Abstract
Streptococcus mutans develops competence for genetic transformation in response to regulatory circuits that sense at least two peptide pheromones. One peptide, known as CSP, is sensed by a two-component signal transduction system through a membrane receptor, ComD. The other, derived from the primary translation product ComS, is thought to be sensed by an intracellular receptor, ComR, after uptake by oligopeptide permease. To allow study of this process in a medium that does not itself contain peptides, development of competence was examined in the chemically defined medium (CDM) described by van de Rijn and Kessler (Infect. Immun. 27:444, 1980). We confirmed a previous report that in this medium comS mutants of strain UA159 respond to a synthetic peptide comprising the seven C-terminal residues of ComS (ComS11-17) by increasing expression of the alternative sigma factor SigX, which in turn allows expression of competence effector genes. This response provided the basis for a bioassay for the ComS pheromone in the 100 to 1,000 nM range. It was further observed that comS+ (but not comS mutant) cultures developed a high level of competence in the late log and transition phases of growth in this CDM without the introduction of any synthetic stimulatory peptide. This endogenous competence development was accompanied by extracellular release of one or more signals that complemented a comS mutation at levels equivalent to 1 μM synthetic ComS11-17.
INTRODUCTION
Over 70 bacterial species are known to have the capacity for natural genetic transformation (10). In some, such as Neisseria gonorrhoeae, competence for DNA uptake and incorporation is described as constitutive. In many others, competence in laboratory cultures depends on coordinately regulated expression of gene sets encoding effectors of DNA transport and recombination. Among the streptococci, a number of species develop competence transiently during laboratory culture under conditions that are not completely understood. Central to this regulation in each case, however, is a conserved streptococcal alternative sigma factor, SigX or ComX, that directs the transcription of more than a dozen unlinked operons encoding competence effector proteins (3, 12, 14, 24, 26, 31).
S. mutans, a common human commensal associated with dental caries, has been the subject of intensive genetic characterization. Particularly valuable for this work has been the discovery of several strains that are capable of natural genetic transformation, facilitating directed genetic manipulations. In these strains, competence for genetic transformation is not constitutively expressed but develops during growth in certain media and environments in the laboratory. Competence has been variously reported to develop transiently in lag phase (26) or during exponential growth (16, 20, 23, 28, 29). The conditions that favor such development of competence remain poorly characterized, but a common feature of competence protocols for Streptococcus mutans has been growth in the rich medium Todd-Hewitt Broth (THB) supplemented with heat-inactivated horse serum. A major advance toward understanding competence development in S. mutans was the discovery that a peptide pheromone, called CSP (competence-stimulating peptide), both coordinates the production of several nonlantibiotic bacteriocins known as mutacins and strongly upregulates sigX and the competence cascade (15, 24). The peptide is processed posttranslationally, both during export (at a consensus GG site of proteolysis) and after export, where removal of three C-terminal residues increases the specific activity of the mature pheromone 10-fold (25). CSP acts through a two-component signal transduction system receptor, ComD, and a cognate response regulator, ComE, which in turn directly stimulates transcription from the promoters of three unlinked mutacin genes (bsmA, bsmB, and bsmC), a related transporter gene (nmlA), and a putative mutacin immunity protein gene (immB) (9, 29). However, the pathway transmitting this signal to stimulate expression of sigX is unclear, beyond the fact that it requires mutacin V (also known as CipB, SMU.1914, NlmC, or BsmA), intact comS and comR genes, and a functional oligopeptide permease (4, 17, 24).
Mashburn-Warren et al. (17) recently reported that a second peptide pheromone receptor system, known as type II ComR/ComS, is required to link the CSP response to sigX induction and competence in S. mutans. Type I ComR proteins, characteristic of the salivarius group of streptococci, recognize a peptide with the sequence PYF(A/T)GCL that is produced as a C-terminal fragment of the product of the gene comS and that regulate transcription of comS and sigX (5), while the type II ComR proteins, found in all species of the pyogenic, bovis, and mutans groups of streptococci for which genome sequences are available, are proposed to recognize peptides with a different consensus sequence, XXDWWXX (17). Since a synthetic S. mutans ComS peptide (specifically, the seven C-terminal residues of ComS, designated ComS11-17) induces robust expression of sigX and high levels of transformation in the absence of ComE and appears to be at the core of competence regulation in S. mutans, it is of interest to characterize this pheromone system in more detail. The observation that response to ComS11-17 requires the opp oligopeptide permease suggests that a critical step in this pathway is the uptake of a small peptide pheromone signal and suggests that this part of the pathway could be sensitive to competition by the peptides that are abundant in peptone-containing media, such as THB, much as was reported for the type I ComR/ComS system of Streptococcus thermophilus (6). Thus, we were especially interested in the possibility of using a chemically defined medium (CDM) devoid of exogenous peptides for such studies. Indeed, endogenous competence development is reported to be superior in CDM compared to THB with horse serum (27). However, the resulting optimal, but short-lived, competence condition was obtained at a low cell density (the maximum was at an optical density at 550 nm [OD550] of ∼0.04) during the lag phase of culture growth in anaerobic CDM and allowed transformation of only 1/1,000 or fewer of the cells (27). In contrast, Mashburn-Warren et al. (17) reported that synthetic ComS11-17 can induce sigX expression and development of a high level of competence in exponential CDM cultures and noted that expression of the sigX promoter is low during exponential growth but increases during the transition to stationary phase in such CDM cultures.
To facilitate further studies of the nature and production of the native signal of the type II ComR/ComS system of S. mutans, we investigated the development of competence in a chemically defined, peptide-free culture medium and employed bacterial LuxAB reporter fusions to directly monitor expression from the sigX promoter. Here, we report that CDM cultures at low density respond to ComS11-17 by switching to a pervasive and persistent state of competence. We further describe endogenous competence development in CDM in the late exponential or transitional phase of growth and show that it is preceded by elaboration of a secreted signal that complements a comS defect.
MATERIALS AND METHODS
Strains, plasmids, and media.
Strain UA159 was kindly supplied by Lin Tao. Other strains and plasmids are described in Table 1. Liquid cultures were grown in closed screw-cap 13-mm- or 18-mm-diameter glass tubes at 37°C in CDM or THB (Difco). Stocks were stored at −80°C after supplementation with 1/9 volume glycerol. DNA ligase, Taq polymerase, and restriction enzymes were obtained from Invitrogen. Synthetic ComS11-17 prepared by custom synthesis was obtained from NeoPeptide (Cambridge, MA) and was stored at −20°C in dimethyl sulfoxide (DMSO). Peptides specific for Streptococcus agalactiae 2603, Streptococcus porcinus, and Streptococcus parauberis (MGWWNMG, KDWWHIG, and NDWWYIG, respectively [17]) were obtained from the same source. CDM (30) was prepared from concentrated stock solutions as described by Chang et al. (1), sterile filtered, and stored at 4°C. For selection, samples were embedded in THB containing 0.75% agar on a 1.5% THB agar base and covered with a layer of 1.5% THB agar, followed by THB agar containing selective antibiotics. Each agar layer was 3 ml (for 60-mm-diameter plates) or 10 ml (for 100-mm-diameter plates). The plates were incubated at 37°C for 30 to 40 h in 5% CO2 or in a candle jar. Selective levels of antibiotics were 1.5 μg erythromycin/ml, 200 μg spectinomycin/ml, 7.5 μg chloramphenicol/ml, or 200 μg kanamycin sulfate/ml.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype (phenotype) or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| BH10C | E. coli cloning vector | 8 |
| UA159 | Transformable S. mutans isolate | 28 |
| MW04 | UA159 ΔoppD::spc (Spcr) | 17 |
| MW07 | UA159 ΔcomR::cat pWAR300 (Cmr Ermr) | 17 |
| MW17 | UA159 ΔcomS::spc pWAR304 (Spcr Ermr) | 17 |
| MW30 | UA159 pWAR312 (Ermr) | This study |
| Plasmids | ||
| pWAR304 | pFED761 derivative carrying PsigX-luxAB between the SalI and NotI sites; 6,466 bp (Ermr) | 17 |
| p7INT | Shuttle-suicide vector that integrates at the streptococcal bacteriophage T12 attB site (Ermr) | 18 |
| pWAR312 | Integrative derivative of p7INT carrying PsigX-luxAB between the XbaI and BamHI sites; 7,129 bp (Ermr) | This study |
Construction of MW30.
The pWAR312 reporter plasmid (Fig. 1) was assembled by amplification of PsigX-luxAB from pWAR304 using primers LMW26/LMW27 (LMW26, TCTAGATCGACTTGAATCGGGTAGCATA; LMW27, GGATCCGCGATATCAAAATTATACATGT), followed by insertion into p7INT using the XbaI and BamHI sites (in boldface) and cloning in BH10C. To generate the integrated PsigX-luxAB reporter strain MW30, pWAR312 was transferred into S. mutans UA159 as described previously (17).
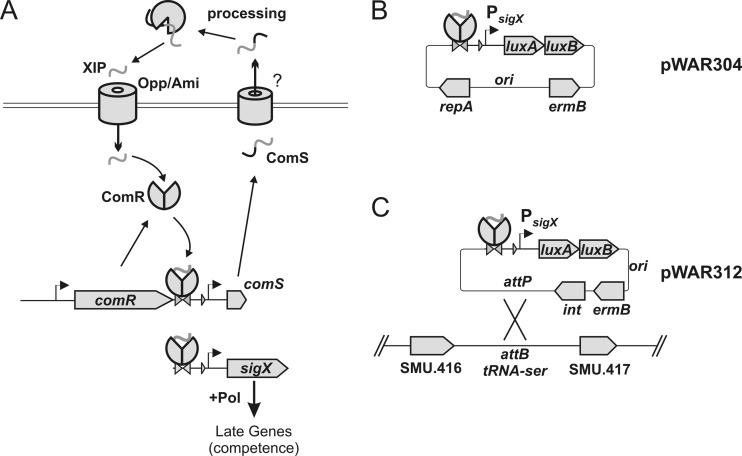
Fig 1.
Design of reporter plasmids used to monitor expression of competence regulons. (A) Elements of the type II ComR cell-to-cell communication circuit proposed as the proximal regulator of sigX expression. ComR is proposed to be a direct regulator of the transcription of comS and sigX. ComS acts as a cell-cell signal, but the steps of its processing into a mature form (XIP) and its export are unknown. SigX is the proximal regulator of late competence effector genes. (Reproduced, with permission, from part of a figure in reference 17.) (B) Organization of the replicative plasmid pWAR304, a luxAB transcriptional reporter of expression from the sigX promoter. ori, replication origin from plasmid pWVO1 from Lactococcus lactis (11); PsigX, fragment upstream of sigX; RepA, repA gene from pWVO1. (C) Organization of the integrative plasmid pWAR312, a luxAB transcriptional reporter of expression from the sigX promoter. attP, integrase target; attB, insertion site in the genome of UA159; int, integrase gene; ermB, rRNA methyl transferase from pAMβ1; ori, replication origin from plasmid pUC18 (18).
Transformation assay.
Unless otherwise indicated, the cultures monitored for development of competence were initiated by 1:50 dilution of a stock prepared by freezing a CDM culture at an OD550 of 0.5. Transformation was carried out by adding donor DNA to 0.5-ml culture samples and incubating them for a fixed time at 37°C in Eppendorf tubes before selective plating. When appropriate, DNA exposure was terminated by adding pancreatic DNase I (Sigma Chemical Co., St. Louis, MO) to 7 μg/ml.
Bioassay of pheromone/activator.
Indicator cells of strain MW17 were frozen with 10% glycerol after growth to an OD550 of 0.5 in CDM. For assay of the activator, cells were thawed at 0°C and used within 2 h. Each assay reaction mixture contained 200 μl cells and 200 μl CDM containing synthetic ComS11-17 or culture filtrate. After incubation at 37°C in open 500-μl Eppendorf tubes for 50 min, samples of 50 to 100 μl were transferred to wells of white 96-well plates (BD 353296; Becton Dickinson, Franklin Lakes, NJ), exposed to vapor from decanal spread on a plate lid for 2 min, and promptly examined for luminescence in a scintillation counter. ComS11-17 equivalents in culture filtrates were estimated by comparison of serial dilutions with standard curves determined using serial dilutions of synthetic ComS11-17.
Donor DNA was prepared by purification of genomic DNA from a lysate of cells that had been treated with 3% glycine in THB (60 min; 37°C), collected by centrifugation at 4°C, washed in cold distilled water, treated with 0.1% lysozyme in 1/50 volume Tris (10 mM, pH 8)-EDTA (1 mM) buffer containing 25% glucose (60 min; 37°C), and held for 20 min at 55°C after addition of sodium dodecyl sulfate to 1%.
RESULTS
Synthetic ComS11-17 induces development of a persistent highly competent state in low-density CDM cultures of UA159.
To begin to characterize the development of competence in CDM further, the effect of synthetic ComS11-17 on low-density cultures was examined in more detail than previously reported by varying critical parameters of the experiment. Following introduction of ComS11-17 in a concentration of 0.1, 1, or 10 μM, expression of a LuxAB reporter linked to the sigX promoter increased dramatically, up to 300-fold above the signal for untreated controls. The luminescence signal continued to increase linearly for up to 4 h in all three cases. After a lag of about 30 to 60 min, competence for transformation began to increase dramatically, as well (Fig. 2). As measured by production of transformants following brief exposures to donor DNA, the resulting competent state persisted for approximately 2 h, after which the rate of transformation dropped to about 20% of its maximum but remained well above the background in untreated controls. This pattern of persistent competence contrasts with the typical streptococcal transient expression of competence; in Streptococcus pneumoniae, for example, competence induced by CSP peaks by 20 min and disappears altogether by 50 min (7). However, it is consistent with the temporal patterns reported previously for S. mutans (16, 20). Synthetic ComS11-17 also had a dramatic physiological effect, reducing the growth rate significantly in a dose-dependent manner (Fig. 2 and 3A), indicating a pervasive response to ComS11-17 throughout the culture. These responses to ComS11-17 did not reflect a nonspecific effect of small peptides or small peptides encompassing adjacent tryptophanyl residues, as three similar double-tryptophan heptapeptides failed to stimulate competence or sigX expression or to have any depressive effect on growth (Fig. 2). Maximal competence was achieved using synthetic ComS11-17 at a level of 1 μM or higher (Fig. 3B). The yield of transformants depended linearly on the DNA concentration, reaching a half-maximal yield above 107/ml at ∼5 μg of genomic DNA per ml (Fig. 3C). To ask whether components of a rich culture medium might compromise the response to ComS11-17 by the S. mutans type II ComR/ComS circuit, as is true for the type I ComR/ComS cell-to-cell communication circuits (6), responses to ComS11-17 were compared in cultures in CDM amended with various amounts of THB. Sensitivity to ComS11-17 was reduced by the addition of small amounts of THB but was not completely abolished even in full-strength THB medium (Fig. 3D).
Fig 2.
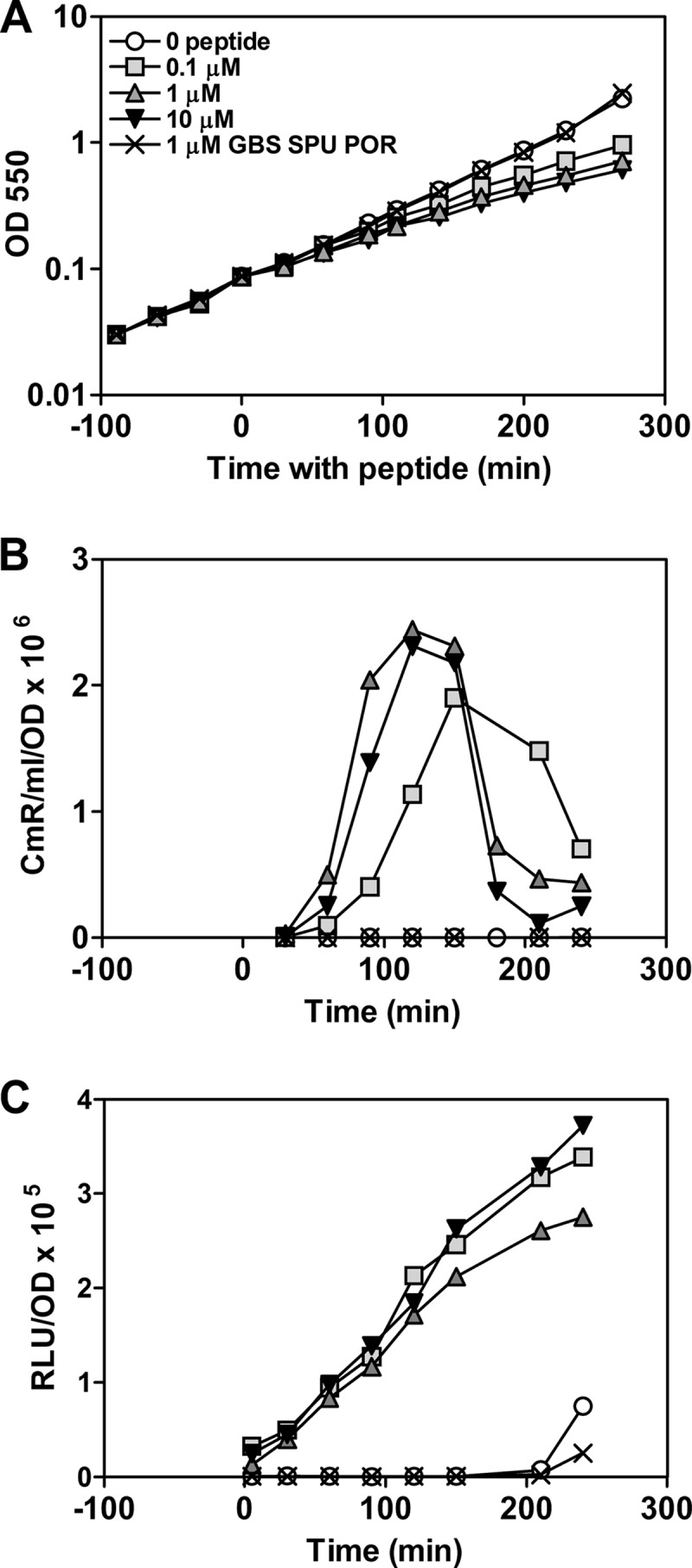
Effect of synthetic ComS11-17 peptide on growth, sigX expression, and competence in S. mutans cultured in CDM at low density. Five parallel 22-ml cultures of MW30 growing in CDM at an OD550 of 0.1 were treated with 0.1 μM, 1 μM, or 10 μM ComS11-17; 1 μM (each) XIP peptides predicted for S. agalactiae 2603 (GBS), S. porcinus (POR), and S. parauberis (SPU); or vehicle alone at time zero. (A) Growth was monitored as the OD550. (B) To determine competence at 30-min intervals, MW07 donor DNA (5 μg) was added to 500-μl samples from the cultures; after 8 min at 37°C, DNase was added, and dilutions were plated to determine Cmr transformants after 60 min of further incubation. (C) To determine expression from the sigX promoter, triplicate 50-μl samples from each tube were exposed to decanal vapor in a 96-well plate for 2 min before determining luminescence.
Fig 3.
Dose dependence of responses to ComS11-17. (A) Reduction of the growth rate in CDM by added ComS11-17. Twelve 6-ml cultures of UA159 were grown in CDM after dilution of an overnight CDM culture to an OD550 of 0.012. At 155 min, ComS11-17 was added at 0.01, 0.03, 0.1, or 1 μM, and growth was monitored for an additional 350 min. The values shown are the averages of duplicate parallel cultures. (B) Titration of competence response to ComS11-17. Samples (1 ml) of a culture of UA159 growing in CDM at an OD550 of 0.1 were treated with the indicated concentrations of ComS11-17 for 60 min and then exposed to MW07 DNA at 10 μg/ml for 60 min before plating to determine Cmr transformants. (C) Donor DNA dose-response. A culture of UA159 growing in CDM at an OD550 of 0.1 was treated with 10 μM ComS11-17 for 60 min. Samples were then exposed for 20 min to the indicated amounts of MW07 DNA at 37°C, diluted, and plated to determine Cmr transformants. (D) Reduced sensitivity to ComS11-17 in rich media. A culture of UA159 growing in CDM at an OD550 of 0.1 was treated with the indicated amounts of ComS11-17 for 60 min after supplementation with THB as indicated. Finally, competence was assayed by a further 30-min incubation with 0.1 μg/ml MW04 DNA before plating to determine Spcr transformants. A parallel culture of UA159 in THB was treated at an OD550 of 0.1 in the same way.
Bioassay of ComS pheromone.
The robust response to ComS11-17 was used to develop a convenient bioassay for the comS pheromone by using a comS mutant tester strain, MW17, in which a LuxAB reporter was linked to the sigX promoter. This strain was chosen because its comS deficiency would ensure a low background level of PsigX-luxAB expression, even at high cell densities, as reported previously (17). The comS mutation also blocks the operation of any autocatalytic ComR/ComS loop, which might have complicated the bioassay. Noncompetent MW17 cells, prepared as frozen stocks (OD550 = 0.5), were used directly after thawing to assay pheromone activity. The level of luminescence exhibited by the PsigX-directed LuxAB reporter in the MW17 suspensions increased at a constant rate for more than 100 min during exposure to synthetic ComS11-17 at 37°C. This rate depended directly on the level of ComS11-17 in the 0.1 to 1.0 μM range (Fig. 4) and on the density of the tester cells (data not shown).
Fig 4.

Bioassay of ComS11-17. (A) Kinetics of response of the comS mutant to ComS11-17. Samples (250 μl) of MW17 (ΔcomS PsigX-luxAB) growing in CDM at an OD550 of 0.4 were mixed with 250-μl volumes of CDM containing ComS11-17 at twice the indicated final concentrations. During incubation at 37°C, 60-μl portions were removed at the indicated times for exposure to decanal and determination of luminescence. (B) ComS11-17 bioassay standard curve. The titration curve was constructed using the MW17 luminescence values at 80 min from the experiment in panel A.
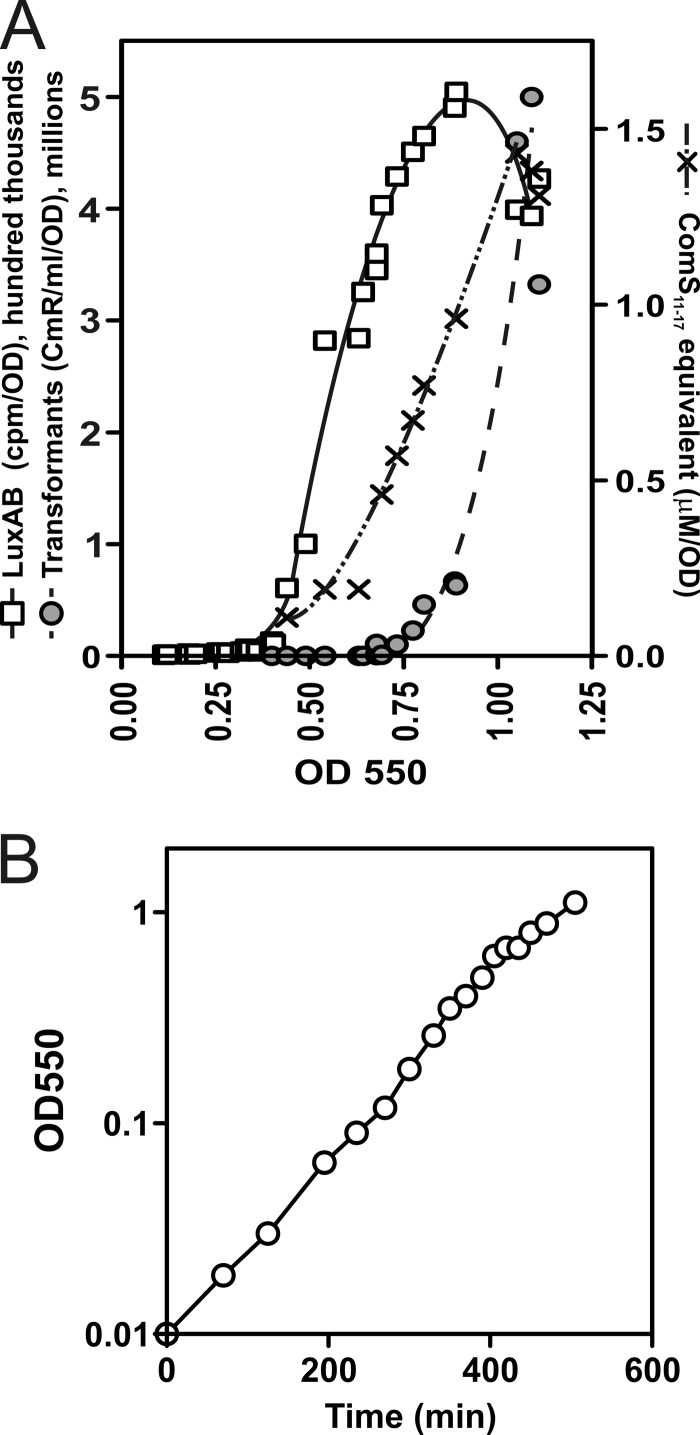
Endogenous competence development occurs at high cell densities (OD550 > 0.4) in CDM without stimulation by ComS11-17. As Mashburn-Warren et al. (17) noted that the level of sigX expression in CDM increased at high cell densities, the behavior of S. mutans in CDM culture without added peptide was investigated in more detail by examining both expression of sigX and competence in the reporter strain MW30 over a wide range of culture densities. As shown in Fig. 5, expression of a sigX transcriptional reporter increased abruptly by more than 100-fold as the cultures reached densities above 0.4 to 0.8, while the development of competence for uptake of DNA followed a similar, but somewhat delayed, course. Development of competence in the parental wild-type (WT) strain UA159 during growth in parallel cultures in CDM followed a similar course, indicating that this behavior was not a consequence of the reporter construct itself (data not shown). The level of competence achieved was very high, contrasting strongly with the typical behavior of cultures in THB, which exhibit maximal competence at densities below an OD550 of 0.1 (16, 20, 23, 27, 28, 29).
Fig 5.
Endogenous competence development in CDM. (A) Development of competence, expression from PsigX, and pheromone secretion by strain MW30 (PsigX-luxAB) during growth in CDM in parallel closed culture tubes. The tubes were opened on reaching the indicated OD550 values, and their contents were distributed for three assays. MW07 donor DNA (0.5 μg) was added to one 500-μl sample from each culture; after 15 min at 37°C, DNase was added and dilutions were plated for Cmr transformants after 60 min of further incubation. Triplicate 150-μl samples from each tube were exposed to decanal vapor in a 96-well plate for 2 min before determining luminescence. A 4-ml filtrate of the remainder (0.2-μm filters) was prepared for determination of XIP as comS-complementing activity (ComS11-17 equivalents) by bioassay, as described in Materials and Methods. (B) Growth kinetics of cultures assayed for panel A.
Endogenous competence development is accompanied by elaboration of an extracellular activator.
Endogenous development of competence in this CDM offered an opportunity to directly test the idea, proposed earlier (17), that a ComS-derived peptide signal, designated XIP (sigX-inducing peptide), is secreted during competence development by using the new bioassay to determine the level and timing of accumulation of XIP or another pheromone in CDM cultures of MW30 growing to high density. As shown in Fig. 5, filtrates prepared from cultures at densities above an OD550 of 0.4 did indeed exhibit PsigX-stimulating activity in such an assay. Parallel controls confirmed the absence of such activator activity in cultures of the comS mutant MW17, whereas the WT UA159 behaved like MW30 in producing similar levels of activator at high cell densities (data not shown). From the temporal pattern of these events, we conclude that as CDM cultures of UA159 approach stationary phase, endogenous development of competence follows two preliminary phases in which sigX and comS promoters are activated and high levels of one or more derivatives of ComS accumulate in the extracellular fluid.
DISCUSSION
S. mutans has been recognized as a naturally transformable species for over 30 years, and it has been understood since the earliest studies that its competence is a regulated trait. However, the mechanism by which competence is controlled in the species remains unclear. The standard method for achieving competence in the laboratory is culture in THB with added horse serum, where competence develops transiently at low cell densities (16, 20, 23, 27–29). The active components of the medium that promote development of competence, if any, are unknown, and while a variety of bacterial proteins have been reported to modulate such competence development, few of their specific relevant targets have been identified. Intercellular signaling is thought to play an important role in competence regulation, and two chromosomally encoded peptides that have strong and specific stimulatory effects are already known. One, CSP, controls the expression of several of the bacteriocins known as mutacins via a two-component signal transduction system, ComDE; the other, XIP, is thought to mediate a type II ComR/ComS autocatalytic signaling loop. Both CSP and a more active C-terminally shortened derivative of CSP have been recovered from culture filtrates after CSP stimulation (25), but the native XIP molecule has not yet been characterized chemically. Indeed, neither of these peptide pheromones has yet been isolated from cultures developing competence without stimulation by synthetic peptides.
Members of a new family of Rgg proteins, designated type II ComR regulators, were recently proposed to act as part of a quorum-sensing circuit that includes ComR-regulated promoters that direct expression of the comS and sigX genes in a pattern conserved throughout the pyogenic, bovis, and mutans groups of streptococci (17). In the case of S. mutans, both comR and comS are required for competence development in response to the CSP pheromone, and a synthetic ComS derivative both stimulates competence and complements a comS defect. It was hypothesized that endogenous competence development depends on a secreted type II ComS-derived signal, termed XIP, but no direct evidence for production of this signal was available. The present data support that hypothesis by showing the production of a comS-dependent secreted signal that complements a comS mutation and that appears in CDM cultures just before development of competence for DNA uptake. These results set the stage for isolating and identifying the native signal by providing conditions for endogenous development of competence in a culture medium that itself is devoid of extraneous peptides (see the accompanying article by Khan et al. [10a]). It is interesting that maximal expression of sigX was achieved somewhat before XIP activity reached its maximum. We interpret this as a reflection of the autocatalytic nature of the ComS/ComR circuit and conclude that an understanding of the details of initiation of the expression of this circuit may require more sensitive assay tools than were used here.
The present results, showing endogenous development of competence in CDM as cultures approach stationary phase, suggest that in S. mutans, in contrast to streptococci of the mitis, anginosus, and salivarius groups, competence may be a characteristic of certain high-density culture conditions. This interpretation is consistent with the report by Li et al. (15) that cells in S. mutans biofilms either are themselves highly competent or rapidly become so when exposed to fresh medium and donor DNA. The more recent discovery that competence development is stimulated strongly by the hdrRM system, which itself is activated at very high cell densities (19, 21, 22), is also consistent with this view.
Competence for genetic transformation is expressed transiently in multiple well-studied streptococci, including species of the mitis, anginosus, and salivarius groups. In S. pneumoniae, for example, competence development can be coordinated so that all cells of a culture suddenly and simultaneously upregulate the competence regulon and then downregulate expression of the same genes, creating a temporary but pervasive state of competence lasting little more than 15 to 20 min (2). An unidentified product of the pneumococcal competence regulon itself appears responsible for this rapid negative feedback (13), independent of the continued high levels of CSP. Endogenous competence development by S. mutans in THB has also been commonly described as a transient state, appearing during early or mid-log phase. Development of competence in S. mutans in CDM in response to synthetic ComS11-17 contrasts with all these behaviors, as competence persists for hours, with a reduced growth rate that is reminiscent of the competent state of Bacillus subtilis. Indeed, the present results could be interpreted as indicating that in S. mutans competence represents a stable alternative mode of growth. It is possible that this state is also characteristic of the competent subpopulation expressing sigX after CSP stimulation in rich media, as described by Lemme at al. (14).
The apparent reduction in the growth rate that we found to be one aspect of the response to ComS11-17 in CDM is reminiscent of the response to CSP in rich media, where an apparently lower growth rate is accompanied by the death of a significant fraction of the population (14, 24). In that case, the cell death has been attributed to the CSP-induced bacteriocin CipB when it overcomes immunity provided by CipI. It is therefore interesting that activation of competence by ComR/ComS, thought to act downstream of CipB, produces a similar effect, suggesting that some of the slowed growth occasioned by CSP may be an indirect effect of the CipB pathway.
ACKNOWLEDGMENTS
L.M.-W. is a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation. Some of this work was supported by grants NIH AI091779 from the NIAID (to M.J.F.) and MCB1020863 from the NSF (to D.A.M.).
We thank Indranil Biswas for plasmid pGH9:ISS1, Kevin McIver for plasmid pLZI2Spe, and Alketa Zyka for assistance with many of the experiments reported here. We are grateful to Lin Tao for gifts of strains and for helpful technical advice.
Footnotes
Published ahead of print 18 May 2012
REFERENCES
- 1. Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ. 2011. Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathog. 7:e1002190 doi:10.1371/journal.ppat.1002190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Claverys JP, Havarstein LS. 2002. Extracellular-peptide control of competence for genetic transformation in Streptococcus pneumoniae. Front. Biosci. 7:d1798–d1814 [DOI] [PubMed] [Google Scholar]
- 3. Dagkessamanskaia A, et al. 2004. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol. Microbiol. 51:1071–1086 [DOI] [PubMed] [Google Scholar]
- 4. Dufour D, Cordova M, Cvitkovitch DG, Levesque CM. 2011. Regulation of the competence pathway as a novel role associated with a streptococcal bacteriocin. J. Bacteriol. 193:6552–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fontaine L, et al. 2010. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J. Bacteriol. 192:1444–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardan R, Besset C, Guillot A, Gitton C, Monnet V. 2009. The oligopeptide transport system is essential for the development of natural competence in Streptococcus thermophilus strain LMD-9. J. Bacteriol. 191:4647–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Havarstein LS, Coomaraswamy G, Morrison DA. 1995. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 92:11140–11144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howell-Adams B, Seifert HS. 2000. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol. Microbiol. 37:1146–1158 [DOI] [PubMed] [Google Scholar]
- 9. Hung DC, et al. 2011. Characterization of DNA binding sites of the ComE response regulator from Streptococcus mutans. J. Bacteriol. 193:3642–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnsborg O, Eldholm V, Havarstein LS. 2007. Natural genetic transformation: prevalence, mechanisms and function. Res. Microbiol. 158:767–778 [DOI] [PubMed] [Google Scholar]
- 10a. Khan R, et al. 2012. Extracellular identification of a processed type II ComR/ComS pheromone of Streptococcus mutans. J. Bacteriol. 194:3781–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kok J, van der Vossen JM, Venema G. 1984. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl. Environ. Microbiol. 48:726–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kreth J, et al. 2007. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology 153:1799–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee MS, Morrison DA. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181:5004–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I. 2011. Subpopulation-specific transcriptome analysis of competence-stimulating-peptide-induced Streptococcus mutans. J. Bacteriol. 193:1863–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindler LE, Macrina FL. 1986. Characterization of genetic transformation in Streptococcus mutans by using a novel high-efficiency plasmid marker rescue system. J. Bacteriol. 166:658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mashburn-Warren L, Morrison DA, Federle MJ. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McShan WM, McLaughlin RE, Nordstrand A, Ferretti JJ. 2004. Vectors containing streptococcal bacteriophage integrases for site-specific gene insertion Methods Cell Sci. 20:51–57 [Google Scholar]
- 19. Merritt J, Zheng L, Shi W, Qi F. 2007. Genetic characterization of the hdrRM operon: a novel high-cell-density-responsive regulator in Streptococcus mutans. Microbiology 153:2765–2773 [DOI] [PubMed] [Google Scholar]
- 20. Murchison HH, Barrett JF, Cardineau GA, Curtiss R., III 1986. Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect. Immun. 54:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okinaga T, Niu G, Xie Z, Qi F, Merritt J. 2010. The hdrRM operon of Streptococcus mutans encodes a novel regulatory system for coordinated competence development and bacteriocin production. J. Bacteriol. 192:1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okinaga T, Xie Z, Niu G, Qi F, Merritt J. 2010. Examination of the hdrRM regulon yields insight into the competence system of Streptococcus mutans. Mol. Oral Microbiol. 25:165–177 [DOI] [PubMed] [Google Scholar]
- 23. Perry D, Kuramitsu K. 1981. Genetic transformation of Streptococcus mutans. Infect. Immun. 32:1295–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perry JA, Jones MB, Peterson SN, Cvitkovitch DG, Levesque CM. 2009. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol. Microbiol. 72:905–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petersen FC, Fimland G, Scheie AA. 2006. Purification and functional studies of a potent modified quorum-sensing peptide and a two-peptide bacteriocin in Streptococcus mutans. Mol. Microbiol. 61:1322–1334 [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez AM, et al. 2011. Physiological and molecular characterization of genetic competence in Streptococcus sanguinis. Mol. Oral Microbiol. 26:99–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah GR, Caufield PW. 1993. Enhanced transformation of Streptococcus mutans by modifications in culture conditions. Anal. Biochem. 214:343–346 [DOI] [PubMed] [Google Scholar]
- 28. Tao L, MacAlister TJ, Tanzer JM. 1993. Transformation efficiency of EMS-induced mutants of Streptococcus mutans of altered cell shape. J. Dent. Res. 72:1032–1039 [DOI] [PubMed] [Google Scholar]
- 29. van der Ploeg JR. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van de Rijn I, Kessler RE. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vickerman MM, Iobst S, Jesionowski AM, Gill SR. 2007. Genome-wide transcriptional changes in Streptococcus gordonii in response to competence signaling peptide. J. Bacteriol. 189:7799–7807 [DOI] [PMC free article] [PubMed] [Google Scholar]