Abstract
Crenarchaeotal genomes encode the 3-hydroxypropionate/4-hydroxybutyrate (3-HP/4-HB) cycle for carbon dioxide fixation. Of the 13 enzymes putatively comprising the cycle, several of them, including methylmalonyl-coenzyme A (CoA) epimerase (MCE) and methylmalonyl-CoA mutase (MCM), which convert (S)-methylmalonyl-CoA to succinyl-CoA, have not been confirmed and characterized biochemically. In the genome of Metallosphaera sedula (optimal temperature [Topt], 73°C), the gene encoding MCE (Msed_0639) is adjacent to that encoding the catalytic subunit of MCM-α (Msed_0638), while the gene for the coenzyme B12-binding subunit of MCM (MCM-β) is located remotely (Msed_2055). The expression of all three genes was significantly upregulated under autotrophic compared to heterotrophic growth conditions, implying a role in CO2 fixation. Recombinant forms of MCE and MCM were produced in Escherichia coli; soluble, active MCM was produced only if MCM-α and MCM-β were coexpressed. MCE is a homodimer and MCM is a heterotetramer (α2β2) with specific activities of 218 and 2.2 μmol/min/mg, respectively, at 75°C. The heterotetrameric MCM differs from the homo- or heterodimeric orthologs in other organisms. MCE was activated by divalent cations (Ni2+, Co2+, and Mg2+), and the predicted metal binding/active sites were identified through sequence alignments with less-thermophilic MCEs. The conserved coenzyme B12-binding motif (DXHXXG-SXL-GG) was identified in M. sedula MCM-β. The two enzymes together catalyzed the two-step conversion of (S)-methylmalonyl-CoA to succinyl-CoA, consistent with their proposed role in the 3-HP/4-HB cycle. Based on the highly conserved occurrence of single copies of MCE and MCM in Sulfolobaceae genomes, the M. sedula enzymes are likely to be representatives of these enzymes in the 3-HP/4-HB cycle in crenarchaeal thermoacidophiles.
INTRODUCTION
It has become clear over the past decade that microorganisms fix CO2 into cellular biomass through a more diverse set of pathways than was previously thought (8, 9, 22). In particular, genomes within the crenarchaeal order Sulfolobales encode a pathway that purportedly converts CO2 into 3-hydroxypropionate and 4-hydroxybutyrate as part of a cycle that, in some cases, ultimately forms a molecule of acetyl-CoA from two molecules of CO2 (10). The identification and biochemical characteristics of many of the enzymes in this cycle have been reported by Fuchs and coworkers (1, 28, 30, 44, 50, 54), mostly focusing on a member of the Sulfolobales, Metallosphaera sedula (27). Supporting genome sequence information (6) and complementary transcriptomic data for growth of M. sedula under autotrophic and heterotrophic conditions helped elucidate the components of this cycle (5, 25). This extremely thermoacidophilic archaeon, which grows optimally at 73°C and pH 2, can utilize organic carbon (peptides) or CO2 as its carbon source and metal sulfides, organic carbon, and/or H2 as an energy source (4). Recently, the relationship of the so-called “3-hydroxypropionate/4-hydroxybutyrate cycle” to central carbon metabolism in M. sedula was determined, indicating that succinyl-CoA and acetyl-CoA were the only two intermediates removed from the cycle to form precursor metabolites (17). Whether this is the case for the other members of the Sulfolobales harboring the enzymes making up this cycle has not been determined.
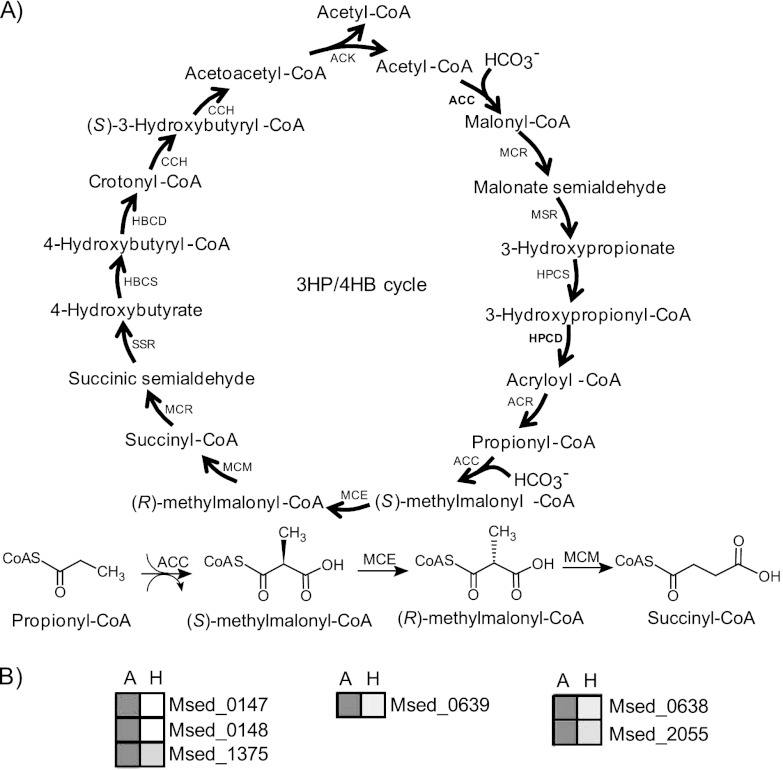
Although activities corresponding to all enzymes in the proposed 3-HP/4-HB pathway have been detected in M. sedula cell extracts, not all of them have been confirmed and characterized biochemically (see Table 1 and Fig. 1A). Of the 13 enzymes catalyzing the 16 steps in the cycle, 3 enzymes (ACC, MCR, and CCH) are bifunctional; 8 of the 13 (ACC [28, 44], MCR [30], MSR [30], HPCS [1], HPCD [54], ACR [54], SSR [30], and CCH [50]) have been characterized in purified form, and activities corresponding to the remaining 5 (MCE [9], MCM [9], HBCS [9], HBCD [9], and ACK [9]) have been detected in cell extracts. Of interest here are the identity and biochemical properties of the methylmalonyl-CoA epimerase and methylmalonyl-CoA mutase in the M. sedula 3-HP/4-HB cycle. Versions of these two enzymes have been identified in the genomes of bacteria (2, 34, 37–39, 43, 52, 56), animals (13, 20, 33, 53, 55), and algae (47), as well as in archaea (14), but have not been implicated in CO2 fixation pathways. Previous studies on MCEs and MCMs showed that these enzymes catalyze the reversible conversion of (S)-methylmalonyl-CoA to succinyl-CoA, which is a key intermediate in the tricarboxylic acid cycle. In animals, MCE and MCM are involved in glyoxylate regeneration (31) and metabolism of propionate, branched-chain amino acids, and odd-chain fatty acids, where propionyl-CoA is converted to succinyl-CoA via (R,S)-methylmalonyl-CoA (23, 29). The reverse pathway is found in prokaryotes, i.e., synthesis of propionate from succinyl-CoA in the TCA cycle via propionyl-CoA and (R,S)-methylmalonyl-CoA (3, 23, 42). In this report, we confirm the identity of MCE and MCM in M. sedula and present the biochemical properties of the enzymes as these relate to the 3-HP/4-HB cycle.
Table 1.
Enzymes in the 3-HP/4-HB cycle in Metallosphaera sedula
| 3-HP/4-HB pathway step(s) | Enzyme reference no. | ORF | Enzyme | Comment(s) (reference[s]); source |
|---|---|---|---|---|
| 1, 7 | ACCα | Msed_0147(α) | Acetyl-CoA/propionyl-CoA carboxylase | Native (28, 44) |
| ACCβ | Msed_0148(β) | |||
| ACCγ | Msed_1375(γ) | |||
| 2, 11 | MCR | Msed_0709 | Malonyl-CoA/succinyl-CoA reductase | Recombinant (30) |
| 3 | MSR | Msed_1993 | Malonate semialdehyde reductase | Recombinant (30) |
| 4 | HPCS | Msed_1456 | 3-Hydroxypropionyl-CoA synthetase | Native (1) |
| 5 | HPCD | Msed_2001 | 3-Hydroxypropionyl-CoA dehydratase | Native, recombinant (54) |
| 6 | ACR | Msed_1426 | Acryloyl-CoA reductase | Native (54) |
| 8 | MCE | Msed_0639 | Methylmalonyl-CoA epimerase | Native (cell extracts) (9); this work |
| 9 | MCMα | Msed_0638 | Methylmalonyl-CoA mutase (catalytic subunit) | Native (cell extracts) (9); this work |
| MCMβ | Msed_2055 | Methylmalonyl-CoA mutase (coenzyme B12-binding subunit) | Native (cell extracts) (9); this work | |
| 10 | SSR | Msed_1424 | Succinate semialdehyde reductase | Native, recombinant (30) |
| 12 | HBCS | Unknown | 4-Hydroxybutyrate-CoA synthetase | Native (cell extracts) (9) |
| 13 | HBCD | Msed_1321 | 4-Hydroxybutyrl-CoA dehydratase | Native (cell extracts) (9) |
| 14, 15 | CCH | Msed_0399 | Crotonyl-CoA hydratase/(S)-3-hydroxybutyrl-CoA dehydrogenase | Recombinant (50) |
| 16 | ACK | Msed_0656 | Acetoacetyl-CoA β-ketothiolase | Native (cell extracts) (9) |
Fig 1.
Proposed autotrophic 3-hydroxypropionate/4-hydroxybutyrate cycle in M. sedula. (A) Enzymes ACC, MCR, MSR, HPCS, HPCD, ACR, MCE, MCM, SSR, HBCS, HBCD, CCH, and ACK (see Table 1). (B) Heat plot showing ACC (Msed_0147, Msed_0148, Msed_1375), MCE (Msed_0639), and MCM (Msed_0638, Msed_2055) under autotrophic (columns A) and heterotrophic (columns H) conditions. Normalized transcription levels are depicted in grayscale, with darker shading correlating with higher transcription levels.
MATERIALS AND METHODS
Abbreviations.
Methylmalonyl-coenzyme A (CoA) epimerase (MCE); the gene encoding methylmalonyl-CoA epimerase (mce); methylmalonyl-CoA mutase (MCM); the catalytic subunit of methylmalonyl-CoA mutase (MCM-α); the gene encoding the catalytic subunit of methylmalonyl-CoA mutase (mcm-α); coenzyme B12-binding subunit of methylmalonyl-CoA mutase (MCM-β); the gene encoding coenzyme B12-binding subunit of methylmalonyl-CoA mutase (mcm-β); holoenzyme MCM (holo-MCM); 3-hydroxypropionate/4-hydroxybutyrate (3-HP/4-HB) cycle; amino acid (aa); open reading frame (ORF); polyacrylamide gel electrophoresis (PAGE); sodium dodecyl sulfate (SDS); base pair (bp); kilobase (kb); high-performance liquid chromatography (HPLC); molecular mass cutoff (MMCO); fast protein liquid chromatography (FPLC); isobutyryl-CoA mutase (ICM); glutamate mutase (GLM); kilodalton (kDa); isopropyl-β-d-thiogalactopyranoside (IPTG); Metallosphaera sedula (Msed); enterokinase/ligation-independent cloning (Ek/LIC); bovine serum albumin (BSA); Protein Data Bank (PDB); immobilized metal ion affinity chromatography (IMAC); methylmalonyl-CoA (MM-CoA); succinyl-CoA (SC-CoA); tricarboxylic acid cycle (TCA); acetyl-CoA/propionyl-CoA carboxylase (ACC); malonyl-CoA/succinyl-CoA reductase (MCR); malonate semialdehyde reductase (MSR); 3-hydroxypropionyl-CoA synthetase (HPCS); 3-hydroxypropionyl-CoA dehydratase (HPCD); acryloyl-CoA reductase (ACR); succinic semialdehyde reductase (SSR); 4-hydroxybutyryl-CoA synthetase (HBCS); 4-hydroxybutyryl-CoA dehydratase (HBCD); crotonyl-CoA hydratase/(S)-3-hydroxybutyryl-CoA dehydrogenase (CCH); acetoacetyl-CoA β-ketothiolase (ACK); 4-hydroxybutyrate-CoA ligase (HBCL).
Materials.
M. sedula (DSM 5348) was grown at 70°C on a chemically defined medium (DSMZ 88, pH 2.0), and genomic DNA was purified, as reported previously (6). Strains and vectors used included a pET-46b EK/LIC cloning kit, a pRSF-2 Ek/LIC vector kit, and NovaBlue GigaSingles Escherichia coli competent cells (Novagen, San Diego, CA) and Rosetta (DE3) E. coli competent cells (Stratagene, La Jolla, CA). Other reagents and chemicals used included a Quickload DNA ladder (New England BioLabs, Ipswich, MA) (100 bp); a QlAquick gel extraction kit and a QIAprep Spin Miniprep kit (Qiagen, Inc., Valencia, CA); Amicon Ultra 10k centrifugal filter units (Millipore, Billerica, MA); isopropyl β-d-thiogalactopyranoside, antibiotics, agar, agarose, sodium chloride, EDTA, tryptone, sodium acetate, acetic acid, methanol, and K2HPO4 and KH2PO4 (Fisher Scientific, Pittsburgh, PA); imidazole (ACROS Organics, Geel, Belgium); a HiTrap column (GE Healthcare Bio-Sciences Corp., Piscataway, NJ); a BenchMark protein ladder (10 to 220 kDa) and NativeMark unstained protein standard (Invitrogen); methylmalonyl coenzyme A tetralithium salt hydrate, succinyl coenzyme A sodium salt, and adenosyl-cobalamin (Sigma Chemical Co., St. Louis, MO); GelCode Blue stain reagent (Thermo Fisher Scientific Inc., Rockford, IL); and Bio-Rad protein assay dye reagent (Hercules, CA).
Transcriptional response analysis.
To identify the genes encoding MCE and MCM in the M. sedula genome, a whole-genome oligonucleotide microarray for M. sedula was used to follow the transcriptomes for growth under autotrophic and heterotrophic conditions (5, 25). Briefly, M. sedula cells were grown aerobically at 70°C in a shaking oil bath (70 rpm) under autotrophic or heterotrophic conditions on a chemically defined medium (DSM 88) at pH 2.0. Cell growth was scaled up to 2 liters in a stirred benchtop glass fermentor (Applikon Biotechnology, Foster City, CA). For autotrophic conditions, M. sedula was grown on DSM 88 (pH 2), with gas feed 1 at 1 ml/min of H2 (80%) and N2 (20%) and gas feed 2 at 100 ml/min of air (78% N2, 21% O2, 0.03% CO2). Heterotrophically grown cells were cultivated on DSM 88 (pH 2), supplemented with 0.1% tryptone, with gas feed 1 at 1 ml/min of N2 (80%) and CO2 (20%) and gas feed 2 at 100 ml/min of air (78% N2, 21% O2, 0.03% CO2). Cells were harvested at mid-exponential phase by rapid cooling with dry ice and ethanol and then centrifuged at 6,000 rpm for 15 min at 4°C. RNA was extracted, purified, and reverse transcribed, as described elsewhere (6), and then used for hybridization with microarray slides. Microarray slides were scanned with a GenePix 4000B microarray scanner (Molecular Devices, Sunnyvale, CA).
Cloning, expression, and purification of MCE and MCM.
Genes encoding putative MCE and MCM in the M. sedula genome were identified by amino acid sequence alignment with enzymes of similar function in the NCBI database, in conjunction with transcriptomic analysis (5). Msed_0638 and Msed_2055 were predicted to be the catalytic subunit (MCM-α) and coenzyme B12-binding subunit (MCM-β), respectively, of MCM, while Msed_0639 corresponded to MCE. These genes (mce, mcm-α, and mcm-β) were amplified using genomic DNA as the template (primers synthesized by Integrated DNA Technologies [Coralville, IA] are listed in Table S1 in the supplemental material). mce and mcm-α were ligated into pET46; mcm-β was ligated into pRSF-2 Ek/LIC, according to the protocol of an EK/LIC cloning kit. Plasmids pET46-MCE, pET46-MCM-α, and pRSF-MCM(β) transformed into NovaBlue GigaSingles E. coli cells were extracted with a QIAprep Spin Miniprep kit and with the sequence confirmed by Eton Bioscience Inc. (Durham, NC). Plasmid pET46-MCE was transformed into E. coli Rosetta 2 (DE3) by heat shock and selected by overnight growth on Luria-Bertani (LB) agar medium supplemented with the two antibiotics (ampicillin, 100 μg/ml; chloramphenicol, 50 μg/ml) at 37°C. For coexpression, pET46-MCM-α and pRSF-MCM(β) were transformed into E. coli Rosetta 2 (DE3) and selected by growth on LB agar medium supplemented with three antibiotics (ampicillin, 100 μg/ml; streptomycin; 50 μg/ml; chloramphenicol, 50 μg/ml).
Recombinant protein was produced and purified as reported elsewhere (24) with minor modification. E. coli Rosetta 2 (DE3) cells harboring the recombinant plasmids were induced with IPTG (final concentration 0.1 mM) at an optical density at 600 nm (OD600) of 0.4 at 16°C and then cultured for another 15 h at 16°C. Cells were harvested by centrifuging at 5,000 rpm for 10 min at 4°C and then resuspended in binding buffer (50 mM K2HPO4-KH2PO4, 300 mM NaCl, pH 7.5) and lysed by sonication (S-4000; Misonix Ultrasonic Liquid Processors, Farmingdale, NY) for 10 min with 10-s off/on pulses. The cell extract was then centrifuged at 8,000 × g for 30 min at 4°C. The heat-sensitive proteins in the supernatant were removed by incubating for 10 min at 65°C and centrifuging at 8,000 × g for 30 min at 4°C. The supernatant containing MCE and MCM was applied to a HiTrap column for purification, eluted (50 mM K2HPO4-KH2PO4, 300 mM NaCl, 300 mM imidazole, pH 7.5), and monitored by A280 analysis using FPLC (BioLogic DuoFlow system; Bio-Rad, Hercules, CA). Protein purity was determined by SDS-PAGE. Samples containing MCE and MCM were concentrated and exchanged into phosphate buffer (50 mM K2HPO4-KH2PO4, 150 mM NaCl, pH 7.0) by the use of Amicon Ultra-10 centrifugal filter units (10,000 MMCO). MCE was then further purified by size exclusion chromatography (Superdex 75 10/300 GL; GE Healthcare); the column was preequilibrated with buffer (50 mM K2HPO4-KH2PO4, 150 mM NaCl, pH 7.0), and the sample was added and eluted at a flow rate of 0.5 ml/min and monitored at A280 in FPLC. Protein concentrations were determined using the method of Bradford with protein assay dye reagent from Bio-Rad (Hercules, CA) at 595 nm with BSA as a standard.
Homogeneity of coexpressed MCM (α and β) from native PAGE.
Homogeneity of coexpressed MCM subunits (α and β) was analyzed with acidic and basic gel electrophoresis, respectively. For basic electrophoresis, the coexpressed MCM (α or β) was loaded onto a 4% to 12% (wt/vol) Bis-Tris Novex polyacrylamide gel, using running buffer (pH 8.8) according to the Invitrogen protocol. Acidic electrophoresis (12% [wt/vol]) was done with a stacking gel (3% acetate–KOH, pH 6.8) and separating gel (10% acetate-KOH, pH 4.3), using a running buffer of β-alanine–acetic acid (pH 4.6). The electrophoresis was conducted at 100 V until the front Bromo Phenol Blue (basic) or Methyl Green (acidic) indicator reached the bottom, and then the reaction mixture was stained with GelCode Blue stain reagent and destained with water. In basic electrophoresis, NativeMark unstained protein standards (1,048, 720, 480, 242, 146, 66, and 20 kDa) were used.
Molecular mass determination for MCE and MCM.
The apparent molecular masses of MCE and MCM were determined by size exclusion chromatography. MCE in K2HPO4-KH2PO4 buffer was separated using Bio-Rad FPLC and a Superdex 75 column. For MCM (α and β); the enzymes were first incubated with coenzyme B12 (63 μM), dithiothreitol (2 mM), and KCl (10 mM) for 12 h at 4°C and then separated using Bio-Rad FPLC and a Superdex 200 10/300GL column (GE Healthcare). In both cases, columns were preequilibrated with buffer (50 mM K2HPO4-KH2PO4, 150 mM NaCl, pH 7.0), after which MCE or coexpressed MCM (α and β) (each at 0.3 mg/ml, 200 μl) was loaded and washed with same buffer at a flow rate of 0.5 ml/min; the elution was monitored at 280 nm. Fractions were collected at 1 ml/tube and analyzed by SDS-PAGE. Calibration curves for the Superdex 200 and Superdex 75 were done using the same buffer and protein standards: blue dextran (2,000 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), BSA (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.4 kDa). Calibration curves were generated by plotting the retention volume versus the logarithm of molecular masses of protein standards (24). The apparent molecular masses of MCE and coexpressed MCM (α and β) were determined based on elution volume using the calibration curves.
Measurement of MCE and MCM activity.
Holo-MCM was prepared in the dark by incubating coexpressed MCM (1.6 μM) and coenzyme B12 (63 μM) in dithiothreitol (2 mM), HEPES (10 mM, pH 7), NaCl (25 mM), and KCl (10 mM) and kept at 4°C until assayed. MCM activity was determined by incubating (R,S)-methylmalonyl-CoA with holo-MCM (final concentration 20 nM) at a final volume of 200 μl at 65°C for 5 min, with the reaction being terminated by the addition of acetic acid (1 M, 50 μl). MCE activity was measured by using a coupled assay described by Bobik and Rasche (12), with minor modification. In the coupled assay, (S)-methylmalonyl-CoA was converted to (R)-methylmalonyl-CoA by MCE, and the latter was then converted to succinyl-CoA by MCM. Initially, (R,S)-methylmalonyl-CoA was incubated with holo-MCM (in K2HPO4-KH2PO4 [pH 7, 50 mM]–NaCl [25 mM]) at 65°C with shaking at 300 rpm. MCE (final concentration 20 nM) and NiCl2 (final concentration 2 mM) were then added and incubated for another 5 min at a final volume of 200 μl. The reaction was then terminated by the addition of acetic acid (1 M, 50 μl). The disappearance of methylmalonyl–CoA and production of succinyl-CoA was measured using a Waters HPLC apparatus (2487 dual-absorbance detector and a 717 Plus autosampler) and a NovaPak C18 column (3.9 by 150 mm) equipped with a C18 Sentry guard column (Waters, Milford, MA), as described by Bobik and Rasche (12). The elution profile was monitored at 260 nm and analyzed by the use of buffers as follows: for min 0 to 12, 0% to 60% buffer B (10% sodium acetate [100 mM, pH 4.6], 90% methanol); for min 13 to 16, 60% buffer B; and for min 17 to 20, 100% buffer A (90% sodium acetate [100 mM, pH 4.6], 10% methanol). For quantification, calibration curves for (R,S)-methylmalonyl–CoA (20, 40, 80, and 120 μM) and succinyl-CoA (20, 40, 80, and 120 μM) were prepared by plotting the area versus concentration.
Determination of MCE and MCM specific activities.
The specific activity of holo-MCM was determined by incubating an appropriate amount of enzyme with (R,S)-methylmalonyl–CoA (0.2 mM) or succinyl-CoA (0.15 mM) at 75°C in K2HPO4-KH2PO4 buffer. The specific activity of MCE was determined using a coupled assay with (R,S)-methylmalonyl–CoA (0.2 mM) as the substrate. The reactions were initiated by the addition of excess holo-MCM (80 nM) in K2HPO4-KH2PO4 buffer, and the reaction mixtures were then incubated for 5 min at 75°C, after which MCE was added to the mixture. The reaction was stopped by the addition of acetic acid (1 M), and the amount of methylmalonyl–CoA was determined by HPLC. A linear reaction curve was obtained for both enzymes.
Effects of divalent metals on MCE activity.
To determine the effects of divalent metals on MCE activity, assays were carried out, as described above, with a few modifications. In the initial 5 min, (R,S)-methylmalonyl-CoA (50 μM) was incubated with holo-MCM in K2HPO4-KH2PO4 buffer (pH 7, 50 mM) at 65°C. MCE (5 nM) was then added to the mixture, with or without the addition of divalent metals (Ni2+, Co2+, Mg2+) and reacted for another 2.5 min. The divalent metals were provided as chloride or sulfate salts at a final concentration of 2 mM in the reaction mixture. The reaction was terminated by acetic acid, and the amount of methylmalonyl–CoA was assayed by HPLC.
Thermal activity profile for MCE and MCM.
The activity of MCE and coexpressed MCM (α and β) was determined as a function of temperature (50 to 80°C). The substrate (R,S)-methylmalonyl-CoA (75 μM) was incubated with holo-MCM (20 nM) for 5 min at each temperature, MCE (5 nM) and NiCl2 (2 mM) were added, and the mixture was incubated for another 5 min at each temperature. The final concentration of methylmalonyl-CoA was measured by HPLC, as described above.
Alignments of MCE, MCM-α, and MCM-β and protein structure prediction.
For secondary structural and conserved amino acid sequence analysis, multiple-sequence alignment of MCE, MCM-α, and MCM-β was generated from different organisms, using ClustalW (http://www.genome.jp/tools/clustalw/) and the ESPript V2.2 alignment program (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). The three-dimensional homology models of MCE, MCM-α, and MCM-β were constructed using the ModWeb online server (http://modbase.compbio.ucsf.edu/ModWeb20-html/modweb.html), based on sequence similarity (18, 19), and the structural model visualization was then analyzed using UCSF Chimera (http://www.cgl.ucsf.edu/chimera/). The predicted structural model of MCE was created by using the hypothetical protein BH1468 from Bacillus halodurans C-125 (PDB accession no. 3OA4) as the template. The structural models of MCM-α and MCM-β were created using MCM from Homo sapiens (PDB accession no. 2XIJ_A) (21) and MCM-β of Aeropyrumpernix K1 (PDB accession no. 2YXB), respectively, as the templates.
RESULTS AND DISCUSSION
Identification of M. sedula MCE and MCM from the genome sequence and transcriptional response analysis.
All members of the Sulfolobaceae with sequenced genomes appear to contain the genes for a CO2 fixation cycle that, when primed with acetyl-CoA, generates an additional acetyl-CoA by fixing two molecules of CO2 (see Fig. 1A; see also Table S2 in the supplemental material). In support of this, Fuchs and coworkers have provided convincing biochemical evidence for the proposed steps in the M. sedula 3-HP/4-HB carbon fixation cycle by characterization of individual, purified enzymes in native and/or recombinant forms (1, 28, 30, 44, 54), by carbon flux analysis (17), or by activity assays using partially purified cell extracts (9, 50) (see Table 1 and Fig. 1A). However, the specific genes that encode the enzymes responsible for certain steps have yet to be confirmed and characterized biochemically (15, 50). Of interest here are those steps that catalyze the conversion of (S)-methylmalonyl-CoA to succinyl-CoA. The M. sedula genome encodes a putative methylmalonyl-CoA epimerase (Msed_0639; MCE) collocated with but oriented divergently from a putative catalytic subunit of methylmalonyl-CoA mutase (Msed_0638) (see Table S3 in the supplemental material). The relative locations of these two genes are conserved in other members of the Sulfolobaceae but not in all thermophilic archaea (see Table S3 and Table S4 in the supplemental material). In M. sedula, the genes adjacent to those encoding MCE and MCM (catalytic subunit) are unrelated and are not involved in the 3-HP/4-HB cycle. MCM homologs in other organisms typically contain a coenzyme B12-binding subunit encoded with the catalytic subunit, but this is not the case in M. sedula; rather, Msed_2055 is homologous to the expected coenzyme B12-binding subunit. No other gene proximate to Msed_2055 appears to be directly related to the 3-HP/4-HB cycle. However, Msed_2056 is annotated as a putative LAO/AO transport system ATPase and could play a role, as has been suggested, in converting hydroxy-B12 to coenzyme B12 (13) or in protecting the MCM complex from inactivation during catalysis (32).
Transcriptomic analyses of M. sedula grown under autotrophic versus heterotrophic conditions indicated that the expression of Msed_0639 (MCE), Msed_0638 (MCM-α), and Msed_2055 (MCM-β) is significantly upregulated during CO2 fixation (Fig. 1B; see also Table S5 in the supplemental material). Genes adjacent to Msed_2055, namely, Msed_2056 and Msed_2057, were upregulated under autotrophic conditions, while Msed_0637, Msed_0641, and Msed_2054 were downregulated (see Table S5 in the supplemental material). Based on this information, the genes encoding the putative MCE and MCM were investigated further to determine if their gene products catalyzed the relevant reactions in the cycle.
Molecular assembly of MCE (Msed_0639) and MCM (Msed_0638, Msed_2055).
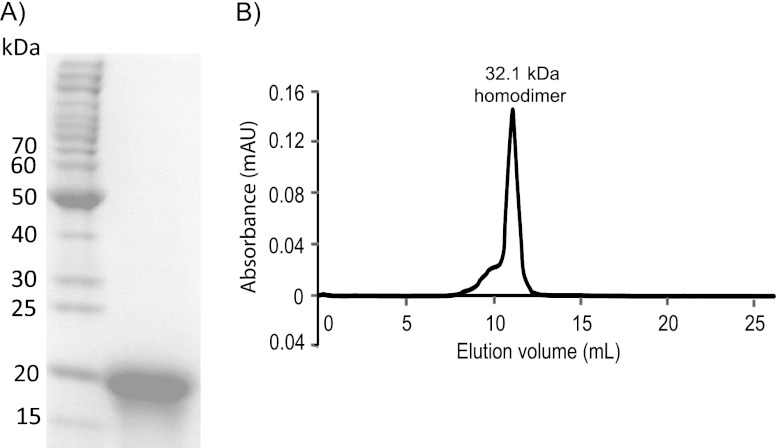
MCE, encoded by Msed_0639, was produced recombinantly in E. coli with an N-terminal hexahistidine tag, yielding an ∼20-kDa protein (SDS-PAGE), consistent with the expected Mr of 17.3 (tag plus protein; see Fig. 2A). As shown in Fig. 2B, MCE eluted as a single peak from a size exclusion chromatography column with an apparent Mr of 32.1. These data indicate that the enzyme is a homodimer, consistent with reports on MCEs from microorganisms unable to fix CO2, such as the hyperthermophilic archaeon Pyrococcus horikoshii and the anaerobic mesophilic bacterium Propionibacterium shermanii (14, 34).
Fig 2.
Molecular assembly of recombinant M. sedula MCE and quaternary structure analysis. (A) The recombinant MCE purified by heat treatment and IMAC was analyzed by SDS-PAGE. (B) The quaternary structure of MCE was assayed by using size exclusion chromatography (Superdex 75).
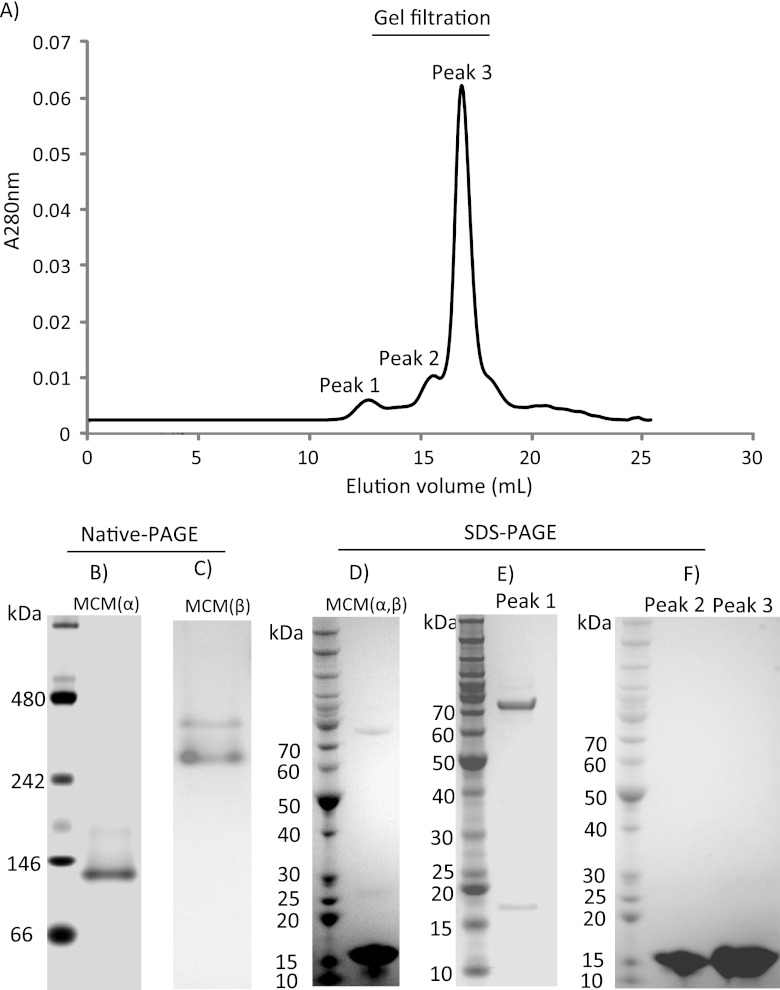
The catalytic (MCM-α; 64.6-kDa) and coenzyme-binding (MCM-β; 17.0-kDa) subunits (each with hexahistidine affinity tags) of MCM from M. sedula were coexpressed in E. coli. Attempts to express soluble forms of MCM-α separately were unsuccessful. Two bands corresponding to MCM-α and MCM-β were observed after SDS-PAGE for MCM purified by heat treatment and IMAC (Fig. 3D). Since the predicted isoelectric points (pI) of MCM-α and MCM-β were 5.8 and 8.8, respectively, basic and acidic native PAGE experiments were used to examine assembly of coexpressed subunits. MCM-α gave rise to a protein band of ∼140 kDa (Fig. 3B), indicating a dimer. For MCM-β, two bands were observed in acidic native PAGE (Fig. 3C). When the two coexpressed subunits incubated with coenzyme B12 were subjected to size exclusion chromatography for MCM assembly analysis, three peaks (173.7 kDa, 36.2 kDa, and 17.9 kDa) corresponding to the fully assembled enzyme, dimeric MCM-β, and monomeric MCM-β were observed (see Fig. 3A). The three peaks were collected and examined by SDS-PAGE. For the 173.7-kDa peak, two bands were observed at approximately 70 kDa and 17 kDa (Fig. 3E), suggesting that the MCM holoenzyme assembly is heterotetrameric (α2β2). Peak 2 and peak 3 yielded only a single band of 17 kDa by SDS-PAGE (Fig. 3F), indicating that MCM-β is a homodimer (peak 2) and monomer (peak 3), consistent with the result of the acidic native gel analysis for coexpressed MCM.
Fig 3.
Molecular assembly of M. sedula MCM and quaternary structure analysis. (A) Size exclusion chromatography (Superdex 200) of recombinant MCM purified through heat treatment and IMAC. (B) Basic native PAGE of recombinant coexpressed MCM. (C) Acidic native PAGE of recombinant coexpressed MCM (no Mr ladder available). (D) SDS-PAGE of recombinant coexpressed MCM purified through heat treatment and IMAC. (E and F) SDS-PAGE of elution peaks for recombinant coexpressed MCM from size exclusion chromatography.
Relationship of M. sedula MCE and MME to homologs in other organisms.
All characterized MCEs are homodimeric enzymes, with Mr values comparable to that of the M. sedula enzyme (∼30). The specific activity of M. sedula MCE (217.9 ± 1.6 μmol/min/mg) is comparable to that of P. horikoshii, lower than those of the MCEs from Homo sapiens (833 μmol/min/mg) and P. shermanii (608 μmol/min/mg), and much lower than that of the enzyme from Mus musculus (8,400 μmol/min/mg). Phylogenetic analysis of the MCE versions listed in Table 2 (see Fig. 4) indicates that the M. sedula enzyme is most closely related to this enzyme from other thermophilic microorganisms.
Table 2.
Features of biochemically characterized MCE and MCM
| Source | Mr (kDa) | Structure (Mr subunit[s]) | Specific activity (μmol/min/mg)a |
Function(s)b | Source or reference | |
|---|---|---|---|---|---|---|
| MM-CoA | SC-CoA | |||||
| MCE | ||||||
| Metallosphaera sedula | 32.1 | α2 (17.3) | 217.9 | N/Ac | A, B | This work |
| Pyrococcus horikoshii | 31.7 | α2 (16) | 162 | N/A | B | (14) |
| Propionibacterium shermanii | 33 | αi (17) | 33.4/607.5 | N/A | B | (2, 34, 41) |
| Thermoanaerobacter tengcongensis | N/A | α2 | N/A | N/A | B | (52) |
| Homo sapiens | N/A | N/A (15) | 833 | N/A | B | (13) |
| Caenorhabditis elegans | N/A | N/A (15) | N/A | N/A | B | (33) |
| Mus musculus | 32 | α2 (16) | 8,400 | N/A | B | (53) |
| Bacillus halodurans C-125 | N/A | N/A (18.2) | N/A | N/A | N/A | N/A |
| MCM | ||||||
| Metallosphaera sedula | 174 | α2 β2 (65/17) | 2.2 | 40.7 | A, B | This work |
| Pleurochrysis carterae | 150 | α2 (80) | 11.9 | N/A | B | (47) |
| Sinorhizobium meliloti | 165 | α2 (80) | 10.9 | N/A | B | (46) |
| Propionibacterium shermanli | 165 | αβ (80/69) | N/A | N/A | B | (37–39, 43, 56) |
| Methylobacterium extorquens | 150 | αβ (85/70) | N/A | N/A | B | (48) |
| Streptomyces cinnamonensis | 144 | αβ (79/65) | N/A | N/A | B | (11) |
| Escherichia coli | N/A | N/A (80) | 4.1 | N/A | B | (12) |
| Homo sapiens | 150 | α2 (78) | N/A | N/A | B | (20) |
| Mus musculus | N/A | N/A | N/A | N/A | B | (55) |
| Euglena gracilis Z | 149 | α2 (75) | 21 | N/A | B | (45) |
| Aeropyrum pernix | N/A | N/A (Mrβ, 18.2) | N/A | N/A | N/A | N/A |
MM-CoA, (R,S)-methylmalonyl-CoA; SC-CoA, succinyl-CoA.
A, autotrophic carbon fixation; B, metabolism of branched amino acids (valine, isoleucine, and methionine) and propanoate.
N/A, not available.
Fig 4.
Phylogenetic tree of characterized MCE. The organisms include Propionibacterium shermanii (P. shermanii), Pyrococcus horikoshii (P. horikoshii), Metallosphaera sedula DSM 5348 (M. sedula), Thermoanaerobacter tengcongensis (T. tengcongensis), Caenorhabditis elegans (C. elegans), Homo sapiens (H. sapiens), and Rattus norvegicus (R. norvegicus).
Crystal structures exist for microbial MCEs from the moderately thermophilic bacterium Thermoanaerobacter tengcongensis (52) and the mesophilic bacteria P. shermanii (41) and Bacillus halodurans C-125 (PDB accession no. 3OA4). Each of these enzymes is 30% to 40% identical at the amino acid level to the M. sedula MCE (see alignments in Fig. S1-A in the supplemental material). The putative three-dimensional structure of the M. sedula MCE was modeled using hypothetical protein BH1468 (PDB accession no. 3OA4) (see Fig. S1-B in the supplemental material), which is composed of the motif β1-α1-β2-β3-β4-α2-β5-α3-α4-β6-β7. MCEs are typically activated by divalent metal ions. The metal binding residues for the P. shermanii MCE were identified as H12, Q65, H91, and E141, which, as demonstrated by amino acid sequence alignment, correspond to H8, E57, H84, and E134 in M. sedula; these would be located at the bottom of a deep cleft formed by β1, β4, β5, and β7 (see Fig. S1-C in the supplemental material). MCE catalyzes the epimerization reaction through an acid/base mechanism, and E141 and E48 of P. shermanii MCE were predicted to be the proton donor and acceptor, respectively (42). Based on this, the proton donor for M. sedua MCE should be E134, while the corresponding proton acceptor is predicted to be V44. The crystal structures of MCE from P. shermanii (41) and T. tengcongensis (52) are similar, with both containing a conserved metal-binding/active site, which is present in the corresponding model of M. sedula MCE shown in Fig. S2 in the supplemental material. In fact, very low epimerase activity was detected in the M. sedula enzyme without addition of cations, but significant activity was measured with Ni2+, Co2+, or Mg2+ addition. This result is consistent with MCEs from organisms that do not fix CO2 (34, 41).
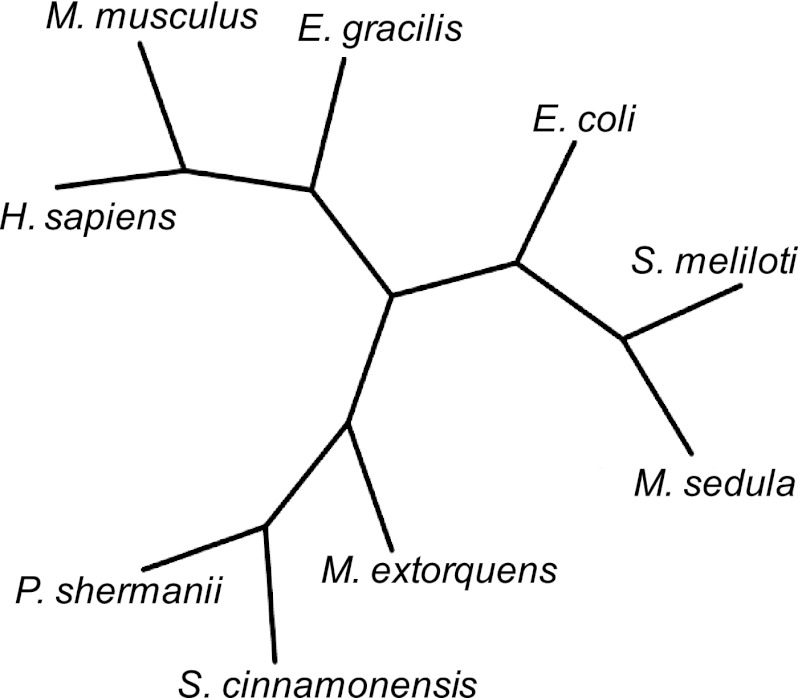
Only limited information is available on the biochemical properties of MCM in organisms that do not fix CO2. MCMs from Streptomyces cinnamonensis (11), P. shermanii (37), and Methylobacterium extorquens (48) are heterodimers, based on the presence of 80-kDa (MCM-α) and 65-kDa (MCM-β) subunits. All other characterized MCMs (from Pleurochrysis carterae [47], Sinorhizobium meliloti [46], Homo sapiens [20], and Euglena gracilis Z [45]) are homodimers based on the presence of ∼80-kDa subunits. The M. sedula MCM is heterotetrameric (α2β2) and, therefore, somewhat different in molecular assembly, especially since the B12-binding subunit is ∼15 kDa. A phylogenetic analysis of the catalytic subunit of MCMs revealed that the M. sedula enzyme branches with the E. coli and S. meliloti versions (see Fig. 5). Only limited kinetic data have been reported for MCMs, but the M. sedula enzyme activity on methylmalonyl-CoA is comparable to MCM activity from Pleurochrysis carterae, Sinorhizobium meliloti, and E. coli (2.2 ± 0.002 compared to 11.9, 10.9, and 4.1 μmol/min/mg, respectively). Note that the M. sedula MCM was also active on succinyl-CoA, with a specific activity of 40.7 ± 0.7 μmol/min/mg (see Table 2).
Fig 5.
Phylogenetic tree of characterized MCM. The organisms include Metallosphaera sedula (M. sedula), Sinorhizobium meliloti (S. meliloti), Propionibacterium shermanii (P. shermanii), Methylobacterium extorquens (M. extorquens), Streptomyces cinnamonensis (S. cinnamonensis), Escherichia coli (E. coli), Homo sapiens (H. sapiens), Mus musculus (M. musculus), and Euglena gracilis Z (E. gracilis).
The amino acid sequences of MCM-α (see Fig. S2 in the supplemental material) and MCM-β (see Fig. S3-A in the supplemental material) of M. sedula were aligned with versions encoded in genomes of Sulfolobaceae, Thermoproteales, Acidilobales, Desulfurococcales, Euryarchaeotes, and Chloroflexales and with those of other coenzyme B12-dependent enzymes (e.g., isobutyryl-CoA mutase of S. cinnamonensis and glutamate mutase of Clostridium tetanomorphum). While the MCMs from H. sapiens, M. musculus, and P. shermanii are composed of one subunit assembled into a homodimer, the others are heteromultimers. The M. sedula MCM-α is 45% identical to the P. shermanii MCM-α, but no coenzyme B12 binding domain could be identified in the M. sedula MCM-α (see Fig. S2 in the supplemental material). In this case, MCM-β is required to bind coenzyme B12 to form an active MCM with coenzyme B12 and MCM-α. In P. shermanii MCM, three aromatic residues (Y89, Y243, and H244) are present in the active site (35) which are conserved in other MCM-α homologs and correspond to Y81, Y235, and H236 in M. sedula MCM-α (see Fig. S3-B in the supplemental material). The three-dimensional structure of MCM-α was predicted using the MCM from Homo sapiens (PDB accession no. 2XIJ_A) as a model (21) (note the similarity of the N-terminal region of 2XIJ_A to that of M. sedula MCM-α [see Fig. S3-B in the supplemental material]). The α2β2-heterotetrameric structure of M. sedula MCM is different from those of other MCMs but similar to that of the B12-requiring ICM from Streptomyces cinnamonensis and GLM from Clostridium tetanomorphum. The S. cinnamonensis ICM has two subunits (catalytic subunit, 62.5 kDa; coenzyme B12-binding subunit, 14.3 kDa), but icmA and icmB are not adjacent in the genome (51). The S. cinnamonensis GLM has two subunits (catalytic subunit, ∼50 kDa; coenzyme B12-binding subunit, ∼17 kDa) (26).
In most coenzyme B12-binding proteins, such as MCM, GLM, methyleneglutarate mutase, and ICM (7, 16, 40), the motif “DXHXXG-SXL-GG” (where X is any amino acid) has been proposed as the coenzyme B12-binding domain (23); this motif was found in all MCM-βs in the alignment (see Fig. S3-A in the supplemental material). In the M. sedula MCM-β, the corresponding residues for this motif are D19, H21, G24, S56, L58, G96, and G97 (see Fig. S3-A in the supplemental material). The hydrogen-bonding network for coenzyme B12 binding in P. shermanii MCM was A610-D608-Y604 (36), and the corresponding residues in M. sedua MCM-β were predicted to be H21-D19-Y15 through alignment. The three-dimensional structure of M. sedula MCM-β was predicted using MCM-β of A. pernix K1 (PDB accession no. 2YXB) as a model (21) and is shown in Fig. S3-C in the supplemental material.
Physiological role of MCE and MCM in M. sedula.
In the proposed 3-HP/4-HB cycle in M. sedula, MCM converts (R)-methylmalonyl-CoA to succinyl-CoA. The maximum flux through the cycle requires the S-isomer to be epimerized to the R-isomer, and we propose that this occurs through the action of the MCE characterized here. For example, MCM was specific for (R)-methylmalonyl-CoA such that, when (R,S)-methylmalonyl-CoA was incubated with MCM, the R-isomer was converted to succinyl-CoA (see Fig. 6). However, significant amounts of the racemic mixture of MM-CoA were not converted (12). However, after (R,S)-mM-CoA was incubated with MCE, thereby converting the S- to the R-isomer, the amount of succinyl-CoA produced increased significantly. Based on the coupled assay, the optimum temperature (at pH 7.0) for the coupled MCE and MCM reactions was 75°C under the assay conditions, corresponding to specific activities of MCE and MCM of 218 and 2.2 μmol/min/mg, respectively.
Fig 6.
Conversion of (R,S)-methylmalonyl–CoA to succinyl-CoA by M. sedula MCE and MCM at 75°C and pH 7.0. From bottom to top: succinyl-CoA; methylmalonyl-CoA; formation of succinyl-CoA from (R,S)-methylmalonyl-CoA catalyzed by MCM; and formation of succinyl-CoA from (R,S)-methylmalonyl-CoA, with the S-isomer present first epimerized to the R-isomer by MCE.
In M. sedula, acetyl-CoA and succinyl-CoA are intermediates connecting the 3-HP/4-HB cycle and general carbon metabolism (17). Therefore, MCE and MCM are responsible for producing succinyl-CoA from (S)-methylmalonyl-CoA. As is the case in other organisms, MCE and MCM may also be involved in degradation of propionate and branched-chain amino acids (valine, isoleucine, methionine) (49) (see Fig. S4 in the supplemental material). The genomic locations of MCE, MCM-α, and MCM-β are common among sequenced Sulfolobaceae genomes; i.e., MCE and MCM-α clustered together in a location distant from that of MCM-β. None of the genes encoding MCE and MCM in members of the Chloroflexales are colocated, which is interesting since members of the bacterial order Chloroflexales fix CO2 through the 3-hydroxypropionate bicycle (10). Only MCM-α and MCM-β, and not MCE, were identified in genomes of the archaeal orders Thermoproteales, Acidilobales, and Desulfurococcales; all three of the genes are found in members of the Thermococcales, where MCM-β and MCE are clustered together. The function of MCE and MCM from members of the Thermoproteales, Acidilobales, Desulfurococcales, and Thermococcales would appear to be related to the metabolism of organic carbon and not involved in CO2 fixation, although closer examination of this is warranted.
With this study, the identity and biochemical characteristics of 2 more of the 13 enzymes proposed to make up the 3-HP/4-HB cycle in M. sedula (and, perhaps, in other members of the Sulfolobales) are now determined. Of the remaining 10 enzymes in the pathway, 3 (HBCS, HBCD, and ACK) have yet to be characterized, and there is only one (HBCS, a putative 4-hydroxybutyrate-CoA ligase) for which a leading candidate has not been identified. Efforts along these lines are now under way.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the U.S. Department of Energy ARPA-E Electrofuels Program (DE-AR0000081).
We thank Thomas A. Bobik of Iowa State University for kindly providing the plasmid harboring methylmalonyl-CoA mutase from E. coli.
Footnotes
Published ahead of print 29 June 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Alber BE, Kung JW, Fuchs G. 2008. 3-Hydroxypropionyl-coenzyme A synthetase from Metallosphaera sedula, an enzyme involved in autotrophic CO2 fixation. J. Bacteriol. 190: 1383–1389 doi:10.1128/JB.01593-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen SHG, Kellermeyer R, Stjernholm R, Jacobson B, Wood HG. 1963. The isolation, purification, and properties of methylmalonyl racemase. J. Biol. Chem. 238: 1637–1642 [PubMed] [Google Scholar]
- 3. Allen SHG, Kellermeyer RW, Stjernholm RL, Wood HG. 1964. Purification and properties of enzymes involved in the propionic acid fermentation. J. Bacteriol. 87: 171–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Auernik KS, Kelly RM. 2010. Impact of molecular hydrogen on chalcopyrite bioleaching by the extremely thermoacidophilic archaeon Metallosphaera sedula. Appl. Environ. Microbiol. 76: 2668–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auernik KS, Kelly RM. 2010. Physiological versatility of the extremely thermoacidophilic archaeon Metallosphaera sedula supported by transcriptomic analysis of heterotrophic, autotrophic, and mixotrophic growth. Appl. Environ. Microbiol. 76: 931–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Auernik KS, Maezato Y, Blum PH, Kelly RM. 2008. The genome sequence of the metal-mobilizing, extremely thermoacidophilic archaeon Metallosphaera sedula provides insights into bioleaching-associated metabolism. Appl. Environ. Microbiol. 74: 682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee R, Ragsdale SW. 2003. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 72: 209–247 doi:10.1146/annurev.biochem.72.121801.161828 [DOI] [PubMed] [Google Scholar]
- 8. Bar-Even A, Noor E, Lewis NE, Milo R. 2010. Design and analysis of synthetic carbon fixation pathways. Proc. Natl. Acad. Sci. U. S. A. 107: 8889–8894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berg IA, Kockelkorn D, Buckel W, Fuchs G. 2007. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 318: 1782–1786 doi:10.1126/science.1149976 [DOI] [PubMed] [Google Scholar]
- 10. Berg IA, et al. 2010. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol. 8: 447–460 [DOI] [PubMed] [Google Scholar]
- 11. Birch A, Leiser A, Robinson JA. 1993. Cloning, sequencing, and expression of the gene encoding methylmalonyl-coenzyme A mutase from Streptomyces cinnamonensis. J. Bacteriol. 175: 3511–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bobik TA, Rasche ME. 2003. HPLC assay for methylmalonyl-CoA epimerase. Anal. Bioanal. Chem. 375: 344–349 [DOI] [PubMed] [Google Scholar]
- 13. Bobik TA, Rasche ME. 2001. Identification of the human methylmalonyl-CoA racemase gene based on the analysis of prokaryotic gene arrangements. J. Biol. Chem. 276: 37194–37198 [DOI] [PubMed] [Google Scholar]
- 14. Bobik TA, Rasche ME. 2004. Purification and partial characterization of the Pyrococcus horikoshii methylmalonyl-CoA epimerase. Appl. Microbiol. Biotechnol. 63: 682–685 [DOI] [PubMed] [Google Scholar]
- 15. Cracan V, Padovani D, Banerjee R. 2010. IcmF is a fusion between the radical B12 enzyme isobutyryl-CoA mutase and its G-protein chaperone. J. Biol. Chem. 285: 655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drennan CL, Huang S, Drummond JT, Matthews RG, Lidwig ML. 1994. How a protein binds B12: a 3.0 A X-ray structure of B12-binding domains of methionine synthase. Science 266: 1669–1674 doi:10.1126/science.7992050 [DOI] [PubMed] [Google Scholar]
- 17. Estelmann S, et al. 2011. Labeling and enzyme studies of the central carbon metabolism in Metallosphaera sedula. J. Bacteriol. 193: 1191–1200 doi:10.1128/JB.01155-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eswar N, et al. 2003. Tools for comparative protein structure modeling and analysis. Nucleic Acids Res. 31: 3375–3380 doi:10.1093/nar/gkg543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eswar N, et al. 2007. Comparative protein structure modeling using Modeller. Curr. Protoc. Protein Sci. 50: 2.9.1–2.9.31. [DOI] [PubMed] [Google Scholar]
- 20. Fenton WA, Hack AM, Willard HF, Gertler A, Rosenberg LE. 1982. Purification and properties of methylmalonyl coenzyme A mutase from human liver. Arch. Biochem. Biophys. 214: 815–823 [DOI] [PubMed] [Google Scholar]
- 21. Froese DS, et al. 2010. Structures of the human GTPase MMAA and vitamin B12-dependent methylmalonyl-CoA mutase and insight into their complex formation. J. Biol. Chem. 285: 38204–38213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fuchs G. 2011. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu. Rev. Microbiol. 65: 631–658 doi:10.1146/annurev-micro-090110-102801 [DOI] [PubMed] [Google Scholar]
- 23. Gruber K, Kratky C. 2001. Methylmalonyl CoA mutase, p 995–1009 In Messerschmidt A, Huber R, Poulos T, Wieghardt K. (ed), Handbook of metallosproteins. John Wiley & Sons, Ltd., Chichester, United Kingdom [Google Scholar]
- 24. Han Y, et al. 2010. Comparative analyses of two thermophilic enzymes exhibiting both β-1,4 mannosidic and β-1,4 glucosidic cleavage activities from Caldanaerobius polysaccharolyticus. J. Bacteriol. 192: 4111–4121 doi:10.1128/JB.00257-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hawkins AS, et al. 2011. Extremely thermophilic routes to microbial electrofuels. ACS Catal. 1: 1043–1050 doi:10.1021/cs2003017 [Google Scholar]
- 26. Holloway DE, Marsh EN. 1994. Adenosylcobalamin-dependent glutamate mutase from Clostridium tetanomorphum. Overexpression in Escherichia coli, purification, and characterization of the recombinant enzyme. J. Biol. Chem. 269: 20425–20430 [PubMed] [Google Scholar]
- 27. Huber G, Spinnler C, Gambacorta A, Stetter KO. 1989. Metallosphaera sedula gen. and sp. nov. represents a new genus of aerobic, metal-mobilizing, thermoacidophilic archaebacteria. Syst. Appl. Microbiol. 12: 38–47 [Google Scholar]
- 28. Hügler M, Krieger RS, Jahn M, Fuchs G. 2003. Characterization of acetyl-CoA/propionyl-CoA carboxylase in Metallosphaera sedula. Eur. J. Biochem. 270: 736–744 [DOI] [PubMed] [Google Scholar]
- 29. Kamoun P. 1992. Valine is a precursor of propionyl-CoA. Trends Biochem. Sci. 17: 175–176 [DOI] [PubMed] [Google Scholar]
- 30. Kockelkorn D, Fuchs G. 2009. Malonic semialdehyde reductase, succinic semialdehyde reductase, and succinyl-coenzyme A reductase from Metallosphaera sedula: enzymes of the autotrophic 3-hydroxypropionate/4-hydroxybutyrate cycle in Sulfolobales. J. Bacteriol. 191: 6352–6362 doi:10.1128/JB.00794-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korotkova N, Chistoserdova L, Kuksa V, Lidstrom ME. 2002. Glyoxylate regeneration pathway in the methylotroph Methylobacterium extorquens AM1. J. Bacteriol. 184: 1750–1758 doi:10.1128/JB.184.6.1750-1758.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Korotkova N, Lidstrom ME. 2004. MeaB is a component of the methylmalonyl-CoA mutase complex required for protection of the enzyme from inactivation. J. Biol. Chem. 279: 13652–13658 [DOI] [PubMed] [Google Scholar]
- 33. Kühnl J, et al. 2005. Functional analysis of the methylmalonyl CoA epimerase from Caenorhabditis elegans. FEBS J. 272: 1465–1477 doi:10.1111/j.1742-4658.2005.04579.x [DOI] [PubMed] [Google Scholar]
- 34. Leadlay PF. 1981. Purification and characterization of methylmalonyl-CoA epimerase from Propionibacterium shermanii. Biochemical J. 197: 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maiti N, Widjaja L, Banerjee R. 1999. Proton transfer from histidine 244 may facilitate the 1,2 rearrangement reaction in coenzyme B12-dependent methylmalonyl-CoA mutase. J. Biol. Chem. 274: 32733–32737 [DOI] [PubMed] [Google Scholar]
- 36. Mancia F, et al. 1996. How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 Å resolution. Structure 4: 339–350 [DOI] [PubMed] [Google Scholar]
- 37. Marsh EN, Harding SE. 1993. Methylmalonyl-CoA mutase from Propionibacterium shermanii: characterization of the cobalamin-inhibited form and subunit-cofactor interactions studied by analytical ultracentrifugation. Biochem. J. 290: 551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marsh EN, Harding SE, Leadlay PF. 1989. Subunit interactions in Propionibacterium shermanii methylmalonyl-CoA mutase studied by analytical ultracentrifugation. Biochem. J. 260: 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marsh EN, McKie N, Davis NK, Leadlay PF. 1989. Cloning and structural characterization of the genes coding for adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. Biochem. J. 260: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marsh ENG, Holloway DE. 1992. Cloning and sequencing of glutamate mutase component S from Clostridium tetanomorphum Homologies with other cobalamin-dependent enzymes. FEBS Lett. 310: 167–170 doi:10.1016/0014-5793(92)81321-C [DOI] [PubMed] [Google Scholar]
- 41. McCarthy AA, et al. 2001. Expression, crystallization and preliminary characterization of methylmalonyl coenzyme A epimerase from Propionibacterium shermanii. Acta Crystallogr. D Biol. Crystallogr. 57(Pt 5): 706–708 [DOI] [PubMed] [Google Scholar]
- 42. McCarthy AA, Baker HM, Shewry SC, Patchett ML, Baker EN. 2001. Crystal structure of methylmalonyl-coenzyme A epimerase from P. shermanii: a novel enzymatic function on an ancient metal binding scaffold. Structure 9: 637–646 [DOI] [PubMed] [Google Scholar]
- 43. McKie N, Keep NH, Patchett ML, Leadlay PF. 1990. Adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. Active holoenzyme produced from Escherichia coli. Biochem. J. 269: 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menendez C, Bauer Z, Huber H, Stetter KO, Fuchs G. 1999. Presence of acetyl coenzyme A (CoA) carboxylase and propionyl-CoA carboxylase in autotrophic Crenarchaeota and indication for operation of a 3-hydroxypropionate cycle in autotrophic carbon fixation. J. Bacteriol. 181: 1088–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyamoto E, et al. 2010. Characterization of methylmalonyl-CoA mutase involved in the propionate photoassimilation of Euglena gracilis Z. Arch. Microbiol. 192: 437–446 [DOI] [PubMed] [Google Scholar]
- 46. Miyamoto E, et al. 2003. Purification and characterization of homodimeric methylmalonyl-CoA mutase from Sinorhizobium meliloti. Arch. Microbiol. 180: 151–154 [DOI] [PubMed] [Google Scholar]
- 47. Miyamoto E, Watanabe F, Yamaguchi Y, Takenaka H, Nakano Y. 2004. Purification and characterization of methylmalonyl-CoA mutase from a photosynthetic coccolithophorid alga, Pleurochrysis carterae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 138: 163–167 [DOI] [PubMed] [Google Scholar]
- 48. Miyamoto E, et al. 2002. Purification and characterization of methylmalonyl-CoA mutase from a methanol-utilizing bacterium, Methylobacterium extorquens NR-1. J. Nutr. Sci. Vitaminol. 48: 242–246 doi:10.3177/jnsv.48.242 [DOI] [PubMed] [Google Scholar]
- 49. Ogata H, et al. 1999. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 27: 29–34 doi:10.1093/nar/27.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramos-Vera WH, Weiss M, Strittmatter E, Kockelkorn D, Fuchs G. 2011. Identification of missing genes and enzymes for autotrophic carbon fixation in Crenarchaeota. J. Bacteriol. 193: 1201–1211 doi:10.1128/JB.01156-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ratnatilleke A, Vrijbloed JW, Robinson JA. 1999. Cloning and sequencing of the coenzyme B12-binding domain of isobutyryl-CoA mutase from Streptomyces cinnamonensis, reconstitution of mutase activity, and characterization of the recombinant rnzyme produced in Escherichia coli. J. Biol. Chem. 274: 31679–31685 [DOI] [PubMed] [Google Scholar]
- 52. Shi L, Gao P, Yan XX, Liang DC. 2009. Crystal structure of a putative methylmalonyl coenzyme a epimerase from Thermoanaerobacter tengcongensis at 2.0 Å resolution. Proteins 77: 994–999 doi:10.1002/prot.22528 [DOI] [PubMed] [Google Scholar]
- 53. Stabler SP, Marcell PD, Allen RH. 1985. Isolation and characterization of methylmalonyl-coenzyme A racemase from rat liver. Arch. Biochem. Biophys. 241: 252–264 [DOI] [PubMed] [Google Scholar]
- 54. Teufel R, Kung JW, Kockelkorn D, Alber BE, Fuchs G. 2009. 3-Hydroxypropionyl-coenzyme A dehydratase and acryloyl-coenzyme A reductase, enzymes of the autotrophic 3-hydroxypropionate/4-hydroxybutyrate cycle in the Sulfolobales. J. Bacteriol. 191: 4572–4581 doi:10.1128/JB.00068-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilkemeyer MF, Crane AM, Ledley FD. 1990. Primary structure and activity of mouse methylmalonyl-CoA mutase. Biochem. J. 271: 449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zagalak B, Retey J, Sund H. 1974. Studies on methylmalonyl-CoA mutase from Propionibacterium shermanii. Eur. J. Biochem. 44: 529–535 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.