Abstract
Background
Many lung carcinogens activate mitogen activated protein kinase (MAPK) pathways and DNA methyltransferases (DNMTs) are under investigation as therapeutic targets for lung cancer. Our goal is to determine whether C10 type II alveolar epithelial cells are a sensitive model to investigate ERK-dependent transformation and DNMT expression patterns in experimental lung cancer.
Findings
Ectopic expression of an extracellular signal regulated kinase (ERK)-green fluorescent protein (ERK1-GFP) induces acquisition of growth in soft agar that is selectively associated with latent effects on the expression of DNA methyl transferases (DNMT1 and 3b), xeroderma pigmentosum complementation group A (XPA), DNA-dependent protein kinase catalytic subunit (DNA-PKcs), increased phosphatase activity and enhanced sensitivity to 5-azacytidine (5-azaC)-mediated toxicity, relative to controls.
Conclusions
Ectopic expression of ERK alone is sufficient to promote phenotypic conversion of C10 cells associated with altered DNMT expression patterns and sensitivity to DNMT inhibitor. This model may have applications for predicting sensitivity to DNMT inhibitors.
Keywords: Epigenetics, ERK, DNMT
Findings
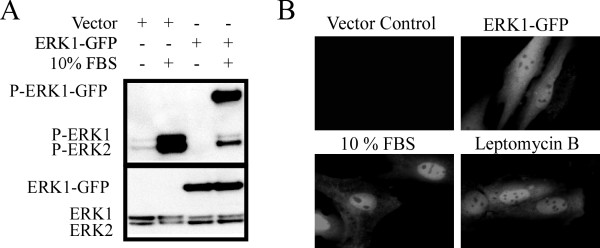
Many lung carcinogens activate the extracellular signal regulated kinase (ERK) [1] and in some model systems constitutive overexpression of ERK can induce transformation [2-6]. However, it is unclear whether ERK alone can modulate cell transformation responses in lung type II alveolar epithelial cells. In addition, cell transformation induced by ERK overexpression does not correlate with ERK activity [7], suggesting an important role for secondary regulatory events. Murine C10 type II alveolar epithelial cells have been used as an in vitro model to investigate molecular determinants of lung cell physiology and pathophysiology [8-11]. C10 type II alveolar epithelial cells are a non-tumorigenic cell line derived from normal BALB/c mouse lung tissue and do not contain native Ras mutations [12,13]. Type II features include the presence of lamellar bodies, the biosynthesis of surfactant, proliferation that is contact inhibited and anchorage-dependent growth [14]. Here we ectopically expressed an ERK1-GFP chimera in C10 cells using retroviral technology as previously described [15] and asked whether ectopic ERK expression induced phenotypic conversion. Thus, our use of the terms “transformation or phenotypic conversion” are constrained to observable changes in cell behavior linked to carcinogenesis in vitro, such as loss of cell density-dependent growth arrest, anchorage-independent growth and morphological changes associated with an epithelial to mesenchymal transition. ERK1-GFP protein expression was confirmed by Western blot (Figure 1, panel A bottom). The phosphorylation of both ERK1-GFP and endogenous ERKs was increased in response to growth factor treatment (10% FBS; 10 min), relative to respective quiescent controls (Figure 1, panel A top). ERK1-GFP translocated to the nuclear compartment following growth factor stimulation (Figure 1, panel B), consistent with nuclear translocation of ERK upon activation [16]. ERK is exported from the nucleus by the chromosome region maintenance protein 1 (CRM1) exporter [17] and treatment with a CRM1 inhibitor (20 nM LB; 30 min) resulted in nuclear retention of ERK1-GFP. Therefore, ERK1-GFP displays expected regulatory patterns in C10 cells.
Figure 1.
Panel A: Expression of ERK1-GFP chimera was confirmed by Western blot analysis (bottom). Phosphorylation of both ERK1-GFP and endogenous ERKs is increase by growth factor stimulation (FBS), relative to quiescent controls (top). Panel B: Epifluorescent microscopy confirmed ERK1-GFP nuclear translocation following stimulation with FBS, relative to unstimulated ERK1-GFP cells. Blocking nuclear export with leptomycin B (30 min treatment) resulted in retention of ERK1-GFP in the nucleus as expected. Similar results were observed in two independent experiments. Methods for retroviral expression of ERK1-GFP can be found in [15].
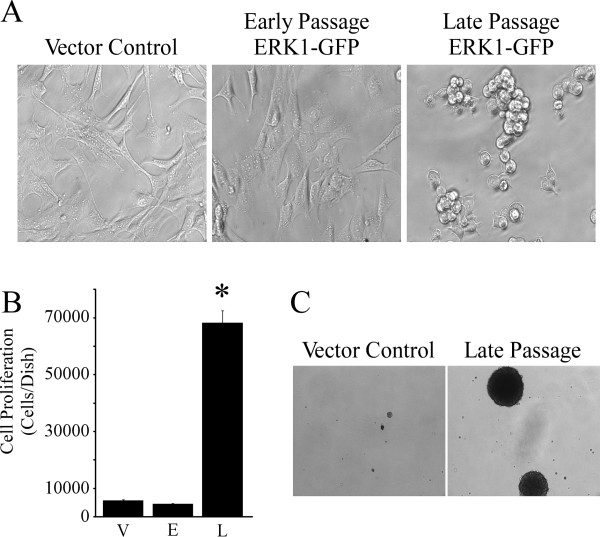
ERK1-GFP transduced cells exhibit a morphological change upon prolonged passaging (Figure 2A, compare early and late passage cells) which often accompanies cell transformation in vitro [18]. Total cell number was increased by approximately 11 fold in 5 day postconfluent cultures of late passage ERK1-GFP cells, relative to postconfluent vector control or early passage ERK1-GFP cells (Figure 2B), indicating loss of growth inhibition by cell-cell contact. Late passage ERK1-GFP cells grow in soft agar, while vector controls do not show significant anchorage-independent growth potential (Figure 2C), defined as previously described [19]. Collectively, late passage ERK1-GFP cells display multiple phenotypic alterations that suggest they have transformed to a malignant state.
Figure 2.
Phenotypic conversion of C10 cells induced by ERK1-GFP.Panel A: Late passage ERK1-GFP cells exhibit rounded morphology, relative to early passage ERK1-GFP cells and vector controls. Panel B: The proliferation of late passage ERK1-GFP cells (L) is not inhibited by cell-cell contact at confluence, while proliferation of early passage ERK1-GFP cells (E) and vector controls (V) are contact inhibited. *Significantly different from vector control. Values represent the mean ± se (n = 3). Similar results were observed in three independent experiments. Panel C: Late passage cells exhibit a qualitative change in their capacity to grow in soft agar, while vector controls show marginal anchorage-independent growth responses. Methods for the soft agar assay can be found in [20]. Similar results were observed in two independent experiments.
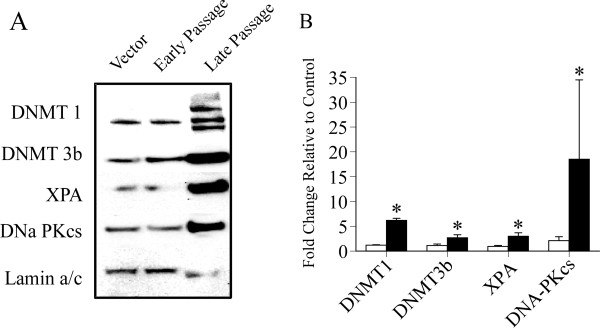
ERK can regulate DNMT expression [21,22] which could impact epigenetic programming. Altered epigenetic programming is an attractive candidate in carcinogenesis because alterations in methylation of DNA are heritable and can lead to transcriptional dysregulation linked to neoplastic cellular changes [23]. Upon examination of DNMT expression patterns in our model, we observed a marked increase in DNMT1 and 3b isoforms in late passage ERK1-GFP cells, relative to early passage cells and vector controls (Figure 3A). DNMT3a was not detected by Western blot in our experiments (data not shown). DNMT1 was consistently characterized by the appearance of multiple bands immunoreacting with anti-DNMT1 antibody that were absent in early passage ERK1-GFP cells and vector controls. At present we do not know whether these bands represent alternative splice variants, degradation products, post-translational modifications or some combination. Thus, increased DNMT expression is latent in ERK transduced C10 cells (Figure 3A), suggesting that ERK is not directly regulating DNMT expression in this model, or that compensatory mechanisms prevent significant increases in DNMT expression patterns in early passage cells.
Figure 3.
Panel A: DNMT1, DNMT3b, XPA, and DNA-PKcs levels were increased in nuclear extracts prepared from late passage ERK1-GFP cells, relative to early passage ERK1-GFP cells and vector controls. DNMT1 consistently exhibited multiple bands immunoreacting with anti-DNMT1 antibody in late passage ERK1-GFP cells. Lamin a/c was used as a loading control. Panel B: Graph illustrates pooled results from three independent experiments for early (white) and late (black) passage ERK1-GFP cells, relative to vector control (referenced as fold change value of 1). Values represent mean ± se (n = 3). *Significantly different from vector control. General methods for Western blot analysis can be found in [24].
In previous studies we have used xeroderma pigmentosum complementation group A (XPA) as a loading control for nuclear extracts because the expression of this protein generally showed little change under a variety of experimental conditions. However, we observed a marked increase in the expression of XPA in late passage ERK1-GFP cells, relative to early passage cells and vector controls (Figure 3A). We subsequently defined the expression of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) in late passage ERK1-GFP cells as an additional index for DNA damage signaling which was also increased (Figure 3A). Lamin a/c levels were not increased under these conditions and served as loading control. The combined results of three independent experiments are illustrated in Figure 3B. The reason for increased expression of DNA repair proteins is unclear. One possible interpretation may relate persistent ERK activation to genomic instability, which is a common feature of human cancers [25]. Genomic instability encompasses a broad array of chromosomal rearrangements and DNA damage events [26] that could generate signals leading to the regulation of repair proteins such as XPA and DNA-PKcs. ERK regulates NADPH oxidase activity [27], which is associated with a significant generation of oxygen free radicals [28] and chronic oxidative stress can induce genomic instability [29]. Alternatively, DNA-PKcs is hypothesized to play an important role in maintaining genomic stability [30] and the increase in DNA-PKcs may reflect effort to maintain stability in an unstable environment.
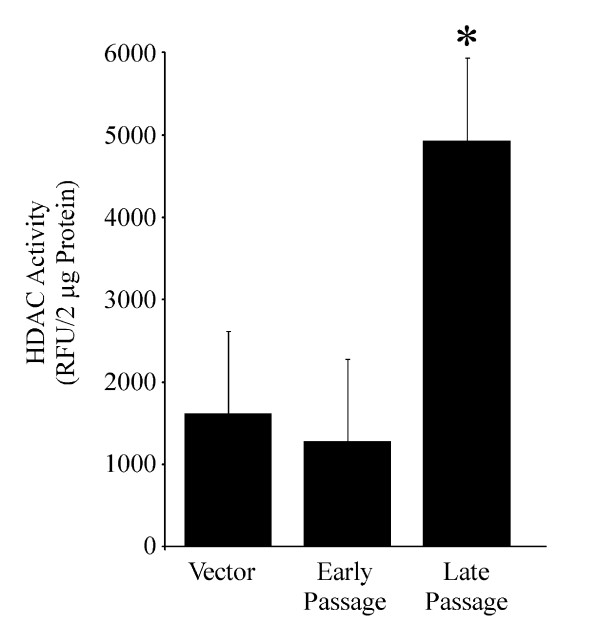
DNMTs possess HDAC binding domains [31] and DNMT/HDAC systems are believed to be interdependent [32]. Therefore, we surveyed whether HDAC activity was altered under these conditions as an additional index for altered epigenetic programming that may be directly influenced by HDAC binding domains on DNMTs [31,32]. HDAC activity was significantly increased in late passage ERK1-GFP cells, relative to early passage cells and vector control (Figure 4). The abrupt increase in HDAC activity in late passage, but not early passage ERK1-GFP cells, is consistent with increased DNMT expression patterns and coupled HDAC regulation [31]. There is precedence for an ERK-DNMT-HDAC linkage in fear conditioning [33] and we hypothesize that these activities may also be aligned in carcinogenesis. Additional studies are required to dissect the relative amount of HDAC activity that is dependent on DNMTs in late passage cells.
Figure 4.
HDAC activity in nuclear extracts from vector control, early and late passage ERK1-GFP cells. Increased relative fluorescence units (RFU) is an index for increased HDAC activity, which was measured using the Fluor de Lys – Green kit (Enzo Life Sciences, Plymouth Meeting, PA) according to manufacturer’s directions. HDAC activity was increased in late passage ERK1-GFP cells, relative to early passage ERK1-GFP cells and vector control. *Significantly different from vector control. Values represent the mean ± se (n = 3). Similar results were observed in three independent experiments.
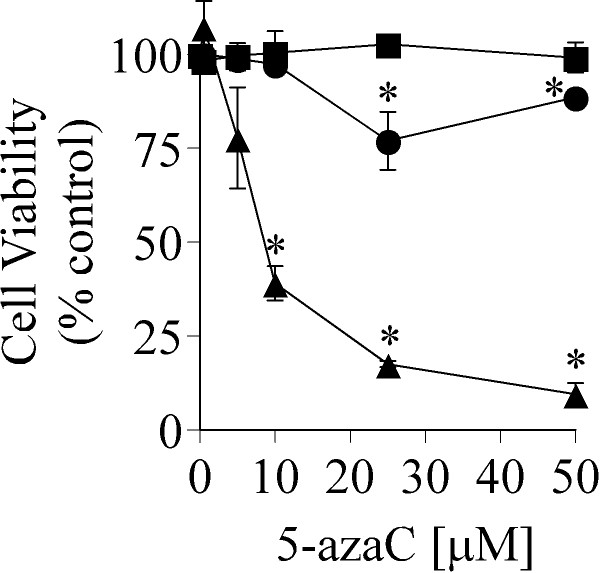
To determine if increased DNMT expression was linked to cell fate regulation, we asked whether vector control, early and late passage ERK1-GFP cells were differentially sensitive to a DNMT inhibitor (5-azaC). Cells were treated with 0.5–50 μM 5-azaC for 7 days and cell viability was determined using a neutral red assay as previously described [34]. Cell viability was reduced in a dose-dependent manner by 5-azaC in late passage ERK1-GFP cells, but not in vector controls (Figure 5). Early passage ERK1-GFP cells displayed a small reduction in cell viability at the highest concentrations of 5-azaC (25–50 μM) employed. DNMT’s are under investigation as therapeutic targets for lung cancer [31]. Biomarkers that can predict when DNMT inhibitors may exhibit high efficacy could significantly aid in this effort. Because the C10 model developed here is sensitive to DNMT inhibitors, it may provide insight into molecular features that may serve as biomarkers, to the extent that such features are conserved in human cancers.
Figure 5.
5-azaC toxicity in ERK1-GFP transduced cells. Vector control (square), early passage ERK1-GFP (circle) and late passage ERK1-GFP (triangle) were maintained in media supplemented with 5-azaC at the indicated concentrations for 7 days at which time cell viability was measured using a neutral red assay as described [34]. Values represent the mean ± se (n = 3). *Significantly different from vector control. Similar results were observed in three independent experiments.
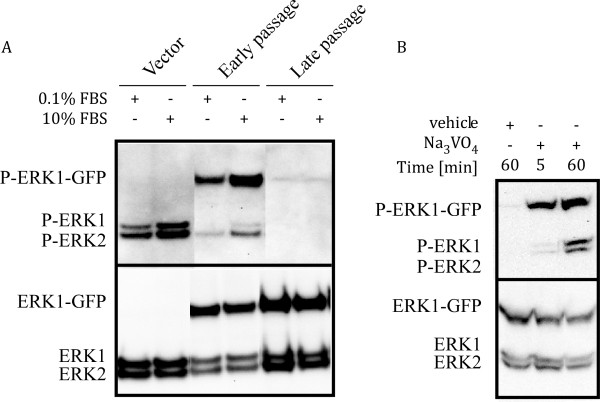
We consistently observed that late passage ERK1-GFP cells exhibited a marked reduction in phospho-ERK (P-ERK) levels, but not total ERK protein levels, relative to vector controls and early passage ERK1-GFP cells (Figure 6A). Treatment of serum starved cells (0.1% FBS) with 10% FBS for 5 min resulted in increased P-ERK levels in vector controls and early passage ERK1-GFP cells, which is the expected response to serum stimulation. Lack of P-ERK levels in late passage cells could result from either a general lack of signal transduction leading to ERK activation or an increase in phosphatase activity. We treated late passage ERK1-GFP cells with 1 mM sodium orthovanadate (Na3VO4) to determine whether a broad spectrum phosphatase inhibitor could restore P-ERK levels. P-ERK levels were restored within minutes of Na3VO4 treatment (Figure 6B), suggesting that the decrease in P-ERK levels associated with late passage ERK1-GFP cells was due to increased phosphatase activity.
Figure 6.
Evidence for increased phosphatase activity in late passage ERK1-GFP cells.Panel A: Western blot analysis of P-ERK levels in serum starved (0.1% FBS) vs 10% FBS stimulated cells. Treatment of cells with 10% FBS for 5 min results in increased P-ERK levels in vector control and early passage ERK1-GFP cells, as expected, while late passage ERK1-GFP cells display diminished P-ERK, but not total ERK levels. Panel B: Treatment of late passage ERK1-GFP cells with a broad spectrum phosphatase inhibitor (1 mM Na3VO4) for 5 or 60 min resulted in reappearance of P-ERK levels, suggesting that the decrease in P-ERK levels is due to increased phosphatase activity. Similar results were observed in two independent experiments
Conclusions
Ectopic expression of ERK alone is sufficient to induce phenotypic conversion of C10 cells and this model may provide insight into the underlying molecular determinants of this response. The window between early and late passage ERK-transduced variants that encompasses phenotypic conversion (approximately 15 passages) is a reasonable time frame to enable interrogative studies to define molecular determinants. Our expectation is that causal molecular processes will precede the appearance of the transformed phenotype and will be observed in early passage cells. At present, we have characterized changes in DNMT, DNA damage recognition and repair proteins and phosphatase activities that are selectively altered in late, but not early passage cells, suggesting they are secondary to transformation. Additional studies, perhaps with a more global screening approach, may provide insight into those molecular processes perturbed by ERK overexpression in early passage cells. Alternatively, because DNMTs are under investigation as therapeutic targets for lung cancer, the C10 model may provide insight into the molecular processes that confer sensitivity to DNMT inhibitors and regulate their aberrant expression.
Abbreviations
5-azaC = 5-azacytidine; CRM1 = chromosome region maintenance protein 1; DNA-PKcs = DNA-dependent protein kinase catalytic subunit; DNMT = DNA methyl transferase; EMT = epithelial-mesenchymal transition; ERK = extracellular signal regulated kinase; ERK1-GFP = ERK1-green fluorescent protein; HDAC = histone deacetylase; LB = leptomycin B; MAPK = mitogen activated protein kinase; Na3VO4 = sodium orthovanadate; P-ERK = phospho-ERK; XPA = xeroderma pigmentosum complementation group A.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RS completed all experiments described under supervision by TW. All authors read and approved the final manuscript.
Contributor Information
Ryan L Sontag, Email: ryan.sontag@pnnl.gov.
Thomas J Weber, Email: Thomas.Weber@pnnl.gov.
References
- Grozio A, Catassi A, Cavalieri Z, Paleari L, Cesario A, Russo P. Nicotine, lung and cancer. Anticancer Agents Med Chem. 2007;7:461–466. doi: 10.2174/187152007781058587. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lodish HF. Constitutive activation of the MEK/ERK pathway mediates all effects of oncogenic H-ras expression in primary erythroid progenitors. Blood. 2004;104:1679–1687. doi: 10.1182/blood-2004-04-1362. [DOI] [PubMed] [Google Scholar]
- Chung E, Hsu CL, Kondo M. Constitutive MAP kinase activation in hematopoietic stem cells induces a myeloproliferative disorder. PLoS One. 2011;6:e28350. doi: 10.1371/journal.pone.0028350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Khavari PA. Effects of active MEK1 expression in vivo. Cancer Lett. 2005;230:1–5. doi: 10.1016/j.canlet.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Boucher MJ, Jean D, Vezina A, Rivard N. Dual role of MEK/ERK signaling in senescence and transformation of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G736–G746. doi: 10.1152/ajpgi.00453.2003. [DOI] [PubMed] [Google Scholar]
- Welch DR, Sakamaki T, Pioquinto R, Leonard TO, Goldberg SF, Hon Q, Erikson RL, Rieber M, Rieber MS, Hicks DJ. et al. Transfection of constitutively active mitogen-activated protein/extracellular signal-regulated kinase kinase confers tumorigenic and metastatic potentials to NIH3T3 cells. Cancer Res. 2000;60:1552–1556. [PubMed] [Google Scholar]
- Alessandrini A, Greulich H, Huang W, Erikson RL. Mek1 phosphorylation site mutants activate Raf-1 in NIH 3 T3 cells. J Biol Chem. 1996;271:31612–31618. doi: 10.1074/jbc.271.49.31612. [DOI] [PubMed] [Google Scholar]
- Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, Vacek P, Mossman BT. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000;23:182–187. doi: 10.1165/ajrcmb.23.2.4035. [DOI] [PubMed] [Google Scholar]
- Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- Lounsbury KM, Stern M, Taatjes D, Jaken S, Mossman BT. Increased localization and substrate activation of protein kinase C delta in lung epithelial cells following exposure to asbestos. Am J Pathol. 2002;160:1991–2000. doi: 10.1016/S0002-9440(10)61149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24:405–413. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines–tools for the study of differentiation and the neoplastic phenotype. Toxicology. 1997;123:53–100. doi: 10.1016/S0300-483X(97)00108-X. [DOI] [PubMed] [Google Scholar]
- Wardlaw SA, Zhang N, Belinsky SA. Transcriptional regulation of basal cyclooxygenase-2 expression in murine lung tumor-derived cell lines by CCAAT/enhancer-binding protein and activating transcription factor/cAMP response element-binding protein. Mol Pharmacol. 2002;62:326–333. doi: 10.1124/mol.62.2.326. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Le Mesurier SM, de Montfort ML, Lykke AW. Development and characterization of type 2 pneumocyte-related cell lines from normal adult mouse lung. Pathology. 1984;16:401–405. doi: 10.3109/00313028409084730. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Onishi M, Kinoshita S, Shibuya A, Miyajima A, Nolan GP. Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci U S A. 1995;92:9146–9150. doi: 10.1073/pnas.92.20.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerbraun BS, Shapiro RA, Billiar TR, Tzeng E. RhoA influences the nuclear localization of extracellular signal-regulated kinases to modulate p21Waf/Cip1 expression. Circulation. 2003;108:876–881. doi: 10.1161/01.CIR.0000081947.00070.07. [DOI] [PubMed] [Google Scholar]
- Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- Weber TJ, Markillie LM, Chrisler WB, Vielhauer GA, Regan JW. Modulation of JB6 mouse epidermal cell transformation response by the prostaglandin F2alpha receptor. Mol Carcinog. 2002;35:163–172. doi: 10.1002/mc.10079. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Weber TJ, Colburn NH. AP-1, NF-kappa-B, and ERK activation thresholds for promotion of neoplastic transformation in the mouse epidermal JB6 model. Environ Health Perspect. 2002;110:865–870. doi: 10.1289/ehp.02110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Yang J, Scott J, Hanash S, Richardson BC. Role of the ras-MAPK signaling pathway in the DNA methyltransferase response to DNA hypomethylation. Biol Chem. 1998;379:1113–1120. doi: 10.1515/bchm.1998.379.8-9.1113. [DOI] [PubMed] [Google Scholar]
- Scheinbart LS, Johnson MA, Gross LA, Edelstein SR, Richardson BC. Procainamide inhibits DNA methyltransferase in a human T cell line. J Rheumatol. 1991;18:530–534. [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Weber TJ, Shankaran H, Wiley HS, Opresko LK, Chrisler WB, Quesenberry RD. Basic fibroblast growth factor regulates persistent ERK oscillations in premalignant but not malignant JB6 cells. J Invest Dermatol. 2010;130:1444–1456. doi: 10.1038/jid.2009.383. [DOI] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:NADEOE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pandey D, Fulton DJ. Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am J Physiol Heart Circ Physiol. 2011;300:H1336–H1344. doi: 10.1152/ajpheart.01163.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Morgan WF, Swarts SG, Jones GD, Hyun W. Persistent oxidative stress in chromosomally unstable cells. Cancer Res. 2003;63:3107–3111. [PubMed] [Google Scholar]
- Marampon F, Gravina GL, Di Rocco A, Bonfili P, Di Staso M, Fardella C, Polidoro L, Ciccarelli C, Festuccia C, Popov VM. et al. MEK/ERK inhibitor U0126 increases the radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by downregulating growth and DNA repair signals. Mol Cancer Ther. 2011;10:159–168. doi: 10.1158/1535-7163.MCT-10-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Xu W, Wang Q, Xiao W, Xu R. Potential of DNMT and its Epigenetic Regulation for Lung Cancer Therapy. Curr Genomics. 2009;10:336–352. doi: 10.2174/138920209788920994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiman TM, Sankpal UT, Robertson AK, Zhao Y, Robertson KD. DNMT3B interacts with hSNF2H chromatin remodeling enzyme, HDACs 1 and 2, and components of the histone methylation system. Biochem Biophys Res Commun. 2004;318:544–555. doi: 10.1016/j.bbrc.2004.04.058. [DOI] [PubMed] [Google Scholar]
- Monsey MS, Ota KT, Akingbade IF, Hong ES, Schafe GE. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS One. 2011;6:e19958. doi: 10.1371/journal.pone.0019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber TJ, Monks TJ, Lau SS. PGE2-mediated cytoprotection in renal epithelial cells: evidence for a pharmacologically distinct receptor. Am J Physiol. 1997;273:F507–F515. doi: 10.1152/ajprenal.1997.273.4.F507. [DOI] [PubMed] [Google Scholar]