Abstract
The diversity of bacteria nodulating Aeschynomene americana L. in Thailand was determined from phenotypic characteristics and multilocus sequence analysis of the 16S rRNA gene and 3 housekeeping genes (dnaK, recA, and glnB). The isolated strains were nonphotosynthetic bacteria and were assigned to the genus Bradyrhizobium, in which B. yuanmingense was the dominant species. Some of the other species, including B. japonicum, B. liaoningense, and B. canariense, were minor species. These isolated strains were divided into 2 groups—nod-containing and divergent nod-containing strains—based on Southern blot hybridization and PCR amplification of nodABC genes. The divergent nod genes could not be PCR amplified and failed to hybridize nod gene probes designed from B. japonicum USDA110, but hybridized to probes from other bradyrhizobial strains under low-stringency conditions. The grouping based on sequence similarity of nod genes was well correlated with the grouping based on that of nifH gene, in which the nod-containing and divergent nod-containing strains were obviously distinguished. The divergent nod-containing strains and photosynthetic bradyrhizobia shared close nifH sequence similarity and an ability to fix nitrogen in the free-living state. Surprisingly, the strains isolated from A. americana could nodulate Aeschynomene plants that belong to different cross-inoculation (CI) groups, including A. afraspera and A. indica. This is the first discovery of bradyrhizobia (nonphotosynthetic and nod-containing strain) originating from CI group 1 nodulating roots of A. indica (CI group 3). An infection process used to establish symbiosis on Aeschynomene different from the classical one is proposed.
INTRODUCTION
The symbiotic interaction between leguminous plants and rhizobia (nitrogen-fixing bacterial symbionts) leads to the formation of a specialized plant organ called a nodule, where the bacteria fix atmospheric dinitrogen to the benefit of the plants. The genus Aeschynomene comprises about 175 to 180 species of legumes distributed throughout the tropical and subtropical regions of the world (33). Taxonomically, Aeschynomene belongs to the Dalbergioid clade, which is in subfamily Papilionoideae of the Fabaceae (23). Aeschynomene establishes a symbiotic relationship with bacteria belonging to the genus Bradyrhizobium, and the nitrogen-fixing nodules are formed on roots and/or stems (28, 29). Three cross-inoculation (CI) groups among Aeschynomene species have been described according to their symbiotic bacteria (27). Members of CI group 1, such as A. americana and A. elaphroxylon, are nodulated only on their roots by nonphotosynthetic bradyrhizobia. Members of CI group 2, such as A. afraspera and A. nilotica, and CI group 3, such as A. indica and A. sensitiva, are nodulated on their roots and stems. CI group 2 is nodulated by both nonphotosynthetic and photosynthetic bradyrhizobia, which are also able to nodulate CI group 3 (27). CI group 3 is only nodulated by photosynthetic bradyrhizobia, such as Bradyrhizobium sp. strains BTAi1 and ORS278.
The infection process in Aeschynomene species is rather primitive, as it occurs via “crack entry” by intercellular infection of epidermal fissures generated by the emergence of lateral roots (1, 3). Two different nodulation processes used to establish a symbiotic interaction with Aeschynomene have been described (3). First, a nodulation process between A. afraspera and its specific symbiont Bradyrhizobium sp. strain ORS285 occurs via a Nod-factor-dependent mode. This mode is a multistep process requiring lipochitooligosaccharide signal molecules (Nod factors) produced by the bacteria to initiate symbiosis, as described in the model legumes Lotus japonicus and Medicago truncatula (11, 30). The bacteria contain nodulation (nod) genes, which encode proteins involved in the biosynthesis and secretion of the Nod factor (31). Another process, via the Nod-factor-independent mode, has been observed in A. indica nodulated by photosynthetic bradyrhizobia, including Bradyrhizobium sp. strains BTAi1 (12), ORS278 (4), and ORS285 (3). The strains BTAi1 and ORS278 do not possess the canonical nodABC genes required for the synthesis of the core structure of Nod factors, but use other mechanisms of signaling to the plant (12). Mutation analyses of the nod genes in the strain ORS285 did not affect nodulation of A. indica and A. sensitiva but blocked nodulation of the original host plant, A. afraspera (3, 9, 12). Therefore, the nod-containing photosynthetic strain ORS285 is able to use both modes, depending on the host plant. It was hypothesized that a purine derivative, such as cytokinin, might play a role in triggering nodule formation instead of the Nod factor (2, 12).
Phylogenetic analyses revealed that the bacterial symbionts associated with CI group 3 Aeschynomene belonged to the genus Bradyrhizobium but form a separate subbranch distinct from the nonphotosynthetic species B. japonicum and B. elkanii (28). Multilocus sequence analyses (MLSA) of a number of housekeeping genes were used to study the evolutionary relationships of and delineate species in the genus Bradyrhizobium (29, 34, 45). The dnaK, glnB, and recA produced a well-revolved phylogeny of photosynthetic bradyrhizobia and have been suggested for MLSA analyses in the genus Bradyrhizobium. However, only a little information from the nonphotosynthetic bradyrhizobia isolated from Aeschynomene in CI group 1 was obtained (28, 29), and their infection process has not been elucidated so far. Therefore, in this study, phylogenetic analyses of the 16S rRNA gene and a combination of the 3 housekeeping genes (dnaK, glnB, and recA) were used to elucidate the taxonomic relationships of the strains isolated from A. americana, and their symbiotic evolutions were explored by analyses of a nitrogenase gene (nifH) and nodulation genes (nodA and nodB). In addition, β-glucuronidase (GUS)- and green fluorescent protein (GFP)-labeled strains were used to examine the infection process on Aeschynomene species. This study could provide more information how the bacteria establish a symbiotic interaction with A. americana.
MATERIALS AND METHODS
Soil samples, collection sites, and Bradyrhizobium isolation and screening.
Bradyrhizobial strains were isolated from nodules of A. americana, which is a local Thai variety confirmed by matK sequence (see Fig. S1 in the supplemental material). The A. americana was grown in soil collected from 11 rice field areas, where A. americana is found, in Thailand. These areas are located in different regions in northern and central Thailand, including Phitsanulok (17°31′4.77N, 100°19′3.92E and 17°31′31.82N, 100°19′11.11E), Uttaradit (17°18′7.97N, 100°13′53.33E, 17°47′33.99N, 100°6′58.96E, 17°47′48.81N, 100°6′57.12E, and 17°41′22.21N, 100°8′34.04E), Chiangmai (19°3′44.04N, 98°56′26.53E and 19°4′17.28N, 98°56′13.93E), Lampang (17°31′13.50N, 99°11′6.34E), and Lopburi (17°22′8.99N, 99°48′2.13E). After 45 days of growth, nodules were harvested, surface sterilized by immersion in 70% ethanol for 30 s and then in 3% hydrogen peroxide for 3 min, and washed five times with sterilized water. Each nodule was crushed and then streaked and purified on yeast extract-mannitol (YEM) agar (40) plates. These isolated strains were reinoculated onto the host plant under sterilized conditions to verify their nodulation ability. The isolated strains showing different BOXAIR1-genomic patterns were selected for further study. BOXAIR1 fingerprints were obtained by using a BOXA1R primer (5′-CTACGGCAAGGCGACGCTGAC-3′) (44).
Bacterial strains, plasmids, and growth conditions.
The 40 isolated strains and the plasmids used in this study are listed in Table 1. Bradyrhizobial strains were cultured at 28°C in HM medium (7) for further analyses. Escherichia coli strains were cultured at 37°C in LB medium (36). Media were supplemented with the following antibiotics when appropriate: for E. coli, 25 μg/ml kanamycin, 15 μg/ml tetracycline, and 25 μg/ml gentamicin; and for Bradyrhizobium, 200 μg/ml streptomycin and 200 μg/ml tetracycline.
Table 1.
Strains and plasmids used in this study, sampling sites, and relevant characteristics
| Strain or plasmid | Geographical origin (province in Thailand) | Relevant characteristic(s) | Source or reference |
|---|---|---|---|
| Strains | |||
| Bradyrhizobium sp. | |||
| SUTN1-2 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-3 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-4 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-6 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-7 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-8 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-9 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-12 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN1-13 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN2-1 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN2-2 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN2-3 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN2-4 | Pitsanulok | A. americana nodule isolate | This study |
| SUTN3-1 | Uttradit | A. americana nodule isolate | This study |
| SUTN4-1 | Uttradit | A. americana nodule isolate | This study |
| SUTN4-3 | Uttradit | A. americana nodule isolate | This study |
| SUTN5-1 | Uttradit | A. americana nodule isolate | This study |
| SUTN5-3 | Uttradit | A. americana nodule isolate | This study |
| SUTN5-5 | Uttradit | A. americana nodule isolate | This study |
| SUTN5-6 | Uttradit | A. americana nodule isolate | This study |
| SUTN6-1 | Uttradit | A. americana nodule isolate | This study |
| SUTN6-2 | Uttradit | A. americana nodule isolate | This study |
| SUTN7-1 | Chiangmai | A. americana nodule isolate | This study |
| SUTN7-2 | Chiangmai | A. americana nodule isolate | This study |
| SUTN8-2 | Chiangmai | A. americana nodule isolate | This study |
| SUTN8-3 | Chiangmai | A. americana nodule isolate | This study |
| SUTN9-1 | Lampang | A. americana nodule isolate | This study |
| SUTN9-2 | Lampang | A. americana nodule isolate | This study |
| SUTN9-3 | Lampang | A. americana nodule isolate | This study |
| SUTN9-4 | Lampang | A. americana nodule isolate | This study |
| SUTN9-5 | Lampang | A. americana nodule isolate | This study |
| DOA1 | Lopburi | A. americana nodule isolate | This study |
| DOA2 | Lopburi | A. americana nodule isolate | This study |
| DOA3 | Lopburi | A. americana nodule isolate | This study |
| DOA4 | Lopburi | A. americana nodule isolate | This study |
| DOA6 | Lopburi | A. americana nodule isolate | This study |
| DOA7 | Lopburi | A. americana nodule isolate | This study |
| DOA8 | Lopburi | A. americana nodule isolate | This study |
| DOA9 | Lopburi | A. americana nodule isolate | This study |
| DOA10 | Lopburi | A. americana nodule isolate | This study |
| DOA9GUS | DOA9 marked with mTn5SSgusA20 (pCAM120); Smr Spr | This study | |
| DOA9GFP | DOA9 containing pBZ1; Tcr | This study | |
| BTAi1 | Photosynthetic bacterium isolated from A. indica nodule | 10 | |
| ORS278 | Photosynthetic bacterium isolated from A. sensitive nodule | 28 | |
| ORS285 | Photosynthetic bacterium isolated from A. afraspera nodule | 28 | |
| B. japonicum USDA110 | Wild type, soybean isolated | 19 | |
| E. coli S17-1 | pro recA RP4-2(Tcs∷Mu) (Kms∷Tn7); Mob+ | 38 | |
| Plasmids | |||
| pEGFP | Expression vector of GFP | Clontecha | |
| pRK404 | Broad-host-range vectors | 37 | |
| pCAM120 | mTn5SSgusA20 in pUT/mini-Tn5 | S. Okazakib; 47 | |
| pBZ1 | pRK404 containing HindIII/EcoRI fragment (gfp) from pEGFP | This study |
Clontech Laboratories, Inc., Mountain View, CA.
Department of International Environmental and Agricultural Science, Graduate School of Agriculture, Tokyo University of Agriculture and Technology, Tokyo, Japan.
Phenotypic characterization.
Colony color and morphology were observed during cultivation on agar medium. The production of photosynthetic pigment from the bradyrhizobial strains was observed on the HM agar medium. In order to detect bacterial chlorophyll, bacterial strains were grown aerobically at 28°C for 7 days under a 12-h-light/12-h-dark cycle. Cell pellets were extracted in the dark with cold acetone-methanol (7:2 [vol/vol]) for 30 min (24). Absorbance of the supernatant was observed at a wavelength range from 350 to 800 nm. The nitrogen-fixing activity of the bacterial cultures was examined by an acetylene reduction assay as described previously (32). The reaction was carried out in a 21-ml test tube containing 7 ml of bacterial culture in LG medium (42) supplemented with arabinose and 0.005% yeast extract and incubated at 28°C for 5 days.
Plant test.
Bradyrhizobial strains were grown for 5 days in HM broth. All plants were grown in a growth room with controlled environmental conditions of 25°C on a 12-h-light/12-h-dark cycle. Peanut (Arachis hypogaea), mungbean (Vigna radiata), soybean (Glycine max cv. SJ5), siratro (Macroptilium atropurpureum), and Sesbania rostrata seeds were sterilized as described previously (40). A. americana (a local Thai variety), A. afraspera (provided by Eric Giraud), and A. indica (ecotype Tottori, Japan) seeds were sterilized by incubation in concentrated sulfuric acid for 25 min. The seeds were washed with sterilized water and then soaked in sterilized water overnight at ambient temperature. All seeds were germinated on 0.8% water agar. Cross-nodulation experiments were performed in plastic pouches (40). Symbiotic abilities of bradyrhizobial strains were determined in Leonard jars containing sterilized vermiculite and inoculated with 1 ml of bacterial culture, equivalent to 107 cells. N-free Hoagland's solution (17) was added to each jar as required. After 6 weeks, plants were harvested and the entire plant was used for analysis of nitrogenase activity by measurement of acetylene reducing activity (ARA) (40). After the ARA assay, nodules were detached from the roots and the number of the nodule was scored. The root, shoot, and nodule dry weights were determined after drying at 70°C for 72 h.
DNA extraction, PCR amplification, and sequencing.
Bradyrhizobial genomic DNA was prepared as described previously (26). The primers used are listed in Table 2. The thermal cycler was programmed as follows: an initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 94°C for 1 min, annealing at the appropriate temperature (Table 2) for 45 s, and extension at 72°C for 1 min, with a final extension at 72°C for 10 min. Gene fragments were amplified using the Go Taq Flexi DNA polymerase kit (Promega, Germany). The PCR products were purified using the Wizard SV gel and PCR clean-up system (Promega, Germany) and sequenced using the same primers as those for the PCR. DNA sequencing was carried out by Macrogen, Inc. (Seoul, South Korea). In order to extract bacterial DNA from nodules, the nodule was surface sterilized by immersion in 70% ethanol for 1 min, followed by rinsing in sterile water and then in 3% sodium hypochlorite for 5 min, followed by rinsing in sterile water. The nodule was crushed in 100 μl of phosphate buffer, and the suspension was taken up for extraction of bacterial DNA as described above.
Table 2.
Primers used in this study
| Target gene | Primer name | Primer sequence (5′→3′) | Reference or description of design | Annealing temp used (°C) |
|---|---|---|---|---|
| 16S rRNA | fD1 | AGAGTTTGATCCTGGCTCAG | 46 | 55 |
| rP2 | ACGGCTACCTTGTTACGACTT | |||
| dnaK | TSdnaK4 | GGCAAGGAGCCGCAYAAGG | 29 | 53 |
| TSdnaK2 | GTACATGGCCTCGCCGAGCTTCA | |||
| glnB | TSglnBF | AAGCTCGAGTACATCTGGMSCTCGACGG | 29 | 53 |
| TSglnBR | SGAGCCGTTCCAGTCGGTGTCG | |||
| recA | recA41F | TTCGGCAAGGGMTCGRTSATG | 45 | 53 |
| recA640R | ACATSACRCCGATCTTCATGC | |||
| nodA | nodAF28 | GAAGGATCTTCTGGGCGCG | Designed from nodA of B. japonicum | 45 |
| nodAR627 | CTCAGGCCCGTTACGATCG | USDA110 (NC_004463) | ||
| nodAYF46 | GCTCAAGTGCAGTGGAGCCTTC | Designed from nodA of B. yuanmingense | 53 | |
| nodAYR595 | CCGGCCATTCGCTTATCGAGCG | CCBAU10071 (AM117557) | ||
| nodAF25-ORS285 | GTATGCTGGGAGAGTGATCTCG | Designed from nodA of Bradyrhizobium | 46 | |
| nodAR584-ORS285 | GGCCCATTCCTCTCAATCGTTG | sp. strain ORS285 (AF284858) | ||
| nodA-CI1F45 | CTCGC(C/T)(G/A)AGTTCTTCCGCAA(G/A)AG | Designed from nodB of Bradyrhizobium | 52 | |
| nodA-CI1R498 | CCACCACGATCACGTCTTC(G/A)ATG | sp. strain ORS301 (AJ437608) and ORS304 (AJ437610) nodulating CI group 1 Aeschynomene | ||
| nodAf.brad | GTYCAGTGGAGSSTKCGCTGGG | 6 | 53 | |
| nodAr.brad | TCACARCTCKGGCCCGTTCCG | |||
| nodA1f | TGCRGTGGAARNTRBVYTGGGAAA | 6 | 45 | |
| nodAb1r | GGNCCGTCRTCRAASGTCARGTA | |||
| nodABrMf | CARSTKMGRTGGAGYSTDYGCTGGGA | 27 | 60–50 | |
| nodAb1r | GGNCCGTCRTCRAASGTCARGTA | (touchdown PCR) | ||
| nodB | nodBF26 | CTGTCCGCTGCGACTACGC | Designed from nodB of B. japonicum | 47 |
| nodBR625 | CGCGCCGTTGTAGTGCTGG | USDA110 (NC_ 004463) | ||
| nodBF6-BY | CAGAGGTGCTCGATGTGCTGGC | Designed from nodB of B. yuanmingense | 55 | |
| nodBR536-BY | GACGGATTACAAACCCGCGCCG | CCBAU10071 (AY923050) and LMTR28 (AY923048) | ||
| nodBF73-ORS285 | CTGACATTTGACGATGGGCCCG | Designed from nodB of Bradyrhizobium | 53 | |
| nodBR622-ORS285 | GCCCATGAAGAGCTGGGATCAG | sp. strain ORS285 (AF284858) | ||
| nodC | nodCF195 | CGCCGAATGTCTGGAGTCG | Designed from nodC of B. japonicum | 45 |
| nodCR1394 | CCTGAGTCATCAGCCGACC | USDA110 (NC_004463) | ||
| nodCF197-ORS285 | TGGCGTGCCTAGAGTCGATTGC | Designed from nodC of Bradyrhizobium | 53 | |
| nodCR1196-ORS285 | CACGGTGATTTGCGCGACAACC | sp. strain ORS285 (AF284858) | ||
| nodCF108-Can | AACGCAAGGCGCAG(T/A)TCGC(A/T)GC | Designed from nodC of B. canariense | 53 | |
| nodCR860-Can | ATCGG(G/T)GTGTG(C/G)AGCGAGAAGC | BTA-1 (AJ560653) and CCBAU51257 (GU433570) | ||
| nodCF | AYGTHGTYGAYGACGGTTC | 22 | 41 | |
| nodCI | CGYGACAGCCANTCKCTATTG | |||
| nifH | nifHF | TACGGNAARGGSGGNATCGGCAA | 22 | 48 |
| nifHI | AGCATGTCYTCSAGYTCNTCCA |
Southern blot hybridization of nodulation genes.
The nodulation gene probes were obtained through a PCR amplification using genomic DNA of bradyrhizobial strains as a template. These bradyrhizobial strains included B. japonicum USDA110, Bradyrhizobium sp. strain ORS285, the isolated strain SUTN6-2 (99% identity to B. yuanmingense based on 16S rRNA gene sequence JN578798), and the isolated strain SUTN7-2 (99% identity to B. canariense based on the 16S rRNA gene sequence JN578800). The strains SUTN6-2 and SUTN7-2 were used as representatives of B. yuanmingense and B. canariense, respectively. DNA fragments of nodA, nodB, and nodC of B. japonicum USDA110 were amplified from the primer pairs nodA28/nodA627, nodB26/nodB625, and nodCF/nodCI (Table 2), respectively. DNA fragments of nodA, nodB, and nodC of the isolated strains SUTN6-2 and SUTN7-2 were amplified from the primer pairs nodAYF46/nodAYR595, nodB26/nodB625, and nodCF/nodCI (Table 2), respectively. Each nod gene probe was verified by DNA sequencing. The nodA probe obtained from B. japonicum USDA110 (600 bp) shared 100% identity with that of B. japonicum USDA110, that from strain SUTN6-2 (550 bp) shared 95% identity with that of B. yuanmingense, and that from strain SUTN7-2 shared 93 to 95% identity with that of various strains of Bradyrhizobium sp. The nodB probe obtained from B. japonicum USDA110 (600 bp) shared 100% identity with that of B. japonicum USDA110, that from strain SUTN6-2 (530 bp) shared 95% identity with that of B. yuanmingense, and that from strain SUTN7-2 (530 bp) shared 87% identity with that of B. yuanmingense. The nodC probe (1,000 bp) obtained from B. japonicum USDA110 shared 100% identity with that of B. japonicum USDA110, that from strain SUTN6-2 shared 93% identity with that of B. yuanmingense, and that from strain SUTN7-2 shared 96% identity with that from B. canariense. DNA probes were labeled overnight by random priming and hybridized using the digoxigenin (DIG) High Prime DNA labeling and detection starter kit I (Roche). Bradyrhizobial genomic DNAs were digested with EcoRI and separated in a 1% agarose gel. Southern blotting was carried out by capillary transfer to a Hybond-N+ nylon membrane (Amersham, Cardiff, United Kingdom), as described previously (36). The membranes were hybridized at 44°C for 18 h. Sequentially, membranes were twice washed in 2× SSC–0.1% SDS at 25°C for 5 min and in 0.5× SSC–0.1% SDS at 65°C for 15 min (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). For the low-stringency condition, the membranes were hybridized at 40°C for 18 h and then twice washed in 2× SSC–0.1% SDS at 25°C for 5 min and in 0.5× SSC–0.1% SDS at 60°C for 15 min.
Phylogenetic analyses.
DNA sequences of gene fragments generated in this study were subjected to the algorithm BLASTN to identify the most similar sequences available in the database. DNA sequences of each gene from relative strains in the family Bradyrhizobiaceae, of other rhizobia, and of outgroups were obtained from the GenBank database. Multiple alignments were performed with MUSCLE from phylogeny.fr (8). Unaligned regions and gaps were excluded from the analyses. In total, 1,351, 677, 458, 587, 643, 480, and 534 nucleotide positions were used for the phylogenetic analyses of 16S rRNA genes, dnaK, glnB, recA, nifH, nodA, and nodB, respectively. Phylogenetic trees were reconstructed by the maximum likelihood method using PhyML (15), and confidence levels were estimated for 1,000 replicates. In comparison, phylogenetic trees were also reconstructed by the distance neighbor-joining method (35) using the MEGA 4.1 package (21).
Construction of GUS- and GFP-labeled Bradyrhizobium strains.
To obtain GUS-labeled strains, plasmid pCAM120 (47) was transferred into bradyrhizobial strains by biparental mating. Blue colonies expressing GUS activity were selected in HM agar medium supplemented with streptomycin and gentamicin. In order to construct GFP-expressing strains, plasmid pEGFP (Clontech), containing a gfp fragment, was digested with HindIII and EcoRI. The HindIII/EcoRI fragment was cloned into a broad-host-range vector pRK404 (37) to give vector pBZ1. The pBZ1 vector was introduced into E. coli S17-1 using electroporation (18 kv/cm, 100 Ω, and 25 μF) and was transferred into bradyrhizobial strains by biparental mating. Transconjugants were selected on HM agar medium supplemented with tetracycline and gentamicin.
Biparental spot mating for transfer of the recombinant plasmids to the bradyrhizobial strains was carried out using E. coli S17-1 as a donor with some modification (20). E. coli S17-1 containing the recombinant plasmid and bradyrhizobial strains were cultured to stationary phase in LB and HM medium, respectively, with appropriate antibiotics added. Bacteria obtained from 1 ml of each culture were pelleted, and cultures of E. coli and bradyrhizobial strains were resuspended in 1 ml and 0.1 ml 0.85% NaCl, respectively, and mixed at a volume ratio of 1:5. The mixture (40 μl) was dropped on HM medium agar and incubated for 4 days at 28°C. The cells were harvested and then resuspended in 1 ml of 0.85% NaCl. The cell suspensions were plated onto the HM agar with appropriate antibiotics.
Microscopic study of infection process.
To study the invasion process and localization of the bradyrhizobial strains, A. americana, A. afraspera, and A. indica seeds were inoculated with GUS- and GFP-labeled strains. The plants inoculated with GUS-labeled strains were monitored daily for 10 days. GUS staining was performed as described previously (26), and GUS expression was observed under light microscopy. The localization of A. americana by GFP-labeled strains was observed using fluorescence microscopy at 6 and 24 h after inoculation. The fresh stems, roots, and nodules were embedded in 5% agarose gel, and the 90-μm-thick sections were prepared using a vibratome (Microm HM 650V).
Statistical analysis.
The data were subjected to analysis of variance (ANOVA) using the program SPSS version 11.5. Duncan's multiple range test was used to identify differences between means at P ≤ 0.05.
RESULTS
Bacterial isolation and phenotypic characteristics.
A total of 246 bacterial strains were obtained after nodule isolation and renodulation of A. americana. Identical BOXAIRI profiles were found with the isolated strains from different geographic origins. Therefore, the 40 isolated strains showing different BOXAIR1 fingerprints (data not shown) were chosen for further study. When grown on HM medium, most of the strains formed 1.5- to 2-mm-diameter colonies within 4 to 5 days of incubation. Photosynthetic pigment was observed neither from growth on plate culture nor from extraction of broth culture with acetone-methanol. However, the peaks of bacterial chlorophyll (780 nm) and carotenoids (460 to 490 nm) of photosynthetic Bradyrhizobium sp. strains BTAi1 and ORS278 could be detected and used as positive controls (see Fig. S2 in the supplemental material).
Phylogenetic analysis of 16S rRNA genes.
Sequences of 16S rRNA genes were determined for the 40 representative strains obtained from A. americana. Sequences from various reference strains were added, which consisted of different Bradyrhizobium species as well as Rhodopseudomonas palustris. Mesorhizobium loti MAFF303099 was chosen as an outgroup strain to root the tree. On the basis of 16S rRNA gene sequence similarity, there are 2 major phylogenetic lineages, including lineage 1 of B. elkanii and lineage 2, in which other species of Bradyrhizobium and photosynthetic bradyrhizobia were included, with strong bootstrap support (98%) (Fig. 1). All of the strains isolated belonged to lineage 2, including various Bradyrhizobium species (B. yuanmingense, B. japonicum, B. liaoningense, and B. canariense), and also R. palustris. In lineage 2, photosynthetic bradyrhizobial strains formed a separate branch with strong bootstrap support (98%), supporting that the isolated strains were nonphotosynthetic Bradyrhizobium. Using BLASTN, the isolated strains shared only 94% sequence identity to the photosynthetic bradyrhizobial strains.
Fig 1.
Neighbor-joining trees based on sequences of 16S rRNA genes showing classification of the Bradyrhizobium strains isolated from nodules of A. americana. Bootstrap values are expressed as percentages of 1,000 replications. The bar represents 1 estimated substitution per 100 nucleotide positions. The evolutionary distances were computed using the Kimura two-parameter method and are shown in the units representing the number of base substitutions per site.
Cross-nodulation test.
The nodulation test on the original host A. americana showed that most of the isolated strains could produce up to 100 nodules per plant after 45 days of inoculation. Besides nodulating their original host, all isolated strains could efficiently nodulate A. hypogaea, V. radiata, and M. atropurpureum, but they failed to nodulate S. rostrata and G. max (Table 3). B. japonicum USDA110 had the ability to form root nodules on all plants tested, except A. americana and S. rostrata.
Table 3.
Nodulation test on various leguminous plants and genotypic characterization by Southern hybridization of nodulation genes
| Bradyrhizobium strains | Nodulation ona: |
nod gene hybridizationb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A. americana | G. max | Ar. hypogaea | V. radiata | M. atropurpureum | S. rostrata | nodA | nodB | nodC | |
| Divergent nod-containing strains | |||||||||
| SUTN1-2 | + | 0 | + | + | + | 0 | YL | CL | JL |
| SUTN1-3 | + | 0 | + | + | + | 0 | Y | CL | JL |
| SUTN1-4 | + | 0 | + | + | + | 0 | YL | CL | JL |
| SUTN1-6 | + | 0 | + | + | + | 0 | YL | CL | JL |
| SUTN1-8 | + | 0 | + | + | + | 0 | YL | CL | J |
| SUTN1-12 | + | 0 | + | + | + | 0 | J | CL | JL |
| SUTN1-13 | + | 0 | + | + | + | 0 | YL | CL | JL |
| SUTN2-1 | + | 0 | + | + | + | 0 | YL | CL | J |
| SUTN2-2 | + | 0 | + | + | + | 0 | YL | CL | JL |
| SUTN2-3 | + | 0 | + | + | + | 0 | YL | J | JL |
| SUTN2-4 | + | 0 | + | + | + | 0 | YL | CL | JL |
| SUTN4-3 | + | 0 | + | + | + | 0 | YL | CL | J |
| SUTN5-6 | + | 0 | + | + | + | 0 | YL | CL | JL |
| DOA1 | + | 0 | + | + | + | 0 | YL | CL | JL |
| DOA2 | + | 0 | + | + | + | 0 | YL | CL | JL |
| DOA3 | + | 0 | + | + | + | 0 | YL | CL | JL |
| DOA4 | + | 0 | + | + | + | 0 | YL | CL | JL |
| DOA6 | + | 0 | + | + | + | 0 | N | CL | JL |
| DOA7 | + | 0 | + | + | + | 0 | YL | CL | JL |
| DOA8 | + | 0 | + | + | + | 0 | N | CL | JL |
| DOA9 | + | 0 | + | + | + | 0 | N | CL | JL |
| DOA10 | + | 0 | + | + | + | 0 | YL | CL | JL |
| nod-containing strains | |||||||||
| SUTN1-7 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN1-9 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN3-1 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN4-1 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN5-1 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN5-3 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN5-5 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN6-1 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN6-2 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN7-1 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN7-2 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN8-1 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN8-2 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN9-1 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN9-2 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN9-3 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN9-4 | + | 0 | + | + | + | 0 | J | J | J |
| SUTN9-5 | + | 0 | + | + | + | 0 | J | J | J |
| USDA110 | 0 | + | + | + | + | 0 | J | J | J |
| nod-independent strains | |||||||||
| BTAi1 | 0 | 0 | + | 0 | 0 | 0 | N | N | N |
| ORS278 | 0 | 0 | + | 0 | 0 | 0 | N | N | N |
+, positive with effective nodule; 0, negative.
Southern blot hybridization signals are presented in Fig. S3 in the supplemental material. J, signal obtained from B. japonicum USDA110 under a stringent condition; YL, signal obtained from strain SUTN6-2 (99% 16S rRNA similarity to B. yuanmingense) under a low-stringency condition; CL, signal obtained from strain SUTN7-2 (99% 16S rRNA similarity to B. canariense) under a low-stringency condition; JL, signal obtained from B. japonicum USDA110 under a low-stringency condition and from strains SUTN6-2 and SUTN7-2 under a stringent condition; N, not detected.
Presence of nodulation genes.
Southern blots of EcoRI-digested DNA derived from the isolated strains were hybridized to probes of nodA, nodB, and nodC from different bradyrhizobial strains. The Southern blot hybridization results are shown in Table 3 (see Fig. S3 in the supplemental material). Based on Southern blot hybridization results, the isolated strains were divided into 2 groups—nod-containing strains and divergent nod-containing strains. Of 40 strains, 18 strains, showing signals of nodA, nodB, and nodC after hybridization to the probes designed from B. japonicum USDA110, were called “nod-containing strains.” The remaining 22 strains failed to hybridize all nod gene probes from B. japonicum USDA110 under stringent conditions, except for strains SUTN1-8, SUTN1-12, SUTN2-1, SUTN2-3, and SUTN4-3, which showed signals of some nod genes. However, under low-stringency conditions, the remaining 22 strains showed weak signals of nodC after hybridization to probes obtained from B. japonicum USDA110 and of nodA and nodB after hybridization to probes obtained from the other bradyrhizobial strains (SUTN6-2 and SUTN7-2). Therefore, these strains were called “divergent nod-containing strains.” In detail, the divergent nod-containing strains failed to hybridize to nodA probe from strain USDA110 (under either stringent or low-stringency conditions), but hybridized to nodA probe from strain SUTN6-2 under the low-stringency condition. For Southern blot hybridization of nodB, the divergent nod-containing strains failed to hybridize to nodB probes designed from either strain USDA110 or strain SUTN6-2. Therefore, the nodB probes from strain SUTN7-2 were used, and the divergent nod-containing strains showed the signals of nodB under the low-stringency condition. For Southern blot hybridization of nodC, the signals were obtained after hybridization to probes obtained from strain USDA110 under the low-stringency condition and also from strains SUTN6-2 and SUTN7-2 under stringent conditions. Taken together, these results show that the canonical nodABC genes of the divergent nod-containing strains differed from the classical one and each nod gene might diverge from various origins.
In addition, detection of nodulation genes (nodA, nodB, and nodC) was carried out by PCR amplification and sequencing. B. japonicum USDA110 and Bradyrhizobium sp. strain BTAi1 were used as positive and negative controls, respectively. Several primer sets designed from different species of Bradyrhizobium were used for PCR amplification of nodulation genes. Using the primer pairs designed from B. japonicum USDA110, only the PCR product of nodB was obtained from the nod-containing strains, but none of the PCR products of nodA and nodC was obtained from these strains. However, the PCR products of nodA and nodC of the nod-containing strains were obtained by using primer pair nodAYF46/nodAYR595 (designed from the nodA sequence of B. yuanmingense) (Table 2) and degenerate primer pair nodCF/nodCI, respectively (data not shown). In contrast, none of the PCR product of nodA, nodB, and nodC was obtained from the divergent nod-containing strains, even though several primer sets (Table 2) were used. These results indicated that the canonical nodABC genes of the divergent nod-containing strains were unusual compared to those of the other rhizobia.
Phylogenetic analyses of nodulation genes.
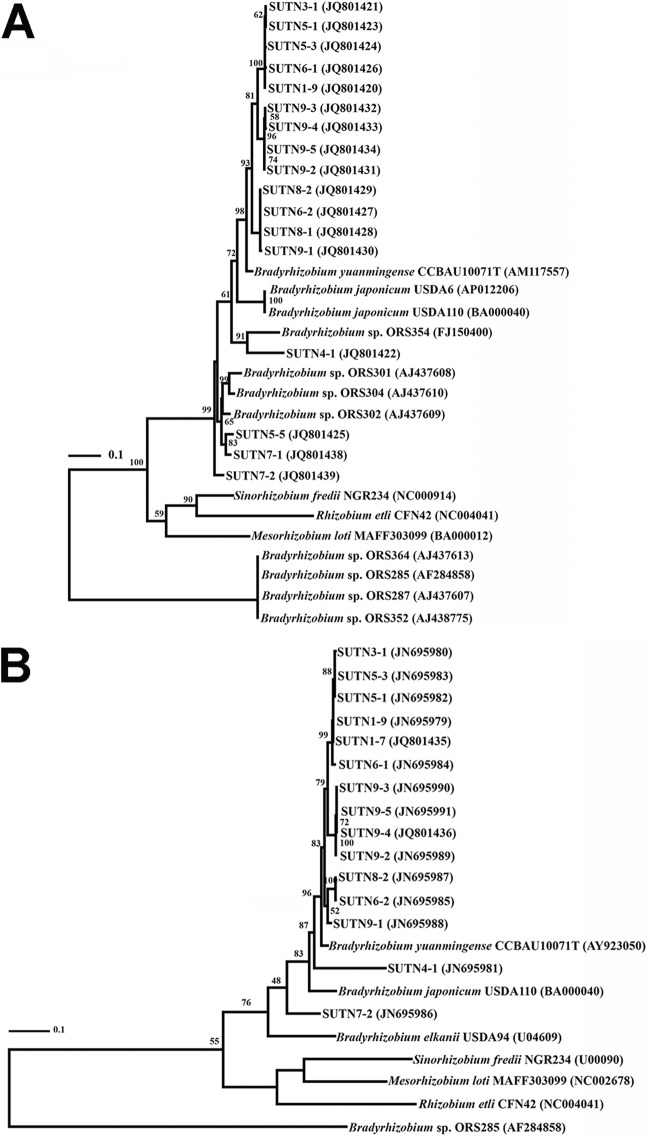
PCR product of nodA, nodB, and nodC could not be obtained from the divergent nod-containing strains. Therefore, only sequences of nodulation genes obtained from nod-containing strains were used to construct the phylogenetic trees. The taxonomic positions of the nod-containing strains in trees constructed from nodA and nodB were congruent (Fig. 2). Analyses of nodA and nodB sequences showed that these genes from all of the nod-containing strains were similar to those from B. yuanmingense (Fig. 2A and B), and the strains were clustered with nonphotosynthetic bradyrhizobia (either from CI group 1 or from CI group 2) in the nodA tree (Fig. 2A). Comparison of nodA sequences revealed that most of the nod-containing strains shared 91 to 95% sequence identity with B. yuanmingense, but they shared only 88% and 80% sequence identity with B. japonicum and B. elkanii, respectively. The exceptions were found for the strains SUTN5-5, SUTN7-1, SUTN7-2, and SUTN4-1; the nodA sequences of strains SUTN5-5, SUTN7-1, SUTN7-2, and SUTN4-1 shared 85 to 91% identity with that of B. yuanmingense and 84 to 87% identity with that of B. japonicum. These strains were placed on unclassified branches with the nonphotosynthetic bradyrhizobia originating from nodules of CI group 1 (ORS301, ORS302, and ORS304) and CI group 2 (ORS354) (Fig. 2A). In the same way, comparison of nodB sequences showed that most of the nod-containing strains shared 96% sequence identity with that of B. yuanmingense but shared only 90% and 89% sequence identity with those of B. japonicum and B. elkanii. The nodB sequences of strains SUTN4-1 and SUTN7-2 shared 85 to 86% identity with that of B. yuanmingense and 83 to 84% identity with that of B. japonicum. The sequences of nodA and nodB from the photosynthetic CI group 2 strains (ORS364, ORS285, ORS287, and ORS352) were divergent from those of the other bradyrhizobia, sharing only 55 to 58% identity with those of the A. americana strains, and were placed as a distinct cluster (Fig. 2A and B).
Fig 2.
Maximum likelihood trees based on sequences of nodA (A) and nodB (B) of the nod-containing Bradyrhizobium strains isolated from nodules of A. americana. Bootstrap values are expressed as percentages of 1,000 replications. The bar represents 1 estimated substitution per 100 nucleotide positions.
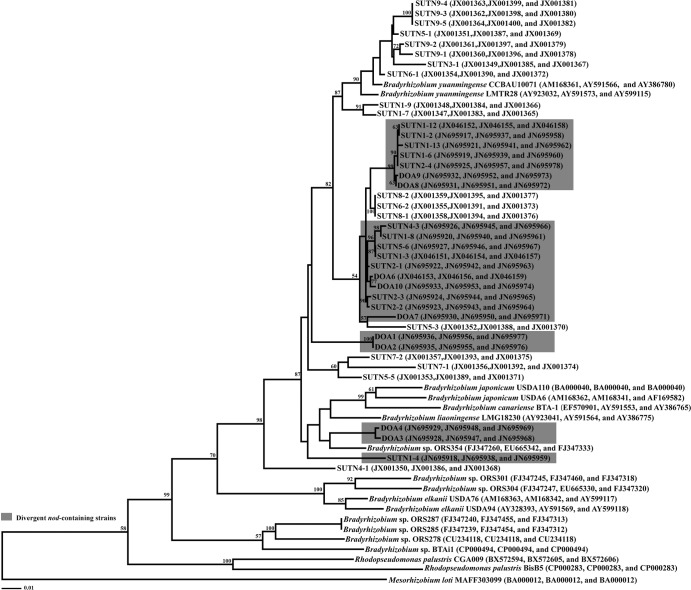
Phylogenetic analyses of dnaK, recA, and glnB.
In order to determine the taxonomic position of the isolated strains, the phylogenetic trees based on sequences of dnaK, recA, and glnB were constructed. The taxonomic positions of the isolated strains in the combination tree (Fig. 3) were almost congruent with their taxonomic positions in the 16S rRNA gene tree (Fig. 1). In the combination tree, the isolated strains were clustered with B. yuanmingense and B. japonicum and separated from the group of B. elkanii and CI group 1 strains (ORS301 and ORS304) with 98% bootstrap support. In addition, the nonphotosynthetic bradyrhizobia isolated from CI group 1 were clearly distinguished from the bradyrhizobia isolated from CI groups 2 and 3 with 99% bootstrap support. Moreover, in the B. yuanmingense cluster, the number of divergent nod-containing strains formed a separate subcluster distinct from the majority of nod-containing strains, except for strains SUTN6-2, SUTN8-1, and SUTN8-2. In the B. yuanmingense cluster, the combination of dnaK, recA, and glnB sequence analyses showed that the nod-containing strains shared 98 to 99% sequence identities with that of B. yuanmingense, but the divergent nod-containing strains shared only 96 to 97% sequence identities with that of B. yuanmingense. Taken together, the grouping based on the similarity of dnaK, recA, and glnB correlated with the grouping based on the type of nodulation gene.
Fig 3.
Maximum likelihood tree based on combined sequences of housekeeping genes (dnaK, recA, and glnB), showing classification of divergent nod-containing Bradyrhizobium strains isolated from nodules of A. americana. Bootstrap value is expressed as percentages of 1,000 replications. The bar represents 1 estimated substitution per 100 nucleotide positions.
Phylogenetic analysis of nifH.
The partial DNA sequences of PCR-amplified nifH fragments were determined from the 40 strains isolated. On the basis of nifH sequence similarity, there are 2 major phylogenetic lineages, including nifH lineage 1, in which the photosynthetic bradyrhizobial strains were a majority, and nifH lineage 2, in which the nonphotosynthetic bradyrhizobial strains were a majority (Fig. 4). Interestingly, it was found that the nifH tree was well correlated with the grouping based on the type of nodulation genes (Table 3). In nifH lineage 2, the strains isolated from A. americana were divided into 2 major clusters, including a cluster of nod-containing strains and a cluster of divergent nod-containing strains. The divergent nod-containing strains were distinguished from the nod-containing strains with 61% bootstrap support. The exceptions were found for divergent nod-containing strains DOA1 and DOA2. The DOA1 and DOA2 strains were placed in the cluster of nod-containing strains, but they formed a separate subbranch distinct from the nod-containing strains and the other nonphotosynthetic species. In addition, the nitrogenase activity was correlated with the grouping based on nifH sequence similarity, because the divergent nod-containing strains tended to have higher nitrogen-fixing ability than the nod-containing strains in the free-living state (see Fig. S4 and Table S1 in the supplemental material). However, the acetylene reduction activities in the symbiosis state (see Fig. S5 and Table S1 in the supplemental material) and the dry weights of A. americana inoculated with the divergent nod-containing and nod-containing strains (see Fig. S6 and Table S1 in the supplemental material) were not significantly different.
Fig 4.
Maximum likelihood tree based on sequences of nifH genes showing classification of the Bradyrhizobium strains isolated from nodules of A. americana. Bootstrap values are expressed as percentages of 1,000 replications. The bar represents 1 estimated substitution per 100 nucleotide positions.
Infection and nodulation on Aeschynomene.
In order to determine the ability of nodulation on Aeschynomene isolates belonging to different CI groups, the strains DOA9 and SUTN9-2 were chosen as divergent nod-containing and nod-containing representatives, respectively. The strains were inoculated on A. indica (CI group 3) and A. afraspera (CI group 2), with strains ORS278 and ORS285 used as positive controls for A. indica and A. afraspera, respectively. Figure 5 shows the nodule morphologies of A. americana (CI group 1), A. afraspera (CI group 2), and A. indica (CI group 3) nodulated by the isolated strains. Tested on A. americana, the root nodules appeared 7 to 9 days after inoculation, and 3 weeks later, they were fully developed in vitro. Nodules were restricted to 2 main locations—the root and the lowest part of the stem (root primordial zone) located at the junction between the stem and the root. The root nodules were the aeschynomenoid type associated with the lateral root, established at the junctions of the parent roots and the lateral roots (Fig. 5A). The root nodules of A. americana were generally small, about 1 to 2 mm in diameter. The nodules were soft pink on external appearance and contained a red-pigmented central tissue, suggesting the presence of leghemoglobin and the effective bacteria.
Fig 5.
Infection and nodulation of Aeschynomene by the isolated strain DOA9 and SUTN9-2. (A) Nodules of A. americana inoculated with the strain DOA9. (B and C) Infection and accumulation in epidermis cells and root hairs of A. americana by GUS-tagged DOA9 (B) and GUS-tagged SUTN9-2 (C) 5 days after inoculation. (D and E) Nodules of A. afraspera inoculated with strain DOA9 (D) and strain SUTN9-2 (E) 21 days after inoculation. (F) One-kilobase DNA marker (lane1) and BOXAIR PCR fingerprints compared between bacterial culture of strain DOA9 (lane 2) and A. afraspera nodule inoculated with strain DOA9 (lane 3) and culture of strain SUTN9-2 (lane 4) and A. afraspera nodule inoculated with strain SUTN9-2 (lane 5). (G and H) Accumulation at the lateral root base of A. indica by GUS-tagged DOA9 (G) and GUS-tagged SUTN9-2 (H) 5 days after inoculation. (I) Nodules of A. indica inoculated with the strain SUTN9-2 21 days after inoculation. (J) One-kilobase DNA marker (lane 1) and BOXAIR PCR fingerprints compared between inoculated culture of strain SUTN9-2 (lane 2) and A. indica nodule inoculated with strain SUTN9-2 (lane 3).
In addition, strains DOA9 and SUTN9-2 were able to nodulate roots of A. afraspera, but only SUTN9-2 was able to nodulate roots of A. indica. None of the stem nodules of A. afraspera and A. indica was found after inoculation with both strains. The root nodules of A. afraspera appeared within 2 weeks, and those of A. indica appeared within 3 weeks after inoculation. Similar to A. americana, the nodules that formed on A. afraspera (Fig. 5D and E) and A. indica (Fig. 5I) were the aeschynomenoid type associated with the lateral root and located on 2 main parts, including the root and the lower and submerged part of the stem. BOXAIR1 fingerprints confirmed the ability to form root nodules on A. afraspera and A. indica by the bradyrhizobial strains originating from CI group 1 A. americana. Similar BOXAIR1 fingerprints were found from the pure cultures of the isolated strains (DOA9 and SUTN9-2) and from the nodules of A. afraspera inoculated with these strains (Fig. 5F). In the same way, the similar BOXAIR1 fingerprints were found from the pure cultures of the isolated strain SUTN9-2 and from the nodules of A. indica inoculated with these strains (Fig. 5J).
The divergent nod genes were unusual; therefore, it would be interesting to observe the infection process of the divergent nod-containing strain DOA9. Strain DOA9 was labeled with GUS and GFP and inoculated on seedlings of A. american, A. afraspera, and A. indica. Infected areas were observed every day for 10 days. Strain DOA9 was able to infect all of the tested plants (A. americana, A. afraspera, and A. indica) belonging to 3 different CI groups, and the infection features in each plant were similar. For example, the bacterial accumulation was found in root hairs and epidermis cells surrounding the lateral root origins of A. americana (Fig. 5B) and A. indica (Fig. 5G). The infection feature of strain SUT9-2 was also observed, and it was similar to that of strain DOA9 in all plants tested (Fig. 5C and H). Figure 6 shows A. americana root infected by strain DOA9. At 6 h after inoculation, DOA9 was found to attach at root hairs and epidermis cells surrounding the lateral root origins (Fig. 6B and C). Later, at 1 to 3 days after inoculation, accumulation of the bacterial cells was observed in root hairs and epidermis cells around lateral root primordia (Fig. 6D to F). No infection thread was observed. After 3 days, intercellular bacterial spreading was observed, and the infected area spread to the root cortex (Fig. 6G). The intracellular infection in cortex cells and the intercellular spreading in endodermis and steles were observed after 4 days (Fig. 6H). The expression of GUS in the nodules located at lateral root bases was observed after 2 weeks of inoculation (Fig. 6I and J). Thin sections of nodules collected from 2-week-old plants showed a high density of GUS-expressing bacterial cells in the infected area, and the bacterial cells were also seen in inner cortex cells surrounding the infected area of the nodule.
Fig 6.
(A to G) Micrographs of control uninoculated A. americana root (A) and GFP-tagged (B and C) and GUS-tagged (D to G) Bradyrhizobium strain DOA9 in A. americana roots. (B and C) Dense colonization of epidermal cell (B) and lateral root base (C) at 1 day after inoculation. (D) Close-up view of the root hair at 2 days after inoculation. (E) Bacterial entrapment within root hairs and epidermis cells 3 days after inoculation. (F) Bacterial cells cluster at the junction of root epidermal cells. (G) Infection on the root cortex and steles area 4 days after inoculation. (H) Intracellular infection in root cortex 4 days after inoculation. (I) A nodule establishing at the lateral root base. (J) Accumulation of bacteria in the infection zone of the nodule. Arrows indicate the intensities of GFP (B and C) and GUS (F and G) activities.
DISCUSSION
Bradyrhizobia have been described in the nodules of Aeschynomene (39), and most studies have focused on photosynthetic bradyrhizobial strains nodulating on Aeschynomene that belong to CI group 2 and CI group 3 (27). Extensive studies have been done for photosynthetic Bradyrhizobium sp. strains BTAi1 and ORS278, which nodulate A. indica and A. sensitiva belonging to CI group 3, respectively. These strains lack classical nodulation genes, so they are called “nod-independent strains” (27). In addition, a photosynthetic Bradyrhizobium strain, ORS285, from A. afraspera (CI group 2) was found to have coexistence of 2 nodulation mechanisms—a classical nod-dependent one with A. afraspera and a nod-independent one with A. indica (29). In this study, A. americana, which is a species belonging to CI group 1, was focused on. We found that all of the strains isolated from A. americana belonged to the genus Bradyrhizobium, based on their molecular characterization. B. yuanmingense was found to be the dominant species, and some of the other species, including B. japonicum, B. liaoningense, and B. canariense, were the minor species. The bradyrhizobial strains isolated from A. americana were nonphotosynthetic bacteria; therefore, they were distinguished from the photosynthetic strains nodulating the other Aeschynomene species belonging to CI group 2 and CI group 3 (27). In addition, all of the strains isolated from A. americana were able to nodulate M. atropurpureum, A. hypogaea, and V. radiata, and some strains were able to nodulate A. afraspera (e.g., DOA9 and SUTN9-2) and A. indica (e.g., SUTN9-2). However, the photosynthetic strains BTAi1 and ORS278 were more specific, with a narrow host range; they were not able to nodulate A. americana (28). These results support the occurrence of nonspecific and specific bradyrhizobial strains among Aeschynomene symbionts, with the photosynthetic strains being highly specific, and A. americana plants are commonly nodulated by Bradyrhizobium spp. of the cowpea miscellaneous group (43).
After detection of the canonical nodulation genes (nodA, nodB, and nodC), the isolated strains were divided into 2 groups: nod-containing strains and divergent nod-containing strains. The strains in both groups comprised various nodulation gene patterns based on the size of each hybridized signal (see Fig. S3 in the supplemental material). This result indicated the genomic variation of these strains. The taxonomic positions of the divergent nod-containing strains in the housekeeping gene trees were congruent with their positions in the 16S rRNA gene tree, pointing out that the divergent nod-containing strains were closely related to B. yuanmingense. Interestingly, based on nifH similarity, the nod-containing and divergent nod-containing strains were obviously distinguished (Fig. 4). The grouping based on nifH similarity was well correlated with the grouping based on types of nodulation genes (Table 3), Also, this finding correlated with the ability to fix nitrogen in the free-living state detected from the most of divergent nod-containing strains, which was reported to be restricted among rhizobia to photosynthetic bradyrhizobia (14). Moreover, we found that the A. americana strain SUTN9-2 could nodulate all CI group representatives of Aeschynomene, but ORS285 could nodulate only CI groups 2 and 3. This might be explained by the nod genes of the A. americana strains having broadened their host range. This was supported by the similar sequences of nodA and nodB to nonphotosynthetic bradyrhizobial species, separating them from the photosynthetic strains belonging to CI group 2. Alternatively, it was possible that the A. americana strains use another simple mode for invasion of the Aeschynomene species. For example, the invasion might involve direct invasion at the site of emerging lateral roots, and other signals (such as cytokinin) instead of the Nod factor might play a role in triggering infection and nodule formation (1, 2, 12). We found that the divergent nod-containing strain DOA9 could infect both A. afraspera and A. indica but could only establish nodules in A. afraspera. Possibly, the divergence of the divergent nod genes might impair the nodulation ability in A. indica. Altogether from these results, gene loss and lateral transfer of nodulation genes might explain this finding. It was hypothesized that the A. americana strains arose from a photosynthetic ancestor, and the adaptation of the bacteria in different environments led to loss of photosynthetic ability and acquisition of nodulation genes to broaden their host range to diverse Aeschynomene species. Two groups of the strains isolated from A. americana, nod-containing strains (nifH lineage 2) and divergent-nod containing strains (nifH lineage 1), possibly indicated independent acquisition events of nif and nod genes through lateral gene transfer. For the nod-containing strains, their evolution might involve the loss of a photosynthetic trait and nif genes of the photosynthetic bradyrhizobial ancestor, followed by acquisition of nif and nod genes from the other nonphotosynthetic species. For the divergent nod-containing strains, the nif genes of photosynthetic bradyrhizobia were still retained, and the sequence divergence of the acquired nod-genes from the other nonphotosynthetic species was driven later. However, the role of nodulation genes of the A. americana strains in the infection and nodulation of Aeschynomene species is still unclear. One or both possibly different infection modes, including divergent nod-dependent and nod-independent modes, might be involved. Therefore, it would be interesting to determine the role of the divergent nod gene and Nod-factor produced from the divergent nod gene products in nodulation ability.
This is the first explanation of the diversity of the nonphotosynthetic bradyrhizobial strains nodulating the CI group 1. In addition, we found that the canonical nodABC genes of the nod-containing strains differed from those of the other bradyrhizobia, and those of the divergent nod-containing strains were more diverse. We hypothesized that each nod gene might diverge from various origins. Therefore, the infection process of the divergent nod-containing strains with different CI group plants is attractive to study. Different rhizobial invasion processes have been described, of which two have been studied in detail (5, 16, 25, 41). The best-known entry mechanism is the root hair curling process (18). Upon recognition of proper rhizobia using Nod factor, the bacteria are entrapped within the curling root and induce the formation of an intracellular infection thread within the root hair. The infection thread proceeds intracellularly toward the cells of the nodule primordia. Another entry mechanism is lateral root base invasion (13). This process starts with colonization of the bacteria at intercellular spaces between cortical cells and then induces the formation of infection pockets, from which intercellular and intracellular infection threads guide the bacteria to the target cells for nitrogen fixation within the nodule primordia. From our study, we found that the A. americana nodulating strains used a different process that mixes up these 2 described processes to establish symbiosis on A. americana, A. afraspera, and A. indica. The proposed steps of infection on A. americana are shown in Fig. 7. In the early step, the bradyrhizobia attach at root hair and lateral root primordial origins and none of infection thread was observed. This early step is similar to those observed in A. indica and A. afraspera infected by Bradyrhizobium sp. strain ORS285 (3). Then, the bacterial invasion occurs via crack entry of the lateral root instead of using the epidermal root hair track. The invasion induces the formation of an intracellular accumulation of bacteria within the root hair and epidermis. Within the root epidermis, the dense bacterial cells cluster at the junction and intercellular spaces of root epidermis cells (Fig. 6E and F). Next, the bacteria spread further through the root cortex (Fig. 6H) in the intercellular matrix and then through the endodermis and stele area (Fig. 6G), and the same event was also found in both A. indica and A. afraspera inoculated with strains DOA9 and SUTN9-2 (data not shown). The cortex cells of these 3 species of Aeschynomene filled with bacterial cells also appeared without the infection thread formation (data not shown). It might be speculated that a phagocytic event might take place and that the plants initially utilized some of these functions to accidentally internalize bacteria through a direct mechanism resembling phagocytic events (16). At both ends of each epidermis cell, heavily clustered bradyrhizobial cells were found; however, it is still unclear whether bradyrhizobial cells first invaded at root hairs or epidermis cells, or whether they invaded both during the same period of time. Interestingly, detection of highly clustered bacterial cells at the both ends of epidermis cells implies the sink of nutrients for cell propagation prior to invasion of adjacent epidermis cells and intercellular movement downward to the cortex layer. The mechanisms behind this scenario will be further investigated.
Fig 7.
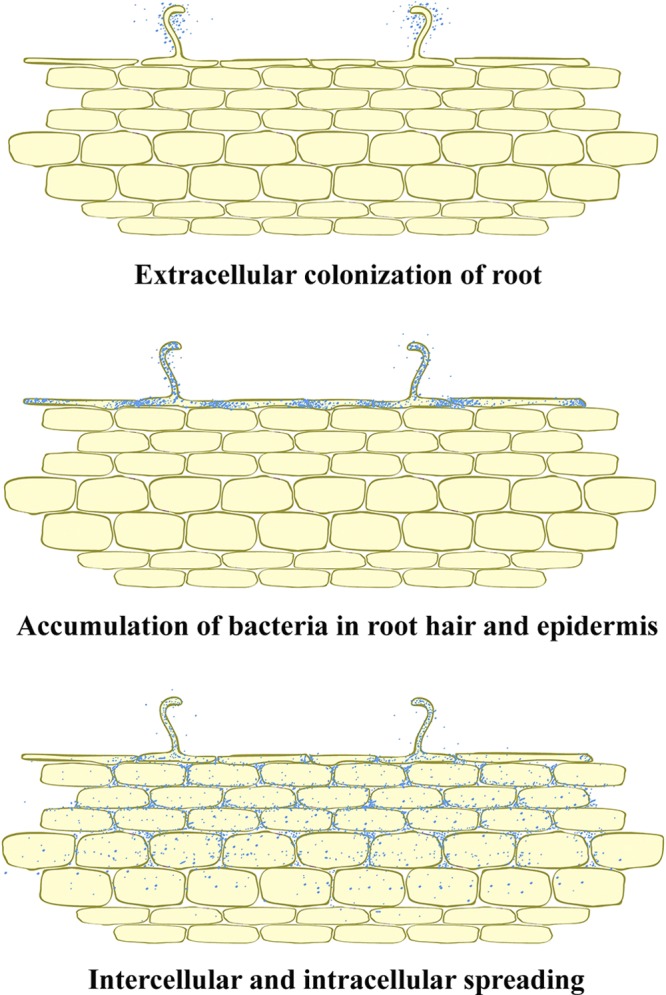
Proposed model of the infection process of A. americana by the divergent nod-containing bradyrhizobia.
The discovery in this study raises several interesting questions. It seems that the divergent nod genes (from both nod-containing and divergent nod-containing strains) play a role in the interaction between the bradyrhizobial strains and Aeschynomene species. What is or are the bacterial signals that initiate nodule formation in this system? What are the plant signal molecules that induce bacterial attachment and colonization on the root? Is there the molecular interaction between the bradyrhizobia and development of lateral root? The molecular level of the invasion pathways, including bacterial accumulation in root epidermis and the lateral root base process, would be attractive to study to provide understanding of infection pathways in leguminous plants. If the simple mode rather than the complex one via root hair curling and infection thread formation is used for invasion, it is possible to transfer these bacteria to nonleguminous plants.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Suranaree University of Technology, by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, and by a Ronpaku scholarship.
Footnotes
Published ahead of print 29 June 2012.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Alazard D, Duhoux E. 1990. Development of stem nodules in a tropical forage legume, Aeschynomene afraspera. J. Exp. Bot. 41: 1199–1206 [Google Scholar]
- 2. Boiero L, et al. 2007. Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl. Microbiol. Biotechnol. 74: 874–880 [DOI] [PubMed] [Google Scholar]
- 3. Bonaldi K, et al. 2011. Nodulation of Aeschynomene afraspera and A. indica by photosynthetic Bradyrhizobium sp. strain ORS285: the Nod-dependent versus the Nod-independent symbiotic interaction. Mol. Plant Microbe Interact. 24: 1359–1371 [DOI] [PubMed] [Google Scholar]
- 4. Bonaldi K, et al. 2010. Large-scale transposon mutagenesis of photosynthetic Bradyrhizobium sp. strain ORS278 reveals new genetic loci putatively important for Nod-independent symbiosis with Aeschynomene indica. Mol. Plant Microbe Interact. 23: 760–770 [DOI] [PubMed] [Google Scholar]
- 5. Capoen W, Oldroyd G, Goormachtig S, Holsters M. 2010. Sesbania rostrata: a case study of natural variation in legume nodulation. New Phytol. 186: 340–345 [DOI] [PubMed] [Google Scholar]
- 6. Chaintreuil C, Boivin C, Dreyfus B, Giraud E. 2001. Characterization of the common nodulation genes of the photosynthetic Bradyrhizobium sp. ORS285 reveals the presence of a new insertion sequence upstream of nodA. FEMS Microbiol. Lett. 194: 83–86 [DOI] [PubMed] [Google Scholar]
- 7. Cole MA, Elkan GH. 1973. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 4: 248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dereeper A, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36: W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Downie JA. 2007. Infectious heresy. Science 316: 1296–1297 [DOI] [PubMed] [Google Scholar]
- 10. Eaglesham ARJ, Szalay AA. 1983. Aerial stem nodules on Aeschynomene spp. Plant Sci. Lett. 29: 265–272 [Google Scholar]
- 11. Geurts R, Bisseling T. 2002. Rhizobium Nod factor perception and signalling. Plant Cell 14: S239–S249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giraud E, et al. 2007. Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316: 1307–1312 [DOI] [PubMed] [Google Scholar]
- 13. Goormachtig S, Capoen W, Holsters M. 2004. Rhizobium infection: lessons from the versatile nodulation behaviour of water-tolerant legumes. Trends Plant Sci. 9: 518–522 [DOI] [PubMed] [Google Scholar]
- 14. Gourion B, et al. 2011. Bacterial RuBisCO is required for efficient Bradyrhizobium/Aeschynomene symbiosis. PLoS One 6: e21900 doi:10.1371/journal.pone.0021900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52: 696–704 [DOI] [PubMed] [Google Scholar]
- 16. Held M, et al. 2010. Common and not so common symbiotic entry. Trends Plant Sci. 15: 540–545 [DOI] [PubMed] [Google Scholar]
- 17. Hotter GS, Scott DB. 1991. Exopolysaccharide mutants of Rhizobium loti are fully effective on a determinate nodulating host but are ineffective on an indeterminate nodulating host. J. Bacteriol. 173: 851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. 2007. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat. Rev. Microbiol. 5: 619–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jordan DC. 1982. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int. J. Syst. Bacteriol. 32: 136–139 [Google Scholar]
- 20. Krause A, Doerfel A, Göttfert M. 2002. Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium japonicum. Mol. Plant Microbe Interact. 15: 1228–1235 [DOI] [PubMed] [Google Scholar]
- 21. Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinformatics 9: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laguerre G, et al. 2001. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 147: 981–993 [DOI] [PubMed] [Google Scholar]
- 23. Lavin M, et al. 2001. The dalbergioid legumes (Fabaceae): delimitation of a pantropical monophyletic clade. Am. J. Bot. 88: 503–533 [PubMed] [Google Scholar]
- 24. Lorquin J, Molouba F, Dreyfus B. 1997. Identification of the carotenoid pigment canthaxanthin from photosynthetic Bradyrhizobium strains. Appl. Environ. Microbiol. 63: 1151–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madsen LH, et al. 2010. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 1: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manassila M, Nuntagij A, Kotepong S, Boonkerd N, Teaumroong N. 2007. Characterization and monitoring of selected rhizobial strains isolated from tree legumes in Thailand. Afr. J. Biotechnol. 6: 1393–1402 [Google Scholar]
- 27. Miché L, et al. 2010. Diversity analyses of Aeschynomene symbionts in Tropical Africa and Central America reveal that nod independent stem nodulation is not restricted to photosynthetic bradyrhizobia. Environ. Microbiol. 12: 2152–2164 [DOI] [PubMed] [Google Scholar]
- 28. Molouba F, et al. 1999. Photosynthetic bradyrhizobia from Aeschynomene spp. are specific to stem-nodulated species and form a separate 16S ribosomal DNA restriction fragment length polymorphism group. Appl. Environ. Microbiol. 65: 3084–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nzoué A, et al. 2009. Multilocus sequence analysis of bradyrhizobia isolated from Aeschynomene species in Senegal. Syst. Appl. Microbiol. 32: 400–412 [DOI] [PubMed] [Google Scholar]
- 30. Oldroyd GED, Downie JA. 2004. Calcium, kinases and nodulation signalling in legumes. Nat. Rev. Mol. Cell Biol. 5: 566–576 [DOI] [PubMed] [Google Scholar]
- 31. Perret X, Staehelin C, Broughton W. 2000. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 64: 180–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prakamhang J, Minamisawa K, Teamtaisong K, Boonkerd N, Teaumroong N. 2009. The communities of endophytic diazotrophic bacteria in cultivated rice (Oryza sativa L.). Appl. Soil Ecol. 42: 141–149 [Google Scholar]
- 33. Queiroz LP, Cardoso DBOS. 2008. A new species of Aeschynomene L. (Leguminosae, Papilionoideae) from a continental sand dune area in north, eastern Brazil. Bot. J. Linn. Soc. 157: 749–753 [Google Scholar]
- 34. Rivas R, Martens M, de Lajudie P, Willems A. 2009. Multilocus sequence analysis of the genus Bradyrhizobium. Syst. Appl. Microbiol. 32: 101–110 [DOI] [PubMed] [Google Scholar]
- 35. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425 [DOI] [PubMed] [Google Scholar]
- 36. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed, vol 1 Cold Spring Harbor Laboratory Press, New York, NY [Google Scholar]
- 37. Scott HN, Laible PD, Hanson DK. 2003. Sequences of versatile broad-host-range vectors of the RK2 family. Plasmid 50: 74–79 [DOI] [PubMed] [Google Scholar]
- 38. Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1: 784–791 [Google Scholar]
- 39. So RB, Ladha JK, Young JPW. 1994. Photosynthetic symbionts of Aeschynomene spp. form a cluster with bradyrhizobia on the basis of fatty acid and rRNA analyses. Int. J. Syst. Bacteriol. 44: 392–403 [DOI] [PubMed] [Google Scholar]
- 40. Somasegaran P, Hoben HJ. 1994. Handbook for rhizobia: methods in legume-rhizobium technology. Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 41. Spaink HP. 2000. Root nodulation and infection factors produced by rhizobial bacteria. Annu. Rev. Microbiol. 54: 257–288 [DOI] [PubMed] [Google Scholar]
- 42. Turner GL, Gibson AH. 1980. Measurement of nitrogen fixation by indirect means, p 111–139 In Bergensen FJ. (ed), Methods for evaluating biological nitrogen fixation. Wiley, Chichester, United Kingdom [Google Scholar]
- 43. van Berkum P, Tully RE, Keister DL. 1995. Nonpigmented and bacteriochlorophyll-containing bradyrhizobia isolated from Aeschynomene indica. Appl. Environ. Microbiol. 61: 623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Versalovic J, Schneider M, De Bruijn FJ, Lupski JR. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5: 25–40 [Google Scholar]
- 45. Vinuesa P, Silva C, Werner D, Martinez-Romero E. 2005. Population genetics and phylogenetic inference in bacterial molecular systematics: the roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol. Phylogenet. Evol. 34: 29–54 [DOI] [PubMed] [Google Scholar]
- 46. Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173: 697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson KJ, et al. 1995. β-Glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other Gram-negative bacteria. Microbiology 141: 1691–1705 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.