Abstract
Enterococci, recommended at the U.S. federal level for monitoring water quality at marine recreational beaches, have been found to reside and grow within beach sands. However, the environmental and ecological factors affecting enterococcal persistence remain poorly understood, making it difficult to determine levels of fecal pollution and assess human health risks. Here we document the presence of enterococci associated with beach sediment biofilms at eight south Florida recreational beaches. Enterococcal levels were highest in supratidal sands, where they displayed a nonlinear, unimodal relationship with extracellular polymeric secretions (EPS), the primary component of biofilms. Enterococcal levels peaked at intermediate levels of EPS, suggesting that biofilms may promote the survival of enterococci but also inhibit enterococci as the biofilm develops within beach sands. Analysis of bacterial community profiles determined by terminal restriction fragment length polymorphisms showed the bacterial communities of supratidal sediments to be significantly different from intertidal and subtidal communities; however, no differences were observed in bacterial community compositions associated with different EPS concentrations. Our results suggest that supratidal sands are a microbiologically unique environment favorable for the incorporation and persistence of enterococci within beach sediment biofilms.
INTRODUCTION
Fecal indicator bacteria (FIB), like Enterococcus faecalis, are used to assess the health risk of recreational waters. Water quality monitoring of FIB resulted in more than 18,000 recreational beach closings and advisory days in 2009 and over 24,000 in 2010 (51, 52). The validity of these closings is based on the positive correlation of FIB in the water column to both human pathogens (31, 41) and swimmer illness (68), which has been well established for sewage sources. However, the detection of high levels of FIB in beach sands (3, 4, 15, 19, 21, 24, 26, 28, 39) has called into question these monitoring efforts and initiated a number of studies aimed at understanding FIB persistence and growth in sand (10, 32, 37, 48, 55, 69, 70, 71) and/or their possible relation to pathogen presence (1, 61). Recent reports have shown a positive relationship between sand contact activities and enteric illness as a function of concentrations of fecal microbial pollution in beach sand (33, 34). However, the risk of enteric illness varies between beaches, leading investigators to propose that risk level might be site specific and may depend on characteristics such as mineralogy, particle size, moisture content, organic matter, and nutrient availability (34).
The reported sources of FIB contamination responsible for beach closing events in Florida waters include wildlife (73%), storm water runoff (67%), sewage spills (61%), other sources (27%), and unknown sources (41%) (52). Once seeded into beach sand, these bacteria are able to persist (3, 4, 14, 15, 18, 21, 32, 37, 48, 71). The persistence and growth of FIB have been investigated extensively to better understand contamination at recreational beaches (10, 12, 14, 15, 32, 48, 55, 65, 68, 69, 70, 71), identifying important growth requirements in microcosms such as moisture content, temperature, and lack of predators. However, no set of factors can consistently account for the variation and patchy distribution of indicator levels in the natural beach environment. The lack of a set of predictors at the macroscale to account for FIB variability in beach sand led Bonilla et al. in 2007 (10) to conclude that microscale environmental conditions control the survival of these bacteria in beach sands.
Biofilm formation on beach sands could provide a suitable microhabitat for enterococci and other fecal bacteria, including potential pathogens, once they are seeded into the sand. While bacteria can have an independent planktonic existence, they more typically occur within attached, interdependent cooperative populations or communities known as biofilms (17). Extracellular polymeric substances (EPS) are the principal structural component of biofilms and facilitate the attachment of microbes to surfaces or to each other. In doing so, EPS provide protection from the significant physical and biological challenges of the shoreline environment, including frequent fluctuations in temperature, ion concentration, desiccation, UV radiation, predation, and wave action (12, 16, 17, 64). Human intestinal bacteria, like enterococci, are well adapted for living in biofilms where adhesion to extracellular matrix proteins of the human gut is the first step in colonization and infection (48, 49, 50).
Previous studies have reported elevated levels of enterococci and other fecal indicator bacteria associated with biofilms in shallow marine environments (6, 35, 63). In beach sand, Hartz et al. in 2008 (32) demonstrated that higher numbers of FIB are removed from sand grains by vigorous shaking than by gentle washing, providing preliminary support that these microbes are tightly attached to grains by an EPS matrix. Moreover, Phillips et al. in 2011 (58) found that only 10% of the dislodgeable bacteria in beach sand can be removed through pore water flow. However, there has been little investigation into the relationship between enterococci and biofilms in beach sediments because of the dynamic nature and technical difficulties involved in studying biotic factors in these sediments (23). Biofilms represent complex biological systems where bacteria form structured, functional communities that can communicate and adapt to changing environmental conditions (17). By investigating the influence of biofilms on enterococcal persistence in beach sands, we hope to better understand how these bacteria can be mobilized into the marine environment and to influence water quality monitoring and/or understand the potential for pathogen incorporation into biofilms as well.
In this study, we tested the hypothesis that the recommended fecal indicator bacteria at marine recreational beaches, enterococci, are correlated to biofilm development on beach sand. We examined the relationship between enterococcal levels and biofilm development (measured as EPS) from the supratidal sand (0.25 m landward of the high-tide mark), intertidal sand (halfway between the high-tide and low-tide marks), and subtidal sand (1 m seaward of the low-tide mark) collected at Hobie Beach in June 2010 for 10 consecutive days and on two sampling days at eight beaches in September 2010 and February 2011. Enterococcal and EPS levels were compared to sand moisture content, grain size, mineralogy, and potential fecal sources to identify factors influencing their distribution in these tidal zones. Furthermore, the characterization of microbial communities was conducted to determine whether high levels of FIB are associated with specific changes in the microbial ecology of the biofilms. This study focused on evaluating persistence of enterococci in conjunction with biofilms. We did not measure growth, as this would require measurements of the change in enterococcus density with time.
MATERIALS AND METHODS
Field sampling.
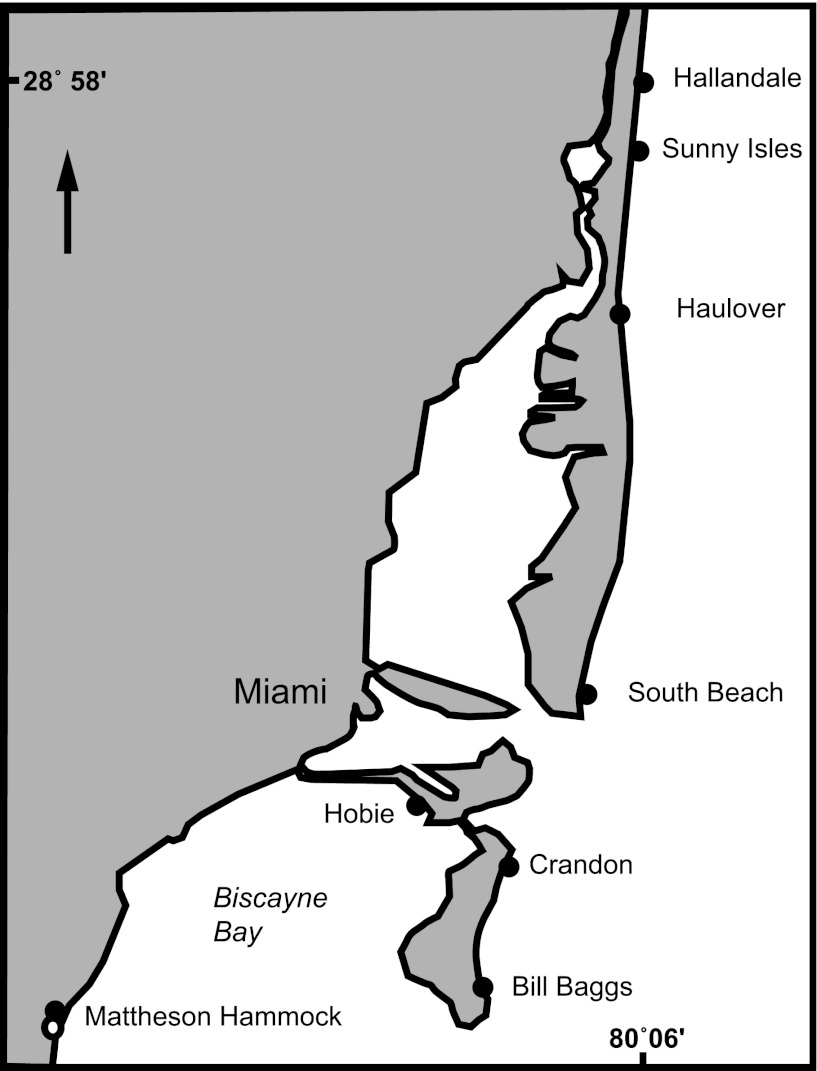
A temporal survey of beach sand, henceforth referred to as “the 10-day study,” was conducted by collecting sand every 6 h over 10 consecutive days (n = 360) at Hobie Beach (also known as Hobie Cat Beach), Virginia Key, FL (Fig. 1), from 1 to 10 June 2010. Additionally, eight beaches, including Hobie Beach (Fig. 1), were sampled on the mornings of 11 September 2010 and 12 February 2011 to assess the spatial variability on beach sand of enterococci, henceforth referred to as “the multiple-beach study” (n = 144). Beaches were selected to represent a range of sediment characteristics and FIB contamination levels recorded from 2009 to 2010 (Table 1). All beaches are similar in width besides Matheson Hammock, an enclosed artificial lagoon, and Hobie Beach, a narrow beach located on Virginia Key. Hobie Beach is the only beach that allows dogs.
Fig 1.
Eight beaches sampled for the south Florida sediment characterization are shown.
Table 1.
South Florida beaches and percentages of samples where FIB exceeded water column densities of 104 CFU/mla
| Beach name | No. of samples taken |
% samples exceeding state standards |
||
|---|---|---|---|---|
| 2009 | 2010 | 2009 | 2010 | |
| Hallandale | 52 | 61 | 0 | 18 |
| Sunny Isles | 61 | 10 | 18 | 0 |
| Haulover | 52 | 53 | 0 | 2 |
| South Beach | 55 | 54 | 5 | 4 |
| Hobie Beach | 55 | 30 | 20 | 3 |
| Crandon Park | 49 | 68 | 5 | 26 |
| Bill Baggs | 54 | 17 | 6 | 6 |
| Matheson Hammock | 58 | 56 | 12 | 7 |
Sample collection consisted of three composite samples (one from each of the following zones: supratidal, intertidal, and subtidal) collected at each beach. Supratidal samples were located 0.25 m above the mean high-tide line, the intertidal samples were located halfway between the high-tide line and low-tide line, and the subtidal samples were located 1 m below the water line in a depth of 0.3 to 0.5 m. Intertidal sediment samples were collected during low tide, when the sediments were exposed, to ensure that conditions within these sediments were consistent across beaches. Sediment collection for the 10-day study consisted of collecting the top 4 cm of sediment from a 20-cm by 20-cm area with a sterilized spoon. Each day, sediment was collected 1 m horizontally adjacent to the previous day's sample in order to collect undisturbed sediment. Approximately 600 g of sediments was placed into a presterilized Whirl-Pak (Nasco, Fort Atkinson, WI) bag and transported back to the lab for analysis in less than 4 h. Multiple-beach study composite-sediment samples consisted of a series of 31 cores (2.54-cm diameter, 4 cm in length) to collect the top 4 cm of sediment every 20 cm along a 6-m transect parallel to the shoreline. The sample transects and small-core sampling tubes were used to standardize sample collection at all beaches. Approximately 900 g of sediments was emptied into sterile sample tins, transported back to the laboratory, and analyzed within 4 h.
Environmental parameters.
The presence of humans, dogs, and birds on both water and sand 65 m to the right and to the left of the sampling site was recorded every hour for the 10-day study and at the time of sampling for the multiple-beach study. Rainfall was recorded at a measurement station less than 1 km from the sampling site at Hobie Beach for the 10-day study (NSF NIEHS OHH Center Remote Sensing Facilities Core [http://www.rsmas.miami.edu/etc/download-weatherpak.cgi]).
Quantification of enterococci.
Sediment samples for enterococcus quantification were homogenized for 3 min using a sterilized spoon prior to analysis (1, 70). Ten grams of sediment was asceptically transferred to 100 ml of prepared, autoclaved phosphate-buffered saline (PBS) in a sterilized bottle. The bottle was shaken vigorously for 2 min to extract enterococci from the sediments (8). After the sediment was allowed to settle (2 min), 2 volumes of the eluent (25 ml and 3 ml) were filtered (Pall; GN-6 grid) and placed on mEI agar (Becton, Dickinson and Company, Sparks, MD) plates as per method 1600 (67). Filters on mEI agar plates were incubated at 41°C for 24 ± 2 h. Blue colonies or colonies with a blue halo were recorded as enterococci and converted to CFU g−1 of dry sand (67).
Quantification of EPS on beach sediments.
EPS, consisting of primarily polysaccharides excreted by microorganisms, were extracted using a modified protocol previously described (20, 57) from three replicate subsamples of 3 g each of fresh sediment collected. Each sample was allowed to stand in 0.5 mM EDTA for 15 min at 40°C, with gentle shaking every 5 min for three consecutive treatments. After each treatment, samples were centrifuged at 8,000 × g and the supernatant was pooled. The supernatant was mixed with cold (4°C) ethanol (final concentration of 70%) for 8 h to precipitate extracted EPS. The extraction was performed a total of three times for each sample to ensure that no detectable EPS remained (20). Precipitate of extracted material was collected by centrifugation, dissolved in 1 ml of deionized water, and used for the quantification of EPS by the phenol-sulfuric acid method (20, 22, 29, 73). Each 1-ml sample of dissolved EPS was incubated with 3.2 ml of sulfuric acid for 1 min and cooled to room temperature in a water bath, and 50 μl of 90% phenol was added. The sample was incubated at room temperature for 1 h, and the absorbance was measured at 490 nm using a spectrophotometer (BioPhotometer plus; Eppendorf, Hamburg, Germany). The amount of carbohydrate present was determined by comparison with a calibration curve using d-glucose. The sediments from the samples were washed with deionized water to remove salts and were dried for the determination of dry weight to calculate the measure of EPS · g−1 dry sand (57).
Visualization of sediment EPS.
EPS coatings on the surface of beach sediments were visualized using the lectin stain wheat germ agglutinin (WGA), specific for binding to N-acetylglucosamine in biofilms (13, 59). Sand grains were prepared by incubation with a blocking buffer for 15 min (5% bovine serum albumin [BSA; Jackson ImmunoResearch, West Grove, PA], 0.1% Micr-O-Protect [Rosch Diagnostics, Mannheim, Germany] in PBS). Sediments were then rinsed in PBS and incubated with 10 μg ml−1 of WGA conjugated with Alexa Fluor 647 (Invitrogen, Eugene, OR) in PBS. After 15 min, the sediments were rinsed in PBS and imaged using a Leica SP5 confocal microscope.
Sediment characterization.
Moisture content was measured by weighing an aliquot of the composite sample before and after drying (110°C for 24 h). Grain size was based on standard wet sediment sieving (2). The percent abundance of calcium carbonate grains was determined by incubating 3 g of sediment in 10% HCl solution for 24 h, followed by three rinses in deionized water, incubation of the sediment at 55°C for 3 days, and weighing of the dried sediment (60).
Characterization of microbial communities.
Bacterial communities were characterized using a combined approach of terminal restriction fragment length polymorphism (T-RFLP) and sequencing of clone libraries to determine whether FIB and/or EPS levels were associated with specific changes in microbial ecology within the sand. The FastDNA SPIN kit for soil (MP Biomedicals, Santa Ana, CA) was used to extract genomic DNA from ∼500-mg sediment samples. Genes encoding 16S rRNA in bacteria were amplified by PCR using a Mastercycler gradient thermocycler (Eppendorf). PCR was carried out as previously described (42) using primers U9 (5′-GAGTTTGATYMTGGCTC) and U1509 (5′-GYTACCTTGTTACGACTT) (Integrated DNA Technologies, Coralville, IA).
For T-RFLP analysis, primer U9F was labeled at the 5′ end with phosphoramidite fluorochrome 6-carboxyfluorescein. Pooled PCR replicates (four reactions, each with 50 μl) were run on a low-melting-point agarose gel, and excised bands were purified using the Wizard SV Gel and PCR Clean-Up (Promega, Madison, WI). Restriction digests of purified PCR product were performed independently using three tetrameric enzymes (MspI, RsaI, and HhaI; Promega). For each digest, 250 ng of DNA was incubated at 37°C for 4 h, followed by 15 min at 65°C. T-RFLP fragments were determined by multicapillary electrophoresis on a model 3130xl capillary sequencer (Applied Biosystems, Foster City, CA). Data matrices for sample versus T-RFLP peak were constructed using peaks above a threshold of 50 units above background. Peaks smaller than 20 bp and larger than 1,050 bp were culled from the data set. Abundance data were obtained from the relative peak height following sample standardization whereby each peak for a given sample was normalized against the total peak height from that sample (7). To assess the variation in sample T-RFLP profiles, sample similarity matrices were constructed using the Bray-Curtis coefficient (11). Multidimensional scaling (MDS) was based on the Bray-Curtis similarity of bacterial communities between T-RFLP profiles of the 16S rRNA gene digested with the three tetrameric restriction enzymes HhaI, MspI, and RsaI, which were concatenated as one data set (42). The taxonomic identity of key terminal restriction fragments (TRFs) was determined by virtual digests of the 16S rRNA gene sequence clone library developed from this study, explained below.
Bacterial clone libraries were constructed from 3 samples from supratidal sediments collected from Hobie Beach during the 10-day study and the multiple-beach study as previously described (42). Complete, forward-oriented clones were inoculated into 2-ml 96-well plates filled with Luria broth and 100 μg ml−1 ampicillin (Roche Molecular Biomedicals, Indianapolis, IN) and incubated overnight (42). PCR sequencing reactions were performed using the T7 plasmid primer and BigDye Terminator chemistry (version 2.0) (Applied Biosystems).
One-way analysis of variance (ANOVA) was used to determine significant differences among mean concentrations of enterococci. Results are reported with the F-test statistic (F) as a comparison of the means along with the degrees of freedom between groups, degrees of freedom within groups, and the P value. Enterococcal concentrations were log10 transformed to meet parametric assumptions of equality of variances and normal distributions. These tests were performed by Levene's test for homogeneity of variance and the Shapiro-Wilk test for normality. Post hoc pairwise comparisons were performed by Tukey's test. Pearson correlation analysis was used to determine correlations between enterococcal concentrations and environmental factors. The analysis-of-similarities (ANOSIM) global R statistic was used to test for significant differences in bacterial communities (42). This analysis is based on a nonparametric permutation (n = 999) procedure applied to the rank similarity matrix. If samples within a group are identical, then global R is 1. MDS plots (43) and statistical tests were performed using PRIMER 5, version 5.2.9 (PRIMER-E, Plymouth, United Kingdom) and SPSS (version 16.0 for Mac; SPSS, Chicago, IL).
RESULTS
Sediment enterococcus quantification.
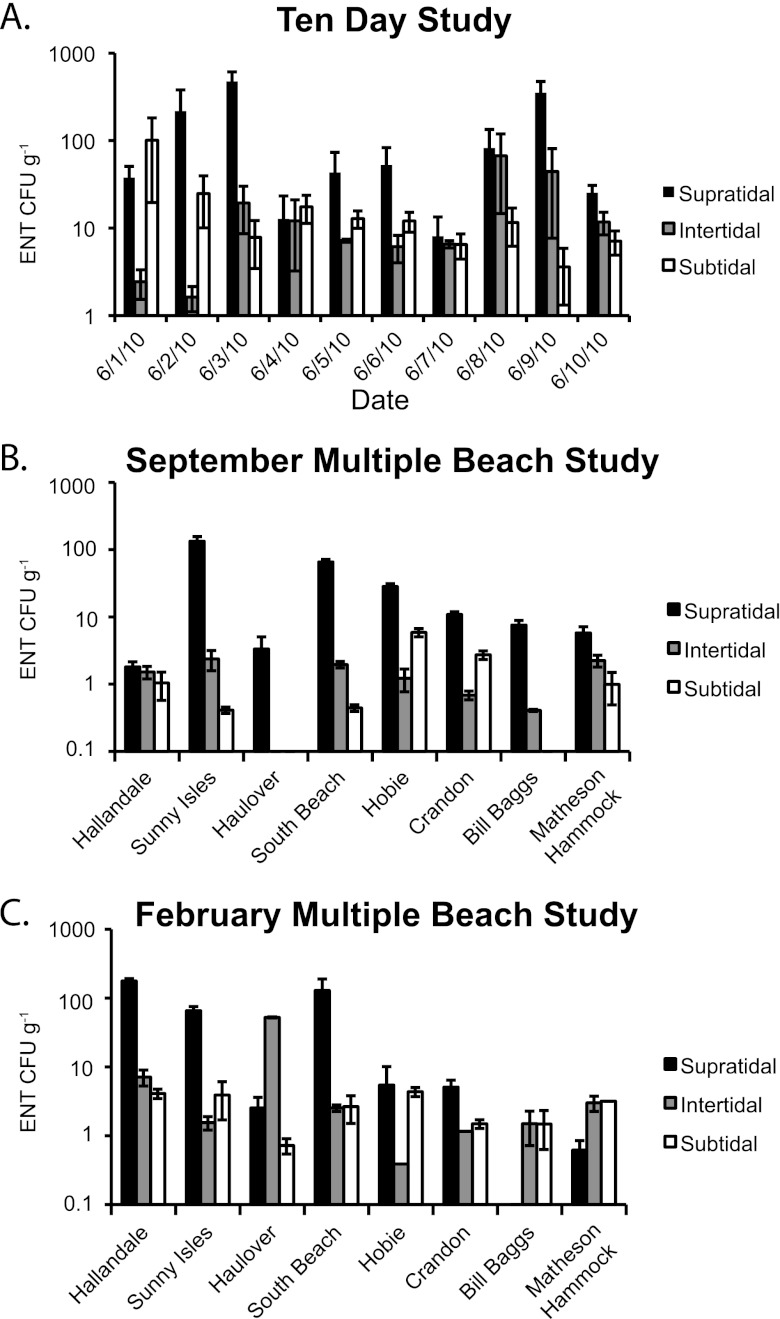
Sediment enterococcal levels were variable but typically higher in supratidal sands than in intertidal and subtidal sands [F(2,75) = 7.8, P < 0.05; Fig. 2]. In the 10-day study, enterococcal levels in supratidal sands were significantly higher than in both intertidal and subtidal sands [F(2,27) = 7.2, P < 0.05]. In the September multiple-beach study, supratidal sediment enterococcal levels were significantly higher than enterococcal levels in intertidal sands and subtidal sands [F(2,18) = 10.4, P < 0.05]. While no significant differences in enterococcal levels between tidal zones in the February multiple-beach study were observed, enterococcal levels ranked highest in the supratidal sediments. Total enterococcal levels from the 10-day study in June were significantly higher than from both the September and February multiple-beach studies [F(2,71) = 11.4, P < 0.05]. There was no significant difference in enterococcal levels between the September and February multiple-beach studies compared across individual tidal zones or with all tidal zone samples combined.
Fig 2.
Concentration of enterococci in beach sediments from the 10-day study conducted at Hobie Beach (A), the September multiple-beach study (B), and the February multiple-beach study (C).
Sediment biofilm visualization.
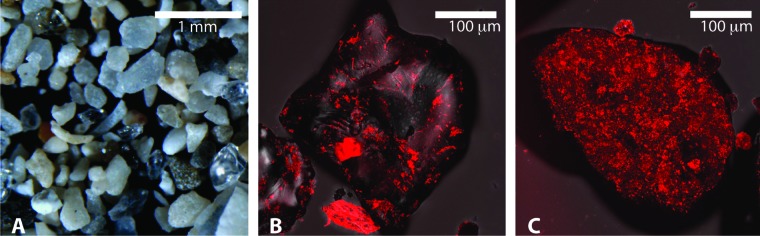
The binding of WGA to both quartz and calcium carbonate grains demonstrates the extensive EPS coatings associated with beach sand. WGA was observed binding to sediments from all tidal zones at all beaches. EPS was concentrated within cracks and crevices of the quartz grains. Calcium carbonate grains appeared to have a more heterogeneous coating of EPS than did quartz grains. Representative samples of both quartz and calcium carbonate grains from Hobie Beach are shown in Fig. 3.
Fig 3.
South Florida beach sand under plane light stereoscope showing a typical mixture of quartz and calcium carbonate grains (A). Biofilms on sand grains were stained with the fluorescent probe wheat germ agglutinin, which binds to EPS (red), and imaged using a confocal laser scanning microscope to examine the EPS coatings on quartz (B) and calcium carbonate (C) grains.
Sediment biofilm quantification.
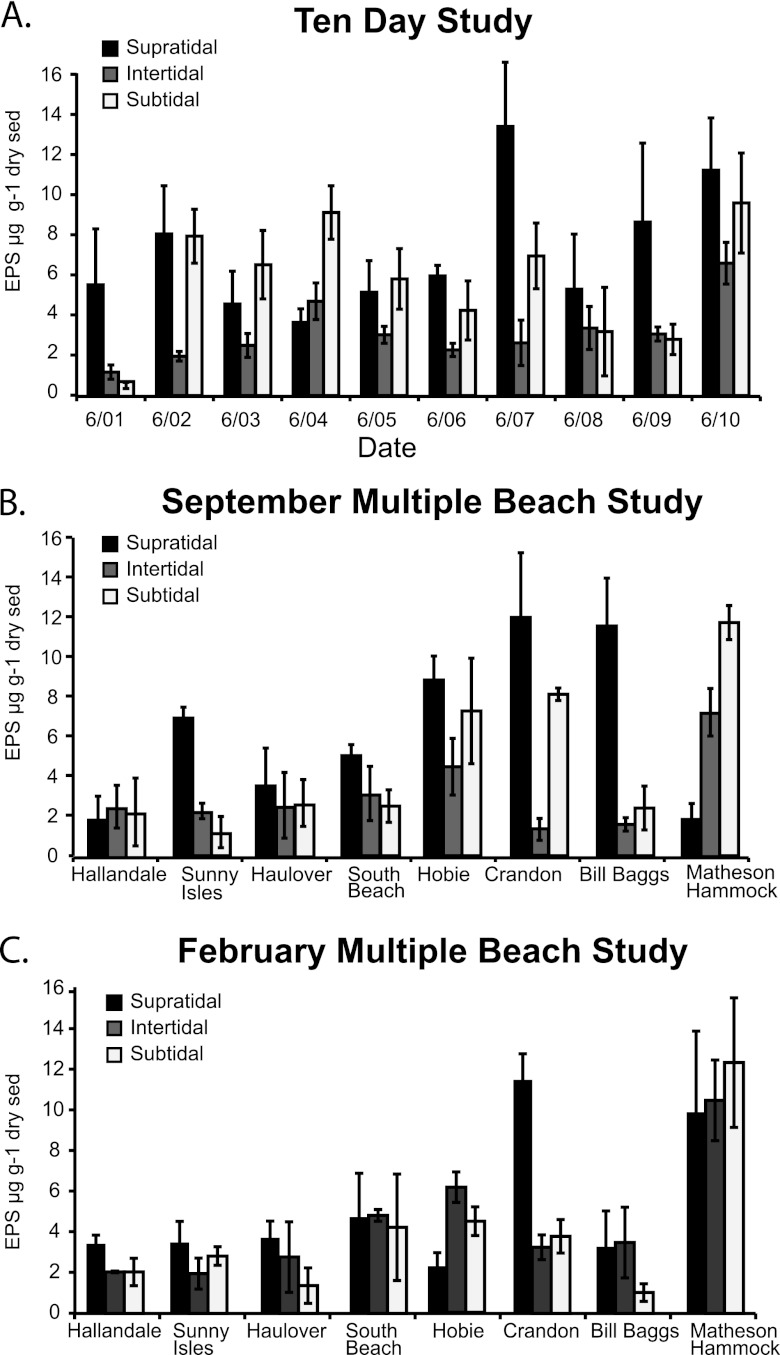
The only significant difference observed between EPS levels was for the 10-day study, in which the EPS level was higher in the supratidal sediment than the intertidal sediment [F(2,27) = 4.0, P < 0.05; Fig. 4A]. For other combinations of tidal zones and beaches, no differences were observed in levels of EPS. September and February multiple-beach study EPS levels (Fig. 4B and C) were not different between the three tidal zones. No differences in EPS levels were found when the data from all studies were grouped together by tidal zone.
Fig 4.
Concentration of EPS extracted from supratidal, intertidal, and subtidal sediments from the 10-day study (A), the September multiple-beach study (B), and the February multiple-beach study (C).
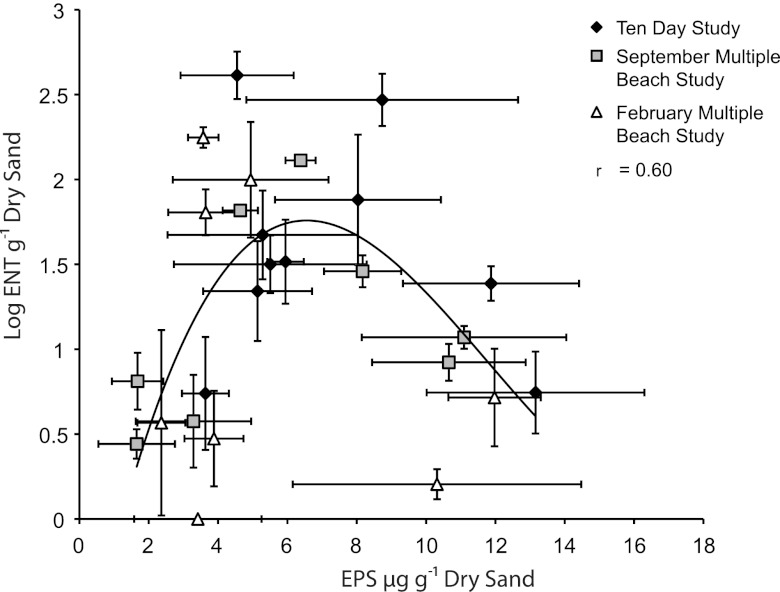
The relationship of enterococcal levels and biofilm development on sediment grains was analyzed from all samples collected across all studies. Results showed a unimodal relationship in the supratidal zone between sediment grain EPS and logarithmic transformed enterococci (Fig. 5; r = 0.60, F = 4.47, P = 0.01, n = 26), with a maximum enterococcal level at EPS levels of 7 μg/g. There was no relationship between enterococcal levels and biofilm development in the intertidal or subtidal zone.
Fig 5.
A cross-plot of the log-transformed enterococcal levels and EPS extracted from supratidal sediments from the 10-day study, the September multiple-beach study, and the February multiple-beach study.
Beach sediment characterization.
The variation in physical sediment characteristics observed among the 8 beaches studied is summarized in Table 2. The mean ratios of wet to dry weight of sand for the supratidal, intertidal, and subtidal zones were 1.11 (n = 40), 1.24 (n = 40), and 1.32 (n = 40), respectively. Supratidal enterococcal levels were not related to any physical characteristic or potential source measured in each study (n = 26 [Table 3]). There was a positive correlation between enterococcal levels and sand moisture content within the intertidal zone (n = 26) and a negative correlation in the subtidal zones (n = 26 [Table 3]). When the data from all three tidal zones from all studies were combined, no relationship was found between enterococcal levels and sand moisture content (n = 78 [Table 3]). Enterococcal levels were negatively correlated to the percentage of calcium carbonate grains in the subtidal zone (n = 78 [Table 3]). EPS were negatively correlated to the percentage of calcium carbonate grains within the intertidal and subtidal zones as well as all the tidal zones combined (n = 78 [Table 3]).
Table 2.
Beach sand characteristics at Florida beachesa
| Study | Moisture (wt%) |
Calcium carbonate (wt%) |
Median grain size (mm) |
Possible sourcesc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supra | Inter | Sub | Supra | Inter | Sub | Supra | Inter | Sub | Human | Dog | Bird | Rain (mm) | |
| Ten-day studyb | |||||||||||||
| 6/1 | 7.0 | 18.4 | 22.0 | 19.6 | 20.4 | 40.5 | 0.4 | 0.7 | 1.0 | 40 | 5 | 8 | 4.7 |
| 6/2 | 8.0 | 19.3 | 26.1 | 25.7 | 20.3 | 42.1 | 0.4 | 0.5 | 0.9 | 50 | 5 | 6 | 0.5 |
| 6/3 | 9.0 | 16.4 | 27.1 | 31.8 | 21.8 | 61.7 | 0.4 | 0.5 | 0.9 | 74 | 4 | 7 | 0.2 |
| 6/4 | 11.6 | 18.6 | 28.0 | 39.2 | 19.6 | 46.6 | 0.4 | 0.5 | 0.7 | 93 | 9 | 0 | 24.3 |
| 6/5 | 12.0 | 20.1 | 21.2 | 42.8 | 34.2 | 37.0 | 0.4 | 0.5 | 1.0 | 249 | 19 | 0 | 3.1 |
| 6/6 | 10.4 | 17.8 | 21.8 | 48.1 | 49.4 | 27.5 | 0.4 | 0.5 | 0.8 | 294 | 20 | 11 | 2.8 |
| 6/7 | 8.2 | 20.2 | 25.3 | 52.1 | 22.5 | 61.3 | 0.4 | 0.4 | 1.2 | 80 | 1 | 0 | 2.1 |
| 6/8 | 12.8 | 21.8 | 28.5 | 43.2 | 36.9 | 68.0 | 0.4 | 0.5 | 1.4 | 73 | 6 | 15 | 24.2 |
| 6/9 | 11.3 | 19.8 | 27.4 | 31.1 | 20.1 | 45.5 | 0.4 | 0.7 | 1.1 | 144 | 12 | 0 | 0 |
| 6/10 | 6.1 | 20.4 | 25.7 | 22.9 | 46.9 | 68.8 | 0.4 | 0.6 | 0.7 | 175 | 6 | 7 | 0 |
| September multiple-beach study | |||||||||||||
| Hallandale | 2.3 | 6.3 | 21.2 | 53.7 | 73.9 | 58.0 | 0.4 | 0.6 | 0.4 | 0 | 0 | 0 | 0 |
| Sunny Isles | 2.1 | 5.2 | 17.2 | 72.8 | 83.2 | 94.9 | 0.5 | 0.8 | 1.5 | 0 | 0 | 0 | 0 |
| Haulover | 2.9 | 4.1 | 18.1 | 94.4 | 95.9 | 98.5 | 0.8 | 1.0 | 1.4 | 0 | 0 | 0 | 0 |
| South Beach | 3.1 | 18.6 | 23.4 | 87.2 | 86.2 | 90.5 | 0.4 | 0.3 | 0.3 | 0 | 0 | 0 | 0 |
| Hobie | 2.1 | 4.2 | 17.9 | 0.6 | 1.2 | 3.8 | 0.7 | 0.7 | 0.6 | 0 | 0 | 0 | 0 |
| Crandon | 3.1 | 17.2 | 21.3 | 40.2 | 60.4 | 61.9 | 0.5 | 0.4 | 0.5 | 0 | 0 | 0 | 0 |
| Bill Baggs | 1.8 | 5.1 | 20.5 | 38.9 | 45.9 | 68.1 | 0.3 | 0.4 | 0.5 | 0 | 0 | 0 | 0 |
| Matheson | 9.0 | 15.6 | 20.0 | 35.4 | 24.8 | 15.5 | 0.6 | 0.4 | 0.7 | 0 | 0 | 0 | 0 |
| February multiple-beach study | |||||||||||||
| Hallandale | 3.5 | 6.6 | 20.4 | 68.9 | 70.3 | 93.9 | 0.6 | 1.5 | 0.7 | 0 | 0 | 0 | 0 |
| Sunny Isles | 1.8 | 6.1 | 21.6 | 60.2 | 65.4 | 67.1 | 0.5 | 0.4 | 0.5 | 0 | 0 | 0 | 0 |
| Haulover | 3.7 | 7.3 | 23.8 | 95.4 | 93.9 | 98.5 | 0.9 | 1.5 | 0.7 | 0 | 0 | 0 | 0 |
| South Beach | 4.5 | 11.2 | 25.1 | 85.8 | 85.8 | 90.7 | 0.3 | 0.4 | 0.3 | 0 | 0 | 0 | 0 |
| Hobie | 0.2 | 6.4 | 20.1 | 0.9 | 1.4 | 1.4 | 0.5 | 0.5 | 0.7 | 0 | 0 | 0 | 0 |
| Crandon | 1.4 | 18.6 | 22.2 | 42.1 | 52.2 | 57.2 | 0.3 | 0.4 | 0.4 | 0 | 0 | 0 | 0 |
| Bill Baggs | 2.1 | 18.7 | 23.8 | 40.5 | 58.0 | 73.7 | 0.4 | 0.5 | 0.6 | 0 | 0 | 0 | 0 |
| Matheson | 11.1 | 15.3 | 22.2 | 23.9 | 17.2 | 13.9 | 0.4 | 0.5 | 0.4 | 0 | 0 | 0 | 0 |
Supra, supratidal; inter, intertidal; sub, subtidal.
6/1, etc., indicate month and day of collection.
Reported as the number of humans, dogs, or birds present at the beach during sampling.
Table 3.
Correlations of enterococci and EPS with sand characteristics
| Parameter | Pearson's R correlation (P value)c |
|||||||
|---|---|---|---|---|---|---|---|---|
| Enterococci |
EPS |
|||||||
| Supraa | Intera | Suba | Combinedb | Supraa | Intera | Suba | Combinedb | |
| Sand moisture | 0.20 (0.34) | 0.40 (0.05)* | −0.22 (0.06)* | 0.20 (0.34) | 0.08 (0.70) | 0.183 (0.37) | 0.20 (0.34) | −0.09 (0.43) |
| Calcium carbonate | 0.07 (0.75) | −0.05 (0.81) | −0.40 (0.05)* | −0.13 (0.30) | −0.28 (0.16) | −0.44 (0.03)* | −0.54 (0.004)* | −0.40 (<0.001)* |
| Grain size | −0.19 (0.34) | −0.14 (0.50) | −0.11 (0.61) | −0.13 (0.26) | 0.08 (0.70) | 0.183 (0.37) | 0.20 (0.34) | −0.09 (0.43) |
| Humans | 0.23 (0.52) | −0.15 (0.68) | −0.15 (0.47) | −0.24 (0.20) | 0.05 (0.90) | −0.19 (0.61) | 0.16 (0.70) | −0.08 (0.70) |
| Dogs | −0.14 (0.70) | −0.60 (0.87) | 0.23 (0.25) | −0.12 (0.55) | −0.30 (0.40) | −0.23 (0.52) | 0.08 (0.72) | −0.18 (0.32) |
| Birds | −0.02 (0.96) | 0.35 (0.32) | 0.43 (0.03)* | 0.30 (0.13) | 0.16 (0.66) | 0.08 (0.82) | −0.02 (0.93) | −0.09 (0.63) |
| Rainfall | −0.36 (0.31) | 0.06 (0.77) | 0.48 (0.16) | 0.28 (0.13) | −0.54 (0.11) | 0.22 (0.27) | 0.13 (0.72) | 0.07 (0.73) |
Data from the 10-day study, September multiple-beach study, and February multiple-beach study combined for each corresponding tidal zone.
Data combined from all tidal zones and all studies.
indicates a significant correlation. Supra, supratidal; inter, intertidal; sub, subtidal.
There was no correlation between enterococcal levels in beach sand and rainfall or the number of humans and dogs at the beach. Sampling was conducted at 7 a.m. for both multiple-beach studies, so the activity of humans and dogs was very limited. There was a positive correlation between subtidal sand enterococcal levels and the number of birds recorded at Hobie Beach in the 10-day study (Table 3).
Variation in bacterial communities.
Bacterial community profiles from beach sediments were analyzed to determine if any differences in community composition existed between tidal zones. The ANOSIM test for differences in beach sediment samples collected within the supratidal, intertidal, and subtidal zones suggested a distinct variation in the TRF composition based on the tidal location sampled (global R = 0.646, P < 0.001). The MDS plots of bacterial community profiles determined by T-RFLP demonstrate a distinct gradation from samples collected in the supratidal zone to the subtidal zone from all three studies (Fig. 6). Significant differences in bacterial communities were detected between supratidal and subtidal (R = 0.956, P = 0.001), supratidal and intertidal (R = 0.413, P = 0.002), and intertidal and subtidal (R = 0.565, P = 0.004) samples from the 10-day study at Hobie Beach. The differences between bacterial community profiles across tidal zones were not as distinct for September (global R = 0.225; P = 0.001). The supratidal bacterial communities were significantly different from the subtidal zone (R = 0.436; P = 0.002) and intertidal zone (R = 0.356; P = 0.002). February bacterial communities were not significantly different between tidal zones.
Fig 6.
Nonmetric multidimensional scaling plot of bacterial communities inhabiting south Florida beach sediments. Plots show the relative similarity of bacterial communities from the supratidal, intertidal, and subtidal zones from the 10-day study (A), the September multiple-beach study (B), and the February multiple-beach study (C).
Clone library.
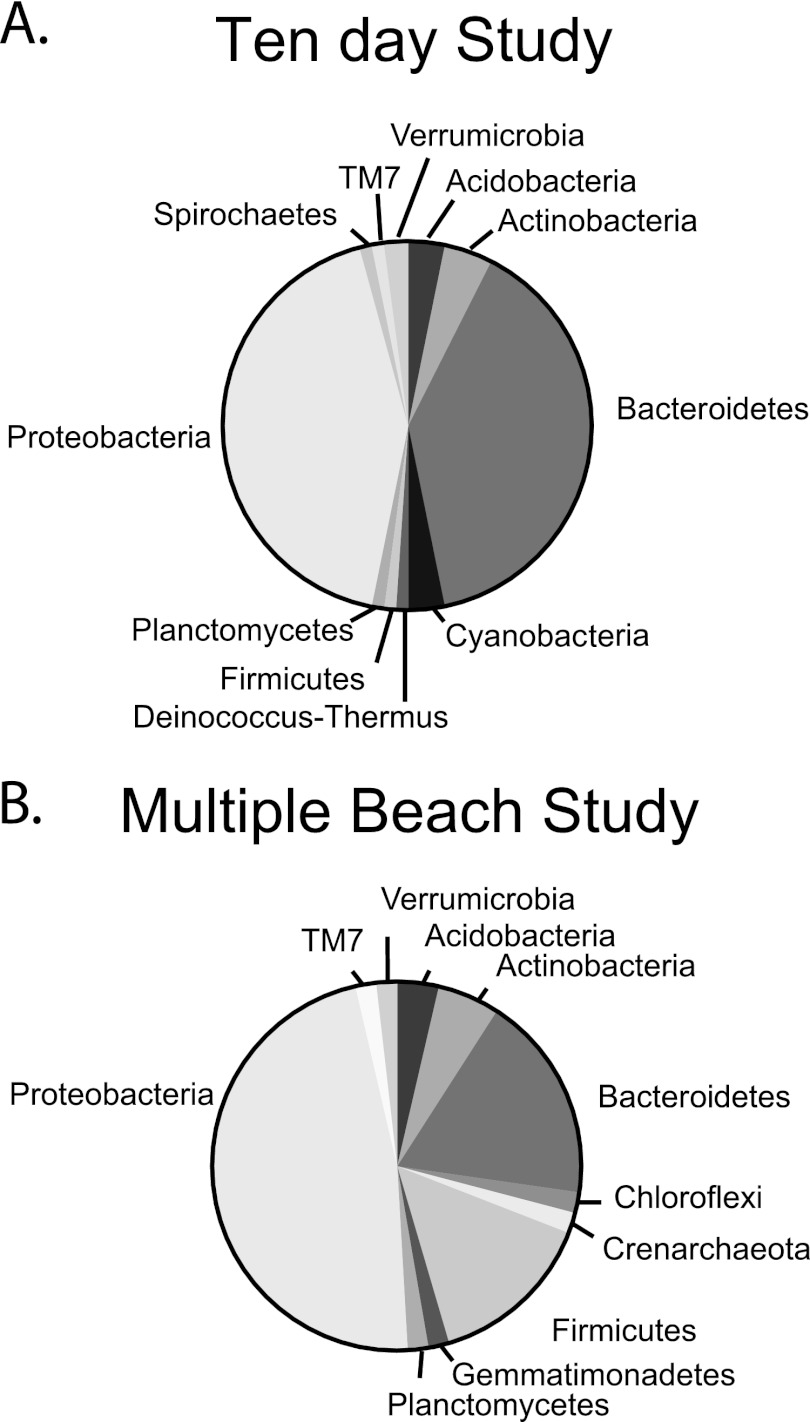
Clone libraries made from bacterial 16S rRNA gene sequences derived from supratidal sediment samples from Hobie Beach during the 10-day study and September multiple-beach study were affiliated with similar dominant bacteria (GenBank accession numbers JX041703 to JX041849; Fig. 7). Proteobacteria and Bacteroidetes were the most abundant of the clones from the 10-day study (42% and 39%, respectively; n = 97) and the multiple-beach study (47% and 18%, respectively; n = 55). While the number of clones in the library was low, a virtual digest of the clone library sequences with the 5 most common TRF cut sites revealed that Halobacillus (GenBank accession number AY0505522.1), Pedobacter (AB461805.1), Alphaproteobacteria (EU373869.1), uncultured Bacteroidetes (GQ850585.1), and uncultured Myxococcales (EF09217.1) may have been the dominant members of the supratidal sand community during this study.
Fig 7.
Bacterial clone libraries of the 16S rRNA gene from the supratidal sediments collected at Hobie Beach during the 10-day study and the multiple-beach study.
DISCUSSION
The risk of enteric illness among nonbathing beachgoers (33, 34) establishes the need for understanding the factors that control FIB survival and growth within beach sands. While persistence of FIB like enterococci in beach sands has been well documented (15, 19, 21, 32, 44), variability among beaches suggests that the risk level might be site specific or depend on characteristics such as mineralogy, particle size, moisture content, organic matter, and nutrient availability (33, 34). The survival of FIB in relation to these factors has been investigated extensively to better understand contamination at recreational beaches (10, 14, 15, 32, 48, 55, 65, 69, 70, 71); however, no set of factors can consistently account for the variation of indicator levels in sands.
Our results suggest that microbial biofilms play a role in enterococcal persistence in supratidal sands. The unimodal relationship between EPS and enterococci raises important questions regarding the nature of sediment biofilms and how they can both positively and negatively affect these bacteria in beach sands. The positive relationship of the unimodal trend between enterococci and biofilm from low to intermediate EPS levels might be expected given that related enteric bacteria such as E. faecalis and Enterococcus faecium are proficient biofilm producers and have been shown to gain protection from antibiotics through adherence to various medical instruments and biomaterials (48, 49). In the natural environment, attachment to surfaces and biofilm formation offer similar protection from stressors (25, 45, 46) and are particularly important for the control of pathogenic microbial contamination (47, 72, 74). Fecal indicator bacteria (6, 35, 63) and pathogens (74) have demonstrated the potential to survive in foreign environments via incorporation into mixed-species biofilms. For example, Listeria monocytogenes, a virulent pathogen widely distributed in moist soils and a notorious contaminator of food processing facilities, was able to avert antilisterial treatment by establishing itself in floor drain biofilm communities (46).
The decline of enterococcal levels in sediments with larger amounts of EPS forms the negative slope of the unimodal relationship. One explanation for this trend is competitive exclusion from the biofilm by bacterial activity. Several studies have demonstrated how the removal of microbiological activity from sediments increased the survival rate of enterococci (5, 21, 44) and Escherichia coli (3, 32). Feng et al. in 2010 (23) identified the resident bacterial community as the predominant biological stress responsible for the die-off of E. coli in sands, while protozoan predation and phage infection were negligible. Biofilm communities at food processing facilities with a recent history of no detectable L. monocytogenes contained antilisterial metabolites and could exclude L. monocytogenes (74). This suggests that the incorporation of pathogens in mixed-species biofilms is not a passive process but controlled by biological activity.
Competition is believed to be the driver of the widely recognized unimodal relationship between species richness and biomass by community ecologists (66). Species richness peaks at intermediate levels of biomass, followed by the exclusion of less competitive species by only a few species that can monopolize resources (30, 36, 53). It was determined that the competitive dominance by a few populations in mature lake biofilms reduced the community richness over time (38). In contrast, we found no differences in bacterial community profiles between samples having different levels of enterococci, suggesting that bacterial community composition did not change along a gradient of low to high EPS levels. However, an absence of change in community composition should be interpreted with caution given that T-RFLP analysis of 16S rRNA genes incorporates both active and inactive members. Dormant bacteria can represent large portions of the microbial community as documented by T-RFLP, and rare bacterial taxa can be disproportionately active compared to common bacterial taxa (40), as well as be active at different times on very short time scales. An understanding of how bacterial community composition influences enterococcal levels could likely be improved by focusing solely on the metabolically active community members.
The variation in enterococcal levels in supratidal sands, where elevated levels typically occur (1, 10, 69, 70, 71), was not related to any potential sources measured at each beach. Enterococcal levels were significantly higher during the 10-day study than during both multiple-beach studies. There was no correlation between enterococcal levels and the densities of humans, birds, or dogs in the supratidal sands. Subtidal sand enterococcal levels were positively correlated to birds, suggesting that wading birds may contribute more to fecal contamination in water than on land. While bird droppings have previously been implicated as sources of indicator bacteria in recreational waters and foreshore sand (56, 69), it is not clear if the bird feces from the current study seeded the sands and/or promoted biofilm development. A more extensive microbial source tracking study of enterococci found in these biofilms would be an interesting area of future investigation.
While moisture content has been attributed to higher indicator densities in the sand (21, 48, 71), we did not find any correlation between enterococci and sand moisture content. However, enterococcal levels were significantly higher in the supratidal zone, which is characteristically drier than intertidal and subtidal sands. This is similar to other studies that compared periodically wet sands and found high levels of enterococci where sand moisture content is low in foreshore sands (21, 27, 56, 65). It has been suggested that this occurs because the low water content in the supratidal sand reduces the predation and grazing pressure from water column microorganisms, leading to higher bacterial densities (10, 19). However, within the supratidal zone, where enterococcal levels are typically the highest, there was no relationship between moisture content and enterococci. There was a positive correlation between intertidal sand enterococcal levels and moisture content of sand. This was likely due to the entrapment of bacteria from the beach water.
Enterococcal levels were not related to any physical characteristics of the sands measured within the supratidal and intertidal sands. However, within the subtidal zone, enterococci were negatively related to calcium carbonate. Previous work showed that FIB were more abundant attached to quartz grains than to calcium carbonate grains when seeded into sterile microcosms saturated with water (32). While no relationship was identified between enterococci and any one physical factor measured here, the influence of a combination of physical factors along with predation, competitive exclusion, and pore water quality on enterococci remains unknown.
The variation in EPS levels was not related to any macroscale environmental factors measured in this study, suggesting that the EPS levels within the supratidal sand are primarily related to biological productivity. However, we cannot rule out that microscale conditions tightly regulated within the interstitial spaces are influential on EPS development. There was a negative correlation between EPS levels and the percentage of calcium carbonate grains in the intertidal and subtidal zones. One explanation could be that the beaches where calcium carbonate grains are abundant (Hallandale, Haulover, and South Beach) are also the most exposed to high wave energy compared to the other beaches, thereby resulting in greater agitation and abrasion which prevent the settlement of bacteria on these grains and EPS development.
While the methodology used in this study to recover enterococci from sand by shaking has been well applied in other studies (44, 58, 61, 70, 71), we acknowledge that tightly bound biofilm enterococcal cells could remain attached to sand grains and that the measured fraction in this study incorporates only those cells dislodgeable by shaking. However, Phillips et al. (58) compared the dislodgeable fractions of enterococci from beach sand between pore water flow and the vigorous-shaking method used in this work. The result was that a 9-fold increase of enterococci was removed through vigorous shaking compared to pore water flow. This suggests that a great number are attached with high affinity through water films bound to particles and organic matter and/or associated with biofilms. However, it is important to note that the relationship observed may be different at other beaches with different sand characteristics such as grain size and organic matter, which can inhibit the release of bacteria from sediment (8, 25).
Since epidemiological studies have shown a positive correlation of enterococcal levels in beach sand with increased illness rates among beachgoers (33), more research is needed to determine the origin of the enterococci in the sands from the different tidal zones at beaches and their impact on the water enterococcus levels. Sand criteria may be needed at beaches, in addition to the water quality criteria, to protect the beachgoers that do not go into the water but do participate in sand activities (62). While the exact mechanism controlling enterococcal levels associated with biofilms is unclear, further investigation could provide insight into key factors influencing the persistence and potential regrowth of enterococci in beach sands.
Given the relationships between biofilms and enterococci, research is also needed to evaluate the possible impacts of biofilms on pathogens. Human pathogens have been isolated from beach sediments (9, 54, 61), and it is important to understand further how these pathogenic microbes interact with biofilm communities in beach sands. It is likely that biofilms can also serve as refugia for pathogens in a manner similar to that for enterococci.
ACKNOWLEDGMENTS
Funding for this project was received through the National Science Foundation (NSF) and the National Institute of Environmental Health Sciences (NIEHS) Oceans and Human Health Center at the University of Miami Rosenstiel School (NSF 0CE0432368/0911373/1127813 and NIEHS P50 ES12736).
We thank the University of Miami students and Miami-Dade Department of Health staff who assisted with sample collection and sample processing. We are especially grateful to Laura Vogel, Amber Enns, Yifan Zhang, Nick Bill, Keiran Swart, and Gaby Toledo, who participated in laboratory analyses, along with numerous students and faculty at the University of Miami and volunteers from the Miami-Dade County Department of Health, who participated in sampling events. We also thank James Baker and Carla Hurt at the University of Miami Molecular and Imaging Core Facility. We also thank the anonymous reviewers who provided comments which improved the quality of the presented material.
REFERENCES
- 1. Abdelzaher AM, et al. 2010. Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Appl. Environ. Microbiol. 76: 724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alekseeva TN, Sval'nov VN. 2005. The refined wet sieving method for the analysis of fine-graded sediments. Lithol. Mineral Resources 40: 564–576 [Google Scholar]
- 3. Alm E, Burke J, Hagan E. 2006. Persistence and potential growth of the fecal indicator bacteria, Escherichia coli, in shoreline sand at Lake Huron. J. Great Lakes Res. 32: 401–405 [Google Scholar]
- 4. Anderson KL, Whitlock JE, Harwood VJ. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71: 3041–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrews RE, Jr, Johnson WS, Guard AR, Marvin JD. 2004. Survival of enterococci and Tn916-like conjugative transposons in soil. Can. J. Microbiol. 50: 957–966 [DOI] [PubMed] [Google Scholar]
- 6. Balzer M, Witt N, Flemming HC, Wingender J. 2010. Faecal indicator bacteria in river biofilms. Water Sci. Technol. 61: 1105–1111 [DOI] [PubMed] [Google Scholar]
- 7. Blackwood CB, Marsh T, Sang-Hoon K, Paul EA. 2003. Terminal restriction fragment length polymorphism data analysis for quantitative comparison of microbial communities. Appl. Environ. Microbiol. 69: 926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boehm AB, et al. 2009. Fecal indicator bacteria enumeration in beach sand: a comparison study of FIB extraction methods in medium to coarse sands. J. Appl. Microbiol. 107: 1740–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolton F, Surman SB, Martin K, Wareing DR, Humphrey TJ. 1999. Presence of campylobacter and salmonella in sand from bathing beaches. Epidemiol. Infect. 122: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonilla TD, et al. 2007. Prevalence and distribution of fecal indicator organisms in south Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar. Pollut. Bull. 54: 1472–1482 [DOI] [PubMed] [Google Scholar]
- 11. Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 34: 77–87 [Google Scholar]
- 12. Brettar I, Holfe M. 1992. Influence of ecosystemic factors on survival of Escherichia coli after large-scale release into lake water mesocosms. Appl. Environ. Microbiol. 58: 2201–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burton E, Yakandawala N, LoVetri K, Madhystha MS. 2007. A microplate spectrofluorometric assay for bacterial biofilms. J. Ind. Microbiol. Biotechnol. 34: 1–4 [DOI] [PubMed] [Google Scholar]
- 14. Byappanahalli MN, Whitman RL, Shively DA, Sadowsky MJ, Ishii S. 2006. Population structure, persistence, and seasonality of autochthonous Escherichia coli in temperate, coastal forest soil from a Great Lakes watershed. Environ. Microbiol. 8: 504–513 [DOI] [PubMed] [Google Scholar]
- 15. Byappanahalli MN, Fujioka RS. 1998. Evidence that tropical soil can support the growth of Escherichia coli. Water Sci. Technol. 38: 171–174 [Google Scholar]
- 16. Craig D, Fallowfield H, Cromar N. 2004. Use of macrocosms to determine persistence of Escherichia coli in recreational coastal water and sediment and validation with in situ measurements. J. Appl. Microbiol. 96: 922–930 [DOI] [PubMed] [Google Scholar]
- 17. Davey ME, O'Toole GA. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64: 847–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davies CM, Bavor HJ. 2000. The fate of storm water-associated bacteria in constructed wetland and water pollution control pond systems. J. Appl. Microbiol. 89: 349–360 [DOI] [PubMed] [Google Scholar]
- 19. Davies CM, Long JAH, Donald M, Ashbolt NJ. 1995. Survival of fecal microorganisms in marine and freshwater sediments. Appl. Environ. Microbiol. 61: 1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Decho AW, Visscher PT, Reid RP. 2004. Production and cycling of natural microbial exopolymers (EPS) within a stromatolite. Palaeogeogr. Palaeoclimatol. Palaeoecol. 219: 71–86 [Google Scholar]
- 21. Desmarais TR, Solo-Gabriele HM, Palmer CJ. 2002. Influence of soil on fecal indicator organisms in tidally influenced subtropical environment. Appl. Environ. Microbiol. 68: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1996. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28: 350–356 [Google Scholar]
- 23. Feng F, Goto D, Yan T. 2010. Effects of autochthonous microbial community on the die-off of fecal indicators in tropical beach sand. FEMS Microbiol. Ecol. 74: 214–225 [DOI] [PubMed] [Google Scholar]
- 24. Ferguson DM, Moore DF, Getrich MA, Zhowandai MH. 2005. Enumeration and speciation of enterococci found in marine and intertidal sediments and coastal water in southern California. J. Appl. Microbiol. 99: 598–608 [DOI] [PubMed] [Google Scholar]
- 25. Flemming HC. 1993. Biofilms and environmental protection. Water Sci. Technol. 27: 1–10 [Google Scholar]
- 26. Fries JS, Characklis GW, Noble RT. 2006. Attachment of fecal indicator bacteria to particles in the Neuse River estuary, N.C. J. Environ. Eng. (NY) 132: 1338–1345 [Google Scholar]
- 27. Fujioka RS, Hashimoto HH, Siwak EB, Young RH. 1981. Effect of sunlight on survival of indicator bacteria in seawater. Appl. Environ. Microbiol. 41: 690–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerba CP, McLeod JS. 1976. Effect of sediments on the survival of Escherichia coli in marine waters. Appl. Environ. Microbiol. 32: 114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerhardt P, Murray RGE, Wood WA, Krieg NR. 1994. Methods for general and molecular bacteriology, p 270–271 American Society for Microbiology, Washington, DC [Google Scholar]
- 30. Grime JP. 1973. Control of species diversity in herbaceous vegetation. J. Environ. Manage. 1: 151–167 [Google Scholar]
- 31. Haile RW, et al. 1999. The health effects of swimming in ocean water contaminated by storm drain runoff. Epidemiology 10: 355–363 [PubMed] [Google Scholar]
- 32. Hartz A, et al. 2008. Survival potential of Escherichia coli and enterococci in subtropical beach sand: implications for water quality managers. J. Environ. Qual. 37: 898–905 [DOI] [PubMed] [Google Scholar]
- 33. Heaney CD, et al. 2012. Fecal indicators in sand, sand contact, and risk of enteric illness among beachgoers. Epidmiology 23: 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heaney CD, et al. 2009. Contact with beach sand among beachgoers and risk of illness. Am. J. Epidemiol. 170: 164–172 [DOI] [PubMed] [Google Scholar]
- 35. Hirotani H, Yoshino M. 2010. Microbial indicators in natural biofilms developed in the riverbed. Water Sci. Technol. 62: 1149–1153 [DOI] [PubMed] [Google Scholar]
- 36. Huston MA, Smith T. 1987. Plant succession: life history and competition. Am. Nat. 130: 168–198 [Google Scholar]
- 37. Ishii S, Ksoll W, Hicks R, Sadowsky M. 2006. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl. Environ. Microbiol. 72: 612–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jackson CR, Churchill PF, Roden EE. 2001. Successional changes in bacterial assemblage structure during epilithic biofilm development. Ecology 82: 555–566 [Google Scholar]
- 39. Jeong Y, et al. 2005. Identifying pollutant sources in tidally mixed systems: case study of fecal indicator bacteria from marinas in Newport Bay, southern California. Environ. Sci. Technol. 39: 9083–9093 [DOI] [PubMed] [Google Scholar]
- 40. Jones SE, Lennon JT. 2010. Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci. U. S. A. 107: 5881–5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kay D, et al. 1994. Predicting likelihood of gastroenteritis from sea bathing: results from randomized exposure. Lancet 344: 905–909 [DOI] [PubMed] [Google Scholar]
- 42. Klaus JS, Janse I, Heikoop JM, Sanford RA, Fouke BW. 2007. Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environ. Microbiol. 9: 1291–1305 [DOI] [PubMed] [Google Scholar]
- 43. Kruskal JB. 1964. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29: 1–27 [Google Scholar]
- 44. Lee CM, et al. 2006. Persistence of fecal indicator bacteria in Santa Monica Bay beach sediments. Water Res. 40: 2593–2602 [DOI] [PubMed] [Google Scholar]
- 45. Massol-Deyá A, et al. 1997. Succession and convergence of biofilm communities in fixed-film reactors treating aromatic hydrocarbons in groundwater. Appl. Environ. Microbiol. 63: 270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McBain AJ, et al. 2003. Microbial characterization of biofilms in domestic drains and the establishment of stable biofilm microcosms. Appl. Environ. Microbiol. 69: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Medema GJ, Schets FM, Teunis PFM, Havelaar AH. 1998. Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. Appl. Environ. Microbiol. 64: 4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mika KB, et al. 2009. Pilot- and bench-scale testing of faecal indicator bacteria survival in marine beach sand near point sources. J. Appl. Microbiol. 107: 72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mohamed JA, Huang DB. 2007. Biofilm formation by enterococci. J. Med. Microbiol. 56: 1581–1588 [DOI] [PubMed] [Google Scholar]
- 50. Nallapareddy SR, Qin X, Weinstock GM, Hook M, Murray BE. 2000. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68: 5218–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. National Resource Defense Council 2009. Testing the waters: a guide to water quality at vacation beaches, 19th annual report. National Resources Defense Council, Washington, DC [Google Scholar]
- 52. National Resource Defense Council 2010. Testing the waters: a guide to water quality at vacation beaches, 20th annual report. National Resources Defense Council, Washington, DC [Google Scholar]
- 53. Newman EI. 1973. Competition and diversity in herbaceous vegetation. Nature 244: 310 [Google Scholar]
- 54. Obiri-Danso K, Jones K. 2000. Intertidal sediments as reservoirs for hippurate negative campylobacters, salmonellae and fecal indicators in three EU recognized bathing waters in northwest England. Water Res. 34: 519–527 [Google Scholar]
- 55. Ortega C, et al. 2009. Correlations between microbial indicators, pathogens, and environmental factors in a subtropical marine estuary. Mar. Pollut. Bull. 58: 1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oshiro R, Fujioka R. 1995. Sand, soil, and pigeon droppings—sources of indicator bacteria in the waters of Hanauma Bay, Oahu, Hawaii. Water Sci. Technol. 31: 251–254 [Google Scholar]
- 57. Perkins RG, Paterson DM, Sun H, Watson J, Player MA. 2004. Extracellular polymeric substances: quantification and use in erosion experiments. Cont. Shelf Res. 24: 1623–1635 [Google Scholar]
- 58. Phillips MC, et al. 2011. Pore water transport of enterococci out of beach sediments. Mar. Pollut. Bull. 62: 2293–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Piggot AM, Fouke BW, Sivaguru M, Sanford RA, Gaskins HR. 2009. Change in zooxanthellae and mucocyte tissue density as an adaptive response to environmental stress by the coral Montastraea annularis. Mar. Biol. 156: 2379–2389 [Google Scholar]
- 60. Pilkey OH, Morton RW, Luternauer J. 1967. The carbonate fraction of beach and dune sands. Sedimentology 8: 311–327 [Google Scholar]
- 61. Shah AH, et al. 2011. Indicator microbes correlate with pathogenic bacteria, yeasts and helminthes in sand at a subtropical beach site. J. Appl. Microbiol. 6: 110 doi:10.1111/j.1365-2672.2011.05013.x [DOI] [PubMed] [Google Scholar]
- 62. Shibata T, Solo-Gabriele HM. 2012. Quantitative microbial risk assessment of human illness from exposure to marine beach sand. Environ. Sci. Technol. 46: 2799–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shikuma NJ, Hadfield MG. 2010. Marine biofilms on submerged surfaces are a reservoir for Escherichia coli and Vibrio cholerae. Biofouling 26: 39–46 [DOI] [PubMed] [Google Scholar]
- 64. Sinton L, Finlay R, Lynch P. 1999. Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 65: 3605–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Solo-Gabriele HM, Wolfert MA, Desmairais TR, Palmer CJ. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 66: 230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tilman D. 1982. Resource competition and community structure. Princeton University Press, Princeton, NJ: [PubMed] [Google Scholar]
- 67. US Environmental Protection Agency 2006. Method 1600: enterococci in water by membrane filtration using membrane-Enterococcus indoxyl-β-D-glucoside agar (Mei), EPA-821-R-06-009. US EPA, Washington, DC [Google Scholar]
- 68. Wade TJ, et al. 2006. Rapidly measured indicators of recreational water quality are predictive of swimming-associated gastrointestinal illness. Environ. Health Perspect. 114: 24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Whitman RL, Nevers MB. 2003. Foreshore sand as a source of Escherichia coli in nearshore water of a Lake Michigan beach. Appl. Environ. Microbiol. 69: 5555–5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yamahara KM, Layton BA, Santoro AE, Boehm AB. 2007. Beach sands along the California coast are diffuse sources of fecal bacteria to coastal waters. Environ. Sci. Technol. 41: 4515–4521 [DOI] [PubMed] [Google Scholar]
- 71. Yamahara KM, Walters SP, Boehm AB. 2009. Growth of enterococci in unaltered, unseeded beach sands subjected to tidal wetting. Appl. Environ. Microbiol. 75: 1517–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yildiz FH, Schoolnik GK. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 96: 4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang X, Bishop PL. 2003. Biodegradability of biofilm extracellular polymeric substances. Chemosphere 50: 63–69 [DOI] [PubMed] [Google Scholar]
- 74. Zhao T, Doyle MP, Zhao P. 2004. Control of Listeria monocytogenes in a biofilm by competitive-exclusion microorganisms. Appl. Environ. Microbiol. 70: 3996–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]