Abstract
Ammonia-oxidizing bacteria (AOB) and archaea (AOA) were quantified in the sediments and roots of dominant macrophytes in eight neutral to alkaline coastal wetlands. The AOA dominated in most samples, but the bacterial-to-archaeal amoA gene ratios increased with increasing ammonium levels and pH in the sediments. For all plant species, the ratios increased on the root surface relative to the adjacent bulk sediment. This suggests that root surfaces in these environments provide conditions favoring enrichment of AOB.
TEXT
Nitrification is a critical process for nitrogen retention in coastal wetlands, which are important transition zones between marine and terrestrial environments. Nitrification rates are commonly higher in the rhizosphere of macrophytes than in the bulk sediment (16, 24), suggesting that nitrifiers are stimulated by rhizosphere conditions of wetland plants. Ammonia oxidation, the first and rate-limiting step in nitrification, is performed by ammonia-oxidizing bacteria (AOB) and archaea (AOA). For freshwater macrophytes, plant-specific differences in the composition and abundance of AOB and AOA have been described both in the rhizosphere (11, 12) and on the epiphyton of submerged shoots (4, 9). The AOA generally outcompete AOB at low ammonia concentrations (10, 13, 14, 26, 29, 30). Also, root exudates may play a role in the relative distribution of AOB and AOA, even though ammonia oxidation is mainly considered an autotrophic process, as mixotrophy at the expense of pyruvate has been proven for a soil AOA isolate (15, 22, 30). Aquatic macrophytes such as Phragmites australis can release up to 70 mg of dissolved organic carbon g−1 (root wet weight) day−1 (31) and may include organic compounds that are needed for AOA dependent on mixotrophy.
Despite the importance of coastal wetlands as nutrient filters, little is known about ammonia oxidizers in these environments. However, based on the above-referenced findings and the fact that AOA dominate marine systems (6, 7), our hypothesis is that AOA are important in coastal wetlands and in particular in the rhizosphere of macrophytes. Our aim was to quantify the relative abundance of AOA and AOB and measure the potential nitrification rates in the sediment and at the root surface of the dominant macrophytes in coastal lagoons to determine if the two groups are differently favored due to their potential interactions with plants. All environments were slightly to moderately alkaline (pH 7.2 to 9.9) and were environments poorly studied in terms of AOB and AOA abundance in plant-associated microbial communities. Samples were obtained from eight coastal lagoons located in two protected areas in Spain with different climatic conditions, covering broad variations in salinity and eutrophication levels.
At the Empordà and Baix Ter wetlands (42°14′N, 3°06′E) (2, 19, 23), two oligohaline lagoons, Ter Vell (TV) and Basses d'en Coll (BC), and two euhaline lagoons, Fra Ramon (FR) and Túries (TU), were sampled. The Doñana National Park (37°01′N, 6°25′W) is located in an arid area with a climatic influence of the Atlantic Ocean and proximity to Africa. Here, two peridunal lagoons, Santa Olalla (SO) and Laguna Dulce (LD), and two salt marshes, Lucio del Cangrejo (LC) and Algaida (AL), were sampled.
For DNA extraction, areas of mono-specific stands of the dominant plant species, Phragmites australis (in BC, LC, and TV), Ruppia maritima (in AL), Ruppia cirrhosa (in FR and TU), and Paspalum distichum (in SO and LD), and the adjacent bulk sediment in each lagoon were sampled in January (non-growing season) and May 2007 (growing season), except for TU and AL, which were sampled only in May (see the descriptions in the supplemental material). Water characteristics at the sampling sites were determined using standard methods (see the supplemental material).
The lagoons differed according to salinity, nutrient content, and dominance of specific plant species (see Table S1 in the supplemental material). The potential nitrification rate (PNR) was measured according to the methods described by Ruiz-Rueda et al. (24) and correlated positively with the N-NO3− (Kendall's r, 0.31; P < 0.001) and N-NH4+ (r, 0.21; P < 0.05) concentrations and negatively with pH (r, −0.20; P < 0.05). These results agreed with those obtained in natural or constructed freshwater systems (24). The rhizospheres had higher PNRs than the bulk sediments, although differences (Welch test, P < 0.05) were only significant in Basses d'en Coll and Ter Vell during January and Lucio del Cangrejo in May (Table 1). These lagoons were dominated by Phragmites australis, which has been shown to support high nitrification activity (24).
Table 1.
Potential nitrification rates in sediment and root samples in January and June
| Date of sampling | Wetland and site | Plant species | PNRa (μg N/g [dry wt]/h) |
|
|---|---|---|---|---|
| Rhizosphere | Sediment | |||
| January 2007 | Empordà | |||
| Basses d'en Coll | Phragmites australis | 13.01 ± 0.99* | 0.05 ± 0.02* | |
| Ter Vell | Phragmites australis | 2.90 ± 0.58* | 0.01 ± 0.00* | |
| Fra Ramon | Ruppia cirrhosa | <0.01 | 0.02 ± 0.00 | |
| Doñana | ||||
| Laguna Dulce | Paspalum distichum | <0.01 | <0.01 | |
| Santa Olalla | Paspalum distichum | 0.24 ± 0.10 | 0.19 ± 0.08 | |
| Lucio del Cangrejo | Phragmites australis | 1.10 ± 0.41 | 0.34 ± 0.02 | |
| May 2007 | Empordà | |||
| Basses d'en Coll | Phragmites australis | 1.24 ± 0.63 | 0.17 ± 0.04 | |
| Ter Vell | Phragmites australis | 1.81 ± 1.43 | <0.01 | |
| Túries | Ruppia cirrhosa | 1.10 ± 0.60 | 0.01 ± 0.00 | |
| Fra Ramon | Ruppia cirrhosa | <0.01 | 0.04 ± 0.02 | |
| Doñana | ||||
| Laguna Dulce | Paspalum distichum | 0.21 ± 0.12 | 0.04 ± 0.02 | |
| Santa Olalla | Paspalum distichum | 0.84 ± 0.59 | 0.47 ± 0.08 | |
| Lucio del Cangrejo | Phragmites australis | 4.34 ± 0.59* | <0.01* | |
| Algaida | Ruppia maritima | <0.01 | 0.02 ± 0.01 | |
Values are means ± standard errors (n = 2 for January and n = 3 for May samples). Significant differences between the rhizosphere and bulk sediment (P < 0.05) are marked with an asterisk.
The 16S rRNA genes of Bacteria and Crenarchaeota and the amoA genes coding for the bacterial and archaeal ammonia monooxygenases were quantified in sediment and rhizosphere samples as described previously (33, 35). No relationship was found between amoA gene abundances and PNR when both root and sediment samples were considered together. However, when sediment and root samples were analyzed separately, a positive correlation between PNR and archaeal amoA (AamoA) gene abundance on roots (r, 0.33; P < 0.05) was established, with high activity and high abundance of AOA in Phragmites australis. This indicated that AOA contribute to nitrification at the root surfaces in these lagoons, but the contribution of bacterial ammonia oxidizers cannot be excluded. With an estimation of AOB and AOA cell densities from gene abundance levels (Table 2) and assuming a mean value of 2.5 and 1 amoA gene copies per genome, respectively (3, 17, 18, 34), the expected AOB abundance on the root surface varied from 1.4 × 106 to 1.9 × 108 cells, which could easily account for the observed PNRs (8, 20).
Table 2.
Gene abundance levels in DNA extracts from root surfaces of the dominant plant species and the adjacent, nonvegetated sediment
| Sampling date | Wetland and site | Gene abundancea in: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Rhizosphere |
Sediment |
||||||||
| 16S rRNA |
amoA |
16S rRNA |
amoA |
||||||
| Bacteria (1010) | Crenarcheota (107) | Bacteria (107) | Archaea (107) | Bacteria (1010) | Crenarcheota (107) | Bacteria (107) | Archaea (107) | ||
| January 2007 | Empordà | ||||||||
| Basses d'en Coll | 73.6 ± 13.2 | 56.1 ± 4.2 | 29.6 ± 9.7 | 166.1 ± 36.7 | 0.9 ± 0.4 | 1.9 ± 0.5 | 0.5 ± 0.2 | 9.2 ± 3.3 | |
| Ter Vell | 7.5 ± 2.6 | 14.3 ± 7.9 | 3.1 ± 0.8 | 41.8 ± 24.2 | 0.5 ± 0.0 | 3.7 ± 2.0 | 0.5 ± 0.1 | 10.8 ± 5.9 | |
| Fra Ramon | 28.2 ± 2.2 | 10.7 ± 1.9 | 8.6 ± 0.6 | 26.5 ± 4.9 | 0.3 ± 0.1 | 1.9 ± 0.5 | 0.3 ± 0.1 | 5.8 ± 1.3 | |
| Doñana | |||||||||
| Laguna Dulce | 0.7 (NA) | 0.7 (NA) | 1.0 (NA) | 1.0 (NA) | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.3 ± 0.0 | 1.5 ± 0.1 | |
| Santa Olalla | 3.0 ± 1.8 | 0.9 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.6 | 0.8 ± 0.7 | 0.2 ± 0.2 | 0.4 ± 0.3 | 1.9 ± 1.6 | |
| Lucio del Cangrejo | 3.3 ± 1.7 | 2.4 ± 1.2 | 1.2 ± 0.5 | 3.6 ± 1.6 | 2.9 ± 1.4 | 4.9 ± 2.9 | 1.0 ± 0.3 | 8.9 ± 4.6 | |
| May 2007 | Empordà | ||||||||
| Basses d'en Coll | 17.8 ± 4.6 | 20.0 ± 0.6 | 10.2 ± 0.9 | 35.1 ± 5.1 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.2 ± 0.1 | 1.4 ± 0.4 | |
| Ter Vell | 32.8 ± 4.8 | 23.3 ± 11.2 | 17.4 ± 3.0 | 52.5 ± 21.9 | 2.1 ± 0.8 | 2.0 ± 0.4 | 0.7 ± 0.1 | 5.1 ± 0.9 | |
| Túries | 5.5 ± 2.8 | 12.9 ± 5.5 | 9.8 ± 5.1 | 28.0 ± 11.8 | 2.4 ± 0.8 | 5.8 ± 0.3 | 1.2 ± 0.4 | 12.1 ± 2.0 | |
| Fra Ramon | 40.7 ± 13.4 | 36.8 ± 3.6 | 14.5 ± 5.2 | 37.8 ± 2.0 | 3.4 ± 1.7 | 8.9 ± 2.7 | 2.3 ± 1.1 | 23.8 ± 5.7 | |
| Doñana | |||||||||
| Laguna Dulce | 3.8 ± 0.8 | 4.0 ± 1.2 | 1.6 ± 0.5 | 1.8 ± 0.4 | 1.6 ± 1.0 | 4.8 ± 1.0 | 1.3 ± 0.7 | 12.6 ± 2.6 | |
| Santa Olalla | 36.3 ± 6.0 | 18.6 ± 1.5 | 14.0 ± 1.2 | 14.9 ± 1.8 | 7.3 ± 1.6 | 24.0 ± 5.0 | 3.8 ± 1.0 | 33.1 ± 2.4 | |
| Lucio del Cangrejo | 13.6 ± 5.5 | 13.0 ± 7.0 | 7.6 ± 3.5 | 28.3 ± 15.8 | 0.3 ± 0.1 | 1.5 ± 0.2 | 0.3 ± 0.0 | 4.3 ± 1.1 | |
| Algaida | 95.9 ± 30.7 | 43.6 ± 6.5 | 25.8 ± 6.6 | 33.6 ± 3.7 | 2.2 ± 1.2 | 5.8 ± 2.0 | 1.1 ± 0.6 | 17.0 ± 4.2 | |
Values are means ± standard errors of the gene abundance levels (in copies/g [dry weight]) obtained from three independent DNA extracts from the root surfaces of the dominant plant species and the adjacent, nonvegetated sediment. Note the differences in the log factors between columns. NA, the standard error was not calculated due to the lack of replicates for this sample.
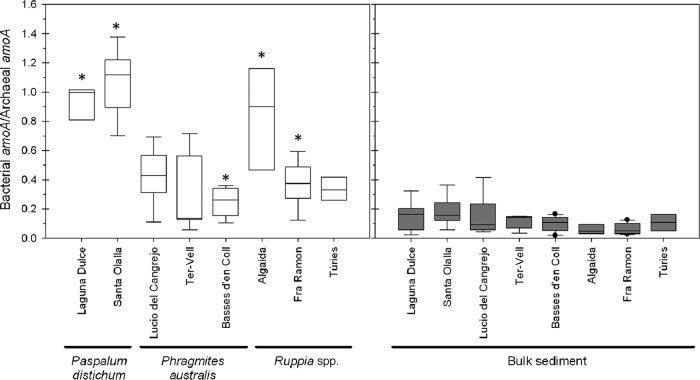
The total bacterial community outnumbered the crenarchaeota, but the AOA were more abundant than the AOB in nearly all samples (Table 2). The exceptions were the roots of P. distichum in Laguna Dulce and Santa Olalla and Ruppia maritima in Algaida, where the two groups were equally abundant (Table 2; Fig. 1). When we compared the abundance of bacterial amoA genes in the rhizosphere and the corresponding sediment, the gene copy numbers in the rhizospheres were significantly higher (P < 0.05) in all lagoons at both sampling occasions, except in Lucio del Cangrejo in January and Laguna Dulce in May (Table 2). For the AOA, a similar pattern was observed in the Empordà samples, whereas it was lagoon dependent in the Doñana. Bacterial-to-archaeal amoA gene ratios (BamoA/AamoA) were calculated as indicators of the relative dominance of one or the other group (Fig. 1). The BamoA/AamoA ratios were significantly higher (P < 0.05) on root surfaces than in the corresponding sediment in five of the eight sampled lagoons. These findings are in contrast with our hypothesis and previous studies, in which the rhizosphere and root surfaces of freshwater macrophytes strongly favored the development of AOA over AOB (11, 12). However, it was recently shown that macroalgal surfaces in marine systems harbor a higher abundance of AOB than AOA (33). This indicates that the presence of plants or specific plant species alone cannot account for the relative differences of one or the other group of ammonia oxidizers.
Fig 1.
Box and whiskers plot of ratios of bacterial amoA versus archaeal amoA gene abundance levels on the root surface (left) of the dominant plant species and in the adjacent bulk sediment (right) in eight coastal wetland lagoons in Spain. The median is represented by the black horizontal line in each box. Boxes cover the 25 and 75% quantiles, and bars show the 10 and 90% quantiles. Outliers are marked as dots. Significant differences between the sediment and rhizosphere are indicated by an asterisk.
Several factors have to be considered to explain the either increased or equal proportion of AOB to AOA in the rhizospheres compared to the adjacent sediments. Different responses of AOA and AOB populations have been reported in relation to oxygen availability in estuarine sediments (1, 32). In those studies, the bacterial amoA gene transcripts increased significantly at higher dissolved oxygen concentrations, whereas the number of amoA archaea transcripts remained unchanged. Oxygen diffusion rates were not measured in our samples, but Ruppia maritima and Phragmites australis have been reported to have high radial oxygen losses at the root surface that may vary from 48 to 144 mg O2 g−1 (dry weight) day−1 and from 15 to 31 mg O2 g−1 (dry weight) day−1, respectively (27, 28). Interestingly, although not significant, BamoA/AamoA ratios were slightly higher on the root surface of Ruppia sp., especially in Algaida. One can also speculate that increased CO2 levels due to increased respiration activity in the rhizosphere potentially favor autotrophic ammonia oxidizers, which could affect the AOB:AOA ratio if mixotrophy were more common among AOA. Other factors known to affect AOA and AOB are pH and ammonia concentration (10, 14, 26). To relate AOB and AOA abundances with these factors, only the bulk sediments were compared, to avoid any confounding root effects (Fig. 2). The BamoA/AamoA ratios were low in environments with low ammonium concentrations and a pH between 7 and 7.5. Positive correlations showed an increased proportion of bacterial amoA genes with alkaline pH and high ammonium concentrations in both freshwater and saline lagoons. pH and ammonium concentration were the only variables that followed a stepwise linear regression model (r2, 0.382; P = 0.018) and significantly contributed to the observed differences. The BamoA/AamoA ratios compared well with previous results obtained in alkaline sandy loam soils (pH 8.4) (25), but they were much higher than those reported for wetland sediments at neutral pH (12). In agreement with our results, increased ammonium supply has been shown to decrease the relative abundance of AOA and their activity (5, 13, 14, 21).
Fig 2.
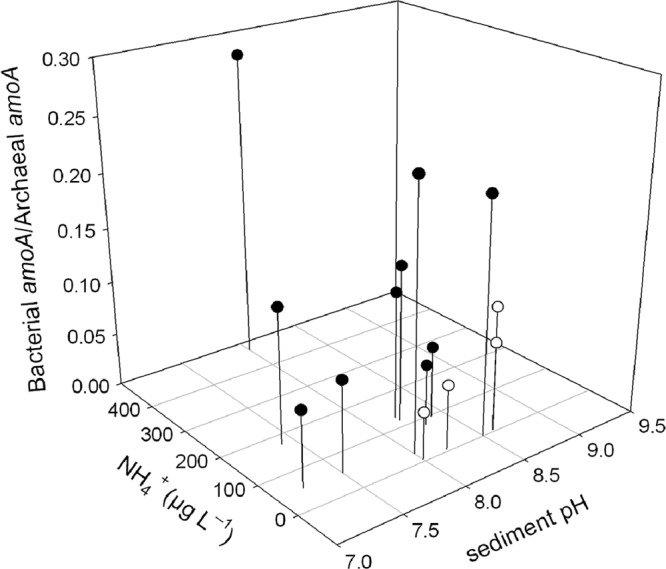
Three dimensional scatter plot showing the relationship between the ratio of bacterial amoA versus archaeal amoA gene abundance in the bulk sediment, the sediment pH, and the ammonium concentration in the water column. Samples obtained from low- and high-conductivity waters are indicated by filled and open symbols, respectively.
In conclusion, macrophytes in coastal wetlands increased the abundance of both total bacteria and crenarchaeota as well as ammonia oxidizers, compared to levels in nonvegetated sediments. However, the plants tended to select for AOB over AOA in the rhizosphere compared to the adjacent sediment in our moderately alkaline lagoons. Maximum relative densities of AOB were found on root surfaces of Paspalum distichum. Reported differences in plant physiology, such as oxygen release rate, could potentially explain differences in the bacterial and archaeal amoA gene ratios. Further research should focus on niche differentiation between ammonia-oxidizing bacteria and archaea with respect to plant physiology in alkaline environments.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the Spanish Ministerio de Ciencia e Innovación (CGL2006-02382, CGL2009-08338, and CGL2011-23907) and by the Spanish Ministerio de Ciencia e Innovación and the Doñana Biological Station (ICTS 24/2007).
The Department of Microbiology, Swedish University of Agricultural Sciences in Uppsala is acknowledged for hosting R. Trias during her research stay.
Footnotes
Published ahead of print 15 June 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abell GCJ, et al. 2011. Effects of estuarine sediment hypoxia on nitrogen fluxes and ammonia oxidizer gene transcription. FEMS Microbiol. Ecol. 75: 111–122 [DOI] [PubMed] [Google Scholar]
- 2. Badosa A, Boix D, Brucet S, López-Flores R, Quintana XD. 2006. Nutrients and zooplankton composition and dynamics in relation to the hydrological pattern in a confined Mediterranean salt marsh (NE Iberian Peninsula). Estuar. Coast. Shelf Sci. 66: 513–522 [Google Scholar]
- 3. Blainey PC, Mosier AC, Potanina A, Francis CA, Quake SR. 2011. Genome of a low-salinity ammonia-oxidizing archaeon determined by single-cell and metagenomic analysis. PLoS One 6: e16626 doi:10.1371/journal.pone.0016626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coci M, et al. 2010. Quantitative assessment of ammonia-oxidizing bacterial communities in the epiphyton of submerged macrophytes in shallow lakes. Appl. Environ. Microbiol. 76: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di HJ, et al. 2010. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 72: 386–394 [DOI] [PubMed] [Google Scholar]
- 6. Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W. 2009. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol. Rev. 33: 855–869 [DOI] [PubMed] [Google Scholar]
- 7. Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. U. S. A. 102: 14683–14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujita M, Tsuji K, Akashi A. 2010. Temporal variation in maximum cell-specific nitrification rate. Water Sci. Technol. 61: 2069–2073 [DOI] [PubMed] [Google Scholar]
- 9. Gorra R, Coci M, Ambrosoli R, Laanbroek HJ. 2007. Effects of substratum on the diversity and stability of ammonia-oxidizing communities in a constructed wetland used for wastewater treatment. J. Appl. Microbiol. 103: 1442–1452 [DOI] [PubMed] [Google Scholar]
- 10. Gubry-Rangin C, et al. 2011. Niche specialization of terrestrial archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. U. S. A. 108: 21206–21211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herrmann M, Saunders AM, Schramm A. 2008. Archaea dominate the ammonia-oxidizing community in the rhizosphere of the freshwater macrophyte Littorella uniflora. Appl. Environ. Microbiol. 74: 3279–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrmann M, Saunders AM, Schramm A. 2009. Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia-oxidizing prokaryotes in freshwater sediments. Appl. Environ. Microbiol. 75: 3127–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herrmann M, Scheibe A, Avrahami S, Kusel K. 2011. Ammonium availability affects the ratio between ammonia-oxidizing bacteria and ammonia-oxidizing archaea in simulated creek ecosystems. Appl. Environ. Microbiol. 77: 1896–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hofferle S, et al. 2010. Ammonium supply rate influences archaeal and bacterial ammonia oxidizers in a wetland soil vertical profile. FEMS Microbiol. Ecol. 74: 302–315 [DOI] [PubMed] [Google Scholar]
- 15. Kirchman DL, Elifantz H, Dittel AI, Malmstrom RR, Cottrell MT. 2007. Standing stocks and activity of Archaea and Bacteria in the western Arctic Ocean. Limnol. Oceanogr. 52: 495–507 [Google Scholar]
- 16. Kirk GJ, Kronzucker HJ. 2005. The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Ann. Bot. 96: 639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klappenbach JA, Saxman PR, Cole JR, Schmidt TM. 2001. rrndb: the Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res. 29: 181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leininger S, et al. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442: 806–809 [DOI] [PubMed] [Google Scholar]
- 19. López-Flores R, Boix D, Badosa A, Brucet S, Quintana XD. 2009. Environmental factors affecting bacterioplankton and phytoplankton dynamics in confined Mediterranean salt marshes (NE Spain). J. Exp. Mar. Biol. Ecol. 369: 118–126 [Google Scholar]
- 20. Lydmark P, et al. 2007. Effects of environmental conditions on the nitrifying population dynamics in a pilot wastewater treatment plant. Environ. Microbiol. 9: 2220–2233 [DOI] [PubMed] [Google Scholar]
- 21. Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. 2009. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461: 976–979 [DOI] [PubMed] [Google Scholar]
- 22. Ouverney CC, Fuhrman JA. 2000. Marine planktonic archaea take up amino acids. Appl. Environ. Microbiol. 66: 4829–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quintana XD, Moreno-Amich R, Comín FA. 1998. Nutrient and plankton dynamics in a Mediterranean salt marsh dominated by incidents of flooding. Part 1: differential confinement of nutrients. J. Plankton Res. 20: 2089–2107 [Google Scholar]
- 24. Ruiz-Rueda O, Hallin S, Bañeras L. 2009. Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol. Ecol. 67: 308–319 [DOI] [PubMed] [Google Scholar]
- 25. Shen J, Zhang L, Zhu Y, Zhang J, He J. 2008. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol. 10: 1601–1611 [DOI] [PubMed] [Google Scholar]
- 26. Stopnisek N, et al. 2010. Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl. Environ. Microbiol. 76: 7626–7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka N, Yutani K, Aye T, Jinadasa KBSN. 2007. Effect of broken dead culms of Phragmites australis on radial oxygen loss in relation to radiation and temperature. Hydrobiologia 583: 165–172 [Google Scholar]
- 28. Thursby GB. 1984. Root-exuded oxygen in the aquatic angiosperm Ruppia maritima. Mar. Ecol. Prog. Ser. 16: 303–305 [Google Scholar]
- 29. Tourna M, Freitag TE, Nicol GW, Prosser JI. 2008. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol. 10: 1357–1364 [DOI] [PubMed] [Google Scholar]
- 30. Tourna M, et al. 2011. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. U. S. A. 108: 8420–8425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toyama T, et al. 2011. Accelerated biodegradation of pyrene and benzo[a]pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions. Water Res. 45: 1629–1638 [DOI] [PubMed] [Google Scholar]
- 32. Treusch AH, et al. 2005. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 7: 1985–1995 [DOI] [PubMed] [Google Scholar]
- 33. Trias R, et al. 2012. Abundance and composition of epiphytic bacterial and archaeal ammonia oxidizers of marine red and brown macroalgae. Appl. Environ. Microbiol. 78: 318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walker CB, et al. 2010. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. U. S. A. 107: 8818–8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wessén E, Nyberg K, Jansson JK, Hallin S. 2010. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl. Soil Ecol. 45: 193–200 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.