Abstract
We isolated a new lytic Pseudomonas aeruginosa phage that requires type IV pili for infection. PA1Ø has a broad bactericidal spectrum, covering Gram-positive and Gram-negative bacteria, and can eradicate biofilm cells. PA1Ø may be developed as a therapeutic agent for biofilm-related mixed infections with P. aeruginosa and Staphylococcus aureus.
TEXT
Bacteriophages (phages) are bacterial viruses that play an important role in bacterial biology, diversity, and evolution (6). Due to their ability to lyse bacteria, attempts have been made to use lytic phages to treat bacterial infections, and some have shown clinical promise as therapeutic antimicrobial agents (2, 11, 16). However, phages are not commonly used therapeutically due to several problems, such as narrow host range, insufficient purity of phage preparations, poor stability or viability of phage preparations, and a lack of understanding of the heterogeneity and mode of action of phages (17, 18). Despite such problems, the worldwide spread of multidrug-resistant (MDR) pathogenic bacteria has led to the revival of this therapeutic approach (12).
In this study, we isolated a novel lytic phage, PA1Ø, which is capable of lysing MDR pathogenic bacteria, especially Pseudomonas aeruginosa and Staphylococcus aureus. From sewage samples collected from poultry and pig farms located in Gyeonggi Province, South Korea, several phages specific to P. aeruginosa were found. Among the isolated phages, one, PA1Ø, was selected, propagated, and purified successfully according to the method of Merabishvili et al. (13). Concentrated phage solutions were prepared as previously described (5) using cesium chloride (CsCl) gradients (9). Approximately 1017 PFU/ml of phage stock solution was obtained and stored at 4°C for future use.
PA1Ø exhibited an icosahedral head and a long noncontractile tail by transmission electron microscopy analysis. The lengths of the icosahedral head and the long tail were approximately 58 and 164 nm, respectively. Morphologically, PA1Ø belongs to the family Siphoviridae (1). To assess the host range of PA1Ø, spot tests and plaque formation assays were performed with various species of bacteria (Table 1). In a spot test, PA1Ø formed clear lytic zones on the lawns of P. aeruginosa, Shigella sonnei, S. aureus, Staphylococcus epidermidis, Staphylococcus hominis, Streptococcus pneumoniae, Streptococcus salivarius, and Listeria monocytogenes. However, no lytic zones were found on the lawns of Escherichia coli, Serratia marcescens, Enterobacter aerogenes, Acinetobacter baumannii, or certain Streptococcus spp. In the plaque formation assays, PA1Ø formed distinct plaques only when used to infect P. aeruginosa or S. sonnei. PA1Ø could not produce typical plaques when used to infect S. aureus, although it formed distinct clear zones on the lawn of S. aureus in spot test with lytic areas two to three times larger than those formed on the lawns of P. aeruginosa or S. sonnei. This result indicates that PA1Ø can infect and propagate in only P. aeruginosa and S. sonnei.
Table 1.
Results of spot tests and plaque formation assays
| Species | Strain or isolate | Spot test resulta | Plaque formation | Source |
|---|---|---|---|---|
| Pseudomonas aeruginosa | PAO1 | + | Yes | Yonsei University |
| ATCC 29853 | + | Yes | ATCCb | |
| PA 1-30 | + | Yes | Laboratory collection | |
| PA 1-67 | + | Yes | Laboratory collection | |
| PA 1-86 | + | Yes | Laboratory collection | |
| PA 2-22 | + | Yes | Laboratory collection | |
| PA 2-35 | + | Yes | Laboratory collection | |
| PA 2-36 | + | Yes | Laboratory collection | |
| PA 2-50 | + | Yes | Laboratory collection | |
| Shigella sonnei | NIH1 | + | Yes | Laboratory collection |
| H9 | + | Yes | Laboratory collection | |
| H114 | + | Yes | Laboratory collection | |
| H131 | + | Yes | Laboratory collection | |
| H151 | + | Yes | Laboratory collection | |
| H1 | − | No | Laboratory collection | |
| H122 | − | No | Laboratory collection | |
| Escherichia coli | ATCC 25922 | − | No | ATCC |
| 03K543 | − | No | Laboratory collection | |
| 04K743 | − | No | Laboratory collection | |
| 03K830 | − | No | Laboratory collection | |
| 03K692 | − | No | Laboratory collection | |
| Serratia marcescens | ATCC 8100 | − | No | ATCC |
| Acinetobacter baumannii | ATCC 19606 | − | No | ATCC |
| Enterobacter aerogenes | EA7 | − | No | Laboratory collection |
| Staphylococcus aureus | ATCC 29213 | ++ | No | ATCC |
| ATCC 25923 | ++ | No | ATCC | |
| MRSA WS-05 | ++ | No | Laboratory collection | |
| MRSA D43-a | ++ | No | Laboratory collection | |
| KNUH-2 | ++ | No | Laboratory collection | |
| KNUH-3 | ++ | No | Laboratory collection | |
| KNUH-5 | ++ | No | Laboratory collection | |
| Staphylococcus epidermidis | KNUH-134 | + | No | Laboratory collection |
| KNUH-174 | + | No | Laboratory collection | |
| KNUH-175 | + | No | Laboratory collection | |
| Staphylococcus hominis | KNUH-328 | ++ | No | Laboratory collection |
| KNUH-329 | ++ | No | Laboratory collection | |
| Streptococcus pneumoniae | 15 | − | No | Laboratory collection |
| 83 | + | No | Laboratory collection | |
| 134 | − | No | Laboratory collection | |
| Streptococcus salivarius | 65 | − | No | Laboratory collection |
| 68 | + | No | Laboratory collection | |
| 84 | + | No | Laboratory collection | |
| 133 | − | No | Laboratory collection | |
| Streptococcus gordonii | 143 | ++ | No | Laboratory collection |
| Streptococcus agalactiae | 188 | − | No | Laboratory collection |
| Listeria monocytogenes | 10403S | + | No | Martin Wiedermann |
++, large clear lysis; +, small clear lysis; −, no lysis.
ATCC, American Type Culture Collection.
The adsorption rate of PA1Ø was determined. About 87% of PA1Ø phage were adsorbed to P. aeruginosa PAO1 cells within 2 min. The rate of PA1Ø adsorption to S. sonnei was similar to that for P. aeruginosa. A one-step growth experiment showed that the latent period of PA1Ø was approximately 10 min. The first burst of PA1Ø was recorded at 15 min, and the burst size was assumed to be approximately 261 PFU/bacterium.
To identify the receptor molecule used by PA1Ø to infect P. aeruginosa, we constructed and screened a library of random transposon (Tn) insertion mutants of P. aeruginosa strain PAO1 for resistance to PA1Ø treatment. A library of random transposon insertion mutants of P. aeruginosa strain PAO1 was constructed by biparental mating using E. coli SM10 (λpir) harboring pBTK30 as a donor strain (4). Gentamicin-resistant (Gmr) transconjugants were pooled, and an aliquot of the pool (∼108 CFU/ml) was inoculated into LB containing 1 × 1011 PFU/ml of PA1Ø. After 16 h growth at 37°C, cultures were diluted 100-fold in fresh LB containing the same titer of PA1Ø to enrich the population of PA1Ø-resistant mutants. This enrichment step was repeated three times, and then culture aliquots were spread on LB agar plates containing 200 μg/ml gentamicin (Gm) to isolate mutants that had potentially acquired resistance to PA1Ø. Under these conditions, three mutants were successfully identified as demonstrating normal growth. As shown in Fig. 1, these mutants exhibited uninterrupted growth in the presence of 1 × 1011 PFU/ml PA1Ø, while the growth of wild-type PAO1 was greatly diminished under the same conditions; the optical cell density of these mutants in the presence of PA1Ø was almost comparable to that of PAO1 in plain LB medium. Further sequence analysis revealed Tn insertion into the pilY1 (PA4554), pilB (PA4526), or pilA (PA4525) gene in each of these selected mutants. Since these genes encode core components of the type IV pilus synthetic machinery, this result strongly suggests that PA1Ø binds to the pilus to infect P. aeruginosa. The adsorption assay using P. aeruginosa mutants revealed that PA1Ø was unable to adsorb to the mutants, confirming that the type IV pilus is the receptor molecule used by PA1Ø to infect P. aeruginosa.
Fig 1.
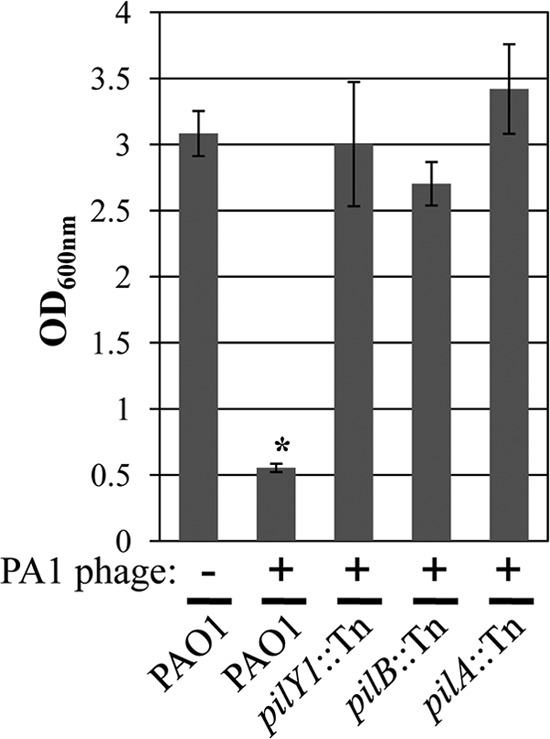
Three pilus mutants grow normally in the presence of PA1Ø. The growth of three pilus-negative strains and wild-type PAO1 was monitored by measuring the optical density (OD) at 600 nm. PAO1 growth in plain LB is included as a control. *, P < 0.001 versus all other growth.
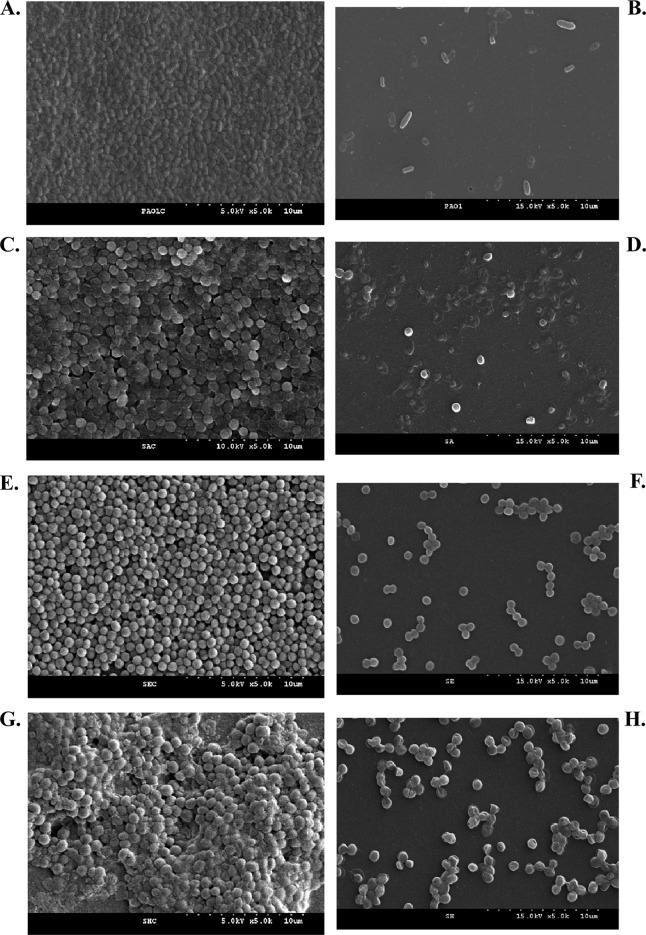
We assessed the bactericidal activity of PA1Ø against eight bacterial strains (S. aureus ATCC 25923, WS-05, and D43-a; P. aeruginosa PAO1; S. hominis KNUH-328 and KNUH-329; and S. epidermidis KNUH-134 and KNUH-174). Overnight bacterial cultures were diluted to an optical density at 600 nm (OD600) of 1, followed by a 10-fold dilution. For treatment of planktonic cultures with phage, 100-μl aliquots (5 × 106 CFU) were inoculated into 96-well microtiter plates, followed by inoculation of 100 μl of PA1Ø samples (1 × 1010 PFU/ml), to a multiplicity of infection (MOI) of 200, into the same wells and a further 6-h incubation at 37°C. Fifty microliters of 0.1% triphenyltetrazolium chloride (TTC) was added, and samples were further incubated at 37°C for 1 h, after which absorbance at 540 nm was determined using an enzyme-linked immunosorbent assay (ELISA) reader (VersaMax; Molecular Devices, Sunnyvale, CA) (7, 9). For biofilm formation, 100 μl of an overnight bacterial culture (5 × 106 CFU) were inoculated into a 96-well microtiter plate and incubated at 37°C for 1 day in a humidified incubator. After removal of planktonic cells and medium by washing with 100 μl of fresh tryptic soy broth (TSB), 100 μl of PA1Ø (1 × 1010 PFU) was inoculated into the biofilm. After incubation at 37°C for 3 h in a humidified incubator, 50 μl of 0.1% TTC was added and incubated for a further 1 h. Absorbance was recorded at 540 nm. PA1Ø significantly inhibited the bacterial growth in all bacterial strains tested (Fig. 2A). After incubation for 6 h with PA1Ø and P. aeruginosa PAO1, the growth of PAO1 was greatly inhibited in comparison with an untreated control. We also measured PA1Ø's biofilm removal activity (Fig. 2B). After treatment of 24-hour-old biofilms of P. aeruginosa, S. aureus, S. epidermidis, and S. hominis with PA1Ø, the numbers of viable cells in phage-treated biofilms were greatly decreased in comparison with those in untreated biofilms. Field emission scanning electron microscopy (FESEM) analysis clearly showed PA1Ø's biofilm removal activities (Fig. 3). In phage-treated samples, lysed cells were obvious, and most of the cells adopted a “ghost” morphology (Fig. 3B, D, F, and H). In contrast, no lysed or killed cells were found in the phage-untreated groups (Fig. 3A, C, E, and G).
Fig 2.
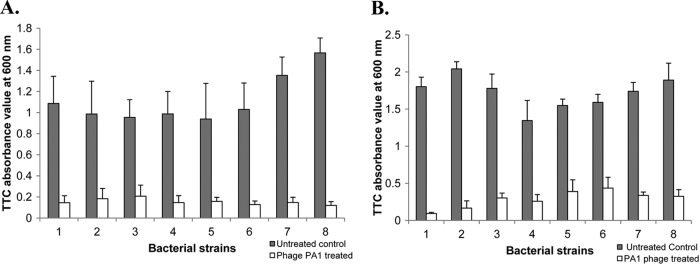
Bactericidal and biofilm removal effects of PA1Ø. Planktonic cultures (A) and 1-day-old biofilms (B) of eight different bacterial species were treated with PA1Ø at an MOI of 200 for 6 h and 1 × 1010 PFU of PA1Ø for 3 h, respectively. Triphenyltetrazolium chloride (TTC) absorbance values at 600 nm were compared with those of non-PA1Ø-treated controls (gray bars). 1, S. aureus ATCC 25923; 2, S. aureus WS-05; 3, S. aureus D43-a; 4, S. epidermidis KNUH-134; 5, S. epidermidis KNUH-174; 6, S. hominis KNUH-328; 7, S. hominis KNUH-329; and 8, P. aeruginosa PAO1. Experiments were performed three times independently, and values are means and standard deviations.
Fig 3.
Biofilm removal capacity of PA1Ø. Non-PA1Ø-treated (A, C, E, and G) and treated (B, D, F, and G) biofilms of four bacterial strains were visualized by scanning electron microscopy. (A and B) P. aeruginosa PAO1; (C and D) S. aureus ATCC 25923; (E and F) S. epidermidis KNUH-134; (G and H) S. hominis KNUH-329.
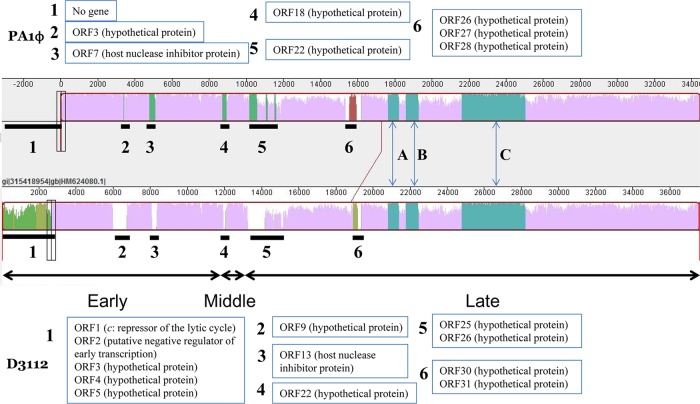
The genome sequence of PA1Ø was deciphered and announced recently (8). The PA1Ø genome consists of 51 putative protein-coding genes. Since the PA1Ø genome showed a high homology to the D3112 phage genome in the study (8), we performed Dot Matrix View and MAUVE genome alignment analyses to compare the two genomes in detail. The analyses revealed six unmatched regions (Fig. 4). Unmatched region 1, spanning ORF1 to ORF5 of D3112 phage including the c repressor protein-coding gene, was not found in the PA1Ø genome. Unmatched region 3 covers the coding regions of host nuclease inhibitor proteins in both phages, and other unmatched regions correspond to genes coding hypothetical proteins of both phages. Three highly conserved regions between PA1Ø and D3112 cover hypothetical proteins (regions A and C), the major head subunit (region B), and the putative tail component (region C).
Fig 4.
Alignment of the PA1Ø and D3112 genomes. This MAUVE-generated diagram shows the degree of sequence similarity between two phages. Both gene maps are measured against a ruler in base pairs. The degree of sequence similarity between two genomes is indicated by the height of the similarity peaks. The six genomic dissimilarity regions are marked with black bold lines and numbered, and affected genes in both phages are listed in numbered boxes. Regions A to C show highly conserved regions of PA1Ø and phage D3112.
In this study, we isolated a novel lytic bacteriophage, PA1Ø, with a broad host range of P. aeruginosa and S. sonnei. Besides these bacteria, PA1Ø could lyse a number of Gram-positive bacterial pathogens. Since PA1Ø could form plaques only when infecting P. aeruginosa and S. sonnei, its bactericidal mechanism against Gram-positive bacteria might be different from that against P. aeruginosa and S. sonnei. One possible explanation is the production of phage-associated lytic enzymes. In fact, Lai et al. reported that Acinetobacter baumannii phage varphiAB2, which shows broad bactericidal activity against Gram-positive and Gram-negative bacteria, produced endolysin (LysAB2), which was responsible for the lysis of S. aureus by degrading bacterial cell wall's peptidoglycan (10). Another feature of PA1Ø is its ability to destroy biofilms of P. aeruginosa, S. aureus, S. epidermidis, and S. hominis. It could significantly lyse those bacterial cells in the established biofilms within 3 h. To our knowledge, the broad bactericidal spectrum and strong lytic activities against both planktonic and biofilm cells exhibited by PA1Ø have never been demonstrated in other phages.
In several aspects, PA1Ø resembles P. aeruginosa phage D3112, which is a well-known temperate phage used for molecular biology studies of P. aeruginosa (3, 19). Both phages are Pseudomonas phages belonging to the family Siphoviridae. Both phages infect P. aeruginosa via the type IV pili (14). The genome of PA1Ø is highly homologous to the genome of D3112 phage (GenBank accession number AY394005.1), with overall nucleotide identity of 90%. The difference of PA1Ø from D3112 is the absence of the unmatched region 1 spanning first five ORFs of phage D3112. Because unmatched region 1 includes a c repressor-encoding gene, known to play a critical role in the maintenance of the lysogenic state of the D3112 phage in P. aeruginosa (15), it seems that PA1Ø lacks the critical lysogenic genes. Thus, it is suggested that PA1Ø is a lytic phage, not a temperate, transposable one.
In conclusion, this study characterized a new lytic P. aeruginosa phage that has a broad bactericidal spectrum covering various species of Gram-positive and Gram-negative bacteria. Moreover, PA1Ø can eradicate biofilm cells. Therefore, PA1Ø can be developed as an alternative antimicrobial agent that may be useful in treatment of biofilm-related infections and mixed infections caused by bacteria such as P. aeruginosa and S. aureus.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea, no. A084442 (to J.K.), and by the National Research Foundation of Korea (NRF), funded by the Korean Government (MEST), no. 2010-0007203 (to J.K.), no. 2009-0087951 (to S.S.Y.), and no. 2011-0016210 (to S.S.Y.).
Footnotes
Published ahead of print 29 June 2012
REFERENCES
- 1. Ackermann HW. 2009. Phage classification and characterization. Methods Mol. Biol. 501: 127–140 [DOI] [PubMed] [Google Scholar]
- 2. Courchesne NM, Parisien A, Lan CQ. 2009. Production and application of bacteriophage and bacteriophage-encoded lysins. Recent Pat. Biotechnol. 3: 37–45 [DOI] [PubMed] [Google Scholar]
- 3. Darzins A, Casadaban MJ. 1989. Mini-D3112 bacteriophage transposable elements for genetic analysis of Pseudomonas aeruginosa. J. Bacteriol. 171: 3909–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodman AL, et al. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7: 745–754 [DOI] [PubMed] [Google Scholar]
- 5. Humphrey SB, Stanton TB, Jensen NS, Zuerner RL. 1997. Purification and characterization of VSH-1, a generalized transducing bacteriophage of Serpulina hyodysenteriae. J. Bacteriol. 179: 323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jessup CM, Forde SE. 2008. Ecology and evolution in microbial systems: the generation and maintenance of diversity in phage-host interactions. Res. Microbiol. 159: 382–389 [DOI] [PubMed] [Google Scholar]
- 7. Kim S, et al. 2010. A simple colorimetric method for testing antimicrobial susceptibility of biofilmed bacteria. J. Microbiol. 48: 709–711 [DOI] [PubMed] [Google Scholar]
- 8. Kim S, Rahman M, Kim J. 2012. Complete genome sequence of Pseudomonas aeruginosa lytic bacteriophage PA1O which resembles temperate bacteriophage D3112. J. Virol. 86: 3400–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knezevic P, Petrovic O. 2008. A colorimetric microtiter plate method for assessment of phage effect on Pseudomonas aeruginosa biofilm. J. Microbiol. Methods 74: 114–118 [DOI] [PubMed] [Google Scholar]
- 10. Lai MJ, et al. 2011. Antibacterial activity of Acinetobacter baumannii phage varphiAB2 endolysin (LysAB2) against both gram-positive and gram-negative bacteria. Appl. Microbiol. Biotechnol. 90: 529–539 [DOI] [PubMed] [Google Scholar]
- 11. Mathur MD, Vidhani S, Mehndiratta PL. 2003. Bacteriophage therapy: an alternative to conventional antibiotics. J. Assoc. Phys. India 51: 593–596 [PubMed] [Google Scholar]
- 12. Matsuzaki S, et al. 2005. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. 11: 211–219 [DOI] [PubMed] [Google Scholar]
- 13. Merabishvili M, et al. 2009. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4: e4944 doi:10.1371/journal.pone.0004944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roncero C, Darzins A, Casadaban MJ. 1990. Pseudomonas aeruginosa transposable bacteriophages D3112 and B3 require pili and surface growth for adsorption. J. Bacteriol. 172: 1899–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salmon KA, Freedman O, Ritchings BW, DuBow MS. 2000. Characterization of the lysogenic repressor (c) gene of the Pseudomonas aeruginosa transposable bacteriophage D3112. Virology 272: 85–97 [DOI] [PubMed] [Google Scholar]
- 16. Sillankorva S, et al. 2011. Efficacy of a broad host range lytic bacteriophage against E. coli adhered to urothelium. Curr. Microbiol. 62: 1128–1132 [DOI] [PubMed] [Google Scholar]
- 17. Sulakvelidze A, Alavidze Z, Morris JG., Jr 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45: 649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verbeken G, et al. 2007. European regulatory conundrum of phage therapy. Future Microbiol. 2: 485–491 [DOI] [PubMed] [Google Scholar]
- 19. Wang PW, Chu L, Guttman DS. 2004. Complete sequence and evolutionary genomic analysis of the Pseudomonas aeruginosa transposable bacteriophage D3112. J. Bacteriol. 186: 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]