistance cassettes (kind gifts from D. A. Morrison) were amplified with the primer pairs FP015-FP016 and FP001-FP068, respectively. The comS flanking regions were amplified with the primer pairs FP649-FP651 and FP652-FP653 and the eep flanking regions with the primer pairs FP658-FP659 and FP660-FP661. The resultant amplicons corresponding to the 5′ or 3′ region flanking the target genes were digested with AscI (New England BioLabs) or FseI (New England BioLabs), respectively, whereas the resistance cassettes were digested with both enzymes. T4 DNA ligase (Fermentas, St. Leon, Germany) was then used to ligate the upstream or the downstream amplicons of the target genes to either the erythromycin or the kanamycin resistance cassettes in separate reactions. The two ligation products were then mixed and PCR amplified with the distal primers. The resultant amplicons were used to transform S. mutans, as described below. The gene deletion in each mutant was confirmed by PCR amplification of the chromosomal DNA extracted from Ermr or Kanr mutants using the distal primers, as well as by using the distal primers in combination with complementary primers internal to the resistance cassette sequences. The correct predicted size of the products was verified by gel electrophoresis. Deletion of comC was also verified by DNA sequencing using the primer pairs FP009-FP037 and FP038-FP012 to generate the amplicons that were sequenced using the primer FP009 or FP012.
Table 2.
Primers used in this study
| Primer | Nucleotide sequencea (5′ to 3′) |
|---|---|
| FP001 | AGGCGCGCCGTTTGATTTTTAATG |
| FP009 | TATGGACCAAGAAATGCTGT |
| FP012 | TGGAGTTGCTTGATTTCATT |
| FP015 | GGCGCGCCCCGGGCCCAAAATTTGTTTGAT |
| FP016 | GGCCGGCCAGTCGGCAGCGACTCATAGAAT |
| FP037 | TCATTTTCTCCCACCAGCTT |
| FP038 | GCGCCTACGAGGAATTTGTA |
| FP068 | AGGCCGGCCTAGGTACTAAAACAATTCATCCAGTA |
| FP419 | AGGATCCCACCTTCAACAGCTGAAAGTGC |
| FP420 | AAAGTCGACTAAAACTTCTGTTAAACAGCCGG |
| FP627 | CGGCCGTCGACGTCCGGATCCATTCCGGCATAGCTCAGTTG |
| FP630 | CATTTACCTCCTCGAGGATCCAACATTCCCTCTTGTTGCCAAT |
| FP649 | CGATTTTGGCGTTGGTATTT |
| FP651 | AGGCGCGCCATCCTGTTATTCTCCTTTCTTTTTGA |
| FP652 | AGGCCGGCCGAGCTTATAATAGACAGC |
| FP653 | AAGATACCGTTCCGCTCCTT |
| FP658 | TCGAAGATGCTGCTTTTCCT |
| FP659 | AGGCGCGCCTTCCCGAACCAAAATTCCTG |
| FP660 | AGGCCGGCCATTCCTGCTCTTGATGGTGGT |
| FP661 | CTTCATGAGTCGGCCCTAAA |
Restriction sites are underlined: BamHI, GGATCC; SalI, GTCGAC; AscI, GGCGCGCC; FseI, GGCCGGCC. Sequences homologous to either side of the BamHI restriction site used to linearize pFW5-luc are in boldface.
For the construction of luciferase promoter reporters, the primer pair FP419-FP420 or FP627-FP630 was used for amplification of the cipB or sigX promoter region, respectively (36). For cloning of the sigX promoter, the CloneEZ PCR cloning kit (GenScript, Piscataway, NJ) was used, following the manufacturer's protocol and using BamHI to linearize the pFW5-luc vector. For cloning of the cipB promoter, SalI and BamHI restriction sites were incorporated at the 5′ ends of the primers. After enzymatic digestion, the plasmid and the amplified promoter regions were ligated, purified, and used to transform One Shot Top 10 chemically competent Escherichia coli (Invitrogen). Plasmids with the correct insert were then purified and used to transform the UA159 wild-type strain, with selection in spectinomycin-containing plates. The chromosomal DNAs from comS, eep, and comC deletion mutants were used to transform the sigX promoter reporter strain (SM068) (Table 1). The comS deletion PsigX-luc reporter SM091 and the PcipB-luc reporter SM059 were used as indicators for XIP- or CSP-like activity in culture supernatants, respectively.
Transformation.
For targeted gene deletion or reporter construction, S. mutans was grown in TSB (Oxoid, Hampshire, United Kingdom), and transformation was conducted as previously described (33). Transformants were selected by growth on THB agar plates containing the selective antibiotics.
Bioassay of pheromone activity in culture supernatants.
The bioassay described in the accompanying article by Desai et al. (8) was used with slight modifications. The PsigX-luc reporter in a comS deletion background (SM091) was used for detection of extracellular XIP-like activity, whereas the cipB promoter reporter (SM059) was used for detection of extracellular CSP-like activity. Each assay reaction mixture contained 40 μl of indicator strain (SM091 or SM059), 40 μl of CDM, 10 μl of culture supernatant or 10 μl synthetic peptides diluted in CDM, and 10 μl of 1 mM d-luciferin (Synchem, Felsberg-Altenberg, Germany) distributed in black 96-well plates with transparent bottoms (Nunc Thermo Scientific). Luminescence and optical density at 600 nm (OD600) were measured by reading the plates in a multidetection microplate reader (Synergy HT; BioTek, Winooski, VT). CDM without synthetic peptides was used to obtain background values that were subtracted from the sample values.
The culture supernatants were collected at different densities during growth. The accumulation of extracellular XIP activity during growth showed a variation between parallel experiments that correlated, at least in part, with the XIP-like activity in the culture used for the inoculum. This carryover of endogenous XIP-like activity introduced variability between the experiments that hampered comparisons between the wild type and the deletion mutants. We then established a protocol for the preparation of supernatants used in comparative activity assays. The protocol used frozen stock cultures in CDM prepared from cultures grown to an OD600 of 0.5 that were centrifuged and resuspended to the same OD in fresh CDM. Ten microliters of the stock cultures was used as the inoculum for overnight cultures prepared in 3 ml CDM. The overnight cultures were then centrifuged, and the pellets were resuspended in fresh CDM to an OD600 of 0.3. Under these conditions, expression of sigX by the PsigX-luc reporter SM068 was reduced to levels close to background. ComS11-17 at 50 nM was then used to reactivate the autocatalytic mechanism of endogenous XIP production.
Synthetic peptide.
The heptapeptide GLDWWSL (ComS11-17) was custom synthesized by GenScript Corporation with an estimated purity of 98%, as determined by high-performance liquid chromatography (HPLC). The lyophilized ComS11-17 was reconstituted with 20 μl dimethyl sulfoxide (DMSO) (Sigma-Aldrich), to which distilled water was added to give a final ComS11-17 concentration of 10 mM. The stock solutions were stored in small aliquots at −20°C. The 18CSP peptide was also synthesized by GenScript and prepared as previously described (31).
LC–MS/MS.
S. mutans UA159 and the isogenic comS (SM065) and eep (SM100) deletion mutants were grown at 37°C in CDM under normal atmospheric conditions. At midexponential growth phase (OD600, ∼1.0), the culture supernatants were recovered by centrifugation (10,000 × g for 5 min at 4°C). A sample of the synthetic ComS11-17 or 18CSP was used as a standard control. The synthetic peptides were resuspended in CDM to a final concentration of 2 μM and concentrated following the same protocol that was used for the preparation of the culture supernatants. To deplete high-molecular-weight proteins, aliquots of 1 ml supernatants with 10% acetonitrile (ACN) (Burdick & Jackson, Germany) were loaded on prewashed Nanosep Centrifugal Devices with a molecular mass cutoff of 3 kDa or 10 kDa, followed by centrifugation (14,000 × g for 15 min at room temperature [RT]). The eluted fractions were then brought to pH 2 using trifluoroacetic acid (TFA) (Sigma-Aldrich) and loaded on Sep-Pak Plus C18 silica cartridges (360 mg silica; Waters, Milford, MA) that were prewashed with 80% ACN-0.1% TFA and equilibrated with 0.1% aqueous TFA. After sample loading, the cartridges were washed with 0.1% TFA. For sample elution, a total volume of 1.6 ml of an aqueous solution with 80% ACN-0.1% TFA was passed through the cartridges. Since the bed volume of the C18 cartridges was approximately 500 μl, we collected the eluents in three serial elution fractions with volumes of 400 μl, 600 μl, and 600 μl. Aliquots of 110 μl corresponding to the different fractions were then dried under vacuum using an Eppendorf (Germany) Concentrator 5301 and resuspended in 5 μl 1% formic acid before liquid chromatography-tandem mass spectrometry (LC–MS/MS) analysis or in CDM for analysis of sigX promoter induction activity using the luciferase reporter system, as described below. LC–MS/MS analysis was conducted by the proteomic facility at the Biotechnology Centre of Oslo. An LC-MS system consisting of a Dionex Ultimate 3000 RSLC nano-UPLC (Sunnyvale, CA) connected to an Orbitrap linear quadrupole ion trap (LTQ Orbitrap XL) mass spectrometer (ThermoElectron, Bremen, Germany) equipped with a nanoelectrospray ion source was used to analyze the peptides. For liquid chromatography separation, an Acclaim PepMap 100 column (C18; 3 μm; 100 Å; Dionex, Sunnyvale CA) capillary with 25-cm bed length was used with a flow rate of 300 nl/min. Two solvents, A (0.1% formic acid) and B (aqueous 90% acetonitrile in 0.1% formic acid), were used in the nanocolumn. The gradient went from 7% to 40% solvent B in 17 min and from 40% to 50% solvent B in 3 min, with a total run time of 33 min. The mass spectrometer was operated in the data-dependent mode to automatically switch between Orbitrap-MS and LTQ–MS/MS acquisition. Survey full-scan MS spectra (from m/z 300 to 2,000) were acquired in the Orbitrap with a resolution (R) of 60,000 at m/z 400 and allowed sequential isolation of up to six of the most intense ions for fragmentation on the linear ion trap using collision-induced dissociation (CID) at a target value of 10,000 charges. For accurate mass measurements, the lock mass option was enabled in MS mode. Other instrument parameters were set as previously described (18).
Data search strategy.
Peptide identifications were obtained using the MASCOT search algorithm (v2.2; Matrix Science, United Kingdom) (28). All acquired MS/MS spectra were searched against the ComS and ComC amino acid sequences, followed by a search of possible ComS or ComC cleavage products in the MS histogram using their corresponding theoretical m/z ion currents. To validate the results, the absence of predicted ComS or a ComS cleavage product(s) in the supernatant of the comS deletion mutant SM065 was examined.
RESULTS
Identification of processed XIP in the supernatants of S. mutans cultures.
To identify the mature form of the secreted XIP peptide, we first conducted experiments to estimate the concentrations of the active factor in the supernatants of S. mutans during growth in CDM, as described in the accompanying paper (8). This medium contains individual amino acids, but no peptides. Concentrations equivalent to 1.5 to 2.5 μM synthetic ComS11-17 were obtained at the midexponential phase at OD600 values between 1 and 2 (data not shown). We selected samples at an OD600 of ∼1 for LC–MS/MS analysis. To estimate the efficiency of the extraction procedures prior to LC–MS/MS analysis, activity recovery from samples using the synthetic ComS11-17 were compared with that from supernatant samples. Recovery of pheromone activity was similar for the synthetic peptide and for the supernatants. Running the samples through the 3-kDa or 10-kDa molecular mass cutoff centrifugal devices resulted in recovery levels above 90%, whereas recovery following the C18 extraction of the filtered samples was approximately 45%. As expected from the cartridge bed volume, a minor fraction of approximately 3% ComS11-17 equivalent activity was found in the first eluted fraction of 400 μl, whereas more than 40% of the pheromone activity was in the second fraction of 600 μl. The third fraction had only 2% of the recovered activity. The second elution was therefore chosen for further analysis. Culture filtrates of the comS deletion mutant had no detectable activity and were therefore used as a negative control in the identification experiments. LC–MS/MS analysis revealed the presence of a peptide corresponding to the seven C-terminal amino acids of the predicted ComS, as observed in the MS histogram (Fig. 1a) and after fragmentation (Fig. 1b). CID–MS/MS fragmentation showed six fragment masses (b4, b5, b6, y2, y3, and y4) that matched the mass of m/z 438.72, which corresponds to the double-protonated form of the heptapeptide (Fig. 1b). In samples of the comS deletion mutant SM065, the heptapeptide was not identified by MS (Fig. 1a), supporting the correct identification of the peptide in the wild type. The complete ComS propeptide was not detected by MS/MS fragmentation.
Fig 1.
Native XIP detection. (a) Extracted ion chromatograms (XICs) of the m/z range 876.40 to 876.44 of samples from the wild type UA159, the ΔcomS mutant SM065, and the synthetic ComS11-17. For relative abundance calculation, the highest peak in the m/z range 876.40 to 876.44 for each sample was set to 100%. (b) CID–MS/MS spectrum showing the protonated b and y ion fragments of the doubly protonated native peptide (m/z 438.72) in the wild-type sample.
Eep is not required for ComS processing or for the import of extracellular XIP.
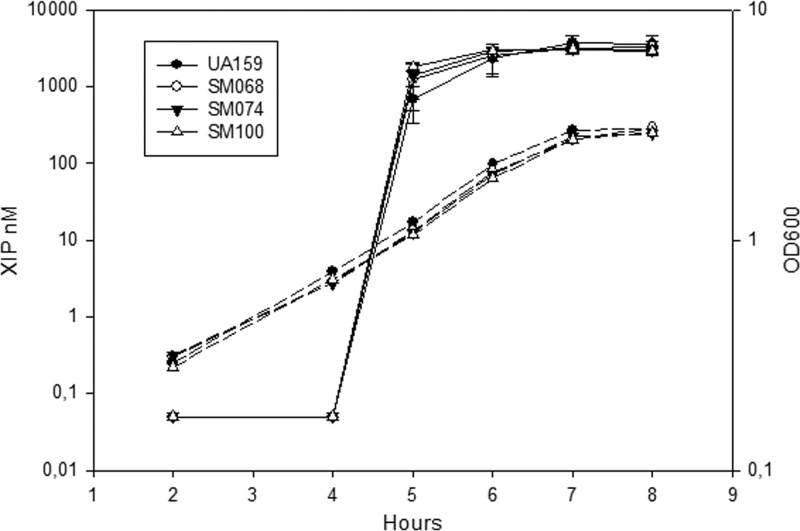
Eep homologues have been implicated in the processing of SHP-like peptides and possibly also in the processing of signal peptides directing SHP-oligopeptide permeases to the bacterial membranes. The S. mutans genome has a single eep homologue showing syntenic arrangement with genes neighboring eep in E. faecalis V583, Streptococcus gordonii Challis, Streptococcus pyogenes NZ131, Streptococcus thermophilus LMD-9, and Streptococcus uberis 0140J (Fig. 2). Comparison of Eep in these strains with S. mutans UA159 Eep revealed identities of 52%, 66%, 70%, 67%, and 68%, respectively. In E. faecalis, Eep processes pheromones involved in plasmid conjugation (3, 4), and in the listed streptococci, it has been associated with pheromone (6, 10, 40) or lipoprotein (7, 9) processing. To assess whether ComS processing involves the Eep homologue in S. mutans, samples prepared from the supernatants of an eep deletion mutant were run in the LC-LTQ-Orbitrap. The MS results revealed a peak corresponding to a peptide with an m/z value similar to that of XIP. The supernatants from the eep deletion mutant SM100 were also investigated for the presence of pheromone activity, using the bioassay for detection of XIP-like activator, and again, no differences from supernatants from the wild type were observed (Fig. 3). Our results indicate, therefore, that Eep is not required for ComS processing.
Fig 2.
Syntenic region surrounding eep. The region shown is 2.2 kb upstream and downstream of the eep gene in S. mutans UA159. The corresponding syntenic regions in E. faecalis V583, S. gordonii Challis, S. pyogenes NZ131, S. thermophilus LMD-9, and S. uberis 0140J are shown as horizontal bars. The identities with S. mutans within the syntenic regions were 66.31%, 69.3%, 71.51%, 70.98%, and 70.78%, respectively. Eep identities were 52%, 66%, 70%, 67%, and 68%, respectively. They represent species or strains in which Eep has been associated with pheromone or lipoprotein processing. SMU.1783 encodes a prolyl-tRNA synthetase, SMU.1785 encodes a putative phosphatidate cytidylyltransferase synthase, SMU.1786 encodes a putative undecaprenyl pyrophosphate synthetase, and SMU.1787c encodes a preprotein translocase subunit, YajC. Syntenic region analysis was conducted using GEVO (27).
Fig 3.
Endogenous XIP activity during growth. Extracellular XIP activity (solid lines) and OD600 (dashed lines) were measured at different time points. The following strains were included: UA159, SM068, the comC deletion mutant SM074, and the eep deletion mutant SM100. The XIP concentration was estimated using a standard curve with serial ComS11-17 dilutions. The tester strain was the comS deletion PsigX-luc reporter SM091. The OD600 values were measured using 1-cm-path-length cuvettes. The results are averages and standard errors (SE) from three independent experiments for the XIP levels and from one experiment for the growth curve.
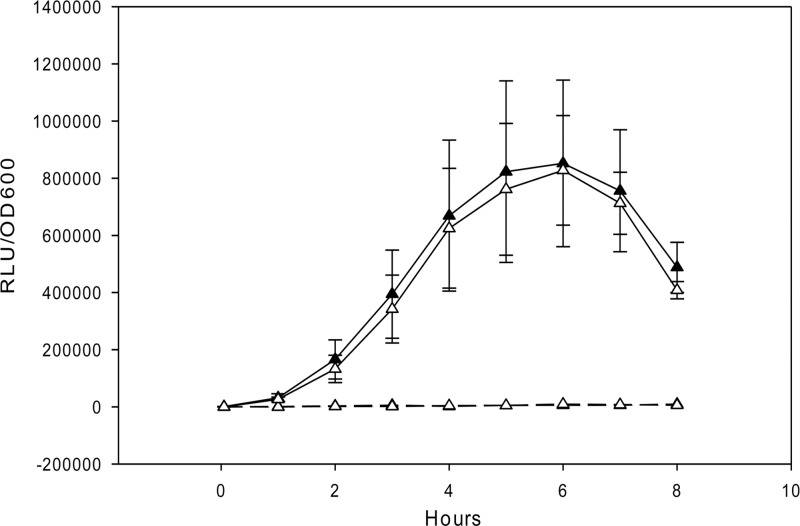
In S. mutans, XIP is proposed to be imported into the cytoplasm by an oligopeptide permease transporter (26). Since Eep has a putative role in processing lipoprotein signal peptides found in oligopeptide permease subunits (7, 9), we investigated whether import of XIP was affected in eep deletion mutants. For this purpose, we measured the expression of sigX in response to ComS11-17 using comS mutants that could respond to XIP but not produce it. The results revealed that sigX induction levels under stimulated conditions using synthetic XIP were similar in the eep-comS mutant SM101 and the comS mutant (SM091) (Fig. 4). We conclude that Eep is not required for processing of an active XIP peptide or for mechanisms involved in the import of XIP.
Fig 4.
Effect of eep deletion on the response to ComS11-17. PsigX-luc reporters were used to measure sigX expression in comS or comS-eep mutants unable to produce their own XIP. The reporter SM091 (ΔcomS PsigX-luc) (black triangles) or SM101 (ΔcomS-Δeep PsigX-luc) (white triangles) was grown in the presence of 1 μM ComS11-17 (solid lines) or without ComS11-17 (dashed line). Relative light unit (RLU) and OD600 values were measured in a 96-well plate using a Multidetection microplate reader. The results are averages and SE from three independent experiments for cultures grown in the presence of ComS11-17 and from one experiment for cultures grown in the absence of ComS11-17.
CSP pheromone activity was not detected in culture supernatants.
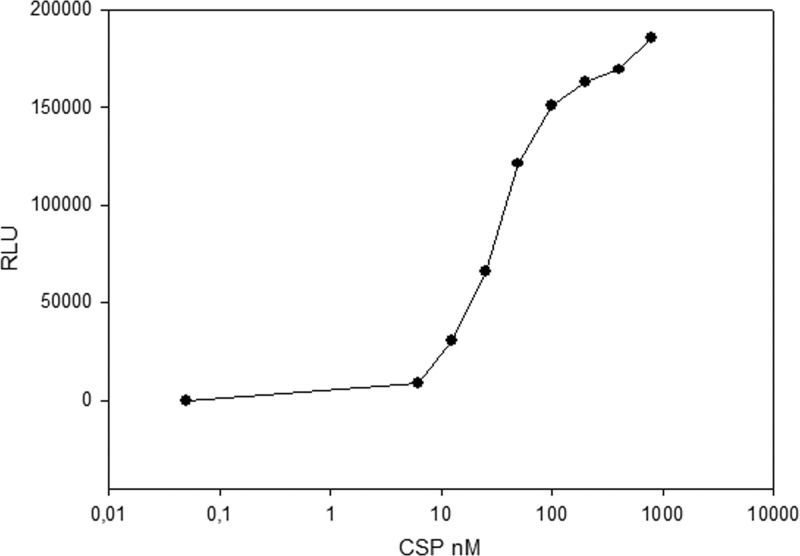
To investigate whether the native CSP pheromone was present in culture supernatants, a bioassay similar to XIP pheromone detection was used, except that the indicator strain, SM059, was a luciferase reporter for the cipB promoter. The cipB promoter has a ComE binding site and shows early activation in response to synthetic CSP (16, 20). The synthetic 18CSP was used at serial dilutions to test the suitability of the assay and to estimate the activity detection range. 18CSP levels as low as 12 nM could be detected (Fig. 5). Since the test samples correspond to 10% of the total volume in the bioassay, this value corresponds to 120 nM in the original tested samples. The CSP-like activity in the culture supernatants of the comC deletion mutant SM074 were compared with the activity in the supernatant of SM068, which has a wild-type background. For both strains, luminescence values for supernatants collected at different densities during growth were below background levels. Exposure of SM068 to 5 nM, 10 nM, or 100 nM 18CSP was then used to test whether low synthetic CSP concentrations could trigger an autocatalytic circuit involving the comDE two-component system. However, no differences between SM068 and the comC deletion mutant were detected.
Fig 5.
Bioassay for detection of CSP-like activity. The indicator strain SM059 (PcipB-luc) was exposed to serial dilutions of synthetic 18CSP. Induction of luciferase activity is presented as RLU.
We also used LC–MS/MS analysis to search for the presence of the ComC processed peptides in the supernatants of the wild type in which the native XIP was identified. Recovery rates of synthetic CSP activity using a 3-kDa or a 10-kDa centrifugal device were approximately 30% and 40%, respectively, and after the C18 cartridge extraction, they were approximately 20% and 30%. However, no ComC or ComC processed peptides from wild-type supernatant extracts were detected by mass spectrometry.
Synthetic CSP has previously been shown to induce comS expression in a subpopulaton of S. mutans competent cells (23). We investigated, therefore, whether the CSP pheromone was produced under the experimental conditions used, based on the hypothesis that CSP may lead to increased production of XIP. We measured the XIP-like activity in the supernatant of the comC mutant SM074 and in the supernatant of SM068, a reporter with a wild-type background. The results showed no differences between the wild type and the deletion mutant. We concluded that endogenous XIP pheromone production is a phenomenon that may occur in the absence of endogenous CSP pheromone activity.
DISCUSSION
S. mutans competence for genetic transformation may be triggered by two pheromones. One is XIP, whose activity was recently described in culture filtrates of S. mutans grown in CDM devoid of peptides (8). The other is CSP, which has not been identified in its native form and which may not always be required for competence, since comC deletion mutants whose competence levels were not affected have been reported (1). In this study, we identified the heptapeptide GLDWWSL in the supernatants of S. mutans and showed that endogenous XIP production occurred in the absence of detectable CSP levels in the supernatants of S. mutans. This is the first report on the direct identification of a ComR/ComS pheromone.
Pheromones have traditionally been identified following the isolation and purification of active fractions from large volumes of bacterial cultures, involving many laborious steps. However, when pheromones are first identified by genomic analysis, the search for posttranslationally processed peptides can be simplified. This is particularly the case for pheromones that are endogenously produced in a growth medium devoid of peptides, as in our study. In these instances, MS analytical tools can be used to advantage for identification of peptides of bacterial origin in supernatant extracts. Recently, the SHP 1358 pheromone of S. thermophilus was identified using such an approach (9). For the S. mutans XIP identification in our study, analysis of the crude extract resulted in few fragments corresponding to XIP in the MS/MS analysis. The analysis of complex mixtures of peptides may be complicated, however, by factors such as ion suppression, in which peptides with a composition that favors ionization may reduce the chances of other peptides to become protonated. Pheromones are also short, and since the aim is to identify the peptide in its native form, trypsinization of samples is omitted. Such factors reduce the power to identify single peptides in complex mixtures, which is based on probability scores, and may have contributed to our difficulties in identifying the processed ComS in crude extracts. This was solved, however, by including a simple filtration step that excluded larger peptides from the supernatants.
Direct identification of intracellular native pheromones bound to their respective regulators has not yet been described for any Rgg system to which ComR belongs. Without such an approach, it is not possible to draw definitive conclusions about the nature of the native pheromone. However, the findings that the peptide identified in our study corresponded to the seven C-terminal amino acids of ComS previously shown to induce high levels of S. mutans transformation in its synthetic form (26) and that active pheromones in Gram-positive bacteria usually represent truncated C-terminal derivatives modified before import (37) indicate that XIP is an active form of the pheromone.
To date, no identified systems for the processing of ComR/ComS pheromones have been reported. Since the Eep membrane metalloprotease plays an important role in the processing of E. faecalis pheromones and pheromone inhibitors and is probably involved in the processing of SHP pheromones in S. thermophilus (9) and S. pyogenes (6), we investigated whether Eep was involved in S. mutans ComS processing. Our results failed, however, to demonstrate its requirement, indicating the presence of an alternative system. We also investigated whether the XIP response was affected in the eep deletion mutant, since its participation in the assembly of Opp systems has been suggested (9). Although XIP import is thought to be mediated by an Opp system, our results did not demonstrate a role of Eep in the response to XIP. The presence of alternative mechanisms to process lipoprotein signal peptides have, however, been reported in streptococci (7). Therefore, we cannot exclude the possibility that functional redundancy may obscure a role for Eep in the assembly of a functional Opp system.
The recent finding that the ComR/ComS system is at the core of the competence response poses the question of how the second pheromone system represented by CSP is involved in competence stimulation (26). Although this mechanism is still unknown, a recent report that comS expression is increased in the presence of synthetic CSP (23) (also supported by our unpublished microarray results), at least in undefined rich media, indicates that CSP may stimulate competence by increasing production of the XIP pheromone. To date, however, there is no direct evidence that native CSP is produced. We therefore investigated whether a CSP-like activator was present in S. mutans supernatants, using a PcipB-luc reporter as the indicator strain. The synthetic 18CSP induced luciferase production by the indicator strain at concentrations as low as 12 nM, with maximal levels at approximately 100 nM. This concentration range of synthetic 18CSP activity is similar to that found in cultures of S. mutans grown in rich medium (31). No CSP-like activity was identified in the supernatants of wild-type or comC deletion strains. MS/MS analysis of wild-type supernatants also failed to demonstrate the presence of ComC processed peptides. Together with the finding that XIP activity in the supernatant of the comC deletion mutant was similar to that in the wild type, the results indicate that CSP either is not produced under the conditions investigated or was produced at concentrations that did not stimulate XIP production. Our results support the previous finding that deletion of comC has no effect on sigX expression or transformation (1). In the previous study, a nutritionally rich medium was used, indicating that the lack of an association between comC and sigX expression is not restricted to the chemically defined medium used in our study.
ComR/ComS type II pheromones are found in at least eight other species of streptococci, including some with high pathogenic potential, such as S. pyogenes (9, 10, 26). It is most likely that, similar to the ComS pheromone in S. mutans and to a variety of other pheromones in Gram-positive bacteria, ComS proteins in other strepococci are also processed into mature peptides. The analysis of crude mixtures of peptide extracts by advanced MS/MS methods has already been used for the identification of native peptides from a variety of sources, including, for instance, serum and neuron extracellular fractions (27, 38). In bacteria, such an “omics” approach to the identification of secreted endogenous peptides is in its infancy. Despite the challenges ahead, such as limitations to the identification of bacterial open reading frames (ORFs) encoding small peptides, with comS as an example, and of possible uncommon amino acid modifications, this is an exciting field for future investigations.
ACKNOWLEDGMENTS
We thank D. A. Morrison and M. J. Federle for communicating results before publication and for valuable comments on the manuscript and Andreas Podbielski for the kind gift of pFW5-luc. Heidi Aarø Åmdal provided excellent technical assistance.
A.P.R.F. is a recipient of a Ph.D. grant, number 2335-11-5, from CAPES.
Footnotes
Published ahead of print 18 May 2012
REFERENCES
- 1. Ahn SJ, Wen ZT, Burne RA. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 74:1631–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ajdic D, et al. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434–14439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. An FY, Clewell DB. 2002. Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis. J. Bacteriol. 184:1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. An FY, Sulavik MC, Clewell DB. 1999. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J. Bacteriol. 181:5915–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhagwat SP, Nary J, Burne RA. 2001. Effects of mutating putative two-component systems on biofilm formation by Streptococcus mutans UA159. FEMS Microbiol. Lett. 205:225–230 [DOI] [PubMed] [Google Scholar]
- 6. Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ. 2011. Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathog. 7:e1002190 doi:10.1371/journal.ppat.1002190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denham EL, Ward PN, Leigh JA. 2008. Lipoprotein signal peptides are processed by Lsp and Eep of Streptococcus uberis. J. Bacteriol. 190:4641–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desai K, Mashburn-Warren L, Federle MJ, Morrison DA. 2012. Development of competence for genetic transformation of Streptococcus mutans in a chemically defined medium. J. Bacteriol. 194:3774–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleuchot B, et al. 2011. Rgg proteins associated with internalized small hydrophobic peptides: a new quorum-sensing mechanism in streptococci. Mol. Microbiol. 80:1102–1121 [DOI] [PubMed] [Google Scholar]
- 10. Fontaine L, et al. 2010. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J. Bacteriol. 192:1444–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Håvarstein LS, Coomaraswamy G, Morrison DA. 1995. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 92:11140–11144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Håvarstein LS, Gaustad P, Nes IF, Morrison DA. 1996. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 21:863–869 [DOI] [PubMed] [Google Scholar]
- 13. Håvarstein LS, Hakenbeck R, Gaustad P. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J. Bacteriol. 179:6589–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heng NC, Tagg JR, Tompkins GR. 2006. Identification and characterization of the loci encoding the competence-associated alternative sigma factor of Streptococcus gordonii. FEMS Microbiol. Lett. 259:27–34 [DOI] [PubMed] [Google Scholar]
- 15. Hui FM, Zhou L, Morrison DA. 1995. Competence for genetic transformation in Streptococcus pneumoniae: organization of a regulatory locus with homology to two lactococcin A secretion genes. Gene 153:25–31 [DOI] [PubMed] [Google Scholar]
- 16. Hung DC, et al. 2011. Characterization of DNA binding sites of the ComE response regulator from Streptococcus mutans. J. Bacteriol. 193:3642–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnsborg O, Eldholm V, Håvarstein L. 2007. Natural genetic transformation: prevalence, mechanisms and function. Res. Microbiol. 158:767–845 [DOI] [PubMed] [Google Scholar]
- 18. Koehler CJ, Strozynski M, Kozielski F, Treumann A, Thiede B. 2009. Isobaric peptide termini labeling for MS/MS-based quantitative proteomics. J. Proteome Res. 8:4333–4341 [DOI] [PubMed] [Google Scholar]
- 19. Kreth J, et al. 2007. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology 153:1799–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kreth J, Merritt J, Shi W, Qi F. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol. Microbiol. 57:392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193–205 [DOI] [PubMed] [Google Scholar]
- 22. Lee MS, Morrison DA. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181:5004–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I. 2011. Subpopulation-specific transcriptome analysis of competence-stimulating-peptide-induced Streptococcus mutans. J. Bacteriol. 193:1863–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin B, Quentin Y, Fichant G, Claverys JP. 2006. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 14:339–345 [DOI] [PubMed] [Google Scholar]
- 26. Mashburn-Warren L, Morrison D, Federle M. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell JW, Atkins N, Jr, Sweedler JV, Gillette MU. 2011. Direct cellular peptidomics of hypothalamic neurons. Front. Neuroendocrinol. 32:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551–3567 [DOI] [PubMed] [Google Scholar]
- 29. Perry JA, Jones MB, Peterson SN, Cvitkovitch DG, Levesque CM. 2009. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol. Microbiol. 72:905–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pestova EV, Havarstein LS, Morrison DA. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21:853–862 [DOI] [PubMed] [Google Scholar]
- 31. Petersen FC, Fimland G, Scheie AA. 2006. Purification and functional studies of a potent modified quorum-sensing peptide and a two-peptide bacteriocin in Streptococcus mutans. Mol. Microbiol. 61:1322–1334 [DOI] [PubMed] [Google Scholar]
- 32. Petersen FC, Pecharki D, Scheie AA. 2004. Biofilm mode of growth of Streptococcus intermedius favored by a competence-stimulating signaling peptide. J. Bacteriol. 186:6327–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petersen FC, Scheie AA. 2010. Natural transformation of oral streptococci. Methods Mol. Biol. 666:167–180 [DOI] [PubMed] [Google Scholar]
- 34. Petersen FC, Tao L, Scheie AA. 2005. DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 187:4392–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peterson SN, et al. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51:1051–1070 [DOI] [PubMed] [Google Scholar]
- 36. Podbielski A, Spellerberg B, Woischnik M, Pohl B, Lutticken R. 1996. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177:137–147 [DOI] [PubMed] [Google Scholar]
- 37. Thoendel M, Horswill AR. 2010. Biosynthesis of peptide signals in gram-positive bacteria. Adv. Appl. Microbiol. 71:91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tinoco AD, Saghatelian A. 2011. Investigating endogenous peptides and peptidases using peptidomics. Biochemistry 50:7447–7461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Ploeg JR. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vickerman MM, et al. 2010. A genetic determinant in Streptococcus gordonii Challis encodes a peptide with activity similar to that of enterococcal sex pheromone cAM373, which facilitates intergeneric DNA transfer. J. Bacteriol. 192:2535–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vickerman MM, Iobst S, Jesionowski AM, Gill SR. 2007. Genome-wide transcriptional changes in Streptococcus gordonii in response to competence signaling peptide. J. Bacteriol. 189:7799–7807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ween O, Gaustad P, Håvarstein LS. 1999. Identification of DNA binding sites for ComE, a key regulator of natural competence in Streptococcus pneumoniae. Mol. Microbiol. 33:817–827 [DOI] [PubMed] [Google Scholar]