Abstract
Two Pseudomonas strains known to utilize furan derivatives were shown to respond chemotactically to furfural, 5-hydroxymethylfurfural, furfuryl alcohol, and 2-furoic acid. In addition, a LysR-family regulatory protein known to regulate furan metabolic genes was found to be involved in regulating the chemotactic response.
TEXT
Furan derivatives are heterocyclic, five-membered aromatic rings, consisting of four carbon atoms and one oxygen, that are used industrially as solvents and chemical precursors (5). Although naturally occurring in the environment (35), they are also formed by the Maillard reaction, and for this reason furan aldehydes are present in biomass sugar preparations that have been pretreated with dilute acid. This pretreatment method, which involves incubation of fibrous biomass in dilute H2SO4 under heat and pressure, generates furfural and 5-hydroxymethylfurfural (5-HMF) from pentose and hexose sugars originating from hemicellulose and cellulose (12). Although furan aldehydes are microbial inhibitors, a number of microbes can utilize these compounds as a source of carbon and energy (2–4, 11, 19, 33–37, 39). The ability of selected microbes to dissimilate furfural and 5-HMF is the basis for a biological abatement strategy for detoxification of microbial inhibitors, including furfural and 5-HMF, in biomass hydrolysates (19, 22, 24, 37).
An aerobic pathway for furfuryl alcohol (FA), furfural, and 2-furoic acid (2-FA) degradation was initially proposed for Pseudomonas putida Fu1 and Pseudomonas F2 (13, 14, 34), and some accessory genes involved in furan metabolism were cloned from P. putida Fu1 (23). The complete complement of genes for conversion of furfural and 5-HMF to 2-oxoglutarate has been cloned from Cupriavidus basilensis HMF14 (15). The pathway includes enzymes for the sequential oxidation of FA and furfural to 2-FA, along with a furoyl-coenzyme A synthetase and a molybdenum-dependent dehydrogenase. Following lactone hydrolysis, which may occur spontaneously, removal of the coenzyme A moiety is catalyzed by 2-oxoglutaroyl coenzyme A thioesterase. Metabolism of 5-HMF also proceeds via furoic acid (15).
Motile bacteria are capable of chemotaxis to a variety of carbon sources. Paradigms were established initially for chemotaxis to sugars and amino acids by Escherichia coli, Salmonella, and Bacillus subtilis (30), with an increasing diversity of chemotactic mechanisms having been described more recently (16, 21). The broad chemotactic capability of pseudomonads, long noted for metabolic diversity (31), has also been recognized (28). Chemotaxis of Pseudomonas has been reported for aromatic acids (8), chlorinated benzoates (7), nitro- and aminobenzoates (26), benzene, toluene, and trichloroethylene (10, 27), naphthalene (6), atrazine (17), 2,4-dichlorophenoxyacetate (2,4-D) (9), and pyrimidines (18), and the importance of chemotaxis in bioremediation efforts has recently been addressed (25, 29, 32). Chemotaxis to furan compounds has not been reported previously; here, we describe the behavioral response of two Pseudomonas strains to furfural, 5-HMF, FA, and 2-FA.
Pseudomonas putida Fu1 (13) and Pseudomonas sp. strain A3 (19), both originally isolated for their ability to degrade furan molecules, were inoculated into 0.3% soft-agar minimal medium swarm plates (28) containing 0.5 mM 5-HMF, 1.0 mM furfural, or 0.5 mM 2-FA and incubated at 30°C for 24 to 36 h. As the cells metabolize the low concentration of attractant present in the soft agar, they follow a self-generated concentration gradient, resulting in the presence of a sharp ring of growth that spreads from the original point of inoculation and indicating a chemotactic response. Swarm plate assays (Fig. 1) demonstrated that strains Fu1 and A3 are attracted to furan molecules or potentially their metabolic intermediates, as metabolism is required for this assay. Subsequently, agarose plug assays (28, 38) were used to assess the ability of these strains to detect furans in a temporal manner over 10 minutes. Overnight cultures of Pseudomonas sp. strain A3 and P. putida Fu1 were grown in 5 mM 2-FA, harvested, and resuspended in chemotaxis buffer (CB) (7) to an A660 of 1.0. Motile cell suspensions were added to a chamber such that they surrounded a low-melting-temperature agarose plug containing CB (negative control), 2% Casamino Acids (CA; positive control), or 1.0 mM 2-FA or FA. As the attractant diffuses out from the agarose plug into the surrounding environment, and if the cells are attracted to the compound, cells accumulate at the optimal attractant concentration, resulting in a characteristic white ring of cells that typically forms around the periphery of the agarose plug. Each white cell ring was assessed microscopically to ensure that the cells that had accumulated were motile. Initial images taken at time zero for both strains and all attractants showed the same diffuse suspension of cells. After a 10-min incubation at room temperature, a white ring of motile cells developed around the agarose plugs containing CA and 2-FA, while areas closest to the plug were devoid of cells. This result demonstrates that the cells can detect 2-FA in a spatial gradient and are not reliant on a self-generated gradient based on growth on 2-FA (Fig. 2A). P. putida Fu1 was also shown to detect FA under the same assay conditions (Fig. 2B).
Fig 1.
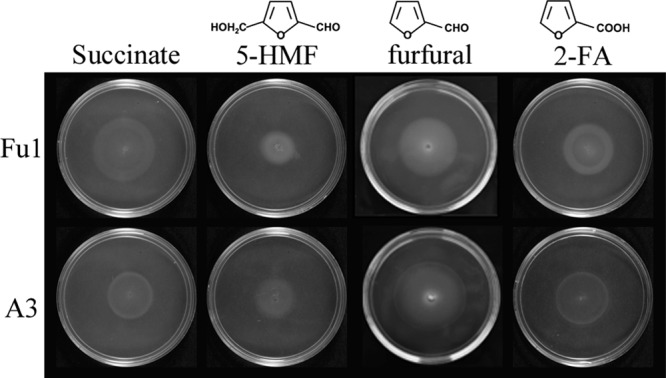
Pseudomonas putida strain Fu1 and Pseudomonas sp. strain A3 are attracted to furan molecules. Soft-agar minimal medium swarm plates containing 0.5 mM 5-HMF, 1.0 mM furfural, or 0.5 mM 2-FA were inoculated with cells at the center of the swarm plate and incubated at 30°C for 24 to 36 h. As cells metabolize the attractant, they follow a self-generated concentration gradient, forming white concentric circles of cellular growth. Succinate (1.0 mM) swarm plates served as positive controls. Images were taken using backlighting to visualize the outward growth patterns generated by attracted cells. Images shown are representative of assays conducted in triplicate.
Fig 2.
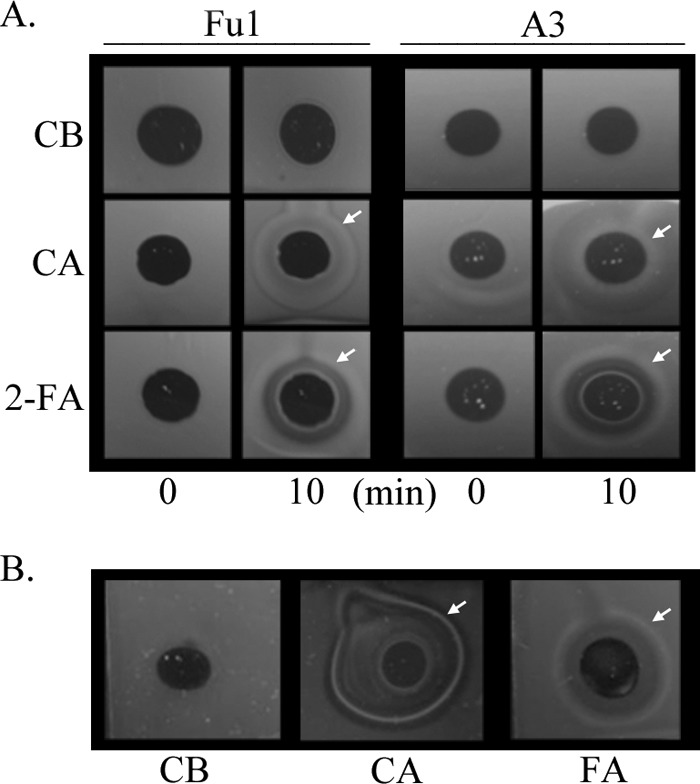
Pseudomonas putida strain Fu1 and Pseudomonas sp. strain A3 grown on 2-FA respond to a spatial gradient of furan molecules. (A). Strains Fu1 and A3 were grown on 5.0 mM 2-FA to provide inducing conditions. Cultures grown overnight with aeration were harvested and resuspended in chemotaxis buffer (CB) at an A600 of 1.0. Low-melting-point agarose (2%, with Coomassie brilliant blue for contrast) contained CB (negative control), 10% Casamino Acids (CA; positive control), or 1.0 mM 2-FA. A characteristic white ring of cells that develops at the optimal attractant concentration diffusing from the agarose plug demonstrates chemotactic behavior and is indicated in these images by a white arrow. Images were documented after 10 min by photography using backlighting. Images shown are representative of assays conducted in triplicate. (B) P. putida strain Fu1 is attracted to furfuryl alcohol (FA) under the same assay conditions.
The inducible nature of chemotactic responses to aromatic hydrocarbons and acids has been well established (29); therefore, quantitative capillary assays (1, 8) were conducted to determine if the response to 2-FA in P. putida Fu1 was inducible. Cells grown in 10 mM pyruvate (PYR) or 2-FA were harvested, washed, and resuspended in CB to an A660 of 1.0. The motile cell suspension was inserted into a chamber containing 1-μl capillary tubes loaded with CB, 2% CA, or 10-fold dilutions of 2-FA ranging from 500 to 0.05 mM. The cells were allowed to chemotactically respond to the attractant diffusing from the capillaries for 30 min at 30°C. The capillary tubes were then removed from the chambers, and the contents were emptied in order to quantitate (by serial dilution and growth on agar plates) the number of bacteria that had responded by swimming toward the attractant and into the capillary. As shown in Fig. 3, the response of P. putida Fu1 to 2-FA is inducible by growth on 2-FA, as the number of cells that responded to 2-FA at any concentration when grown in the presence of PYR was not significantly different from the number responding to buffer alone. However, P. putida Fu1 chemotactically responded to an optimum concentration of 5 mM 2-FA when grown on 2-FA.
Fig 3.
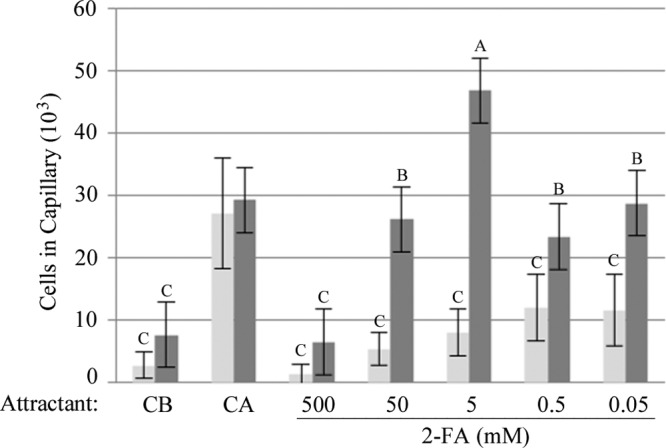
The response of Pseudomonas putida Fu1 to 2-FA is inducible. Aerated cultures were grown in 10 mM pyruvate (light gray bars) or 10 mM 2-FA (dark gray bars) at 30°C for 21 h. Cells were harvested, resuspended in CB, and placed in a U-tube chamber constructed by placing a U-shaped glass tube between a slide and a coverslip. Cells were allowed to chemotactically respond to either CB-, 2% CA-, or 2-FA-filled capillaries for 30 min at 30°C. Error bars represent the standard deviations for at least four independent assays conducted in triplicate. Means with the same letter are not significantly different (P < 0.05; one-way analysis of variance [ANOVA] interaction, Tukey multiple comparison test).
As shown in Fig. 2, the responses of strains Fu1 and A3 were qualitatively different, even when the strains were responding to a positive control such as CA. It has been our experience that different strains can respond differently to the same chemoattractants, as some strains are naturally more motile than others. This was evident in this study, as strain A3 clearly had a weaker overall chemotactic response to both CA and 2-FA in qualitative assays (Fig. 1 and 2). Strain A3 was therefore not tested further in the quantitative capillary assays, because of its overall weaker response.
Because the chemotactic response of P. putida Fu1 to 2-FA was shown to be inducible, we investigated the role in chemotaxis of a regulatory protein, PsfB, known to affect the expression of genes involved in furan metabolism (23). Strain PSF2 is a kanamycin-resistant mutant derived from Fu1 by minitransposon mutagenesis. PSF2 harbors the transposon in psfB, a LysR-type regulatory gene, and is incapable of growth on furfural and 2-FA (23). LysR family regulators typically activate transcription in the presence of an inducing molecule (20, 23). When grown in inducing conditions (PYR plus 2-FA), strain PSF2 did not respond to 2-FA, as assessed by agarose plug assays (Fig. 4.) However, when PsfB was produced in trans in mutant PSF2 under inducing conditions, the chemotactic response was restored to wild-type levels. As shown in Fig. 4A, a slight positive chemotactic response to 2-FA was also detected when the complemented strain was grown under noninducing conditions (absence of 2-FA). This positive response is consistent with the fact that PsfB is homologous to LysR-type positive regulators.
Fig 4.
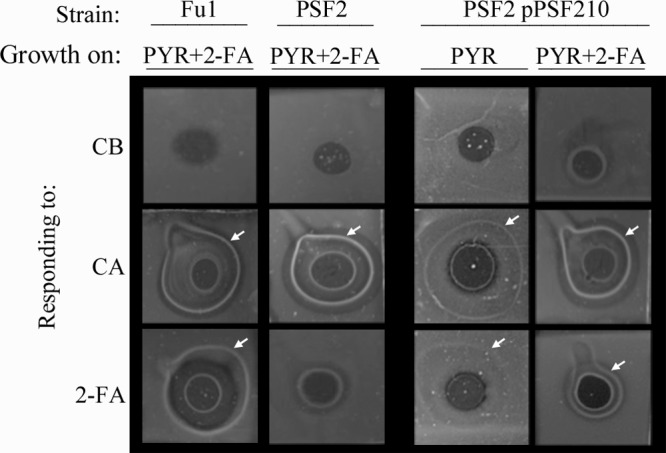
A LysR-family regulatory protein known to be responsible for the regulation of 2-FA metabolic genes regulates the inducible chemotactic response to 2-FA. P. putida strains Fu1, PSF2 (psfB::Km-inactivated strain), and PSF2 pPSF210 (psfB-complementing strain) were grown on 10 mM pyruvate (PYR) and 5.0 mM 2-FA to provide inducing conditions and assessed by the plug assay (see the legend to Fig. 2 for a detailed assay description). Attractants are listed to the left of each row. Descriptions of the psfB mutant PSF2 and the complementing plasmid pPSF210, which carries psfB in trans, can be found in reference 23. Images were documented after 10 min by photography using backlighting. Images shown are representative of assays conducted in triplicate. White arrows indicate positive chemotaxis responses.
The results of this work clearly demonstrate that Pseudomonas strains capable of metabolizing furan molecules also have the capacity to detect and respond chemotactically to furans in their environment. Knowledge of the chemotactic response to furans explains the initial facet of bacterial degradation of these industrially important and naturally occurring compounds. The discovery of bacterial chemotaxis to furfural, 2-FA, FA, and 5-HMF expands the known chemotactic repertoire of pseudomonads and is proof of a behavioral response to a class of compounds, furans, that had not previously been examined. The future identification of microbial furan chemoreceptors will provide a more thorough understanding of the furan chemosensory system and allow comparison to the diversity of chemotaxis pathways and chemoreceptors identified in silico (21).
ACKNOWLEDGMENTS
We are grateful to Jan R. Andreesen for providing P. putida Fu1. The strain is also available as DSM6384 from the German Collection of Microorganisms and Cell Cultures (www.dsmz.de). We thank Lois Fruen, director of the Breck School Advanced Science Research Program, Minneapolis, MN, for supporting the work of C.K.K., R.M.S., and E.J.S.
This study was supported in part by a grant to J.L.D. from the National Science Foundation (MCB-0919930).
Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print 22 June 2012
REFERENCES
- 1. Adler J. 1973. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J. Gen. Microbiol. 74: 77–91 [DOI] [PubMed] [Google Scholar]
- 2. Boopathy R, Bokang H, Daniels L. 1993. Biotransformation of furfural and 5-hydroxymethylfurfural by enteric bacteria. J. Ind. Microbiol. Biotechnol. 11: 147–150 [Google Scholar]
- 3. Brune G, Schoberth SM, Sahm H. 1983. Growth of a strictly anaerobic bacterium on furfural (2-furaldehyde). Appl. Environ. Microbiol. 46: 1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Folkerts M, et al. 1989. Desulfovibrio furfuralis sp. nov., a furfural degrading strictly anaerobic bacterium. Syst. Appl. Microbiol. 11: 161–169 [Google Scholar]
- 5. Gandini A, Belgacem MN. 1997. Furans in polymer chemistry. Prog. Polym. Sci. 22: 1203–1379 [Google Scholar]
- 6. Grimm AC, Harwood CS. 1997. Chemotaxis of Pseudomonas spp. to the polyaromatic hydrocarbon naphthalene. Appl. Environ. Microbiol. 63: 4111–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harwood CS, Parales RE, Dispensa M. 1990. Chemotaxis of Pseudomonas putida toward chlorinated benzoates. Appl. Environ. Microbiol. 56: 1501–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harwood CS, Rivelli M, Ornston LN. 1984. Aromatic acids are chemoattractants for Pseudomonas putida. J. Bacteriol. 160: 622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hawkins AC, Harwood CS. 2002. Chemotaxis of Ralstonia eutropha JMP134(pJP4) to the herbicide 2,4-dichlorophenoxyacetate. Appl. Environ. Microbiol. 68: 968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim HE, et al. 2006. Identification and characterization of the chemotactic transducer in Pseudomonas aeruginosa PAO1 for positive chemotaxis to trichloroethylene. J. Bacteriol. 188: 6700–6702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim T, Hah Y, Hong S. 1983. Toxic effects of furfural on Pseudomonas fluorescens. Korean J. Microbiol. 21: 149–155 [Google Scholar]
- 12. Klinke HB, Thomsen AB, Ahring BK. 2004. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 66: 10–26 [DOI] [PubMed] [Google Scholar]
- 13. Koenig K, Andreesen JR. 1989. Molybdenum involvement in aerobic degradation of 2-furoic acid by Pseudomonas putida Fu1. Appl. Environ. Microbiol. 55: 1829–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koenig K, Andreesen JR. 1990. Xanthine dehydrogenase and 2-furoyl-coenzyme A dehydrogenase from Pseudomonas putida Fu1: two molybdenum-containing dehydrogenases of novel structural composition. J. Bacteriol. 172: 5999–6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koopman F, Wierckx N, De Winde JH, Ruijssenaars HJ. 2010. Identification and characterization of the furfural and 5-(hydroxymethyl)furfural degradation pathways of Cupriavidus basilensis HMF14. Proc. Natl. Acad. Sci. U. S. A. 107: 4919–4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krell T, et al. 2011. Diversity at its best: bacterial taxis. Environ. Microbiol. 13: 1115–1124 [DOI] [PubMed] [Google Scholar]
- 17. Liu X, Parales RE. 2009. Bacterial chemotaxis to atrazine and related s-triazines. Appl. Environ. Microbiol. 75: 5481–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, Wood PL, Parales JV, Parales RE. 2009. Chemotaxis to pyrimidines and identification of a cytosine chemoreceptor in Pseudomonas putida. J. Bacteriol. 191: 2909–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López MJ, Nichols NN, Dien BS, Moreno J, Bothast RJ. 2004. Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl. Microbiol. Biotechnol. 64: 125–131 [DOI] [PubMed] [Google Scholar]
- 20. Maddocks SE, Oyston PC. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154: 3609–3623 [DOI] [PubMed] [Google Scholar]
- 21. Miller LD, Russell MH, Alexandre G. 2009. Diversity in bacterial chemotactic responses and niche adaptation. Adv. Appl. Microbiol. 66: 53–75 [DOI] [PubMed] [Google Scholar]
- 22. Nichols NN, Dien BS, Cotta MA. 2010. Fermentation of bioenergy crops into ethanol using biological abatement for removal of inhibitors. Bioresour. Technol. 101: 7545–7550 [DOI] [PubMed] [Google Scholar]
- 23. Nichols NN, Mertens JA. 2008. Identification and transcriptional profiling of Pseudomonas putida genes involved in furoic acid metabolism. FEMS Microbiol. Lett. 284: 52–57 [DOI] [PubMed] [Google Scholar]
- 24. Nichols NN, et al. 2008. Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzyme Microb. Technol. 42: 624–630 [Google Scholar]
- 25. Pandey G, Jain RK. 2002. Bacterial chemotaxis toward environmental pollutants: role in bioremediation. Appl. Environ. Microbiol. 68: 5789–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parales RE. 2004. Nitrobenzoates and aminobenzoates are chemoattractants for Pseudomonas strains. Appl. Environ. Microbiol. 70: 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parales RE, Ditty JL, Harwood CS. 2000. Toluene-degrading bacteria are chemotactic towards the environmental pollutants benzene, toluene, and trichloroethylene. Appl. Environ. Microbiol. 66: 4098–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parales RE, Harwood CS. 2002. Bacterial chemotaxis to pollutants and plant-derived aromatic molecules. Curr. Opin. Microbiol. 5: 266–273 [DOI] [PubMed] [Google Scholar]
- 29. Parales RE, Ju K-S, Rollefson JB, Ditty JL. 2008. Bioavailability, transport, and chemotaxis towards organic pollutants, p 146–187 In Diaz E. (ed), Microbial biodegradation: genomics and molecular biology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 30. Rao CV, Kirby JR, Arkin AP. 2004. Design and diversity in bacterial chemotaxis: a comparative study in Escherichia coli and Bacillus subtilis. PLoS Biol. 2: E49 doi:10.1371/journal.pbio.0020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stanier RY, Palleroni NJ, Doudoroff M. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43: 159–271 [DOI] [PubMed] [Google Scholar]
- 32. Strobel KL, et al. 2011. Chemotaxis increases vertical migration and apparent transverse dispersion of bacteria in a bench-scale microcosm. Biotechnol. Bioeng. 108: 2070–2077 [DOI] [PubMed] [Google Scholar]
- 33. Thiemer B, Andreesen JR, Schräder T. 2003. Cloning and characterization of a gene cluster involved in tetrahydrofuran degradation in Pseudonocardia sp. strain K1. Arch. Microbiol. 179: 266–277 [DOI] [PubMed] [Google Scholar]
- 34. Trudgill PW. 1969. The metabolism of 2-furoic acid by Pseudomonas F2. Biochem. J. 113: 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trudgill PW. 1984. The microbial metabolism of furans, p 295–308 In Gibson DT. (ed), Microbial degradation of organic compounds. Marcel Dekker, Inc., New York, NY [Google Scholar]
- 36. Wang P, Brenchley JE, Humphrey AE. 2004. Screening microorganisms for utilization of furfural and possible intermediates in its degradative pathway. Biotechnol. Lett. 16: 977–982 [Google Scholar]
- 37. Wierckx N, Koopman F, Bandounas L, De Winde JH, Ruijssenaars HJ. 2010. Isolation and characterization of Cupriavidus basilensis HMF14 for biological removal of inhibitors from lignocellulosic hydrolysate. Microb. Biotechnol. 3: 336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu HS, Alam M. 1997. An agarose-in-plug bridge method to study chemotaxis in the archaeon Halobacterium salinarum. FEMS Microbiol. Lett. 156: 265–269 [DOI] [PubMed] [Google Scholar]
- 39. Zarnt G, Schräder T, Andreesen JR. 1997. Degradation of tetrahydrofurfuryl alcohol by Ralstonia eutropha is initiated by an inducible pyrroloquinoline quinone-dependent alcohol dehydrogenase. Appl. Environ. Microbiol. 63: 4891–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]


