Abstract
The potential for the transport of viable Cryptosporidium parvum oocysts through soil to land drains and groundwater was studied using simulated rainfall and intact soil columns which were applied raw slurry or separated liquid slurry. Following irrigation and weekly samplings over a 4-week period, C. parvum oocysts were detected from all soil columns regardless of slurry type and application method, although recovery rates were low (<1%). Soil columns with injected liquid slurry leached 73 and 90% more oocysts compared to columns with injected and surface-applied raw slurries, respectively. Among leachate samples containing oocysts, 44/72 samples yielded viable oocysts as determined by a dye permeability assay (DAPI [4′,6′-diamidino-2-phenylindole]/propidium iodide) with the majority (41%) of viable oocysts found in leachate from soil columns with added liquid slurry. The number of viable oocysts was positively correlated (r = 0.63) with the total number of oocysts found. Destructively sampling of the soil columns showed that type of slurry and irrigation played a role in the vertical distribution of oocysts, with more oocysts recovered from soil columns added liquid slurry irrespective of the irrigation status. Further studies are needed to determine the effectiveness of different slurry separation technologies to remove oocysts and other pathogens, as well as whether the application of separated liquid slurry to agricultural land may represent higher risks for groundwater contamination compared to application of raw slurry.
INTRODUCTION
Cryptosporidium parvum (42) is a protozoan parasite infecting the gastrointestinal tracts of many vertebrates, including humans. The parasite is among the most common nonbacterial causes of severe human gastroenteritis and diarrhea, which can be life-threatening for immunocompromised individuals (7). Transmission of C. parvum to humans may occur through a number of routes, among which the ingestion of fecal contaminated drinking water is a major source (19, 30). Contamination of drinking water with C. parvum is of particular concern since as few as 10 infective oocysts may be required to cause infection (39). Furthermore, the oocysts are resistant to most commonly used disinfectants, including chlorine, at levels applied during water treatment (24).
Contamination of drinking water with C. parvum originate primarily from surface water (16), where oocysts have been introduced through direct fecal pollution from free-ranging livestock, wildlife or humans, wastewater, or by water runoff from manure-fertilized fields, where the oocysts can remain infective for several weeks (14, 35). Oocysts in fecal pats on rangeland can be released during rainfall and transported to water bodies (41) and have been found throughout the year in streams flowing through areas with livestock production (3, 36).
Besides introduction through malfunctioning boreholes, contamination of groundwater with Cryptosporidium requires that oocysts move through soil to reach the water reservoir. Transportation through soil has usually been considered an insignificant pathway because soil is generally assumed to be an effective filter inhibiting the transport of different pathogens. Thus, the majority of oocysts are typically found in the topsoil (32), but if macropores are present they may facilitate the vertical transport of oocysts. Studies in soil columns do also show that C. parvum oocysts are capable of percolating through up to 50-cm deep sand and soil columns (17, 32, 33). Field surveys of Cryptosporidium spp. in groundwater in Great Britain (29, 30) and United States (34) indicate that contamination with low concentrations of C. parvum in groundwater may be frequent, although it is unknown how the groundwater was contaminated. Limited information is available about the viability and infectivity of oocysts in groundwater, but oocysts have been shown to survive in soils for as long as 22 weeks (22).
Cryptosporidium spp. are capable of infecting virtually every mammal, including humans, but the major reservoir is domestic livestock, almost exclusive young animals, with calves being especially susceptible (19). Other livestock animals, however, have also been shown to excrete large number of Cryptosporidium oocysts, e.g., a study of 50 Danish pig herds demonstrated a crude prevalence of 16, 31, and 100% for sows, piglets, and weaners, respectively (31).
Application of animal slurry to agricultural land is practiced worldwide to fertilize the soil and increase the organic matter content (11). At the same time, animal slurry is also a well-documented source of different pathogens such as Cryptosporidium spp. that may be released into the environment. Conventional slurry management leads to nutrient losses both during storage and when applied to the fields (11, 40), and the slurry has an obnoxious smell. By separating the slurry mechanically as well as chemically into a solid fraction that is typically composted before use and a liquid fraction used to fertilize the paddocks, nutrient losses and smell problems are reduced (12). Little information, however, is available about the impact and fate of pathogens in the separated solid and liquid slurry fractions during storage and when applied on agricultural land, e.g., no information seems to be available concerning transport of Cryptosporidium oocysts in soil water when the separated liquid slurry fraction is used as fertilizer.
The objective of the present study was therefore to assess the transport of C. parvum oocysts in columns of undisturbed soil after the injection of raw slurry and separated liquid slurry into topsoil and raw slurry applied on the soil surface.
MATERIALS AND METHODS
Cryptosporidium oocysts.
Feces from 1- to 3-week-old naturally infected Holstein calves were collected from two Danish dairy farms, Cryptosporidium-positive samples were identified by the modified Ziehl-Neelsen technique (18), and the oocysts were subsequently concentrated (31). Briefly, Cryptosporidium-positive fecal samples were suspended in tap water, filtered through gauze, and centrifuged at 1,540 × g for 10 min; the supernatant was then removed and discarded, and the pellet was resuspended in tap water by vortexing. This washing procedure was repeated two to three times until the supernatant was clear. Depending on the volume of the pellet, tap water was added to the pellet to a total volume of either 5 ml (<2-ml pellet) or 10 ml (>2-ml pellet), and the pellet was then resuspended by vortexing. A total volume of 10 ml was divided into two 50-ml centrifuge tubes before further processing. The fecal solution was underlayered with a gradient consisting of 1.09/1.05/1.01 Percoll (Amersham Biosciences, Australia) prepared with distilled water. Samples were centrifuged at 1,540 × g for 10 min, and oocysts were collected between the 1.09/1.05 Percoll layers. Purified oocysts were washed three times by centrifugation in distilled water at 1,540 × g for 10 min to remove Percoll; all of the samples were then pooled, and the oocysts were then enumerated by immunofluorescence microscopy. Cryptosporidium oocysts were identified as C. parvum by sequencing of the 18S rRNA gene locus and the HSP70 gene (28).
Soil columns.
Soil columns used in the study were collected in October 2009 from the Foulum Experimental Station (56°29′N, 9°34′E), Denmark, from a field rotated with spring barley and winter wheat. When soil was sampled, the field contained some leftover crops which were removed together with 5-cm topsoil before the start of the experiment. No animal manure had been applied to the plot for the previous 2 years. The columns consisted of undisturbed soil, sampled by pushing a 20-cm-long, 20-cm-wide stainless steel column vertically into the soil by a tractor-mounted device. Collected intact columns capped with plastic lids were transported to the laboratory within a few hours and stored at 2°C until initiation of the leaching experiment. Prior to the start of the experiment, the soil was saturated with 0.01 M CaCl2 solution and drained to a soil water potential at −100 hPa. The soil was loamy sand with 8% clay, 13% silt, and 78% sand; further details of the soil are shown in Table 1.
Table 1.
Physicochemical characteristics of the soil
| Characteristic | Before expt |
|
|---|---|---|
| 10-cm depth | 30-cm depth | |
| Organic content (%) | 2.0 | 1.8 |
| Porosity (%) | 41 | 43 |
| Bulk density (kg m3) | 1,534 | 1,489 |
| Ksat (mm/h)a | 61.1 | 43.2 |
| pH | 6.33 | 6.33 |
| Electrical conductivity (mS cm−1) | 0.047 | 0.047 |
Ksat, hydraulic conductivity.
Slurry application.
Raw slurry and chemical-mechanical (Kemira separators; Kemira Water A/S, Denmark) separated liquid slurry fractions collected from a pig farm in Åbɵl, Denmark, were stored at 2°C prior to use. The raw slurry was separated by a mechanical screw press through a sieve (0.2-mm screen size). Details on the physicochemical properties of raw and liquid slurry are shown in Table 2. Before the slurry was spiked with C. parvum oocysts, the presence of naturally occurring oocysts was determined in 2 g of slurry by purifying the oocysts with Percoll as described above. Individual portions of oocysts were prepared in distilled water and mixed with 9 samples of liquid slurry and 15 samples of raw slurry, which were added to individual soil columns. Two different batches of oocysts were added to liquid and raw slurry, respectively. Three portions from each of the two batches of oocysts were used to examine the number and viability of oocysts added to each soil column. The number of C. parvum oocysts in each portion was examined by immunomagnetic separation (IMS) (Dynabeads anti-Cryptosporidium kit; Invitrogen, IDEXX Laboratories, Suffolk, United Kingdom), followed by immunofluorescence microscopy (see below for details). Both slurry types were spiked with C. parvum oocysts and 2,6-difluorobenzoic acid (Sigma-Aldrich, Germany) at 2 g liter−1 as a nonreactive tracer. The total oocysts added per column (i.e., the sum of naturally occurring oocysts and oocysts added by spiking) was 3.1 × 106 (mean of four oocyst counts) with a viability of 58% (36% DAPI+ [4′,6′-diamidino-2-phenylindole positive]/propidium iodide negative [PI−]), 21% DAPI−/PI−) when raw slurry was applied, while 3.0 × 106 oocysts (mean of three oocyst counts) of which 57% were viable (34% [DAPI+/PI−], 24% [DAPI−/PI−]) were inoculated per column when liquid slurry was applied. The oocyst-spiked slurry was added to soil columns either by injection into a slit (10 by 9 by 4 cm) in the middle of the column that was subsequently covered with soil or surface applied at a rate of 50 tha−1 (Fig. 1). The surface application was done by removing the top 2-cm soil from a circular area with a diameter of 17 cm in the center of the column surface, adding the slurry and then covering the slurry again with the soil (Fig. 1). This was done to imitate the normal farmer practices of incorporating the slurry into the soil shortly after its application.
Table 2.
Physicochemical properties of slurry
| Characteristic | Mean ± SDa |
|
|---|---|---|
| Raw slurry | Liquid slurry | |
| Density (g ml−1) | 1.02 | 1.01 |
| Total solids (%) | 5.65 ± 0.08 | 3.14 ± 0.07 |
| Volatile solids (%) | 4.17 ± 0.07 | 1.97 ± 0.03 |
| pH | 7.10 ± 0.01 | 7.56 ± 0.02 |
| Electrical conductivity (mS cm−1) | 19.3 ± 0.01 | 15.8 ± 0.01 |
| NH4-N (g kg−1) | 3.01 ± 0.02 | 2.89 ± 0.01 |
| Total N (g kg−1) | 4.47 ± 0.07 | 4.28 ± 0.02 |
The values are means of 3 to 12 replicates.
Fig 1.
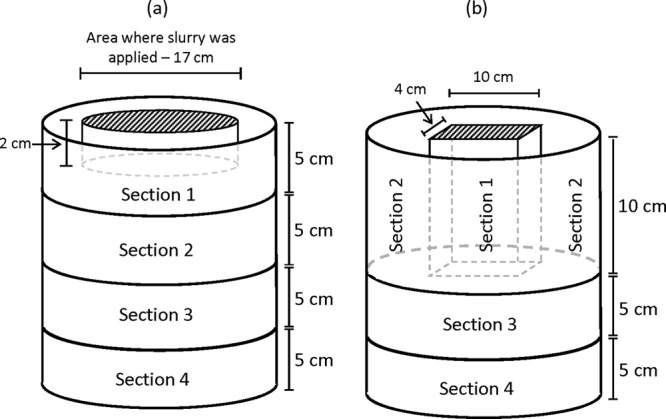
Schematic illustration of soil column sections analyzed for oocysts. Raw slurry was applied to soil columns either on the surface (a) or in a slit (b), while the liquid slurry fraction was injected only in a slit (b).
Experimental setup.
Leaching of oocysts was investigated in intact soil columns (26, 27), where the soil rested on a glass filter disc with a pore size of 60 to 100 μm and a thickness of 1.6 cm (ROBU; Glassfiltergerate GmbH, Germany). The experimental setup is illustrated in Fig. 2. A hypodermic needle guided leachate to a 1,000-ml glass bottle with a suction of −12.5 hPa on the soil columns lower boundary.
Fig 2.
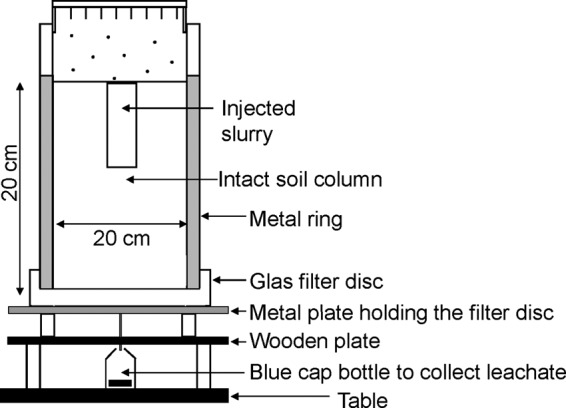
Schematic illustration of the soil column apparatus used in the laboratory experiments.
Before slurry was added to the soil columns, sample portions of oocysts and the chemical tracer were poured into glass bottles containing the liquid or raw slurry to be added to each soil column. The Eppendorf tube that contained oocysts was subsequently washed with ∼2 ml of distilled water to remove any remaining oocysts, and the wash water was also added to the slurry. Oocysts were mixed well with the slurry by turning the bottle 10 times. The effect of the application method on leaching of oocysts was studied in 12 soil columns; to 6 of these, raw slurry was added on the surface, and 6 were injected with raw slurry. Liquid slurry was applied only through injection into six new soil columns to reflect natural conditions. Application of liquid slurry onto the soil surface is not practiced by farmers since this leads to significant emission of nitrogen. All of the above-mentioned columns were irrigated by a rain simulator (Fig. 2). Three soil columns with raw slurry injected and three columns with liquid slurry injected were kept nonirrigated, while three irrigated soil columns with neither slurry nor oocysts applied served as controls. One week after slurry application, the first simulated rainfall was applied at 10 mm h−1 for 3.5 h. Altogether, four rain events occurring 1 week apart were applied through uniformly distributed needles in the rain simulator. After the cessation of each rain event and percolation, leachate was collected, and 50-ml subsamples were analyzed for Cryptosporidium oocysts and the chemical tracer. Subsamples of leachate collected after the first irrigation event were collected after 15, 45, 75, 120, and 180 min and just after percolation had ceased. The leaching experiments were conducted at 10°C. After four rain events and the collection of leachate, the soil columns were sliced into four sections by two different methods depending on the manure application method (Fig. 2), and 0.1% of the dry matter content of each section was analyzed for any remaining oocysts.
Soil samples.
The dry matter content was analyzed in a subsample from each of the soil sections (1) and used to determine the volume (grams) of soil to be analyzed for oocysts in each of the soil sections. This was done to analyze an equal weight of soil particles from each soil section, since the irrigated soil columns had different water content compared to the nonirrigated soil columns. Furthermore, each section of the columns contained different volumes of soil. The dry matter content was measured after drying at 102 to 105°C for 16 h (1). Recovery of oocysts from soil was completed in a single subsample equal to 0.1% of the dry matter content from each section of all soil columns. The weight of 0.1% dry matter was different for each soil section but represented between 1.0 and 5.2 g of soil and was weighed into 50-ml centrifuge tubes. To each tube was added 0.01% Tween 20 (polyoxyethylene sorbitan monolaurate) solution corresponding to 10 times the amount of soil (0.1% dry matter content). These samples were homogenized by vortexing for 1 min, and the sample solutions were then left to sediment for 5 min. The top 10 ml was underlaid with 40 ml of flotation fluid with a specific gravity of 1.27 and centrifuged at 350 × g for 10 min. The top 20 ml was removed and placed in a clean 50-ml centrifuge tube, 40 ml of distilled water was added, and the tube was centrifuged 1,540 × g for 10 min. The supernatant was removed leaving ∼2 ml, and the oocysts were isolated by IMS according to the manufacturer's instructions. All oocysts in each of soil sample were enumerated as described below. An initial pilot study showed that ca. 40% of the oocysts could be recovered from the soil (results now shown). The total number of oocysts in each soil column section was calculated based on the number of oocysts found in 0.1% dry matter.
Enumeration and viability testing of oocysts.
Microscopic detection and quantification of Cryptosporidium oocysts in the leachate was done by IMS. The viability testing of oocysts in the leachate was based on the inclusion or exclusion of two fluorogenic vital dyes, 4′,6 diamidino-2-phenylindole (DAPI) and propidium iodide (PI) (6). The 100-μl oocyst solution obtained from the IMS procedure was incubated with 10 μl of DAPI working solution (2 mg/ml in absolute methanol) and 10 μl of PI working solution (1 mg/ml in 0.1 M phosphate-buffered saline [pH 7.2]) at 37°C for 3 h. Oocysts were washed twice in distilled water by centrifugation at 3,500 × g for 5 min, and the supernatant was removed and discarded down to ∼100 μl. The entire sample volume was placed in a well on a three-well (12 mm) Teflon-printed diagnostic slide (Immono-Cell, Mechelen, Belgium). The slide was air dried, fixed for 5 min with acetone, and left to dry before the addition of 25 μl of anti-Cryptosporidium fluorescein isothiocyanate (FITC)-labeled antibody mix (Crypto-Cell IF test; CellLabs, Australia) according to the manufacturer's instructions. The entire well area of the slide was examined using an epifluorescence microscope at either ×200 or ×400 magnification equipped with appropriate filter blocks (350-nm excitation and 450-nm emission wavelengths for DAPI, 500-nm excitation and 630-nm emission wavelengths for PI, and 519- and 495-nm excitation wavelengths for FITC). The proportion of PI+/DAPI+, DAPI+/PI−, and DAPI−/PI− oocysts were quantified by enumerating up to 100 oocysts in each sample. DAPI+/PI− and DAPI−/PI− oocysts were considered viable as a measure of infectivity (21).
Statistics.
The numbers of oocysts counted in the leachate from the irrigated soil columns with injected liquid slurry, injected raw slurry, and raw slurry applied on the surface were compared. The numbers of oocysts counted in soil sections 2, 3, and 4 from soil columns injected liquid slurry and raw slurry with or without irrigation, and soil columns applied raw slurry on the surface and then irrigated were also compared. The ln(x + 1) transformed oocysts counts were analyzed in a linear normal model with random effects. The normality assumption was validated by quantile-quantile plots, and variance homogeneity was validated by residual plot. Thereafter, the nonsignificant effects were removed by stepwise backward model reduction on a 5% significance level. The final model for the number of oocysts in leachate included (i) each individual column as a random factor, (ii) the leached water volume, pH, and turbidity of the leachate as covariates, (iii) the column type, number of oocysts added, and week as fixed factors, and (iv) the number of interactions column × week. The final model for the number of oocysts counted in the soil included column number as a random factor, while the column type, number of oocysts added, and soil section were fixed factors. The upper section (section 1) of the soil column was left out of the analysis, since the slurry samples with oocysts was added to this section. The purpose of the statistical test was to analyze the differences in oocyst numbers released from the upper section in the different column types.
All mean data and 95% confidence intervals are presented as back-transformed least-significant means, while the total values of oocyst counts were calculated from the raw nontransformed data. The recovery rate was calculated by taking the sum of oocysts found in the leachate from all four weekly samplings and dividing it by the number of oocysts added. All statistical analyses were performed using SAS (version 9.2; SAS Institute. Inc., Cary, NC).
RESULTS
No leachate was observed from any soil column in the period from the application of slurry to the first artificial rain was applied. Visual inspection of the soil columns did not reveal any cracks or holes. A total volume of 1,100 ml of artificial rain was applied weekly to each soil column, and an average of 92.8% ± 2.5% of the irrigated water volume was recovered as leachate from the columns. Before the slurry was spiked with Cryptosporidium oocysts, initial analyses showed that the liquid slurry column contained 9.4 × 103 oocysts and the raw slurry column contained 2.0 × 104 oocysts.
Transport and viability of oocysts in leachate.
Analyses of leachate collected from the six replicate soil columns used for each combination of slurry type and application method (72 leachate samples) yielded a total of 56 (78%) leachate samples that were positive for oocysts. The soil columns with injected liquid slurry and injected raw slurry each yielded 21/24 (88%) positive samples, while 12/24 (50%) leachate samples from soil columns with raw slurry applied to the surface contained oocysts. In general, the recovery rate of oocysts in the leachate was low, with 0.06% oocysts recovered in leachate from soil columns with injected liquid slurry, 0.02% oocysts recovered from columns with injected raw slurry, and 0.01% oocysts found in leachate from soil columns with applied raw slurry on the surface. High-pressure washing of the filter disc located at the bottom end of the soil column (Fig. 1) and subsequent concentration of possible oocysts and IMS analysis of the wash water could not detect any oocysts trapped on the filter disc.
Oocysts were detected in leachate collected at each of the four weekly samplings irrespective of the slurry type and means of application (Table 3), although not all individual replicate soil columns released oocysts. Overall, the mean number of recovered oocysts in the leachate was affected by slurry type and application method (P = 0.033) irrespective of week and soil column number with the highest number of oocysts recovered from the columns with injected liquid slurry. However, the difference was only statistically significant (P = 0.026) between soil columns with injected liquid slurry and soil columns with applied raw slurry on the soil surface. The turbidity and pH value of the leachate showed mean values of 4.1 nephelometric turbidity units and 6.5, respectively but did not have a significant effect on the number of oocysts released.
Table 3.
C. parvum oocysts recovered in leachate following slurry application and irrigation of soil columns
| Slurry type | Application method | Total oocysts recovereda ± SE | Mean oocysts recovered/literb | Mean oocysts/literc |
|||
|---|---|---|---|---|---|---|---|
| Weak 1 | Weak 2 | Weak 3 | Weak 4 | ||||
| Liquid | Injected | 2,651 ± 1,342 | 76.1A (30.7–186.6) | 144 (30–681) | 1,232 (289–5,254) | 20 (4–88) | 9 (1–46) |
| Raw | Injected | 707 ± 173 | 39.0AB (15.1–98.1) | 84 (20–346) | 12 (2–61) | 189 (36–992) | 11 (2–54) |
| Surface | 263 ± 66 | 12.3B (4.3–32.1) | 2 (–0.6–21) | 132 (28–626) | 22 (3–120) | 3 (–0.3–16) | |
That is, the sum of the total oocysts recovered from all leachate samples collected at all four sampling times.
That is, the mean number of oocysts recovered per liter of leachate from six replicate columns at the four weekly samplings (mean of 24 samples). Mean values followed by different superscript capital letter are significantly different at P < 0.05.
Numbers are presented as the back-transformed LS-means estimate, and the 95% confidence interval is indicated in parentheses.
At the initial sampling 1 week after slurry application, leachate from the columns with injected raw and liquid slurry yielded significantly (P = 0.004 and P = 0.009, respectively) more oocysts than leachate from columns where slurry was applied on the surface. In week 2, soil columns with injected liquid slurry yielded the highest number of oocysts observed throughout the study, which was significantly higher than the number of oocysts recovered from columns with raw slurry applied on the surface (P < 0.0001) and injected (P = 0.034). Leachate from any soil column contained the lowest number of oocysts in week 4 (1 to 4%) (Table 3).
The nonreactive chemical tracer added to the slurry was measured in every leachate sample as mg/liter of leachate. The mean percentage of the added tracer measured in the leachate over the 4-week period was almost similar for the 18 irrigated soil columns, with ca. 41% ± 3% of the chemical tracer leached. There was no correlation between the concentration of the chemical tracer and the number of oocysts leached, and the breakthrough curves of the tracer did not follow the same trend as the leached oocysts from the soil columns (results not shown).
The DAPI/PI staining of the oocysts tended to show a positive correlation (r = 0.63) between the number of enumerated oocysts and numbers of viable oocysts with a total of 44/72 (61%) leachate samples containing viable oocysts (DAPI+/PI− and DAPI−/PI−). Leachate from columns injected with liquid slurry released a total of 668 viable oocysts, while columns injected with raw slurry and columns with raw slurry applied on the surface released totals of 123 and 76 viable oocysts, respectively (sum of the four weekly samplings). Viable oocysts were found in leachate collected during all 4 weeks in all column types.
Recovery of oocysts in soil.
After the 4-week study period, the distribution of oocysts retained in the soil column was investigated by slicing the columns into four sections and subsequent analyses of soil samples collected from each of the sections 2, 3, and 4 (Fig. 2). Oocysts were detected in soil from all three sections in all soil columns. Overall, the mean number of recovered oocysts in the five different soil columns was significant different (P = 0.0003), irrespective of whether the columns were irrigated or not, with the highest number of oocysts recovered from the columns with injected liquid slurry.
When comparing the number of oocysts recovered from soil columns with added liquid and raw slurry, the soil columns with injected liquid slurry (with or without irrigation) yielded higher numbers of oocysts than soil columns with applied raw slurry, i.e., injected with or without irrigation, and surface applied with irrigation (Table 4). Furthermore, the total number of oocysts recovered from columns with injected liquid slurry was 38% higher than when the soil columns were irrigated; and 45% more oocysts were recovered from irrigated columns with injected raw slurry compared to nonirrigated columns (Table 4). The distribution pattern of oocysts in the soil sections was similar for all three types of irrigated columns, with the majority of oocysts located in section 2 and numbers of oocysts decreasing with increasing depth (P < 0.05). The oocyst distribution patterns in nonirrigated soil columns with injected raw slurry and injected liquid slurry were different from each other and from the oocyst distribution patterns in the irrigated soil columns. Section 4 had the lowest number of oocysts for all five types of column. Overall, the percentages of oocysts found in the individual soil sections of all soil column types were 62.1% (section 2), 32.7% (section 3), and 5.2% (section 4). The percentages of oocysts in sections 2, 3, and 4 of the soil columns were calculated by dividing the number of oocysts found in each section by the total number of oocysts found in all three sections.
Table 4.
C. parvum oocysts recovered from soil following slurry application and four times irrigation of soil columns
| Slurry type | Application method | Irrigation | Total oocysts recovered per columna ± SE | Mean oocysts (oocysts/0.01% dry matter)b |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Soil section 2 |
Soil section 3 |
Soil section 4 |
|||||||
| LS-ME (95% CI) | % | LS-ME (95% CI) | % | LS-ME (95% CI) | % | ||||
| Liquid | Injection | Yes | 39 ± 13 | 21.5 (10.1–44.7) | 64.5 | 10.4 (5.0–20.6) | 31.3 | 1.4 (0.3–3.5) | 4.2 |
| Raw | Injection | Yes | 20 ± 7 | 12.0 (5.7–24.1) | 69.9 | 3.7 (1.5–7.7) | 21.4 | 1.5 (0.3–3.7) | 8.8 |
| Raw | Surface | Yes | 9 ± 3 | 4.0 (1.7–8.4) | 72.6 | 1.3 (0.2–3.4) | 22.7 | 0.3 (–0.3–1.4) | 4.8 |
| Liquid | Injection | No | 24 ± 10 | 8.4 (2.8–22.4) | 45.2 | 9.6 (3.4–24.4) | 51.7 | 0.6 (–0.4–2.8) | 3.1 |
| Raw | Injection | No | 11 ± 5 | 1.4 (–0.3–7.7) | 86.8 | 0.0 (–0.8–1.1) | 0.0 | 0.2 (–0.5–2.0) | 13.2 |
That is, the sum of total oocysts recovered from the0.01% dry matter content from the three soil sections.
LS-ME (95% CI), back-transformed least-significant mean estimate (95% confidence interval). %, The percent distribution of oocysts in the three soil sections compared to the total numbers of oocysts recovered inall three sections per column type.
Compared to the total number of oocysts applied to the columns, the highest recovery rate of oocysts was seen for soil samples (9.8% ± 3%) compared to the leachate samples (0.03% ± 0.03%). A pilot study made prior to the investigations showed that the method used in the present study recovered ca. 40% of oocysts applied to soil (results not shown).
DISCUSSION
Our findings suggest that field application of the liquid fraction obtained from separation of raw slurry is associated with increased transport of viable C. parvum oocysts in soil compared to the application of raw slurry. Despite the removal of organic particles and nutrients, both chemical and mechanical manure separation seem to cause a limited reduction in the concentration of fecal bacterial indicators and Cryptosporidium oocysts in the liquid fraction compared to raw slurry (D. L. Baggesen, unpublished data).
Other studies have investigated the transport of Cryptosporidium oocysts through soil (4, 8, 9, 15, 25, 32), but none of these studies evaluated the leaching of oocysts following application of separated liquid slurry containing oocysts. In our study, soil columns with injected liquid slurry released more oocysts over the 4-week study period than did soil columns with added raw slurry regardless of the application method, although the difference was not statistically significant. To eliminate differences in soil parameters, such as porosity and clay content that are known to be positively correlated with increased transport of oocysts, all soil columns contained the same type of sandy soil collected at the same field site. The greater recovery of oocysts in leachate from soil columns injected with liquid slurry was probably due to different physicochemical properties of the liquid fraction compared to raw slurry, e.g., the raw slurry contained more than double the amount of volatile solids (organic matter) than the liquid fraction (Table 2). Kuczynska et al. (25) found that more oocysts were attached to soil particles when bovine manure was applied compared to soil where no bovine manure was added, indicating that manure facilitated oocyst attachment, although the factors determining such attachment were not studied. In the present study, it was unfortunately not possible to determine whether slurry alone facilitates oocyst attachment to soil particles, since our study did not include soil columns with added oocysts only. Organic particles have been shown to play a major role in the adsorption of microorganisms in soil, due to their large surface area (20), and Searcy et al. (38) have demonstrated that C. parvum oocysts generally attach to suspended sediments under a wide range of chemical conditions in water. Presumably, an increased number of particles and therefore large surface area in raw slurry will increase the total particle surface area and thereby the number of potential attachment sites for oocysts. Oocysts attached to such manure particles will (due to their increased size) have a higher chance of being retained in soil than nonattached oocysts. The oocysts used in the present study were purified from feces by the use of Percoll and subsequently added to the slurry and the soil columns. Purification of oocysts by using Percoll has been suggested to alter the surface walls of the oocysts (23). Interaction with soil and feces particles can therefore be different in this study than under normal circumstances. Ingestion of only a few infective C. parvum oocysts can cause severe illness in healthy adults (10). Thus, knowledge of the viability of oocysts as an indicator of infectivity is important. Since the relatively low number of leached oocysts did not allow testing of their infectivity through experimental inoculation of mice, we used viability testing by a DAPI/PI dye permeability assay as an indicator of the potential infectivity of C. parvum (21). Although this test has a tendency to overestimate the infectivity compared to mouse infectivity studies (2, 5), our overall finding of 61% viable oocysts in leachate indicates that the consumption of drinking water contaminated with leachate containing such oocysts can cause human infections.
Several studies have looked at the transport of oocysts through soil, but only a few studies have determined the viability and/or infectivity of oocysts in leachate and soil (4, 15, 22). Jenkins et al. (22) used the dye permeability assay to test the viability of oocysts in different soil types kept at different temperatures and found between 12 to 57% viable oocysts after 156 days, with most viable oocysts detected at low temperatures. Boyer and Kuczynska (4) tested the infectivity of C. parvum oocysts released from manure and leached through columns of undisturbed, macroporous karst soil. These researchers found that 20% of the oocysts in leachate were still infective to neonatal mice 12 weeks after the start of the experiment. Since there was a positive correlation between the number of oocysts leached and the number of viable oocysts in our experiments, it was expected that viable oocysts would have been found in leachate from all soil columns after the 4-week study period. Further, our study was based on the application of a relatively small amount (157 g) of slurry compared to the amount of slurry normally added to the agricultural fields as fertilizer. This, in addition to calves typically excreting thousands of viable oocysts per gram of feces when infected (13), suggests that there is a real hazard of leaching substantial numbers of viable oocysts to high level groundwater tables when contaminated slurry from calves is applied to agricultural soil.
Our results support a high retention of oocysts in the surface soil following manure application (32, 43). It was further shown that the type of slurry added to the soil, as well as irrigation of the soil, had an impact on the vertical distribution of oocysts. Thus, more oocysts were found in soil from columns with injected liquid slurry compared to columns with injected raw slurry, regardless of whether the columns were irrigated or not. Similarly, a higher percentage of the oocysts were seen in sections 2 and 3 from columns with added liquid slurry compared to columns with injected raw slurry. These results support the hypothesis that the higher organic particle content in raw slurry compared to liquid slurry increases the number of attachment sites and therefore the retention of attached oocysts in the topsoil. Nevertheless, irrigation seems somehow to interfere with the attachment between oocysts and particles in raw slurry, since 45% more oocysts were found in the soil when columns injected with raw slurry was irrigated. A similar pattern was seen for columns with injected liquid slurry, where 38% more oocysts were found in the soil when the columns were irrigated. Our results corroborate the findings of Ramirez et al. (37), who also found that the amount of irrigation had an effect on the transport of pathogens through the soil, with more oocysts leaching when soil columns were heavily irrigated.
Other studies have shown a similar distribution of microorganisms in soil columns. Wollum and Cassel (43) monitored the transport of bacteria and Streptomycetes conidia in a sandy soil and showed that the majority of microorganisms were retained near the soil surface. Retention of Escherichia coli similar to that found for oocysts in our study was seen for E. coli when using the same soil columns (1). Forslund et al. (15) found that intact soil cores injected with raw pig slurry spiked with C. parvum leached 10 times more oocysts than did soil cores applied with slurry on the surface after 148 days of study under natural climate conditions. However, the amount of oocysts retained in the soil was not determined (15).
It is obvious that laboratory studies can only predict oocysts transport in the field under conditions similar to those used in the laboratory, e.g., only one soil type was studied in the present investigation. A higher recovery rate in leachate and thereby more viable oocysts would be expected if a less sandy soil was used (32). Furthermore, the liquid slurry used in the present study was obtained from only one type of slurry separation technology. Therefore, additional studies are needed on the effectiveness of different slurry separation technologies to remove oocysts and other pathogens before recommendations can be made on the hygiene aspects of using the liquid and solid slurry fractions as fertilizers for different crop types. Nevertheless, we showed here that the application of separated liquid slurry spiked with oocysts to soil columns resulted in the leaching of higher numbers of viable oocysts compared to soil columns with raw slurry applied regardless of the application method. Thus, further studies are needed to assess whether the application of separated liquid slurry may cause groundwater contamination with Cryptosporidium oocysts.
ACKNOWLEDGMENTS
This research was supported by a grant from the PATHOS Project funded by the Strategic Research Council of Denmark (ENV 2104-07-0015). The Ph.D. fellowship of Heidi Huus Petersen was jointly financed by the PATHOS project and the Faculty of Life Sciences, University of Copenhagen and its Ph.D. Research School, RECETO.
We thank staff at the Faculty of Agricultural Sciences of Aarhus University, Denmark and The National Veterinary Institute, Technical University of Denmark for helpful comments and the use of their laboratory facilities. We also thank Anita Forslund, Leif Eiersted, and Cindy Dawn Juel for helpful comments and assistance in the laboratory.
Footnotes
Published ahead of print on 15 June 2012.
REFERENCES
- 1. Amin MGM. 2011. Environmental fate of pathogens and hormones applied to agricultural land in treated slurry. Ph.D. thesis Aarhus University, Aarhus, Denmark [Google Scholar]
- 2. Black EK, Finch GR, Taghi-Kilani R, Belosevic M. 1996. Comparison of assays for Cryptosporidium parvum oocysts viability after chemical disinfection. FEMS Microbiol. Lett. 135: 187–189 [DOI] [PubMed] [Google Scholar]
- 3. Bodley-Tickell AT, Kitchen SE, Sturdee AP. 2002. Occurrence of Cryptosporidium in agricultural surface waters during an annual farming cycle in lowland UK. Water Res. 36: 1880–1886 [DOI] [PubMed] [Google Scholar]
- 4. Boyer DG, Kuczynska E, Fayer R. 2009. Transport, fate, and infectivity of Cryptosporidium parvum oocysts released from manure and leached through macroporous soil. Environ. Geol. 58: 1011–1019 [Google Scholar]
- 5. Bukhari Z, et al. 2000. Comparison of Cryptosporidium parvum viability and infectivity assays following ozone treatment of oocysts. Appl. Environ. Microbiol. 66: 2972–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell AT, Robertson LJ, Smith HV. 1992. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 58: 3488–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Connolly GM, Dryden MS, Shanson DC, Gazzrd BG. 1988. Cryptosporidial diarrhoea in AIDS and its treatment. Gut 29: 593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Darnault CJG, et al. 2003. Preferential transport of Cryptosporidium parvum oocysts in variably saturated subsurface environments. Water Environ. Res. 75: 113–120 [DOI] [PubMed] [Google Scholar]
- 9. Darnault CJG, et al. 2004. Preferential flow and transport of Cryptosporidium parvum oocysts through the vadose zone: experiments and modeling. Vadose Zone J. 3: 262–270 [Google Scholar]
- 10. DuPont HL, et al. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332: 855–859 [DOI] [PubMed] [Google Scholar]
- 11. Eriksen J, Sorensen P, EIsgaard L. 2008. The fate of sulfate in acidified pig slurry during storage and following application to cropped soil. J. Environ. Qual. 37: 280–286 [DOI] [PubMed] [Google Scholar]
- 12. Fangueiro D, Senbayram M, Trindade H, Chadwick D. 2008. Cattle slurry treatment by screw press separation and chemically enhanced settling: effect on greenhouse gas emissions after land spreading and grass yield. Bioresour. Technol. 99: 7132–7142 [DOI] [PubMed] [Google Scholar]
- 13. Fayer R, Santin M, Trout JM, Greiner E. 2006. Prevalence of species and genotypes of Cryptosporidium found in 1- to 2-year-old dairy cattle in the eastern United States. Vet. Parasitol. 135: 105–112 [DOI] [PubMed] [Google Scholar]
- 14. Fayer R, Trout JM, Jenkins MC. 1998. Infectivity of Cryptosporidium parvum oocysts stored in water at environmental temperatures. J. Parasitol. 84: 1165–1169 [PubMed] [Google Scholar]
- 15. Forslund A, et al. 2011. Leaching of Cryptosporidium parvum oocysts, Escherichia coli, and Salmonella typhimurium bacteriophage through intact soil cores following surface application and injection of slurry. Appl. Environ. Microbiol. 77: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fricker CR, Crabb JH. 1998. Water-borne cryptosporidiosis: detection methods and treatment options. Adv. Parasitol. 40: 241–278 [DOI] [PubMed] [Google Scholar]
- 17. Harter T, Wagner S, Atwill ER. 2000. Colloid transport and filtration of Cryptosporidium parvum in sandy soils and aquifer sediments. Environ. Sci. Technol. 34: 62–70 [Google Scholar]
- 18. Henriksen SA, Pohlenz JFL. 1981. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet. Scand. 22: 594–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunter PR, Thompson RCA. 2005. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 35: 1181–1190 [DOI] [PubMed] [Google Scholar]
- 20. Huysman F, Verstraete W. 1993. Water facilitated transport of bacteria in unsaturated soil columns: influence of cell surface hydrophobicity and soil properties. Soil Biol. Biochem. 25: 83–90 [Google Scholar]
- 21. Jenkins MB, Anguish LJ, Bowman DD, Walker MJ, Ghiorse WC. 1997. Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 63: 3844–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenkins MB, Bowman DD, Fogarty EA, Ghiorse WC. 2002. Cryptosporidium parvum oocyst inactivation in three soil types at various temperatures and water potentials. Soil Biol. Biochem. 34: 1101–1109 [Google Scholar]
- 23. Jenkins MB, et al. 2010. Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 76: 1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korich DG, Mead JR, Madore MS, Sinclair NA, Sterling CR. 1990. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 56: 1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuczynska E, et al. 2005. Effect of bovine manure on Cryptosporidium parvum oocyst attachment to soil. Appl. Environ. Microbiol. 71: 6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laegdsmand M, Andersen H, Jacobsen OH, Halling-Sorensen B. 2009. Transport and fate of estrogenic hormones in slurry-treated soil monoliths. J. Environ. Qual. 38: 955–964 [DOI] [PubMed] [Google Scholar]
- 27. Laegdsmand M, Villholth KG, Ullum M, Jensen KH. 1999. Processes of colloid mobilization and transport in macroporous soil monoliths. Geoderma 93: 33–59 [Google Scholar]
- 28. Langkjaer RB, Vigre H, Enemark HL, Maddox-Hyttel C. 2007. Molecular and phylogenetic characterization of Cryptosporidium and Giardia from pigs and cattle in Denmark. Parasitology 134: 339–350 [DOI] [PubMed] [Google Scholar]
- 29. Lisle J, Rose J. 1995. Cryptosporidium contamination of water in the U.S.A. and UK: a mini-review. Aqua (Oxford) 44: 103–117 [Google Scholar]
- 30. MacKenzie WR, et al. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331: 161–167 [DOI] [PubMed] [Google Scholar]
- 31. Maddox-Hyttel C, Langkjaer RB, Enemark HL, Vigre H. 2006. Cryptosporidium and Giardia in different age groups of Danish cattle and pigs: occurrence and management associated risk factors. Vet. Parasitol. 141: 48–59 [DOI] [PubMed] [Google Scholar]
- 32. Mawdsley JL, Brooks AE, Merry RJ. 1996. Movement of the protozoan pathogen Cryptosporidium parvum through three contrasting soil types. Biol. Fertil. Soils 21: 30–36 [Google Scholar]
- 33. Mawdsley JL, Brooks AE, Merry RJ, Pain BF. 1996. Use of a novel soil tilting table apparatus to demonstrate the horizontal and vertical movement of the protozoan pathogen Cryptosporidium parvum in soil. Biol. Fertil. Soils 23: 215–220 [Google Scholar]
- 34. Moulton-Hancock C, et al. 2000. Giardia and Cryptosporidium occurrence in groundwater. J. Am. Water Works Assoc. 92: 117–123 [Google Scholar]
- 35. Olson ME, Goh J, Phillips M, Guselle N, McAllister TA. 1999. Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J. Environ. Qual. 28: 1991–1996 [Google Scholar]
- 36. Ong C, Moorehead W, Ross A, Isaac-Renton J. 1996. Studies of Giardia spp. and Cryptosporidium spp. in two adjacent watersheds. Appl. Environ. Microbiol. 62: 2798–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramirez NE, et al. 2009. Effect of tillage and rainfall on transport of manure-applied Cryptosporidium parvum oocysts through soil. J. Environ. Qual. 38: 2394–2401 [DOI] [PubMed] [Google Scholar]
- 38. Searcy KE, Packman AI, Atwill ER, Harter T. 2005. Association of Cryptosporidium parvum with suspended particles: impact on oocyst sedimentation. Appl. Environ. Microbiol. 71: 1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith HV. 1992. Cryptosporidium and water: a review. J. Inst. Water Environ. Manag. 6: 443–451 [Google Scholar]
- 40. Sommer SG, Hutchings NJ. 2001. Ammonia emission from field applied manure and its reduction: invited paper. Eur. J. Agron. 15: 1–15 [Google Scholar]
- 41. Tate KW, Atwill ER, George MR, McDougald MK, Larsen RE. 2000. Cryptosporidium parvum transport from cattle fecal deposits on California rangelands. J. Range Manage. 53: 295–299 [Google Scholar]
- 42. Tyzzer EE. 1912. Cryptosporidium parvum (sp. nov.), a coccidium found in the small intestine of the common mouse. Arch. Protistenkd. 26: 394–412 [Google Scholar]
- 43. Wollum AG, Cassel DK. 1978. Transport of microorganisms in sand columns. Soil Sci. Soc. Am. J. 42: 72–76 [Google Scholar]


