Abstract
The microbial community of a full-scale, biologically active drinking water filter was surveyed using molecular techniques. Nitrosomonas, Nitrospira, Sphingomonadales, and Rhizobiales dominated the clone libraries. The results elucidate the microbial ecology of biological filters and demonstrate that biological treatment of drinking water should be considered a viable alternative to physicochemical methods.
TEXT
Biologically active filtration is commonly used in Europe and Asia for drinking water treatment (5). Biological processes have the potential to cut operation costs by decreasing the amount of chemicals required for treatment and increasing effectiveness in terms of decreased biological regrowth (e.g., corrosion, nitrification, taste, and odor) in the distribution system (DS) and decreased chlorine demand (6, 17, 30). However, biological processes have not been widely accepted in drinking water in the United States, mainly due to issues arising from the negative perception of microorganisms as well as questionable reliability and effectiveness (2, 8, 17). With the lack of published long-term operational data using biological processes and the complexity of microbial systems, there exists a need to both document biologically active systems and design experimental systems to elucidate the microbial consortia and the effects of operational parameters.
One use of biologically active filters for drinking water treatment involves the regulation of nitrate/nitrite nitrogen levels. Excessive ingestion of nitrite and nitrate can be hazardous (H. I. Shuval and N. Gruener, presented at the Panel of Experts on Effects of Agricultural Production on Nitrates in Food and Water with Particular Reference to Isotope Studies, Vienna, Austria, 1974), so the United States Environmental Protection Agency (U.S. EPA) has set source water maximum contaminant levels (MCLs) for nitrite and nitrate at 1 mg/liter N and 10 mg/liter N, respectively. Yet no MCL exists for ammonia. As utilities are required to monitor for only nitrite and nitrate in the source water, concentrations may build in the DS via uncontrolled partial (nitrite accumulation) or full nitrification. When excessive levels of free ammonia are present in the source water or ammonia is added to form chloramines, nitrification may occur with sufficient dissolved oxygen (DO) (34). Nitrification in the DS, and the pH drop associated with nitrification, can impact the corrosion rates of the DS and premise materials (41). In addition, the increased chlorine demand and growth of heterotrophic biofilms produce undesirable taste and odor issues (30). Excess ammonia itself may interfere (by way of chloramine formation) with the maintenance of a free chlorine residual in the distribution system and the chemical oxidation of arsenic(III) in treatment plants utilizing the iron removal process for arsenic removal (18, 19). In the case of arsenic oxidation, excess ammonia will bind with free chlorine used to oxidize arsenic(III) to arsenic(V), thus decreasing arsenic sorption to iron(III) and, ultimately, arsenic removal via iron(III) filtration.
Lytle et al. (18, 19) reported on the use of biologically active filters to oxidize ammonia and arsenic in a full-scale water treatment plant. Briefly, the treatment train aerates groundwater, which is then filtered (loading rate, 85 liters/min/m2) through dual-medium anthracite over sand filters. The water is finally chlorinated, fluorinated, and distributed. The filter is backwashed every 3 days. They demonstrated that the filters completely and consistently oxidized 1.13 mg/liter of ammonia nitrogen to nitrate nitrogen and 38 μg/liter of arsenic(III) to arsenic(V) without the addition of a chemical oxidant. Preliminary filter analysis and follow-up pilot studies identified bacteria as the probable source of ammonia oxidation and arsenic oxidation (C. N. Green, presented at Rice University, Houston, TX, 19 October 2007). The findings of Green and Lytle were based primarily on culture-dependent methods, with little use of culture-independent (molecular) methods by Green (C. N. Green, presented at Rice University, Houston, TX, 19 October 2007) (18, 19). Molecular microbiological techniques used to characterize microbial communities in wastewater processes are a well-studied field; however, characterization of full-scale, biologically active water treatment systems for drinking water is limited (16, 25, 26, 36, 38). There is a clear need to better identify the diversity of bacterial communities, including the presence of human pathogens, in biologically active drinking water filters to improve the understanding of such a complex system. Identification of the microbial consortia will provide a greater insight into the dynamics of biologically active filters, establish their susceptibility to pathogen growth, and determine their applicability as a viable treatment technology.
The goal of this study was to identify members of the microbial community in a full-scale drinking water filter in Southwest Ohio and identify the specific microorganisms responsible for ammonia oxidation. Specifically, we revisit the microbial oxidation system previously characterized by Lytle et al. (18, 19).
DNA isolations from filter anthracite/sand medium were collected aseptically from a single fluidized filter during a 5-min backwash. Approximately 400 g of medium was collected across a 5.9-m2 filter bed. Samples were taken to the U.S. EPA laboratories in Cincinnati, homogenized via mixing, and processed immediately. A total of 1.2 g of wet medium was placed in 400 μl of lysis buffer (EpiCentre Biosciences, Madison, WI) containing SDS and sonicated three times for 30 s each, vortexing between steps. The tubes were then centrifuged, and the supernatant was removed and placed into a tube containing glass beads. The supernatant was beaten with the beads for 1 min. Two microliters of 50-μg/ml proteinase K (EpiCentre Biosciences, Madison, WI) was added to each tube and incubated at 65°C for 10 min. The supernatant was extracted, and nucleic acids were precipitated in ice-cold isopropanol at 4°C for 30 min. The nucleic acids were pelleted and washed with 70% ice-cold ethanol and desiccated. Samples were rehydrated in 50 μl of sterile water (Chemicon, Temecula, CA) and stored at −20°C.
A PCR was optimized and conducted in a 25-μl volume containing 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.3 μM each primer (16S rRNA gene-Forward, 5′-GTTTGATCCTGGCTCAG-3′, and 16S rRNA gene-Reverse, 5′-ACGGYTACCTTGTTACGACTT-3′), 0.4 U Taq polymerase (TaKaRa, Otsu, Japan), 0.6× Taq buffer, and 1 μl of template DNA. The amoA PCR mixture contained 0.1 μg/μl of nonacetylated bovine serum albumin (BSA). A touchdown cycle was used, starting at 60°C and decreasing 0.5°C each cycle for 10 cycles, followed by 15 cycles at 55°C. PCRs with amoA and archaeal amoA followed the published protocols of Francis et al. and Rotthauwe et al., respectively (10, 31). PCR products were electrophoresed on a 1.8% agarose gel in 0.5× Tris-acetate-EDTA (TAE) buffer. PCR bands of the16S rRNA gene and amoA gene that were used for cloning were excised from the gel using a sterile scalpel and processed using a gel extraction kit (Qiagen, Chatsworth, CA). Products were cloned using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA).
Sequencing reactions were performed on an ABI 3730 using the BigDye Terminator system with T3/T7 primers. Sequences were edited and aligned in MEGA4 and compared to sequences in the NCBI database using the BLAST function. Highest-similarity sequences were downloaded for each sequence. Duplicate and chimeric sequences were discarded. Chimeric sequences were identified using Mallard (3). Type-cultured and previously identified sequences of bacterial species with close BLAST hits were retrieved from GenBank, and operational taxonomic units (OTUs) were identified using 97% sequence identity as a threshold value.
Phylogenetic trees were constructed using MEGA version 4 (35). Phylogeny was inferred using the neighbor-joining algorithm with 2,000 bootstrap replicates assuming pairwise deletion using the maximum composite likelihood distance correction.
A small amount of filter medium was fixed in a pH 7.2 cacodylate-buffered 1% paraformaldehyde-2.5% glutaraldehyde mixture. The medium was postfixed in 1% OsO4 and dehydrated in an ethanol series, dehydrated in hexamethyldisilazane (HMDS) for 1 h, and then air dried in a desiccator. The medium was coated with gold/palladium prior to scanning electron microscopy (SEM). Samples were viewed using a JEOL 6490LV SEM at 30 kV under high vacuum. Energy dispersive X-ray analysis (EDX) was performed for 60 live seconds using a process time of 4 and a working distance of 10 mm.
A detailed water quality analysis can be found in previous studies by this lab (18, 19). Lytle et al. reported that raw and influent ammonia values averaged 1.13 mg/liter N, with nitrate and nitrite below the detection limits of 0.01 and 0.02 mg/liter N, respectively (19). After filtration, prior to the addition of chlorine, ammonia was oxidized to below 0.1 mg/liter N, with nitrite and nitrate measuring 0.02 mg/liter N and 1.11 mg/liter N, respectively (19). Ammonia-oxidizing bacteria (AOB) counts were highest on the filter medium and lowest in the plant effluent. Heterotrophic plate counts (HPC) were highest in raw influent and lowest in the plant effluent (19).
DNA isolations from the filter medium provided a sufficient template for PCR. PCR products of 16S rRNA genes and amoA genes were the correct length (∼1,300 bp and ∼450 bp, respectively) and of suitable yield for cloning. A total of 431 16S rRNA genes and 61 amoA clones were selected and sequenced. After removing duplicate and chimeric sequences, 297 unique 16S rRNA gene sequences and 31 unique amoA sequences were grouped into OTUs and analyzed for phylogeny. Representatives from each OTU were used as query sequences to NCBI BLAST to identify close relatives. These sequences were then downloaded and included in their respective phylogeny.
Unique amoA sequences were grouped into 9 OTUs, 4 of which were singletons, close to the Chao-1 estimate of 17 ± 8. Eight OTUs were clustered within the genus Nitrosomonas, with the remaining single OTU closely resembling Nitrosospira (Fig. 1). Within Nitrosomonas, 7 OTUs, comprising 28 total sequences, fell within the Nitrosomonas oligotropha lineage. The remaining OTU, comprising 2 sequences, fell within the Nitrosomonas europaea lineage. Primers directed to the archaeal amoA gene were used in an attempt to create a clone library for subsequent identification of ammonia-oxidizing Archaea (AOA), but PCR did not produce any amplification after exhaustive optimization efforts.
Fig 1.
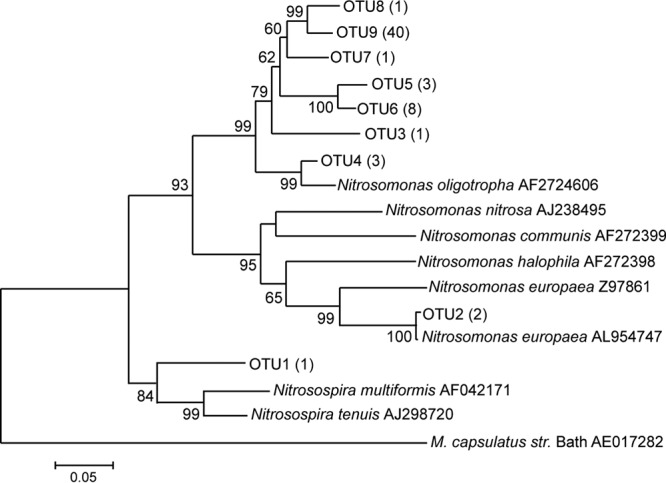
Unrooted neighbor-joining tree of the amoA gene sequences. The numbers in parentheses indicate the numbers of sequences of that OTU. M. capsulatus str. Bath, Methylococcus capsulatus strain Bath.
Unique 16S rRNA gene sequences were grouped into 65 OTUs, 36 of which were singletons. The Chao-1 estimate (157 ± 31) of the 16S rRNA gene library revealed that rare members of the community were undersampled. With nearly 55% of OTUs singletons, diversity was driven by species captured only once, suggesting a highly diverse system. The 65 OTUs were classified into 9 discrete groups (Table 1). Twenty-seven OTUs (together accounting for 21% of all sequences) were closely related to Alphaproteobacteria. Within the Alphaproteobacteria, the orders Rickettsia, Rhodobacterales, Rhizobiales, and Sphingomonadales were represented. Betaproteobacteria accounted for 15% of sequences, with 10 OTUs representing Nitrosomonadales, Methylophilales, and Burkholderiales. Gammaproteobacteria, Deltaproteobacteria, and Epsilonproteobacteria were grouped together and accounted for approximately 3% of the sequences within 8 OTUs. An analysis of 19 sequences representing 6 OTUs identified only unknown bacteria as close relatives. These sequences were placed into the unknown group. Planctomycetes, Bacteroidetes, Verrucomicrobia, and Chloroflexi accounted for approximately 4% of sequences within 9 OTUs.
Table 1.
GenBank relatives to 16S clone library sequences
| Relative in GenBank (accession no.) | Identity (%)b | % of clones in clone library that are relatedc | No. of OTUs |
|---|---|---|---|
| Nitrospira sp. (AF035813) | 96–98 | 29.6 | 2 |
| Nitrospira sp. clone g6 (Nitrospira sp. (AJ224039) | 95–99 | 20.8 | 2 |
| “Candidatus Nitrospira defluvii” (EU559167) | 99 | 0.5 | 1 |
| Rhodospirillaceae bacterium LM22 (FJ455532) | 94–98 | 7.2 | 5 |
| Rhodospirillaceae bacterium L34 (FJ459988) | 92 | 0.8 | 1 |
| Sphingomonas sp. UF010 (AB426571) | 99 | 2.1 | 1 |
| Sphingomonas sp. EZ41 (EU591707) | 95–97 | 2.7 | 4 |
| Sphingomonas sp. MTR-71 (DQ898300) | 95 | 1.1 | 1 |
| Sphingomonadaceae bacterium HINF002 (AB426560) | 95 | 0.5 | 1 |
| Sphingomonas sp. HTCC503 (AY584572) | 97 | 0.3 | 1 |
| Sphingomonas sp. BAC151 (EU131005) | 97 | 0.8 | 2 |
| Hyphomicrobium vulgare (Y14302) | 97 | 2.1 | 1 |
| Hyphomicrobium sp. Ellin112 (AF408954) | 95 | 0.5 | 2 |
| Hyphomicrobium sp. KC-IT-W2 (FJ711209) | 95 | 0.3 | 1 |
| Nordella sp. P-63 (AM411927) | 92 | 0.5 | 1 |
| Bradyrhizobium sp. KC-EP-S3 (FJ711219) | 99 | 0.3 | 1 |
| Other Alphaproteobacteria (FJ203515, AY945895, AF236002, AJ630204, NR 026337, and AF498710) | 87–99 | 5.1 | 7 |
| Nitrosomonas sp. Nm86 (AY123798) | 97–98 | 5.1 | 2 |
| Nitrosomonas sp. Nm84 (AY123797) | 96 | 4.3 | 1 |
| Nitrosomonas sp. Is32 AJ621027) | 97 | 0.3 | 1 |
| “Candidatus Nitrotoga artica” HAM-1 (FJ263061) | 99 | 4.8 | 1 |
| Methylophilus sp. ECd5 (AY436794) | 96–97 | 0.5 | 2 |
| Bacterium TG141 (AB308367) | 93–96 | 1.3 | 3 |
| Other Betaproteobacteria (AJ252690, AB271046, AM412133, and DQ386262) | 97–98 | 1.6 | 4 |
| Gammaproteobacteria (AJ233898) | 93 | 0.5 | 1 |
| Deltaproteobacteria (DQ295890, DQ145534, AB246770, CP001359, AB245340, NR 025348, and NR 024781) | 82–96 | 2.1 | 7 |
| Flavobacterium ferrugineum (AM230484) | 94 | 1.9 | 1 |
| Other Bacteriodetes (EF612324, AF070444, and GQ144415) | 86–90 | 0.8 | 3 |
| Pirellula sp. (X81942) | 92 | 0.8 | 1 |
| Other Planctomycetes (AY162118 and CU925984) | 87–98 | 0.5 | 2 |
| Uncultured Chloroflexi bacterium (FM253645) | 98 | 0.3 | 1 |
| Opitutusa sp. VeSm13 (X99392) | 97 | 0.3 | 1 |
Classified as Verrucomicrobia.
Percent similarity between each relative in the NCBI database and its closest cloned 16S gene.
Total of 375 clones in the clone library.
The phylum Nitrospirae, known to be a nitrite oxidizer (33, 34), dominated the clone library with over 51% of sequences grouped into 5 OTUs. One OTU (16 sequences) was identified as “Candidatus Nitrotoga arctica,” a cold-adapted nitrite-oxidizing bacterium previously isolated from activated sludge (1).
SEM-EDX analysis of the filter medium showed particles coated with an inorganic layer with C, O, S, Cl, Fe, Ca, As, Mn, and P (Fig. 2A, B, and D, bottom). No biofilm was observed on the surface of the medium examined. Differential backscatter imaging identified medium particles lacking the outer coating, presumably from scouring during backwash. Particles devoid of the outer inorganic layer resolved a complex biofilm containing a mix of spirochetes, bacilli, and cocci within an extracellular matrix (Fig. 2C). SEM-EDX analysis of the biofilm revealed the presence of C, O, and S (Fig. 2D, top).
Fig 2.

Scanning electron micrograph and EDX spectra of the filter medium. (A) Backscatter image of a representative anthracite grain. (B) Secondary electron image of the outer inorganic layer on the surface of anthracite. (C) Secondary electron image of the biofilm layer beneath the inorganic layer. (D) EDX spectra of the biofilm layer (top) and outer inorganic layer (bottom).
Lytle et al. (19) observed that raw ground water contained an average of 1.13 mg/liter of ammonia nitrogen prior to filtration and less than 0.1 mg/liter N after filtration (19). Nitrate and nitrite nitrogen levels prior to filtration were below the limit of detection (19). After filtration, nitrate nitrogen was present stoichiometrically with prefiltration ammonia nitrogen. This stoichiometric relationship and presence of nitrate nitrogen after filtration suggested that ammonia was oxidized in the filters (19). As no chemical means of oxidation was included in the treatment train, biological nitrification was determined to be the causative agent. Therefore, the filters were investigated for their microbial diversity.
General congruence was observed between 16S rRNA and amoA gene clone libraries. The dominant nitrifying organisms in the 16S rRNA gene clone library were members of the phyla Proteobacteria and Nitrospirae. Of the sequences in the amoA library, species from the genus Nitrosomonas were found to be the major ammonia oxidizers, accounting for more than 98% of total sequences. Over 95% of the total sequences clustered in the N. oligotropha lineage, and 3% clustered in the N. europaea lineage. One sequence was related to the genus Nitrosospira. These findings are consistent with other research suggesting that Nitrosomonas species, specifically the species N. oligotropha, are better adapted to low ammonia concentrations than other AOB and dominate the drinking water distribution system (28, 29).
The filters operate under relatively limited ammonia concentrations and, thus, limited nitrite concentrations, so Nitrospira species are expected to be the dominant nitrite oxidizers due to their lower half-saturation coefficient for oxygen and nitrite compared to that of Nitrobacter (20, 33). This is supported by the fact that Nitrospirae accounted for approximately 51% of the clone library in this study.
Though many clones were related to the order Rhizobiales, no sequences were related to the genus Nitrobacter. Though Nitrobacter has a higher growth rate, its ability to compete for oxygen and substrate is lower than that of Nitrospira (13, 21).
Moreover, the detection of “Candidatus Nitrotoga artica” may give beneficial operational flexibility to the filter. These organisms have been shown to oxidize nitrite at temperatures near 4°C, possibly adding to the operational temperature range (1). Rhizobiales, though not typically related to aquatic environments, are presumably introduced by aquifer transport and may play a novel role in the nutrient-limiting environment (32).
Recent attention has been focused on the dominance of AOA in the environment (10, 36). Specifically, many studies have shown that Archaea are the dominant ammonia oxidizers in both soil and marine environments. To this end, archaeal-amoA primers were used to determine if AOA were present and to serve as a cloning insert for gene library construction and sequence analysis. No AOA were detected based on the absence of an archaeal-amoA amplicon. The lack of detection of AOA indicates that AOB dominate this system. This may be due to the fact that the raw water chemistry may inhibit the growth or physiology of these organisms or that the ammonia levels in the raw water saturate the AOA amoA enzyme and prevent the ability to oxidize the ammonia, thus selecting for AOB (12, 40).
Sphingomonas and Rhizobiales dominated the 16S rRNA gene clone library. Sphingomonas has been shown to degrade complex organic molecules, such as xenobiotics, chloro/nitro phenolics, and large polymers. They accomplish this via numerous dependent and independent metabolisms that may add to the operational flexibility of biologically active filters (4, 11, 15, 22, 37).
Members of Rhizobiales have demonstrated the ability to utilize a broad range of carbon sources under aerobic conditions (24). Studies on pure cultures of Rhizobiales have shown that they may also be capable of degrading methyl parathion, metolachlor, polyacrylamides, and quaternary ammonium alcohols, all potential source water contaminants (14, 23, 27, 39).
An interesting finding of this study was the fact that no known pathogenic bacteria were identified as the majority of the 16S rRNA gene clone libraries. A primary concern of biologically active filtration is whether or not the filter is hospitable for pathogenic organisms, so this finding is encouraging. Though such organisms may be sensitive to chlorination, the possibility exists for distribution system contamination via slough-off if there is a malfunction in chlorination or the organism is capable of forming endospores. Therefore, there exists a need to further study this question in greater detail.
SEM observations made on the medium indicate that the biofilm formed prior to the deposition of solids during the filtration process. During treatment, the observed outer inorganic layer is constantly forming due to iron(III) filtration and is removed via backwashing every 3 days. This sets up a dynamic environment of constant formation and removal. With the biofilm being the most probable source of nitrification, this layer must allow diffusion to the microorganisms. Based on these observations, it is also worth asking if the outer layer impacts biological activity and if the inorganic layer protects the biofilm from disruption from shear force during backwash. This possible protective function may account for the rapid recovery of biological activity after backwash (7). Previously published culture-dependent studies of this system may support this notion, though a complete analysis is required to draw strong conclusions (9, 19).
The authors acknowledge that phylogenetic identification does not imply physiology, and caution is suggested in interpretation of culture-independent studies of microbial ecology. To this end, the metabolic diversity known to exist in organisms identified in biologically active filters may provide the only means to remove complex contaminants from source waters. With that said, such filters may serve as a unique source for isolation of novel organisms that may be beneficial for bioremediation.
Nucleotide sequence accession numbers.
Sequences of one member from each OTU generated in this study were submitted to GenBank. amoA sequences fall within accession numbers GU596402 to GU596410. 16S rRNA gene sequences fall within accession numbers HM921089 to HM921151 and JX101440 and JX101441.
ACKNOWLEDGMENTS
We acknowledge fellow U.S. EPA staff Daniel Williams and Christy Muhlen for their field assistance and Keith Kelty, Brittany Almassalkhi, and William Kaylor for analytical support. We also thank Mike Watts from the Greene County Sanitary Engineering Department. Finally, we thank Kyle Hawkins of Miami University (Ohio) for editorial comments.
Any opinions expressed in this paper are those of the authors and do not necessarily reflect the official position and policies of the U.S. EPA. Any mention of products or trade names does not constitute recommendation for use by the U.S. EPA.
Footnotes
Published ahead of print 29 June 2012
REFERENCES
- 1. Alawi M, Lipski A, Sanders T, Pfeiffer EM, Spieck E. 2007. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J. 1: 256–264 [DOI] [PubMed] [Google Scholar]
- 2. Andersson A, Laurent P, Kihn A, Prévost M, Servais P. 2001. Impact of temperature on nitrification in biological activated carbon (BAC) filters used for drinking water treatment. Water Res. 35: 2923–2934 [DOI] [PubMed] [Google Scholar]
- 3. Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ. 2006. New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl. Environ. Microbiol. 72: 5734–5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balkwill DL, et al. 1997. Taxonomic study of aromatic-degrading bacteria from deep-terrestrial-subsurface sediments and description of Sphingomonas aromaticivorans sp. nov., Sphingomonas subterranea sp. nov., and Sphingomonas stygia sp. nov. Int. J. Syst. Bacteriol. 47: 191–201 [DOI] [PubMed] [Google Scholar]
- 5. Bower CK, McGuire J, Daeschel MA. 1996. The adhesion and detachment of bacteria and spores on food-contact surfaces. Trends Food Sci. Technol. 7: 152–157 [Google Scholar]
- 6. Camper AK, et al. 2003. Effect of distribution system materials on bacterial regrowth. J. Am. Water Works Assoc. 95: 107–121 [Google Scholar]
- 7. Celmer D, Oleszkiewicz JA, Cicek N. 2008. Impact of shear force on the biofilm structure and performance of a membrane biofilm reactor for tertiary hydrogen-driven denitrification of municipal wastewater. Water Res. 42: 3057–3065 [DOI] [PubMed] [Google Scholar]
- 8. Chung J, Shim H, Park SJ, Kim SJ, Bae W. 2006. Optimization of free ammonia concentration for nitrite accumulation in shortcut biological nitrogen removal process. Bioprocess Biosyst. Eng. 28: 275–282 [DOI] [PubMed] [Google Scholar]
- 9. Clesceri LS, et al. 1996. Standard methods for the examination of water and wastewater, 19th ed supplement American Public Health Association, Washington, DC [Google Scholar]
- 10. Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. U. S. A. 102: 14683–14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fredrickson JK, et al. 1995. Aromatic-degrading Sphingomonas isolates from the deep subsurface. Appl. Environ. Microbiol. 61: 1917–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He JZ, et al. 2007. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 9: 2364–2374 [DOI] [PubMed] [Google Scholar]
- 13. Hill C, Khan E. 2008. A comparative study of immobilized nitrifying and co-immobilized nitrifying and denitrifying bacteria for ammonia removal from sludge digester supernatant. Water Air Soil Pollut. 195: 23–33 [Google Scholar]
- 14. Kaech A, Vallotton N, Egli T. 2005. Isolation and characterization of heterotrophic bacteria able to grow aerobically with quaternary ammonium alcohols as sole source of carbon and nitrogen. Syst. Appl. Microbiol. 28: 230–241 [DOI] [PubMed] [Google Scholar]
- 15. Kawai F. 1999. Sphingomonads involved in the biodegradation of xenobiotic polymers. J. Ind. Microbiol. Biotechnol. 23: 400–407 [DOI] [PubMed] [Google Scholar]
- 16. Kihn A, Laurent P, Servais P. 2000. Measurement of potential activity of fixed nitrifying bacteria in biological filters used in drinking water production. J. Ind. Microbiol. Biotechnol. 24: 161–166 [Google Scholar]
- 17. LeChevallier MW, Becker WC, Schorr P, Lee RG. 1992. Evaluating the performance of biologically active rapid filters. J. Am. Water Works Assoc. 84: 136–146 [Google Scholar]
- 18. Lytle DA, Chen AS, Sorg TJ, Phillips S, French K. 2007. Biological As(III) oxidation in water treatment plant filters. J. Am. Water Works Assoc. 99: 72–86 [Google Scholar]
- 19. Lytle DA, et al. 2007. Biological nitrification in a full-scale and pilot-scale iron removal drinking water treatment plant. J. Water Supply Res. Technol. AQUA 56: 125–136 [Google Scholar]
- 20. Manser R, Gujer W, Siegrist H. 2005. Consequences of mass transfer effects on the kinetics of nitrifiers. Water Res. 39: 4633–4642 [DOI] [PubMed] [Google Scholar]
- 21. Martiny AC, Albrechtsen HJ, Arvin E, Molin S. 2005. Identification of bacteria in biofilm and bulk water samples from a nonchlorinated model drinking water distribution system: detection of a large nitrite-oxidizing population associated with Nitrospira spp. Appl. Environ. Microbiol. 71: 8611–8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nohynek LJ, Suhonen EL, Nurmiaho-Lassila E-L, Hantula J, Salkinoja-Salonen M. 1995. Description of four pentachlorophenol-degrading bacterial strains as Sphingomonas chlorophenolica sp. nov. Syst. Appl. Microbiol. 18: 527–538 [Google Scholar]
- 23. Padden AN, Rainey FA, Kelly DP, Wood AP. 1997. Xanthobacter tagetidis sp. nov., an organism associated with Tagetes species and able to grow on substituted thiophenes. Int. J. Syst. Bacteriol. 47: 394–401 [DOI] [PubMed] [Google Scholar]
- 24. Pang CM, Liu WT. 2007. Community structure analysis of reverse osmosis membrane biofilms and the significance of Rhizobiales bacteria in biofouling. Environ. Sci. Technol. 41: 4728–4734 [DOI] [PubMed] [Google Scholar]
- 25. Park HD, Wells GF, Bae H, Criddle CS, Francis CA. 2006. Occurrence of ammonia-oxidizing Archaea in wastewater treatment plant bioreactors. Appl. Environ. Microbiol. 72: 5643–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin YY, Li DT, Yang H. 2007. Investigation of total bacterial and ammonia-oxidizing bacterial community composition in a full-scale aerated submerged biofilm reactor for drinking water pretreatment in China. FEMS Microbiol. Lett. 268: 126–134 [DOI] [PubMed] [Google Scholar]
- 27. Qiu XH, et al. 2006. Isolation and characterization of a bacterial strain of the genus Ochrobactrum with methyl parathion mineralizing activity. J. Appl. Microbiol. 101: 986–994 [DOI] [PubMed] [Google Scholar]
- 28. Regan JM, Harrington GW, Baribeau H, De Leon R, Noguera DR. 2003. Diversity of nitrifying bacteria in full-scale chloraminated distribution systems. Water Res. 37: 197–205 [DOI] [PubMed] [Google Scholar]
- 29. Regan JM, Harrington GW, Noguera DR. 2002. Ammonia- and nitrite-oxidizing bacterial communities in a pilot-scale chloraminated drinking water distribution system. Appl. Environ. Microbiol. 68: 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rittmann BE, Snoeyink VL. 1984. Achieving biologically stable drinking water. J. Am. Water Works Assoc. 76: 106–114 [Google Scholar]
- 31. Rotthauwe JH, Witzel KP, Liesack W. 1997. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63: 4704–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmeisser C, et al. 2003. Metagenome survey of biofilms in drinking-water networks. Appl. Environ. Microbiol. 69: 7298–7309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R. 1999. Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl. Environ. Microbiol. 65: 3690–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skadsen J. 1993. Nitrification in a distribution system. J. Am. Water Works Assoc. 85: 95 [Google Scholar]
- 35. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- 36. van der Wielen PW, Voost S, van der Kooij D. 2009. Ammonia-oxidizing bacteria and archaea in groundwater treatment and drinking water distribution systems. Appl. Environ. Microbiol. 75: 4687–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White DC, Sutton SD, Ringelberg DB. 1996. The genus Sphingomonas: physiology and ecology. Curr. Opin. Biotechnol. 7: 301–306 [DOI] [PubMed] [Google Scholar]
- 38. Witzig R, et al. 2002. Microbiological aspects of a bioreactor with submerged membranes for aerobic treatment of municipal wastewater. Water Res. 36: 394–402 [DOI] [PubMed] [Google Scholar]
- 39. Yang CF, Lee CM. 2008. Pentachlorophenol contaminated groundwater bioremediation using immobilized Sphingomonas cells inoculation in the bioreactor system. J. Hazard. Mater. 152: 159–165 [DOI] [PubMed] [Google Scholar]
- 40. Zhang LM, Hu HW, Shen JP, He JZ. 2012. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 6: 1032–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Griffin A, Edwards M. 2008. Nitrification in premise plumbing: role of phosphate, pH and pipe corrosion. Environ. Sci. Technol. 42: 4280–4284 [DOI] [PubMed] [Google Scholar]


