Abstract
The composition of the upper respiratory tract microbial community may influence the risk for colonization by the acute otitis media (AOM) pathogens Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. We used culture-independent methods to describe upper respiratory tract microbial communities in healthy children and children with upper respiratory tract infection with and without concurrent AOM. Nasal swabs and data were collected in a cross-sectional study of 240 children between 6 months and 3 years of age. Swabs were cultured for S. pneumoniae, and real-time PCR was used to identify S. pneumoniae, H. influenzae, and M. catarrhalis. The V1-V2 16S rRNA gene regions were sequenced using 454 pyrosequencing. Microbial communities were described using a taxon-based approach. Colonization by S. pneumoniae, H. influenzae, and M. catarrhalis was associated with lower levels of diversity in upper respiratory tract flora. We identified commensal taxa that were negatively associated with colonization by each AOM bacterial pathogen and with AOM. The balance of these relationships differed according to the colonizing AOM pathogen and history of antibiotic use. Children with antibiotic use in the past 6 months and a greater abundance of taxa, including Lactococcus and Propionibacterium, were less likely to have AOM than healthy children (odds ratio [OR], 0.46; 95% confidence interval [CI], 0.25 to 0.85). Children with no antibiotic use in the past 6 months, a low abundance of Streptococcus and Haemophilus, and a high abundance of Corynebacterium and Dolosigranulum were less likely to have AOM (OR, 0.51; 95% CI, 0.31 to 0.83). An increased understanding of polymicrobial interactions will facilitate the development of effective AOM prevention strategies.
INTRODUCTION
Acute otitis media (AOM) is a common pediatric infection and is the leading diagnosis for the prescription of antibiotics in U.S. children (20, 60, 65). In the United States, AOM accounts for close to $1 billion in direct medical expenditures each year (65). Disease etiology and pathogenesis are complex and begin with colonization of mucosal surfaces in the upper respiratory tract by AOM bacterial pathogens (5, 55). Streptococcus pneumoniae is responsible for up to half of all AOM cases (9, 10, 19). Haemophilus influenzae and Moraxella catarrhalis are also frequent causes of AOM in children (10). The majority of AOM episodes occur concurrently with or soon after viral upper respiratory tract infection (URI) (11).
In order for bacterial AOM pathogens to colonize the upper respiratory tract, they must successfully compete with each other and with commensal members of the upper respiratory tract flora. Epidemiologic studies have shown that the risk of S. pneumoniae colonization differs according to whether H. influenzae, M. catarrhalis, and Staphylococcus aureus are also present (7, 29, 46). Bacterium-bacterium interactions may also impact AOM incidence. Simultaneous colonization by multiple AOM pathogens is associated with a greater risk of AOM than that for colonization by one AOM pathogen (39, 50). Members of the normal flora, such as alpha-hemolytic streptococci, inhibit the growth of AOM pathogens in vitro (58). Healthy children are more likely than children with AOM to be colonized by alpha-hemolytic streptococci (18). Collectively, these data indicate that certain commensals influence the risk of AOM pathogen colonization and the subsequent risk of disease. Several hundred different bacterial taxa can potentially colonize the upper respiratory tract of a single individual (1, 38, 40); the majority of these bacterial taxa are not routinely cultured or studied. Thus, the precise role that specific commensal members of the normal upper respiratory tract flora play in modifying the risk for AOM pathogen colonization and AOM is largely unknown.
Overall levels of diversity of the upper respiratory tract microbial community may also influence the risk for AOM pathogen colonization and subsequent AOM. Diversity incorporates both richness (i.e., the number of different species) and evenness (i.e., relative population frequencies) (42). Diverse communities have been shown to be more stable and resistant to invasion by foreign species (8, 61). Reductions in microbial diversity have been associated with Pseudomonas aeruginosa colonization in cystic fibrosis patients (32) and the progression of such diseases as dental caries (21).
Antibiotics are prescribed at up to 80% of clinician visits for otitis media and are among the factors that might influence AOM pathogen colonization and AOM (20). Prior studies have demonstrated that the use of antibiotics shifts the relative prevalence of bacteria within microbial communities and leads to decreased microbial diversity in the oropharynx and gastrointestinal tract (16, 32). Murine models have revealed that antibiotics alter gastrointestinal microbial community composition, leading to increased susceptibility to infection with Salmonella (54) and altered susceptibility to both enteric (36) and respiratory tract viruses (28).
Mounting evidence suggests that microbial community composition has a profound impact on human health (15). Culture-independent approaches are increasingly being used to explore the relationship between microbial community composition, risk of pathogen colonization, and disease. However, these studies have often focused on gastrointestinal, vaginal, or oral microbial communities (33, 48, 56). Prior studies of upper respiratory tract microbial communities have examined infants or a limited number of individuals or have not compared individuals in both health and disease (6, 26, 38, 40). We used 16S rRNA gene pyrosequencing and a taxon-based analytic approach to characterize the nasal microbial communities in nasal swabs collected from 240 healthy children and children experiencing URI with and without concurrent AOM. We hypothesized the following: (i) that specific commensal species play an important role in protecting the upper respiratory tract from colonization by AOM pathogens and reducing the risk of AOM; (ii) that the diversity of the upper respiratory tract flora differs based on colonization by AOM pathogens and health status; and (iii) that antibiotic use alters the diversity of the upper respiratory tract flora, the prevalence of colonizing commensal species, and the prevalence of AOM pathogens.
MATERIALS AND METHODS
Study design and participants.
Demographic data, clinical data, and anterior nasal swabs considered for the current analysis were restricted to those collected from 251 children ages 3 years and under (when the incidence of AOM is highest [31]) who were part of a larger, cross-sectional study of S. pneumoniae colonization in 601 Philadelphia, PA, children, ages 6 months to 6 years. The children were seen for a well child check or for URI symptoms (e.g., cough, head/throat pain, or fever) at one of two primary care clinics (one urban and one suburban in order to increase the racial diversity of the study sample) within the Pediatric Research Consortium of Children's Hospital of Philadelphia during the winter respiratory virus season (26 January to 29 April 2010). Informed consent for participation was obtained during the clinic visit. Demographic and clinical data (including a history of antibiotic prescriptions and pneumococcal conjugate vaccination) were extracted from the electronic medical records at participating sites. The institutional review board (IRB) of the University of Pennsylvania and Children's Hospital of Philadelphia approved the study protocol.
Health outcomes.
Children were classified into one of three health status groups (well, URI alone, and URI with concurrent AOM) using ICD-9 (International Classification of Diseases, 9th revision) codes. Children being seen for a well-child check (V20.2) without any URI or AOM diagnoses or symptoms were classified as well. ICD-9 codes for defining URI included a variety of upper respiratory tract infections (460 to 466 or 381 to 382), as well as URI symptoms, including fever, headache, sore throat, or cough (780.6, 784.0, 784.1, or 786.2, respectively). Children not falling within the well or URI criteria were removed from the analysis (n = 3). In addition, children with documented asthma or asthma symptoms alone (i.e., wheezing) without either a code for a well-child visit or a qualifying URI or AOM ICD-9 code were dropped from the study (n = 8). The children meeting the criteria for URI were further subdivided into those with and without concurrent AOM. ICD-9 codes 381.00 to 381.06 and 382.00 to 382.02 were classified as URI with concurrent AOM. ICD-9 codes 381, 381.4, 382, 382.4, and 382.9 were also classified as URI with concurrent AOM, provided the word “acute” was specified in the descriptor. Data regarding the prescription of antibiotics within the 6 months prior to sample collection were obtained through medical record review and used as a marker of antibiotic use. The 6-month time frame was selected due to the potentially long-term impact antibiotics may have on the normal flora (30). Data from 240 children are included in the current analysis.
Bacterial strains and growth conditions.
Anterior nasal swabs were collected and processed for S. pneumoniae culture and DNA extraction as previously described (38). Strains used for the real-time (RT) PCR assay standard curve described below were S. pneumoniae clinical isolate FG23 (serotype 19A; sequence type 199), H. influenzae strain ATCC 49766, and M. catarrhalis strain ATCC 49143. Growth conditions for S. pneumoniae were 37°C, 5% CO2, on Trypticase soy agar containing 5% sheep blood (BD-Diagnostic Systems, Franklin Lakes, NJ). Growth conditions for H. influenzae and M. catarrhalis were 37°C, 5% CO2, on chocolate agar (BD-Diagnostic Systems).
Real-time PCR assay and otitis media pathogens.
An RT-PCR assay developed and validated by Kais and colleagues was used to identify the presence of the three major AOM pathogens (30a). RT-PCR was performed in 20-μl reaction mixtures containing 2x SYBR green master mix (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA), 0.6 μM primer, and 1 μl of DNA. Cycling conditions for S. pneumoniae quantitative PCR (qPCR) were as follows: 95°C for 15 min, followed by 40 cycles of 94°C for 20 s, 60°C for 20 s, and 72°C for 20 s, with a final extension step at 65°C for 30 s. Cycling conditions for H. influenzae and M. catarrhalis differed only during the annealing step, which was at 50°C. Dissociation curves were completed to check for the absence of primer dimers. PCR was carried out in duplicate, and negative controls were included in each set of reactions. Standard curves were run in parallel to the clinical samples. A cutoff of 500 CFU/swab was used as the limit of detection to determine whether each AOM pathogen was present or absent.
Roche/454 Life Sciences sample preparation.
PCR amplification of hypervariable regions V1 and V2 of the 16S rRNA gene was done using bar-coded 16S rRNA primers targeting 27F and 338R as previously described (38). Samples were pooled in equimolar amounts and submitted to the Environmental Genomics Core Facility (Engencore) at the University of South Carolina for pyrosequencing on the GS FLX Titanium amplicon fusion Roche/454 Life Sciences platform.
Pyrosequencing analysis.
Initial cleaning, binning, and processing of sequence reads were done using the Btrim software program (34). Sequences were scanned for linkers and primers while allowing for two errors, and primer sequences were removed from each sequence read. Reads missing the 5′-end primer were removed from the data set. Bar codes were identified within the first 15 bp of each read, and one error was allowed. Sequence reads were then binned into separate FASTA files, and the bar code sequences were trimmed. Sequences were aligned and analyzed using pipelines and tools available from the RDP database project (13) as previously described (38).
To minimize the number of chimeras, we set strict minimum alignment criteria: all sequences had to align to at least 200 bp of the 16S rRNA gene, and any sequence aligning outside the 27 and/or 338 position of the 16S rRNA gene was discarded. Other methods for chimera detection, such as Bellerophon, ChimeraSlayer, and Pintail, were not used because they are optimized for sequences 400 to 500 bp or longer (22). Taxonomic identification of samples was achieved using the RDP Bayesian classifier tool at 90% confidence (13, 62). Operational taxonomic units (OTUs) were defined in an iterative process by grouping together all sequences of the same genus. When genus-level classification was not possible, sequences were classified and grouped at the next lowest taxonomic level.
Data analysis.
All statistical analyses were done using the software program SAS 9.2 unless otherwise specified. Unadjusted associations between health status and demographic, microbiological, and clinical variables were evaluated by chi-square test and Student's t test. Factors affecting microbial community diversity indices (e.g., health status and antibiotic use) were evaluated with analysis of variance (ANOVA). Differences in the abundances of individual taxa by health outcomes were examined using a modified t test that combines the nonparametric t test, Fisher's exact test, and a false discovery rate (set at 0.05) to produce a q statistic (Metastats statistical software package [63]). The associations between health status and demographic, microbiological, and clinical variables were examined using logistic regression.
Principal component analysis (PCA) was used to group microbial community members into factors representing linear relationships among selected taxa. OTUs more frequent than 0.3% of the microbial community were included as component taxa (n = 26) in the PCA. An eigenvalue of 1 and an orthogonal rotation were specified. PCA factor components had significant loadings of at least ±0.4. Associations between PCA factor scores, PCA component taxa, and health status were examined using Student's t test, correlation, and logistic regression. Rare taxa were not included in PCA analyses.
RESULTS
Study population.
Demographic and clinical characteristics of the study population are shown in Table 1. Seventy percent of the children were experiencing URI symptoms at the time of sample collection. Of the 167 children with URI, 72 (43%) were diagnosed with AOM. Health status did not differ significantly by age, gender, race, or the presence of other children in the home. Compared to healthy children, a higher proportion of children attending daycare experienced URI both with and without concurrent AOM. Fewer children being seen for a healthy visit used antibiotics within the past 6 months (n = 18 [7.5%]) than those being seen for URI alone (n = 43 [17.9%]) or URI with concurrent AOM (n = 39 [16.3%]) (Table 1).
Table 1.
Characteristics of study population and association with health statusa
| Characteristic | No. (%) of subjects | No. (%) with health status |
P valueb | ||
|---|---|---|---|---|---|
| Healthy | URI patient |
||||
| AOM negative | AOM positive | ||||
| Total | 240 | 73 (30.4) | 95 (39.6) | 72 (30.0) | |
| Age at swab (mo) | 0.15 | ||||
| 6–<12 | 55 (22.9) | 13 (23.6) | 22 (40.0) | 20 (36.4) | |
| 12–<24 | 114 (47.5) | 37 (32.5) | 39 (34.2) | 38 (33.3) | |
| 24–36 | 71 (29.6) | 23 (32.4) | 34 (47.9) | 14 (19.7) | |
| Gender | 0.41 | ||||
| Male | 122 (50.8) | 38 (31.2) | 52 (42.6) | 32 (26.2) | |
| Female | 118 (49.2) | 35 (29.7) | 43 (36.4) | 40 (33.9) | |
| Race | 0.25 | ||||
| Caucasian | 101 (42.1) | 26 (25.7) | 38 (37.6) | 37 (36.6) | |
| African-American | 126 (52.5) | 44 (34.9) | 52 (41.3) | 30 (23.8) | |
| Otherc | 13 (5.4) | 3 (23.1) | 5 (38.5) | 5 (38.5) | |
| Day care | 0.0005 | ||||
| Yes | 97 (40.4) | 16 (16.5) | 45 (46.4) | 36 (37.1) | |
| No | 143 (59.6) | 57 (39.9) | 50 (35.0) | 36 (25.2) | |
| Other children in home | 0.39 | ||||
| Yes | 153 (63.8) | 42 (27.4) | 62 (40.5) | 49 (32.0) | |
| No | 87 (36.2) | 31 (35.6) | 33 (37.9) | 23 (26.4) | |
| Days since last antibiotic use | |||||
| None within past 6 mo | 140 (58.3) | 55 (39.3) | 52 (37.1) | 33 (23.6) | 0.007 |
| Current use to 7 days | 24 (10.0) | 5 (20.8) | 6 (25.0) | 13 (54.2) | |
| 8 to 48 | 27 (11.2) | 4 (14.8) | 14 (51.8) | 9 (33.3) | |
| 49–84 | 26 (10.8) | 3 (11.5) | 12 (46.2) | 11 (42.3) | |
| 85–183 | 23 (9.6) | 6 (26.1) | 11 (47.8) | 6 (26.1) | |
n = 240.
P values from χ2 tests of distribution over health status. Boldface indicates a significant result.
“Other” includes biracial (n = 7), Asian (n = 4), and answer missing/refused (n = 2).
Children in this study were recruited from one urban (n = 121) and one suburban (n = 119) site. The distribution of children by race and previous antibiotic use significantly differed by site (P < 0.0001 for both). At the urban site, 118 (97.5%) of the children were African-American. In contrast, 8 (6.7%) of the children at the suburban site were African-American. Antibiotic use was higher at the suburban site; 59% of children at the suburban site were prescribed antibiotics within the 6 months prior to swab collection, compared to 25% at the urban site (P < 0.001). The majority of antibiotic prescriptions were for β-lactam antibiotics. Out of 101 prescriptions, 56 (55.4%) were for amoxicillin, 22 (21.8%) were for amoxicillin and clavulanate, and 16 (15.8%) were for cephalosporins (e.g., cefdinir).
Detection of otitis media pathogens.
Pneumococcal conjugate vaccine-specific data were available for 235 of 240 children. Of these 235 children, all received at least two doses and most, 232 (99%), had received the appropriate number of doses for their age. S. pneumoniae was isolated by culture in 103 (43%) of the samples from the entire study population and in 31(43%) of the samples from the subgroup of children with AOM.
The presence of AOM pathogens, determined by RT-PCR, is given in Table 2. S. pneumoniae culture and RT-PCR results were in significant agreement (kappa statistic, 0.76; P < 0.0001). The proportion of children colonized with S. pneumoniae, H. influenzae, or M. catarrhalis was 39%, 35%, or 43%, respectively. Children in the study who received antibiotics within the past 6 months were less likely to be colonized by S. pneumoniae (Table 2). In contrast, the presence of H. influenzae and M. catarrhalis did not differ significantly by history of antibiotic use. The distribution of S. pneumoniae colonization determined by RT-PCR did not differ significantly by health status (P = 0.58, χ2 test of health status and presence or absence of S. pneumoniae). The proportions colonized with H. influenzae and M. catarrhalis were significantly higher among children with AOM than among healthy children (46% versus 22% [P = 0.002] and 53% versus 30% [P = 0.006], respectively).
Table 2.
Presence of bacterial AOM pathogens detected by RT-PCRa
| Bacterium and result | No. (%) of subjects with result |
P valueb | ||
|---|---|---|---|---|
| All subjects | Antibiotic use in past 6 mo |
|||
| Yes | No | |||
| S. pneumoniae | 0.05 | |||
| Positive | 94 (39.2) | 32 (34.0) | 62 (66.0) | |
| Negative | 146 (60.8) | 68 (46.6) | 78 (53.4) | |
| H. influenzae | 0.58 | |||
| Positive | 84 (35.0) | 37 (44.0) | 47 (56.0) | |
| Negative | 156 (65.0) | 63 (40.4) | 93 (59.6) | |
| M. catarrhalis | 0.72 | |||
| Positive | 104 (43.3) | 42 (40.4) | 62 (59.6) | |
| Negative | 136 (56.7) | 58 (42.6) | 78 (57.4) | |
The distribution of AOM pathogens stratified by antibiotic use in the past 6 months is given.
P values from χ2 tests comparing children with and without antibiotic use in the past 6 months. Boldface indicates a significant result.
Diversity in upper respiratory tract microbial communities.
After processing, a total of 477,327 sequences with an average length of 324 bp were obtained. A mean (standard deviation [SD]) of 1,989 (615) sequences were obtained per nasal swab. Using the RDP pyrosequencing pipeline and a maximum cluster distance cutoff of 3% (97% identity), the mean (SD) Shannon diversity and evenness indices were 3.0 (0.85) and 0.58 (0.12), respectively.
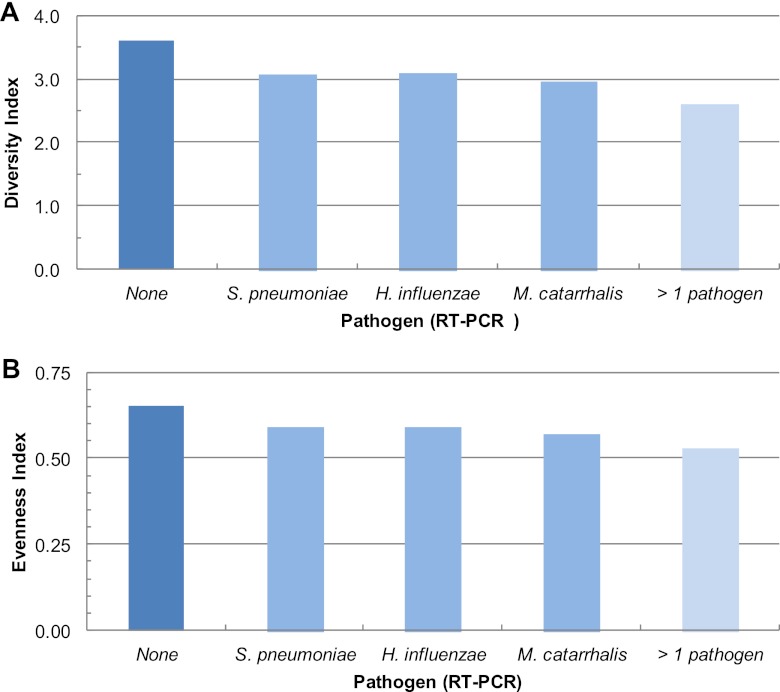
Shannon diversity and evenness indices did not differ significantly by age, gender, race, use of day care, or the presence of other children in the home (data not shown). Shannon diversity and evenness indices differed with the presence of AOM pathogens; diversity indices were lowest when more than one AOM pathogen was present and highest when all three AOM pathogens were absent (Fig. 1). There was a significant interaction between health status and antibiotic use in the past 6 months (ANOVA, P = 0.001 and 0.002 for Shannon and evenness, respectively). Diversity indices were significantly higher for healthy children than for those experiencing URI alone or with concurrent AOM (ANOVA, P = 0.006 for the Shannon diversity index and P = 0.02 for evenness) but only in children who used antibiotics in the past 6 months. The interactions between health status and antibiotic were also significant for children who used antibiotics within the past 7, 14, and 21 days (data not shown). Due to the interactions between health status and antibiotic use, as well as the potential long-term impact of antibiotics on the microbial community, we stratified all subsequent analyses by antibiotic use in the past 6 months.
Fig 1.
Distribution of Shannon diversity and evenness indices for samples as a function of the presence of three acute otitis media pathogens identified by RT-PCR. Diversity indices for samples with no AOM pathogens (dark blue bar) were significantly higher than those for samples with 1 pathogen present (lighter blue), which were significantly higher than those for samples with 2 or 3 AOM pathogens (lightest blue) (Duncan multiple range test, P < 0.05).
Abundance of OTUs.
In total, 541 operational taxonomic units (OTUs) were identified among the 240 children. OTU proportions occurring at ł0.3% in the overall bacterial population (n = 26 OTUs) are listed in Table 3 in order of decreasing frequency. The most prevalent taxa were the AOM-associated genera Streptococcus, Haemophilus, and Moraxella and the commensals Corynebacterium and Dolosigranulum. Mean levels of Dolosigranulum were significantly lower in children who had received antibiotics in the past 6 months (Metastats q value = 0.02) (Table 3). In contrast, mean levels of Rothia and Actinomyces were higher in children who had received antibiotics (q-value = 0.02 and 0.03, respectively).
Table 3.
Frequencies of operational taxonomic units in all samples and grouped by antibiotic use in the past 6 months
| Classification | Mean frequency (SD)a |
qvalueb | ||
|---|---|---|---|---|
| All samples | Antibiotic use within past 6 mo |
|||
| Yes | No | |||
| Genus | ||||
| Moraxella | 22.04 (20.57) | 21.18 (21.67) | 22.65 (19.81) | 0.68 |
| Streptococcus | 16.47 (17.61) | 16.21 (16.82) | 16.66 (18.21) | 0.85 |
| Corynebacterium | 14.59 (15.25) | 13.32 (15.61) | 15.5 (14.97) | 0.38 |
| Dolosigranulum | 11.9 (14.15) | 7.47 (10.01) | 15.06 (15.78) | 0.02 |
| Haemophilus | 5.11 (10.54) | 6.65 (12.57) | 4.02 (8.70) | 0.12 |
| Staphylococcus | 4.11 (8.25) | 4.10 (7.98) | 4.12 (8.46) | 0.89 |
| Lactococcus | 2.25 (3.72) | 2.91 (4.40) | 1.78 (3.07) | 0.12 |
| Anoxybacillus | 2.07 (3.69) | 2.68 (4.31) | 1.63 (3.11) | 0.12 |
| Enhydrobacter | 0.80 (2.35) | 0.82 (2.27) | 0.79 (2.42) | 0.85 |
| Rothia | 0.70 (1.64) | 1.07 (2.33) | 0.44 (0.78) | 0.02 |
| Neisseria | 0.67 (1.59) | 0.94 (1.90) | 0.48 (1.30) | 0.12 |
| Gemella | 0.44 (0.82) | 0.55 (1.01) | 0.36 (0.64) | 0.15 |
| Actinomyces | 0.44 (0.94) | 0.65 (1.27) | 0.28 (0.57) | 0.03 |
| Veillonella | 0.41 (0.81) | 0.56 (1.05) | 0.30 (0.56) | 0.08 |
| Granulicatella | 0.40 (0.70) | 0.52 (0.89) | 0.32 (0.52) | 0.12 |
| Propionibacterium | 0.34 (0.67) | 0.33 (0.40) | 0.35 (0.81) | 0.85 |
| Streptophyta | 0.34 (1.93) | 0.58 (2.92) | 0.16 (0.52) | 0.25 |
| Family | ||||
| Pasteurellaceae | 2.8 (8.39) | 4.08 (11.01) | 1.88 (5.73) | 0.12 |
| Moraxellaceae | 1.68 (4.80) | 1.79 (5.14) | 1.60 (4.56) | 0.85 |
| Enterobacteriaceae | 0.82 (1.39) | 1.01 (1.73) | 0.68 (1.06) | 0.15 |
| Order | ||||
| Actinomycetales | 0.37 (0.27) | 0.40 (0.27) | 0.36 (0.27) | 0.32 |
| Corynebacterineae | 0.31 (0.29) | 0.27 (0.26) | 0.33 (0.30) | 0.12 |
| Class | ||||
| Gammaproteobacteria | 0.98 (2.22) | 0.84 (1.66) | 1.08 (2.54) | 0.50 |
| Betaproteobacteria | 0.78 (2.95) | 0.40 (1.81) | 1.04 (3.53) | 0.12 |
| Phylum | ||||
| Proteobacteria | 0.51 (0.85) | 0.42 (0.63) | 0.57 (0.97) | 0.19 |
| Bacteroidetes | 0.46 (0.72) | 0.46 (0.49) | 0.47 (0.85) | 0.85 |
| Rare taxa combined | 8.21 (8.00) | 9.80 (9.08) | 7.09 (6.94) | 0.06 |
For all samples, n = 240. By antibiotic use within past 6 months: yes, n = 100; no, n = 140.
qvalue from Metastats test of difference in frequencies between antibiotic use groups. Boldface indicates a significant result.
Associations between individual commensal taxa and AOM pathogens.
Because colonization is a critical step in the pathogenesis of AOM, we next examined whether the presence of specific commensals differed by the presence of each bacterial AOM pathogen. Taxa that differed significantly within each group are depicted in Fig. 2. Bars pointing to the left indicate commensals that were negatively associated with colonization by each AOM pathogen, and bars pointing to the right indicate a positive association. As expected, the levels of Streptococcus, Haemophilus, and Moraxella were higher among children colonized by S. pneumoniae, H. influenzae, and M. catarrhalis, respectively. The levels of Streptococcus were also higher in children colonized by M. catarrhalis who had not received antibiotics in the past 6 months. These data indicate a higher propensity for cocolonization by Streptococcus species and M. catarrhalis. Among children given antibiotics in the past 6 months, 2, 6, and 5 taxa identified at the genus level were negatively associated with colonization by S. pneumoniae, H. influenzae, and M. catarrhalis, respectively (Fig. 2). Among children without antibiotics in the past 6 months, 9, 6, and 6 genus-level taxa were negatively associated with colonization by S. pneumoniae, H. influenzae, and M. catarrhalis, respectively (Fig. 2). The abundance of Lactococcus species was negatively associated with the presence of each AOM pathogen regardless of prior antibiotic use.
Fig 2.
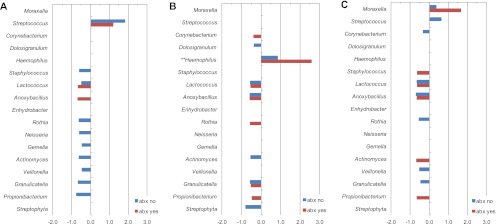
Relative abundances of genus-level taxa by the presence or absence of individual AOM pathogens stratified by antibiotic use. Relative abundance was calculated as the ratio of positive to negative taxon sample frequencies and 1 (i.e., positive/negative − 1) for S. pneumoniae (A), H. influenzae (B), or M. catarrhalis (C). Only ratios significantly different from 1 are shown (q < 0.05). Data are stratified by reported use of antibiotics (abx) within 6 months of sample collection. ∗∗, data for Haemophilus (B) were multiplied by 0.1 to keep all taxa on the same scale (the actual value is 8.7).
Relationships among correlated taxa by AOM pathogen and health status.
Members of the commensal flora may coaggregate or may have a higher propensity for cocolonization based on similar requirements for nutrients or their utilization of waste products and secondary metabolites (51, 64). Therefore, we used PCA to identify groups of correlated commensal taxa (factors). Separate PCAs for each antibiotic use group (with or without antibiotic use in the past 6 months) each produced four independent factors. Factor loadings from the PCAs for each antibiotic use group are shown in Table 4. Positive loadings indicate high levels of a given taxon, and negative loadings indicate low levels of a given taxon. Factor component loadings were similar for children with and without antibiotic use in the previous 6 months, with the following exceptions: (i) Streptococcus sp. positively loaded on factor 1 for children who had used antibiotics in the past 6 months and negatively loaded on factor 4 among children with no antibiotic use in the past 6 months, (ii) members of the phylum Proteobacteria loaded significantly on factor 3 and Dolosigranulum species loaded on factor 4 in children without antibiotic use in the past 6 months; Proteobacteria and Dolosigranulum did not significantly load on any factors in children with antibiotic use in the past 6 months.
Table 4.
Factor loadings for component taxa for factors from principal component analysesa
| PCA factor component | Factor loading by antibiotic use in past 6 months |
|
|---|---|---|
| Yes | No | |
| Factor 1 | ||
| Streptococcus | 51 | [See factor 4] |
| Rothia | 71 | 87 |
| Neisseria | 61 | |
| Gemella | 76 | 65 |
| Actinomyces | 65 | 87 |
| Veillonella | 84 | 88 |
| Granulicatella | 85 | 89 |
| Bacteroidetesb | 45 | |
| Factor 2 | ||
| Lactococcus | 97 | 96 |
| Anoxybacillus | 97 | 94 |
| Propionibacterium | 48 | |
| Enterobacteriaceaec | 89 | 83 |
| Factor 3 | ||
| Enhydrobacter | 93 | 88 |
| Moraxellaceaec | 92 | 85 |
| Gammaproteobacteriad | 91 | 87 |
| Proteobacteriab | 52 | |
| Factor 4 | ||
| Streptococcus | [See factor 1] | −49 |
| Corynebacterium | 79 | 74 |
| Dolosigranulum | 47 | |
| Haemophilus | −53 | −55 |
| Pasteurellaceaec | −56 | −57 |
| Corynebacterineaee | 83 | 77 |
A separate PCA was performed for each antibiotic group. Factor loadings are given for all components. Note that some taxa appear as a component for one group's factor but not for the other, e.g., Neisseria is a component of factor 1 for the antibiotic “yes” group but not for the “no” group.
Phylum.
Family.
Class.
Suborder.
Factor scores for component taxa were used as variables in separate logistic regression analyses to predict the presence of AOM pathogens (Table 5). Factor 1 taxa were negatively associated with the presence of S. pneumoniae among children without antibiotic use in the past 6 months (Table 5). Children with high levels of Rothia, Gemella, Actinomyces, Veillonella, and Granulicatella were 51% less likely to be colonized with S. pneumoniae. Factor 2 and 4 taxa were negatively associated with colonization by each AOM pathogen (S. pneumoniae, H. influenzae, and M. catarrhalis) regardless of antibiotic use (Table 5). There were no significant differences in mean factor scores when children with URI with and without concurrent AOM were compared (data not shown). Logistic regression models were used to predict the odds of an AOM diagnosis using healthy children as the reference group (Table 6). Factor 2 taxa were negatively associated with AOM among children with antibiotic use in the past 6 months. Thus, children with high levels of Lactococcus, Anoxybacillus, members of the Enterobacteriaceae, and Propionibacterium were 54% less likely to have AOM than healthy children (Table 6). In contrast, factor 4 taxa were negatively associated with AOM among children without antibiotic use in the past 6 months (Table 5); those with low levels of Streptococcus, Haemophilus, and members of the Pasteurellaceae and high levels of Corynebacterium and Dolosigranulum were 49% less likely to have AOM.
Table 5.
Association between principal component analysis factor scores and colonization by AOM pathogensa
| AOM pathogen and factor | OR (95% CI) by antibiotic use in past 6 mo |
|
|---|---|---|
| Yes | No | |
| S. pneumoniae | ||
| Factor 1 | 1.03 (0.84, 2.02) | 0.49 (0.27, 0.89) |
| Factor 2 | 0.46 (0.23, 0.91) | 0.63 (0.40, 0.98) |
| Factor 3 | 1.33 (0.83, 2.15) | 1.45 (0.92, 2.27) |
| Factor 4 | 0.58 (0.36, 0.93) | 0.66 (0.45, 0.95) |
| H. influenzae | ||
| Factor 1 | 0.72 (0.44, 1.19) | 0.56 (0.30, 1.05) |
| Factor 2 | 0.43 (0.22, 0.86) | 0.49 (0.26, 0.93) |
| Factor 3 | 0.42 (0.16, 1.13) | 0.70 (0.41, 1.20) |
| Factor 4 | 0.25 (0.13, 0.50) | 0.40 (0.26, 0.93) |
| M. catarrhalis | ||
| Factor 1 | 0.70 (0.44, 1.11) | 0.66 (0.42, 1.03) |
| Factor 2 | 0.42 (0.22, 0.79) | 0.44 (0.24, 0.78) |
| Factor 3 | 1.90 (0.94, 3.86) | 1.23 (0.84, 1.80) |
| Factor 4 | 0.57 (0.36, 0.89) | 0.68 (0.47, 0.97) |
Odds ratios and 95% confidence intervals from logistic regression analyses. Separate analyses were performed for each AOM pathogen and antibiotic use group. Boldface indicates a significant result.
Table 6.
Association between principal component analysis factor scores and AOM
| PCA factor and group | OR (95% CI) by antibiotic use in past 6 monthsa |
|
|---|---|---|
| Yesb | Noc | |
| Factor 1 | ||
| Healthy (refd) | 1.0 | 1.0 |
| AOM | 0.69 (0.39, 1.22) | 1.12 (0.73, 1.72) |
| Factor 2 | ||
| Healthy (ref) | 1.0 | 1.0 |
| AOM | 0.46 (0.25, 0.85) | 1.18 (0.70, 1.99) |
| Factor 3 | ||
| Healthy (ref) | 1.0 | 1.0 |
| AOM | 1.97 (0.65, 6.01) | 0.66 (0.36, 1.21) |
| Factor 4 | ||
| Healthy (ref) | 1.0 | 1.0 |
| AOM | 0.63 (0.32, 1.22) | 0.51 (0.31, 0.83) |
Boldface indicates a significant result. Children with URI alone (without AOM) were excluded from the analysis (n = 43 antibiotic users; n = 52 antibiotic nonusers).
Antibiotic use in past 6 months: healthy group, n = 18; AOM group, n = 39.
No antibiotic use in past 6 months: healthy group, n = 55; AOM group, n = 33.
ref, reference.
DISCUSSION
Specific commensal species may play a role in protecting the upper respiratory tract from colonization by AOM pathogens and AOM. Our data indicate that colonization by AOM pathogens is associated with lower levels of diversity in the upper respiratory tract flora. We identified commensal taxa that were negatively associated with colonization by each AOM bacterial pathogen and with AOM. These negative relationships are suggestive of a competitive relationship between members of the commensal flora, which may in turn alter the risk of AOM. Moreover, the balance of these relationships differed according to the particular AOM pathogen and by antibiotic use. Unlike our earlier studies, where samples from healthy children were not included and the focus was on S. pneumoniae (38), the current study includes samples from healthy children and an examination of all three major bacterial AOM pathogens.
Diversity of the nasal flora.
Ecological studies suggest that diverse bacterial communities should be more resistant to disruption (8, 61). The pathogenesis of AOM is thought to involve overgrowth of AOM pathogens during URI, followed by invasion of the middle ear space. This model of disease is consistent with our observation that AOM pathogen colonization was associated with lower levels of diversity in upper respiratory tract flora. We also observed high levels of diversity in healthy children and lower levels of diversity during URI and AOM. However, these trends were significant only in children with prior antibiotic use. Because our data are cross-sectional, we could not determine whether diverse upper respiratory tract communities are more resistant to colonization by AOM pathogens or whether colonization by AOM pathogens causes a loss of diversity.
Associations between individual commensal taxa and AOM pathogens.
The prevalence of Lactococcus species was significantly lower in children colonized with S. pneumoniae, H. influenzae, or M. catarrhalis. Laufer et al. has previously shown that Lactococcus species were protective for S. pneumoniae colonization in children who were experiencing URI (38). Lactococcus species may play a role in protecting the upper respiratory tract microbial community from colonization by potentially pathogenic species. Lactic acid bacteria have been shown to produce a range of antimicrobial substances, such as organic acids, hydrogen peroxide, fatty acids, and bacteriocins (3, 4, 45). Alternatively, the negative association of Lactococcus and each of the three AOM pathogens may be indicative of a more general mode of competition, such as modulation of host immune responses. A control S. pneumoniae mucosal vaccine vector, comprised of Lactococcus lactis lacking vaccine antigen, stimulated nonspecific host immunity and provided some protection against S. pneumoniae respiratory tract infection in mice (25).
Additional commensal members of the flora were negatively associated with colonization by AOM pathogens. But, the particular balance of these associations differed by individual AOM pathogen examined and by antibiotic use. For example, Staphylococcus and Neisseria species were less prevalent in children colonized by S. pneumoniae. This was the case only in children with no antibiotic use in the prior 6 months. In contrast, Staphylococcus and Neisseria species were not significantly associated with colonization by H. influenzae. Nasal probiotic sprays have been proposed as a means to alter the upper respiratory tract flora to prevent AOM. A streptococcal spray has been shown to reduce the recurrence of AOM (52); however, a separate intervention did not result in decreased colonization with AOM pathogens or protection against AOM (57). Our data suggest that effective probiotic sprays for AOM prevention would have to contain multiple species or be targeted toward specific pathogens.
Relationships between commensal members of the flora and AOM pathogens also differed by antibiotic use. A prior longitudinal study of acute otitis media in children aged 6 months to 3 years linked antimicrobial use in the prior 7 days with a 2.6-fold-increased risk of AOM complicating URI (47). The higher risk of AOM associated with antibiotic use may simply be a marker of heavier antibiotic use in URI- and AOM-prone children. However, our observation of differences in microbial community structure based on antibiotic use raises the intriguing possibility that antimicrobial-induced disruptions of the normal flora alter susceptibility to colonization and infection by URI- and AOM-associated pathogens.
Relationships among correlated taxa by AOM pathogen colonization and health status. (i) Rothia, Neisseria, Gemella, Actinomyces, Veillonella, Granulicatella, and the Bacteroidetes.
Rothia, Neisseria, Gemella, Actinomyces, Veillonella, Granulicatella, and Bacteroidetes were identified as correlated taxa in the first factor identified in both antibiotic use groups. Our previous study indicated that Actinomyces, Rothia, Neisseria, and Veillonella were highly correlated and associated with an increased risk of otitis media among children who were all experiencing URI (38). Levels of these taxa were also higher among children with previous antibiotic use (38). In the current study, these taxa were not associated with an increased risk of AOM. However, mean levels of Rothia and Actinomyces were higher in children with antibiotic use in the past 6 months. Results from these two studies suggest that Actinomyces, Rothia, Neisseria, and Veillonella may preferentially cocolonize and their prevalence is altered by antibiotic use.
(ii) Lactococcus, Anoxybacillus, and the Enterobacteriaceae.
PCA factor 2 taxa included the genera Lactococcus, Anoxybacillus, and members of the family Enterobacteriaceae for both antibiotic use groups. Anoxybacillus bacteria are thermophilic spore-forming facultative anaerobes that may contaminate processed food products and are not generally thought to be human colonizers (49, 53). Anoxybacillus was previously detected in nasopharyngeal samples from children (6). These taxa may represent environmental contamination, or they may be members of the upper respiratory tract community. Propionibacterium were positively correlated with factor 2 taxa in children with antibiotic use in the previous 6 months. Factor 2 taxa were negatively associated with colonization by each of the three bacterial AOM pathogens and with AOM in children who used antibiotics in the past 6 months. These data are consistent with our prior study, which identified Lactococcus and Propionibacterium as highly correlated, associated with decreased colonization by S. pneumoniae, and associated with a decreased risk of otitis media (38).
(iii) Enhydrobacter, Moraxellaceae, Gammaproteobacteria, and Proteobacteria.
As a group, the prevalence of the factor 3 taxa Enhydrobacter, Moraxellaceae, Gammaproteobacteria, and Proteobacteria did not significantly differ by AOM pathogen colonization or presence of AOM.
(iv) Corynebacterium, Haemophilus, Pasteurellaceae, and the Corynebacterineae.
These taxa were components in the fourth factor identified in both antibiotic use groups. Dolosigranulum species were positively correlated with factor 4 taxa in children with antibiotic use in the previous 6 months. Streptococcus species were negatively correlated with factor 4 taxa in children without antibiotic use in the previous 6 months. The observed protective effect of factor 4 taxa for AOM may be due to the lower levels of Haemophilus and Streptococcus or high levels of Corynebacterium and Dolosigranulum. Several lines of evidence suggest that Corynebacterium and Dolosigranulum play a protective role in the upper respiratory tract. In our previous study, mean levels of Corynebacterium and Dolosigranulum were correlated and were protective against S. pneumoniae colonization (odds ratio [OR],0.55; 95% confidence interval [CI], 0.35 to 0.86) and otitis media (OR, 0.54; 95% CI, 0.30 to 0.96) (38). Konno et al. used culture-based approaches to examine the nasopharyngeal flora in patients with acute URI and healthy controls (35). The prevalence of Corynebacterium was lower in children less than 6 years of age with acute URI than with healthy children (26.2% versus 52.9%, P < 0.001) (35). It is also important to note that these taxa may occasionally be pathogenic (12, 27, 37).
We used a taxon-based, rather than phylogenetics-based, approach to examine microbial community composition in the nasal mucosa. Phylogenetics-based methods, such as Fast UniFrac (24), have also been used to explore differences in microbial community structure. In our hands these methods did not perform well in differentiating nasal microbial communities (data not shown). Phylogenetic methods have often been used to compare different tissue sites within the host (40) or differences between microbial community composition due to chronic health conditions (32, 41). The upper respiratory tract is home to a large number of closely related pathogenic and nonpathogenic species (17). Therefore, our inability to detect differences between microbial communities using phylogenetic methods may be due to functional phylogenetic redundancy among colonizing taxa in the nasal passages that is not significantly altered during URI or short-term colonization by AOM pathogens.
There are several limitations to our study. (i) These data were cross-sectional, and longitudinal studies are needed to establish the temporality of these associations. (ii) We did not collect data on specific respiratory viruses, which may alter the composition of the upper respiratory tract flora and differ in their pathogenic mechanisms and propensity to cause AOM (11, 23, 44, 47). (iii) We used data regarding antibiotic prescriptions as a marker of antibiotic use. Some parents may not have filled the prescription and/or children may not have taken the antibiotic. (iv) The majority of sequences could be classified only at the genus level. Increased numbers of alpha-hemolytic streptococci have been isolated from the nasopharynx of healthy children compared to those for OM-prone children (43, 59). We could not achieve species-level identification of alpha-hemolytic streptococci using short-read 16S rRNA pyrosequencing technologies.
An understanding of polymicrobial interactions is necessary for the development of effective AOM prevention strategies. These studies provide the groundwork for improving our comprehension of the complex nasal microbial communities of children. Longitudinal studies are needed to determine causality. Future laboratory studies should examine the underlying mechanisms involved. Corynebacterium, Dolosigranulum, Lactococcus, and Propionibacterium strains could also be studied to assess their impact on AOM pathogenesis using animal models of disease. Such studies may lead to the development of new prevention and treatment methods, including therapies aimed at disrupting interspecies quorum sensing or signaling (2).
ACKNOWLEDGMENTS
Funding for this research was provided by the National Institute on Deafness and Communication Disorders (R21DC011667 [to M.M.P.]) and the National Institute of Allergy and Infectious Diseases (R01 A1068043 [to M.M.P.] and R21 AI078189 and K24 073957 [to J.P.M.]).
We thank Linda Crossette for managing the cross-sectional study of S. pneumoniae colonization from which these data and samples were derived.
Footnotes
Published ahead of print 29 June 2012.
REFERENCES
- 1. Andersson AF, et al. 2008. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One 3: e2836 doi:10.1371/journal.pone.0002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armbruster CE, et al. 2010. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1(3): e00102–10 doi:10.1128/mbio.00102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beasley SS, Saris PE. 2004. Nisin-producing Lactococcus lactis strains isolated from human milk. Appl. Environ. Microbiol. 70: 5051–5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernbom N, et al. 2006. Effects of Lactococcus lactis on composition of intestinal microbiota: role of nisin. Appl. Environ. Microbiol. 72: 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4: 144–154 [DOI] [PubMed] [Google Scholar]
- 6. Bogaert D, et al. 2011. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One 6: e17035 doi:10.1371/journal.pone.0017035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bogaert D, et al. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363: 1871–1872 [DOI] [PubMed] [Google Scholar]
- 8. Burmolle M, et al. 2006. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl. Environ. Microbiol. 72: 3916–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butler JC, Breiman RF, Lipman HB, Hofmann J, Facklam RR. 1995. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978–1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171: 885–889 [DOI] [PubMed] [Google Scholar]
- 10. Casey JR, Adlowitz DG, Pichichero ME. 2010. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 29: 304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chonmaitree T, et al. 2008. Viral upper respiratory tract infection and otitis media complication in young children. Clin. Infect. Dis. 46: 815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cogen AL, Nizet V, Gallo RL. 2008. Skin microbiota: a source of disease or defence? Br. J. Dermatol. 158: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole JR, et al. 2009. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37: D141–D145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reference deleted. [Google Scholar]
- 15. Dethlefsen L, McFall-Ngai M, Relman DA. 2007. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449: 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. U. S. A. 108: 4554–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donati C, et al. 2010. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol. 11: R107 doi:10.1186/gb-2010-11-10-r107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faden H, Stanievich J, Brodsky L, Bernstein J, Ogra PL. 1990. Changes in the nasopharyngeal flora during otitis media of childhood. Pediatr. Infect. Dis. J. 9: 623–626 [PubMed] [Google Scholar]
- 19. Fenoll A, et al. 2000. Streptococcus pneumoniae in children in Spain: 1990–1999. Acta Paediatr. Suppl 89: 44–50 [DOI] [PubMed] [Google Scholar]
- 20. Grijalva CG, Nuorti JP, Griffin MR. 2009. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 302: 758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gross EL, et al. 2010. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J. Clin. Microbiol. 48: 4121–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haas BJ, et al. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21: 494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Håkansson A, Kidd A, Wadell G, Sabharwal H, Svanborg C. 1994. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect. Immun. 62: 2707–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamady M, Lozupone C, Knight R. 2010. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanniffy SB, Carter AT, Hitchin E, Wells JM. 2007. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J. Infect. Dis. 195: 185–193 [DOI] [PubMed] [Google Scholar]
- 26. Hilty M, et al. 2012. Nasopharyngeal microbiota in infants with acute otitis media. J. Infect. Dis. 205: 1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoedemaekers A, Schulin T, Tonk B, Melchers WJ, Sturm PD. 2006. Ventilator-associated pneumonia caused by Dolosigranulum pigrum. J. Clin. Microbiol. 44: 3461–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ichinohe T, et al. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 108: 5354–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacoby P, et al. 2007. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine 25: 2458–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jernberg C, Lofmark S, Edlund C, Jansson JK. 2007. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 1: 56–66 [DOI] [PubMed] [Google Scholar]
- 30a. Kais M, Spindler C, Kalin M, Ortqvist A, Giske CG. 2006. Quantitative detection of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in lower respiratory tract samples by real-time PCR. Diagn. Microbiol. Infect. Dis. 55: 169–178 [DOI] [PubMed] [Google Scholar]
- 31. Klein JO. 2000. The burden of otitis media. Vaccine 19: S2–S8 [DOI] [PubMed] [Google Scholar]
- 32. Klepac-Ceraj V, et al. 2010. Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics and Pseudomonas aeruginosa. Environ. Microbiol. 12: 1293–1303 [DOI] [PubMed] [Google Scholar]
- 33. Koenig JE, et al. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. U. S. A. 108: 4578–4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kong Y. 2011. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98: 152–153 [DOI] [PubMed] [Google Scholar]
- 35. Konno M, et al. 2006. Study of upper respiratory tract bacterial flora: first report. Variations in upper respiratory tract bacterial flora in patients with acute upper respiratory tract infection and healthy subjects and variations by subject age. J. Infect. Chemother. 12: 83–96 [DOI] [PubMed] [Google Scholar]
- 36. Kuss SK, et al. 2011. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334: 249–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laclaire L, Facklam R. 2000. Antimicrobial susceptibility and clinical sources of Dolosigranulum pigrum cultures. Antimicrob. Agents Chemother. 44: 2001–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laufer AS, et al. 2011. Microbial communities of the upper respiratory tract and otitis media in children. mBio 2(1): e00245–10 doi:10.1128/mbio.00245-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leach AJ, Boswell JB, Asche V, Nienhuys TG, Mathews JD. 1994. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australin Aboriginal infants. Pediatr. Infect. Dis. J. 13: 983–989 [DOI] [PubMed] [Google Scholar]
- 40. Lemon KP, et al. 2010. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 1(3): e00129–10 doi:10.1128/mbio.00129-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023 [DOI] [PubMed] [Google Scholar]
- 42. Lozupone CA, Knight R. 2008. Species divergence and the measurement of microbial diversity. FEMS Microbiol. Rev. 32: 557–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marchisio P, et al. 2003. Differences in nasopharyngeal bacterial flora in children with nonsevere recurrent acute otitis media and chronic otitis media with effusion: implications for management. Pediatr. Infect. Dis. J. 22: 262–268 [DOI] [PubMed] [Google Scholar]
- 44. McCullers JA, Bartmess KC. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187: 1000–1009 [DOI] [PubMed] [Google Scholar]
- 45. Oh S, et al. 2006. Effect of bacteriocin produced by Lactococcus sp. HY 449 on skin-inflammatory bacteria. Food Chem. Toxicol. 44: 1184–1190 [DOI] [PubMed] [Google Scholar]
- 46. Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. 2008. Microbial interactions during upper respiratory tract infections. Emerg. Infect. Dis. 14: 1584–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pettigrew MM, et al. 2011. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J. Clin. Microbiol. 49: 3750–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ravel J, et al. 2011. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 108: 4680–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reginensi SM, et al. 2011. RAPD-based screening for spore-forming bacterial populations in Uruguayan commercial powdered milk. Int. J. Food Microbiol. 148: 36–41 [DOI] [PubMed] [Google Scholar]
- 50. Revai K, Mamidi D, Chonmaitree T. 2008. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin. Infect. Dis. 46: e34–7 doi:10.1086/525856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. 2003. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11: 94–100 [DOI] [PubMed] [Google Scholar]
- 52. Roos K, Hakansson EG, Holm S. 2001. Effect of recolonisation with “interfering” alpha streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial. BMJ 322: 210–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saw JH, et al. 2008. Encapsulated in silica: genome, proteome and physiology of the thermophilic bacterium Anoxybacillus flavithermus WK1. Genome Biol. 9: R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sekirov I, et al. 2008. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 76: 4726–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Syrjanen RK, Kilpi TM, Kaijalainen TH, Herva EE, Takala AK. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184: 451–459 [DOI] [PubMed] [Google Scholar]
- 56. Tanner AC, et al. 2011. Cultivable anaerobic microbiota of severe early childhood caries. J. Clin. Microbiol. 49: 1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tano K, Grahn Hakansson E, Holm SE, Hellstrom S. 2002. A nasal spray with alpha-haemolytic streptococci as long term prophylaxis against recurrent otitis media. Int. J. Pediatr. Otorhinolaryngol. 62: 17–23 [DOI] [PubMed] [Google Scholar]
- 58. Tano K, Grahn-Hakansson E, Holm SE, Hellstrom S. 2000. Inhibition of OM pathogens by alpha-hemolytic streptococci from healthy children, children with SOM and children with rAOM. Int. J. Pediatr. Otorhinolaryngol. 56: 185–190 [DOI] [PubMed] [Google Scholar]
- 59. Tano K, Olofsson C, Grahn-Hakansson E, Holm SE. 1999. In vitro inhibition of S. pneumoniae, nontypable H. influenzae and M. catharralis by alpha-hemolytic streptococci from healthy children. Int. J. Pediatr. Otorhinolaryngol. 47: 49–56 [DOI] [PubMed] [Google Scholar]
- 60. Vergison A, et al. 2010. Otitis media and its consequences: beyond the earache. Lancet Infect. Dis. 10: 195–203 [DOI] [PubMed] [Google Scholar]
- 61. Walker BH. 1992. Biodiversity and ecological redundancy. Conserv. Biol. 6: 18–23 [Google Scholar]
- 62. Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73: 5261–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. White JR, Nagarajan N, Pop M. 2009. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 5: e1000352 doi:10.1371/journal.pcbi.1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Willing BP, Russell SL, Finlay BB. 2011. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 9: 233–243 [DOI] [PubMed] [Google Scholar]
- 65. Zhou F, Shefer A, Kong Y, Nuorti JP. 2008. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics 121: 253–260 [DOI] [PubMed] [Google Scholar]