Abstract
Abstracts
Background
Acinetobacter baumannii is well-recognized as an important nosocomial pathogen, however, due to their intrinsic resistance to several antibiotics, treatment options are limited. Synergistic effects between antibiotics and medicinal plants, particularly their active components, have intensively been studied as alternative approaches.
Methods
Fifty-one ethanol extracts obtained from 44 different selected medicinal plant species were tested for resistance modifying agents (RMAs) of novobiocin against A. baumannii using growth inhibition assay.
Results
At 250 μg/ml, Holarrhena antidysenterica, Punica granatum, Quisqualis indica, Terminalia bellirica, Terminalia chebula, and Terminalia sp. that possessed low intrinsic antibacterial activity significantly enhanced the activity of novobiocin at 1 μg/ml (1/8xminimum inhibitory concentration) against this pathogen. Holarrhena antidysenterica at 7.8 μg/ml demonstrated remarkable resistant modifying ability against A. baumannii in combination with novobiocin. The phytochemical study revealed that constituents of this medicinal plant contain alkaloids, condensed tannins, and triterpenoids.
Conclusion
The use of Holarrhena antidysenterica in combination with novobiocin provides an effective alternative treatment for multidrug resistant A. baumannii infections.
Background
An underestimated nosocomial pathogen, Acinetobacter baumannii, is now widely acknowledged as a common bacterium in hospital irrigation and intravenous solutions. It possesses inherent multidrug-resistance (MDR) and the ability to rapidly colonize and infect patients. Moreover, the emergence of acquired MDR by A. baumannii to conventional antibiotics presents a serious therapeutic problem in the treatment of the infections [1,2]. Several investigations suggested that synergy effects of plant secondary metabolites and conventional antibiotics could be an alternative way to increase the bacterial susceptibility [3-6].
Plants, particularly ethnomedicinal plants are important sources of natural products. They are rich in a wide variety of secondary metabolites such as tannins, terpenoids, alkaloids, and flavonoids and have been well-established to possess antimicrobial properties [7]. Many plants have been evaluated not only for their inherent antimicrobial activity, but also for their action as a resistant modifying agent (RMA) [4].
Novobiocin, a Gyr B inhibitor, is an effective aminocoumarin drug for the treatment of Gram-positive bacterial infections. However, its low level of activity against Gram-negative pathogens causes a major limitation [8]. Although, several investigations observed synergy and mechanisms of action between natural products and synthetic drugs in effectively combating Gram positive bacterial infections [5], there are a few RMA effective for use with A. baumannii[9,10]. Therefore, the aim of this study was to further explore the resistant modifying activity of a wide range of medicinal plants according to their ethnobotanical basis in combination with novobiocin against A. baumannii.
Methods
Bacterial strain and culture condition
Acinetobacter baumannii ATCC 19606 was employed in this study as a model reference strain. The strain was susceptible to ciprofloxacin, colistin, imipenem, and tobramycin and resistant to amikacin, ampicillin, azithromycin, erythromycin, and gentamicin which conducted by disc diffusion method [11]. Well-isolated colonies of A. baumannii ATCC 19606 were grown in Mueller Hinton Broth (MHB) (Difco Laboratories, Detroit, MI) at 37°C for 18–24 h. The culture density was adjusted to McFarland standards No. 0.5 and resuspended in MHB to obtain a final concentration of 1 × 106 cfu/ml.
Medicinal plant materials
Tested medicinal plants are shown in Table 1. Fifty-one ethanol extracts of 44 Thai medicinal plant species were kindly provided by the Natural Products Research Center, Prince of Songkla University, Hat Yai, Thailand [12]. Collected plant materials were washed with distilled water and dried at 60°C overnight. Ground plant material was macerated with 95% ethanol (1:2 w/v) for 7 days. The extract was filtered and evaporated using rotary evaporator at 45°C until it became completely dry. A stock solution (200 mg/ml) was prepared by dissolving 0.2 g of the dried extract in 1 ml of dimethylsulfoxide (DMSO) (Merck, Germany) and stored at −20°C.
Table 1.
Intrinsic antibacterial activity and resistant modifying ability of crude extract (250 μg/ml) in combination with novobiocin (1/8xMIC) againstAcinetobacter baumanniiATCC 19606
| |
Botanical names |
Family name |
Part used |
%Growth inhibitiona ± SDb |
Interpretationc |
|
|---|---|---|---|---|---|---|
| PE | PE + NOV | |||||
| 1 |
Aegle marmelos (L.) Corr. Serr. |
Rutaceae |
Fruit |
22.10 ± 0.68 |
27.10 ± 1.38 |
No synergy |
| 2 |
Ardisia colorata Roxb. |
Primulaceae |
Fruit |
30.17 ± 2.56 |
39.00 ± 6.09 |
Synergy |
| 3 |
Asclepias curassavica L. |
Asclepiadaceae |
Wood |
40.81 ± 0.28 |
43.59 ± 1.78 |
No synergy |
| 4 |
Centella asiatica (L.) Urb. |
Apiaceae |
Whole |
19.09 ± 1.06 |
23.93 ± 2.87 |
No synergy |
| 5 |
Cinnamomum bejolghota (Buch.-Ham.) Sweet |
Lauraceae |
Wood |
58.84 ± 1.37 |
59.92 ± 1.78 |
No synergy |
| |
|
|
Bark |
55.62 ± 4.98 |
62.44 ± 2.91 |
No Synergy |
| 6 |
Cinnamomum porrectum (Roxb.) Kosterm. |
Lauraceae |
Wood |
29.72 ± 6.54 |
26.06 ± 5.21 |
No synergy |
| |
|
|
Bark |
56.88 ± 2.14 |
63.31 ± 4.87 |
No synergy |
| 7 |
Curcuma longa L. |
Zingiberaceae |
Rhizome |
86.91 ± 2.64 |
88.78 ± 2.08 |
No synergy |
| 8 |
Curcuma zedoaria (Christm.) Roscoe |
Zingiberaceae |
Rhizome |
77.73 ± 0.48 |
79.59 ± 2.62 |
No synergy |
| 9 |
Derris scandens Benth. |
Leguminosea |
Stem |
49.01 ± 2.37 |
47.31 ± 3.84 |
No synergy |
| 10 |
Dracaena loureiri Gagnep. |
Agavaceae |
Wood |
30.08 ± 0.99 |
29.49 ± 3.19 |
No synergy |
| 11 |
Dryopteris syrmatica (Willd.) Kuntze |
Dryopteridaceae |
Stem |
17.59 ± 0.41 |
26.66 ± 5.32 |
Synergy |
| 12 |
Eleutherine americana (Aubl.) Merr. ex K. |
Iridaceae |
Bulb |
17.87 ± 1.89 |
22.26 ± 3.12 |
No synergy |
| 13 |
Euphorbia thymifolia L. |
Euphorbiaceae |
Whole plant |
53.64 ± 0.90 |
73.99 ± 0.88 |
Synergy |
| 14 |
Garcinia mangostana L. |
Clusiaceae |
Pericarp |
93.25 ± 3.65 |
90.48 ± 3.37 |
No synergy |
| 15 |
Gymnopetalum cochinchinensis (Lour.) Kurz |
Cucurbitaceae |
Fruit |
26.17 ± 0.59 |
32.45 ± 4.39 |
No synergy |
| 16 |
Holarrhena antidysenterica (L.) Wall. ex A. DC. |
Apocynaceae |
Bark |
65.88 ± 0.11 |
94.04 ± 0.59* |
Synergy |
| 17 |
Impatiens balsamina L. |
Balsaminaceae |
Stem |
9.77 ± 0.30 |
12.40 ± 1.56 |
No synergy |
| 18 |
Manilkara achras (Mill.) Fosb. |
Sapotaceae |
Fruit |
56.59 ± 1.02 |
63.06 ± 2.97 |
No synergy |
| 19 |
Millingtonia hortensis L.f. |
Bignoniaceae |
Flower |
28.97 ± 4.30 |
54.08 ± 0.83 |
Synergy |
| 20 |
Mitragyna speciosa Korth. |
Rubiaceae |
Leaf |
43.33 ± 2.40 |
66.15 ± 0.26 |
Synergy |
| 21 |
Momordica charantia L. |
Cucurbitaceae |
Vine |
22.26 ± 0.85 |
25.79 ± 3.10 |
No synergy |
| 22 |
Morinda citrifolia L. |
Rubiaceae |
Fruit |
16.96 ± 0.63 |
25.86 ± 1.22 |
Synergy |
| 23 |
Murdannia loriformis (Hassk.) R. Rao & Kammathy |
Commilinaceae |
Whole plant |
16.42 ± 1.51 |
22.04 ± 1.67 |
No synergy |
| 24 |
Oroxylum indicum (L.) Vent. |
Bignoniaceae |
Leaf |
67.18 ± 1.59 |
71.30 ± 5.28 |
No synergy |
| 25 |
Peltophorum pterocarpum (DC.) Backer ex K. Heyne |
Fabaceae |
Flower |
42.80 ± 0.43 |
47.83 ± 4.49 |
No synergy |
| |
|
|
Bark |
78.26 ± 0.60 |
88.75 ± 6.10 |
Synergy |
| 26 |
Piper betle L. |
Piperaceae |
Leaf |
42.72 ± 0.13 |
39.92 ± 3.43 |
No synergy |
| 27 |
Piper nigrum L. |
Piperaceae |
Fruit |
38.07 ± 1.96 |
42.24 ± 2.60 |
No synergy |
| |
|
|
Seed |
29.07 ± 0.75 |
31.47 ± 3.27 |
No synergy |
| 28 |
Piper retrofractum Vahl |
Piperaceae |
Fruit |
44.02 ± 1.08 |
49.80 ± 4.19 |
No synergy |
| 29 |
Piper sarmentosum Roxb |
Piperaceae |
Leaf |
20.70 ± 0.88 |
25.02 ± 0.62 |
No synergy |
| 30 |
Pluchea indica (L.) Less. |
Asteraceae |
Leaf |
26.64 ± 0.97 |
53.59 ± 3.60* |
Synergy |
| 31 |
Psidium guajava L. |
Myrtaceae |
Leaf |
71.24 ± 2.00 |
81.19 ± 1.50* |
Synergy |
| 32 |
Punica granatum L. |
Puniceaceae |
Pericarp |
72.58 ± 1.20 |
99.29 ± 0.63* |
Synergy |
| 33 |
Quercus infectoria G.Olivier |
Fagaceae |
Gall |
89.09 ± 0.15 |
88.77 ± 1.00 |
No synergy |
| 34 |
Quisqualis indica L. |
Combretaceae |
Flower |
79.22 ± 0.28 |
94.63 ± 2.62* |
Synergy |
| 35 |
Rhizophora mucronata Lam. |
Rhizophoraceae |
Fruit |
44.64 ± 0.59 |
53.35 ± 2.56 |
Synergy |
| |
|
|
Bark |
42.68 ± 8.20 |
53.03 ± 4.95 |
Synergy |
| 36 |
Rhodomyrtus tomentosa (Aiton) Hassk. |
Myrtaceae |
Stem |
77.01 ± 1.28 |
81.81 ± 4.01 |
No synergy |
| 37 |
Sandoricum indicum Cav. |
Meliaceae |
Root |
65.24 ± 1.32 |
66.94 ± 2.13 |
No synergy |
| 38 |
Tamarindus indica L. |
Fabaceae |
Leaf |
19.76 ± 1.55 |
25.03 ± 3.45 |
No synergy |
| 39 |
Terminalia bellirica (Gaertn.) Roxb. |
Combretaceae |
Fruit |
74.79 ± 0.53 |
95.68 ± 1.14* |
Synergy |
| 40 |
Terminalia chebula (Gaertn.) Retz. |
Combretaceae |
Fruit |
61.25 ± 0.42 |
94.33 ± 1.95* |
Synergy |
| 41 |
Terminalia sp. |
Combretaceae |
Fruit |
79.53 ± 0.24 |
95.92 ± 1.10* |
Synergy |
| 42 |
Theobroma cacao L. |
Sterculiaceae |
Pericarp |
17.35 ± 0.74 |
22.81 ± 0.68 |
No synergy |
| |
|
|
Seed |
19.25 ± 1.08 |
29.61 ± 4.13 |
Synergy |
| 43 |
Vitex trifolia L. |
Verbenaceae |
Leaf |
22.12 ± 0.68 |
28.65 ± 3.57 |
No synergy |
| 44 |
Xylocarpus granatum J. Koenig. |
Meliaceae |
Pericarp |
52.39 ± 3.48 |
53.27 ± 1.91 |
No synergy |
| Seed | 44.27 ± 5.13 | 54.55 ± 3.66 | No synergy | |||
Percentage of growth inhibition of novobiocin against A. buamannii ATCC 19606 was 6.67%.
aPercentage of growth inhibition in the present of plant extract (PE) and plant extract in combination with novobiocin (PE + NOV) against A. buamannii ATCC 19606.
bSD Standard Deviation.
cSynergy: (PE + NOV) > (PE) + (NOV); No synergy: (PE + NOV) < (PE) + (NOV) [6].
*P < 0.01: Significantly different from the effect of plant extract.
Determination of minimum inhibitory concentration (MIC) of novobiocin
The MIC of novobiocin was determined by the broth microdilution method as described by the Clinical and Laboratory Standard Institute (CLSI) [13].
Intrinsic antibacterial activity and resistant modifying ability of medicinal plant extracts
Intrinsic antibacterial activities were determined by growth inhibition assays [9]. The bacterial culture (100 μl) was inoculated into a 96-well microtiter plate containing 50 μl of crude extracts (1,000 μg/ml) and 50 μl of MHB and then incubated at 37°C for 18 h. The intrinsic antibacterial activity was exhibited as the percentage of growth inhibition and calculated from the following equation:
| (1) |
Where ODA is Optical density (OD) 595 nm of bacteria culture in MHB supplemented with 1%DMSO as positive control and ODB is OD 595 nm of the bacterial culture in MHB supplemented with plant extracts.
Resistant modifying ability of the extracts was observed by adding of 50 μl novobiocin at a concentration of 1/8xMIC (1 μg/ml) into the tested plate instead of MHB. This biological activity was exhibited as the percentage of growth inhibition as well but calculated from the following equation, where ODC is OD 595 nm of the bacterial culture in MHB supplemented with the plant extract in combination with novobiocin:
| (2) |
Effective medicinal plants that demonstrated a synergistic effect with novobiocin and exhibited bacterial growth inhibition more than 90% were selected for further experiments. The efficacy of combination therapy of the promising medicinal plants with novobiocin was additionally determined by measuring the resistant modifying capabilities of the extracts at varying concentrations ranging from 7.8 to 250 μg/ml.
Phytochemical screening methods
Phytochemical screening tests for alkaloids, condensed tannins, flavonoids, hydrolysable tannins, steroids, and triterpenes were qualitatively analyzed by standard colour tests as previously described [14].
Results and discussion
Intrinsic resistance of A. baumannii to novobiocin was observed with MIC value at 8 μg/ml. As shown in Table 1, 48 out of 51 tested ethanol extracts at concentration of 250 μg/ml had low inherent antibacterial activity (% of bacterial growth inhibition was less than 80%). In combination with the antibiotic, the extracts of 18 medicinal plants demonstrated synergistic interaction against A. baumannii. Interestingly, the bacterial growth inhibition in the presence of novobiocin in combination with the extracts of Holarrhena antidysenterica, Punica granatum, Quisqualis indica, Terminalia bellirica, Terminalia chebula, and Terminalia sp. extracts was significantly higher than the intrinsic antibacterial activity of the extracts (Table 1).
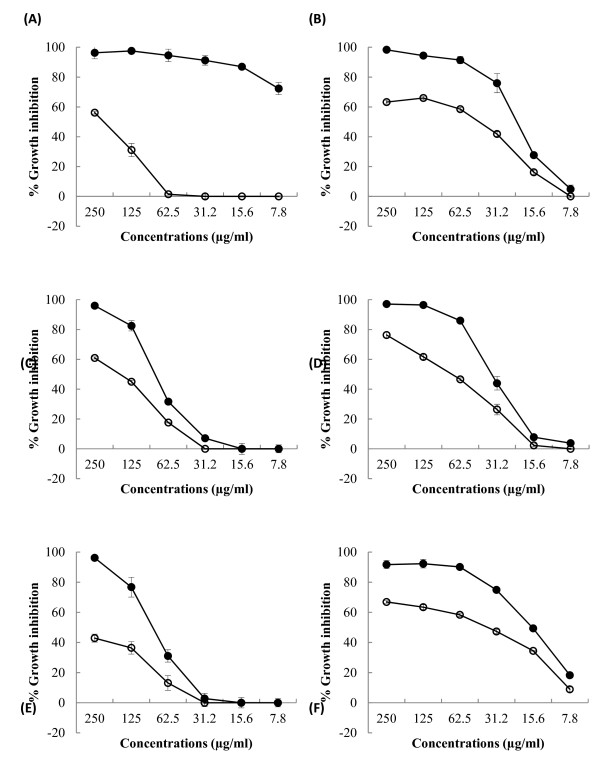
To explore the potential of developing a more powerful combination therapy of these medicinal plants with novobiocin, we determined the resistant modifying ability of varying concentrations of the extracts from 7.8 to 250 μg/ml by growth inhibition assay as illustrated in Figure 1. Holarrhena antidysenterica extract which concentrations ranging from 7.8 to 62.5 μg/ml possessed no intrinsic anti-acinetobacter activity (Figure 1A) was demonstrated to be a powerful RMA in combination with novobiocin against this pathogen.
Figure 1.
Bacterial growth inhibition ofHolarrhena antidysenterica(A),Punica granatum(B),Quisqualis indica(C),Terminalia bellirica(D),Terminalia chebula(E), andTerminaliasp. (F) ethanol extracts (○) and the extracts in combination with 1/8xMIC of novobiocin (●) againstAcinetobacter baumanniiATCC 19606. Percentage of bacterial growth inhibition of 1/8xMIC of novobiocin on this pathogen was 6.67%.
Our preliminary phytochemical test revealed that alkaloids were common principles among the effective extracts. In addition to alkaloids, other compounds including condensed tannins, triterpenoids, flavonoids, hydrolysable tannins, and steroids were detected (Table 2). Although the antibiotic resistant modifying ability of active principles of the effective medicinal plants has never been investigated, plant-derived alkaloids have been well-clarified as efflux pump inhibitors (EPIs) for Gram positive bacteria [15,16]. Recent evaluation of 13 phyto-alkaloids for their EPI potential against staphylococcal isolates revealed that 60% and 30% of the tested compounds exhibited the activity against methicillin resistant Staphylococcus aureus (MRSA) and methicillin susceptible S. aureus (MSSA), respectively [16]. Four plant-derived alkaloids consisting of reserpine, quinine, harmaline, and piperine possessed notable potential EPI activities on both MRSA and MSSA [16]. More importantly, their mechanisms of actions as a RMA have been proposed. Piperine was recorded as an inhibitor of MdeA [17] and NorA [18] efflux pumps of S. aureus and Rv1258c efflux pump of Mycobacterium tuberculosis[19]. Reserpine was found as an inhibitor of Bmr efflux pump in Bacillus subtilis, Tet(K) and NorA efflux pumps of S. aureus[20]. In addition to phyto-alkaloids, several plant-derived polyphenols such as epigallocatechin gallate of Camellia sinesis, tellimagrandin I and rugosin B isolated from Rosa canina have been established as useful RMAs with different mechanisms of actions including inhibitions of adapted drug target sites or enzymatic degradation of drugs [4]. Intensive investigations on plant-derived compounds as RMAs have been performed in Gram-positive, but relatively very few studies have been carried out to evaluated RMA activities of plant-derived compounds on Gram-negative bacteria [21-23].
Table 2.
Extraction yields and phytochemical constituents of tested medicinal plant extracts
| |
Botanical names |
Part used |
Yield (%; w/w)a |
Phytochemical constituentsb |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| 1 |
Aegle marmelos (L.) Corr. Serr. |
Fruit |
5.3 |
+ |
+ |
+ |
- |
+ |
- |
| 2 |
Ardisia colorata Roxb. |
Fruit |
4.4 |
+ |
+ |
- |
- |
+ |
- |
| 3 |
Asclepias curassavica L. |
Wood |
0.9 |
+ |
+ |
- |
- |
- |
- |
| 4 |
Centella asiatica (L.) Urb. |
Whole |
6.0 |
+ |
- |
- |
- |
+ |
- |
| 5 |
Cinnamomum bejolghota (Buch.-Ham.) Sweet |
Wood |
2.2 |
+ |
+ |
- |
- |
+ |
- |
| |
|
Bark |
14.6 |
+ |
- |
- |
+ |
+ |
- |
| 6 |
Cinnamomum porrectum (Roxb.) Kosterm. |
Wood |
11.2 |
- |
- |
- |
- |
+ |
- |
| |
|
Bark |
7.0 |
+ |
+ |
- |
- |
+ |
- |
| 7 |
Curcuma longa L. |
Rhizome |
13.9 |
+ |
+ |
+ |
- |
+ |
- |
| 8 |
Curcuma zedoaria (Christm.) Roscoe |
Rhizome |
13.9 |
+ |
+ |
+ |
- |
- |
+ |
| 9 |
Derris scandens Benth. |
Stem |
3.2 |
- |
+ |
- |
- |
+ |
- |
| 10 |
Dracaena loureiri Gagnep. |
Wood |
16.9 |
- |
- |
- |
- |
- |
+ |
| 11 |
Dryopteris syrmatica (Willd.) Kuntze |
Stem |
4.5 |
+ |
+ |
- |
- |
+ |
- |
| 12 |
Eleutherine americana (Aubl.) Merr. ex K. |
Bulb |
4.8 |
+ |
+ |
- |
- |
- |
- |
| 13 |
Euphorbia thymifolia L. |
Whole plant |
1.3 |
- |
+ |
- |
- |
+ |
- |
| 14 |
Garcinia mangostana L. |
Pericarp |
5.3 |
- |
- |
- |
- |
- |
- |
| 15 |
Gymnopetalum cochinchinensis (Lour.) Kurz |
Fruit |
7.6 |
- |
- |
- |
- |
+ |
- |
| 16 |
Holarrhena antidysenterica (L.) Wall. ex A. DC. |
Bark |
2.1 |
+ |
+ |
- |
- |
- |
+ |
| 17 |
Impatiens balsamina L. |
Stem |
5.2 |
- |
+ |
- |
- |
+ |
- |
| 18 |
Manilkara achras (Mill.) Fosb. |
Fruit |
26.7 |
+ |
- |
+ |
- |
- |
+ |
| 19 |
Millingtonia hortensis L.f. |
Flower |
25.4 |
+ |
+ |
+ |
- |
- |
- |
| 20 |
Mitragyna speciosa Korth. |
Leaf |
5.9 |
+ |
+ |
- |
- |
+ |
- |
| 21 |
Momordica charantia L. |
Vine |
3.0 |
+ |
- |
- |
- |
+ |
- |
| 22 |
Morinda citrifolia L. |
Fruit |
7.3 |
+ |
- |
+ |
- |
+ |
- |
| 23 |
Murdannia loriformis (Hassk.) R. Rao & Kammathy |
Whole plant |
7.6 |
+ |
- |
- |
- |
+ |
- |
| 24 |
Oroxylum indicum (L.) Vent. |
Leaf |
3.7 |
+ |
+ |
- |
- |
+ |
- |
| 25 |
Peltophorum pterocarpum (DC.) Backer ex K. Heyne |
Flower |
7.1 |
+ |
- |
- |
- |
- |
- |
| |
|
Bark |
7.1 |
+ |
+ |
- |
- |
- |
+ |
| 26 |
Piper betle L. |
Leaf |
12.4 |
- |
+ |
- |
- |
+ |
- |
| 27 |
Piper nigrum L. |
Fruit |
4.2 |
+ |
- |
- |
- |
+ |
- |
| |
|
Seed |
4.2 |
+ |
- |
- |
- |
+ |
- |
| 28 |
Piper retrofractum Vahl |
Fruit |
7.0 |
- |
- |
- |
- |
+ |
- |
| 29 |
Piper sarmentosum Roxb |
Leaf |
1.7 |
+ |
- |
- |
- |
+ |
- |
| 30 |
Pluchea indica (L.) Less. |
Leaf |
17.8 |
+ |
+ |
- |
- |
+ |
- |
| 31 |
Psidium guajava L. |
Leaf |
8.0 |
+ |
+ |
- |
- |
+ |
- |
| 32 |
Punica granatum L. |
Pericarp |
13.0 |
+ |
+ |
+ |
- |
- |
+ |
| 33 |
Quercus infectoria G.Olivier |
Gall |
37.8 |
+ |
- |
- |
+ |
- |
- |
| 34 |
Quisqualis indica L. |
Flower |
11.0 |
+ |
- |
+ |
+ |
+ |
- |
| 35 |
Rhizophora mucronata Lam. |
Fruit |
10.7 |
+ |
+ |
- |
- |
- |
+ |
| |
|
Bark |
11.6 |
- |
+ |
- |
- |
- |
+ |
| 36 |
Rhodomyrtus tomentosa (Aiton) Hassk. |
Stem |
7.1 |
+ |
+ |
- |
- |
- |
+ |
| 37 |
Sandoricum indicum Cav. |
Root |
4.0 |
+ |
- |
- |
- |
+ |
- |
| 38 |
Tamarindus indica L. |
Leaf |
4.8 |
+ |
+ |
+ |
- |
+ |
- |
| 39 |
Terminalia bellirica (Gaertn.) Roxb. |
Fruit |
14.8 |
+ |
- |
- |
- |
+ |
- |
| 40 |
Terminalia chebula (Gaertn.) Retz. |
Fruit |
5.9 |
+ |
+ |
- |
- |
- |
+ |
| 41 |
Terminalia sp. |
Fruit |
23.9 |
+ |
- |
- |
+ |
- |
- |
| 42 |
Theobroma cacao L. |
Pericarp |
3.6 |
+ |
+ |
- |
- |
+ |
- |
| |
|
Seed |
5.9 |
- |
+ |
+ |
- |
- |
+ |
| 43 |
Vitex trifolia L. |
Leaf |
NDc |
+ |
+ |
- |
- |
+ |
- |
| 44 |
Xylocarpus granatum J. Koenig. |
Pericarp |
2.6 |
+ |
+ |
- |
- |
+ |
- |
| Seed | 6.7 | + | + | + | - | - | + | ||
aPercentage extract yields of medicinal plants were weight of crude extract per 100 g of dried plant materials.
bPhytochemincal constituents: 1, alkaloids; 2, condensed tannins; 3, flavonoids; 4, hydrolysable tannins; 5, steroids and 6, triterpenoids; ‘-’ indicates absence of phytoconstituents ‘+’ indicates presence of phytoconstituents.
cND Not determined.
In the last decade multidrug resistance in A. baumannii became a serious growing problem worldwide. Colistin, an old antibiotic with risk toxicity, has recently been brought back into use to treat MDR bacteria as a stopgap measure until new antibiotics can be developed [24]. A number of workers have proposed the synergistically action combination of conventional antibiotics with RMA act synergistically against MDR Gram-negative bacteria [4,25,26]. We have demonstrated that certain plant ethanol extracts significantly enhanced the activity of novobiocin against A. baumannii. Holarrhena antidysenterica is of interest since the extract at 7.8 to 62.5 μg/ml possessed no intrinsic antibacterial activity, but in combination with sub-MIC of novobiocin led to a marked decrease in the bacterial growth. Alkaloids were proposed as active principles of the plant that possessed antibacterial activity on S. aureusS. epidermidisStreptococcus faecalisB. subtilisEscherichia coli, and Pseudomonas aeruginosa[27-29]. Some of the alkaloids such as pubadysone, pubescine, norholadiene, pubescimine, puboestrene, pubamide, and naringenin was isolated form bark, seeds, and leaves of this plant [30-32].
Our previous investigation demonstrated that ellagic acid which acts as an efflux pump inhibitor exhibited a synergistic effect with novobiocin and other aminocoumarins against both A. baumannii ATCC 19606 and MDR A. baumannii[9]. Ethylenediaminetetraacetic acid and polyethyleneimine that disturb outer membrane permeability have been reported as RMA for novobiocin against P. aeruginosa and Stenotrophomonas morelense[33,34]. Similarly, berry-derived phenolic compounds that efficiently destabilized outer membrane permeability resulted in increase in novobiocin susceptibility of Salmonella enterica serotype Typhimurium [35].
Since intrinsic novobiocin resistance in A. baumannii is related to the synergistic interaction between limited outer membrane permeability and energy-dependent multidrug efflux pumps [36,37], the RMA for novobiocin possibly acts as a permeabilizer and/or an efflux pump inhibitor.
Conclusion
The RMA activity of Thai medicinal plants in combination with novobiocin against A. baumannii is reported for the first time. These findings led us to the development of a new generation of phytopharmaceuticals that using plant-derived compounds in combination with existing antibiotics to treat MDR A. baumannii that currently are almost untreatable. Its mechanism of action as well as the active constituents of a promising plant, Holarrhena antidysenterica should be further investigated.
Competing interests
The authors declare that they have no competing interests.
Author’ contributions
PN designed and carried out the study. SC and SV supervised in the design of the study and contributed to the writing process. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Pinanong Na Phatthalung, Email: pinanong_np@hotmail.com.
Sasitorn Chusri, Email: s_chusri@yahoo.com.
Supayang P Voravuthikunchai, Email: supayang.v@psu.ac.th.
Acknowledgments
This work was supported by the Thailand research Fund-the Commission on Higher Education (MRG 5480069, Fiscal year 2011–2013) and the Higher Education Research Promotion and National Research University of Thailand, Office of the Higher Education Commission.
References
- Acosta J, Merino M, Viedma E, Poza M, Sanz F, Otero JR, Chaves F, Bou G. Multidrug-resistant Acinetobacter baumannii Harboring OXA-24 Carbapenemase, Spain. Emerg Infect Dis. 2011;17:1064–1067. doi: 10.3201/eid1706.091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsaragakis S, Markogiannakis H, Samara E, Pachylaki N, Theodoraki EM, Xanthaki A, Toutouza M, Toutouzas KG, Theodorou D. Predictors of mortality of Acinetobacter baumannii infections: A 2-year prospective study in a Greek surgical intensive care unit. Am J Infect Control. 2010;38:631–635. doi: 10.1016/j.ajic.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Hemaiswarya S, Doble M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine. 2009;16:997–1005. doi: 10.1016/j.phymed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15:639–652. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Ulrich-Merzenich G, Panek D, Zeitler H, Vetter H, Wagner H. Drug development from natural products: exploiting synergistic effects. Indian J Exp Biol. 2010;48:208–219. [PubMed] [Google Scholar]
- Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Perumal Samy R, Gopalakrishnakone P. Therapeutic potential of plants as aAnti-microbials for drug discovery. Evid Based Complement Alternat Med. 2010;7:283–294. doi: 10.1093/ecam/nen036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampranis SC, Maxwell A. Conversion of DNA gyrase into a conventional type II topoisomerase. Proc Natl Acad Sci U S A. 1996;93:14416–14421. doi: 10.1073/pnas.93.25.14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusri S, Villanueva I, Voravuthikunchai SP, Davies J. Enhancing antibiotic activity: a strategy to control Acinetobacter infections. J Antimicrob Chemother. 2009;64:1203–1211. doi: 10.1093/jac/dkp381. [DOI] [PubMed] [Google Scholar]
- Rosato A, Piarulli M, Corbo F, Muraglia M, Carone A, Vitali ME, Vitali C. In vitro synergistic antibacterial action of certain combinations of gentamicin and essential oils. Curr Med Chem. 2010;17:3289–3295. doi: 10.2174/092986710792231996. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. M02-A10-Performance Standards for Antimicrobial Disk Susceptibility Tests Approved Standard-Tenth Edition. Clinical and Laboratory Standards Institute Wayne, Pennsylvania, USA; 2009. [Google Scholar]
- Voravuthikunchai SP, Limsuwan S, Chusri S. In: Recent Progress in Medicinal Plants: Chronic and Common Diseases-IV. Govil GN, Singh VK, editor. Studium Press, Houstan, Texas, USA; 2006. New Perspectives on Herbal Medicines for Treating Bacterial Infections; pp. 41–101. [Google Scholar]
- Clinical and Laboratory Standards Institute. M07-A8-Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard-Eighth Edition. Clinical and Laboratory Standards Institute Wayne, Pennsylvania, USA; 2009. [Google Scholar]
- Kaur GJ, Arora DS. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med. 2009;9:30. doi: 10.1186/1472-6882-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamicker BJ, Sweeney MT, Kaczmarek F, Dib-Hajj F, Shang W, Crimin K, Duignan J, Gootz TD. Bacterial efflux pump inhibitors. Methods Mol Med. 2008;142:187–204. doi: 10.1007/978-1-59745-246-5_15. [DOI] [PubMed] [Google Scholar]
- Mohtar M, Johari SA, Li AR, Isa MM, Mustafa S, Ali AM, Basri DF. Inhibitory and resistance-modifying potential of plant-based alkaloids against methicillin-resistant Staphylococcus aureus (MRSA) Curr Microbiol. 2009;59:181–186. doi: 10.1007/s00284-009-9416-9. [DOI] [PubMed] [Google Scholar]
- Mirza ZM, Kumar A, Kalia NP, Zargar A, Khan IA. Piperine as an inhibitor of the MdeA efflux pump of Staphylococcus aureus. J Med Microbiol. 2011;60:1472–1478. doi: 10.1099/jmm.0.033167-0. [DOI] [PubMed] [Google Scholar]
- Nargotra A, Sharma S, Koul JL, Sangwan PL, Khan IA, Kumar A, Taneja SC, Koul S. Quantitative structure activity relationship (QSAR) of piperine analogs for bacterial NorA efflux pump inhibitors. Eur J Med Chem. 2009;44:4128–4135. doi: 10.1016/j.ejmech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kumar M, Sharma S, Nargotra A, Koul S, Khan IA. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother. 2010;65:1694–1701. doi: 10.1093/jac/dkq186. [DOI] [PubMed] [Google Scholar]
- Stavri M, Piddock LJV, Gibbons S. Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemother. 2007;59:1247–1260. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- Darwish RM, Aburjai TA. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med. 2010;10 doi: 10.1186/1472-6882-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadli M, Saad A, Sayadi S, Chevalier J, Mezrioui NE, Pagès JM, Hassani L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection - bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;15:464–471. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Fadli M, Chevalier J, Saad A, Mezrioui NE, Hassani L, Pages JM. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int J Antimicrob Agents. 2011;38:325–330. doi: 10.1016/j.ijantimicag.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- Coban AY, Tanriverdi Cayci Y, Erturan Z, Durupinar B. Effects of efflux pump inhibitors phenyl-arginine-beta-naphthylamide and 1-(1-naphthylmethyl)-piperazine on the antimicrobial susceptibility of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J Chemother. 2009;21:592–594. doi: 10.1179/joc.2009.21.5.592. [DOI] [PubMed] [Google Scholar]
- Peleg AY, Adams J, Paterson DL. Tigecycline efflux as a mechanism for nonsusceptibility in Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:2065–2069. doi: 10.1128/AAC.01198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Brantner AH. Antibacterial steroid alkaloids from the stem bark of Holarrhena pubescens. J Ethnopharmacol. 1999;15:339–344. doi: 10.1016/s0378-8741(99)00119-1. [DOI] [PubMed] [Google Scholar]
- Kavitha D, Shilpa PN, Devaraj SN. Antibacterial and antidiarrhoeal effects of alkaloids of Holarrhena antidysenterica WALL. Indian J Exp Biol. 2004;42:589–594. [PubMed] [Google Scholar]
- Kavitha D, Niranjali S. Inhibition of enteropathogenic Escherichia coli adhesion on host epithelial cells by Holarrhena antidysenterica (L.) WALL. Phytother Res. 2009;23:1229–1236. doi: 10.1002/ptr.2520. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ali M. A new steroidal alkaloid from the seeds of Holarrhena antidysenterica. Fitoterapia. 2000;71:101–104. doi: 10.1016/s0367-326x(99)00111-2. [DOI] [PubMed] [Google Scholar]
- Siddiqui BS, Usmani SB, Ali ST, Begum S, Rizwani GH. Further constituents from the bark of Holarrhena pubescens. Phytochemistry. 2001;58:1199–1204. doi: 10.1016/s0031-9422(01)00330-2. [DOI] [PubMed] [Google Scholar]
- Tuntiwachwuttikul P, Pootaeng-on Y, Phansa P, Limpachayaporn P, Charoenchai P, Taylor WC. Constituents of the leaves of Holarrhena pubescens. Fitoterapia. 2007;78:271–273. doi: 10.1016/j.fitote.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Alakomi HL, Paananen A, Suihko ML, Helander IM, Saarela M. Weakening effect of cell permeabilizers on gram-negative bacteria causing biodeterioration. Appl Environ Microbiol. 2006;72:4695–4703. doi: 10.1128/AEM.00142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H, Chen T, Riffon R, Wang R, Wang Z. Synergy between polyethylenimine and different families of antibiotics against a resistant clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:1635–1641. doi: 10.1128/AAC.01071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakomi HL, Puupponen-Pimia R, Aura AM, Helander IM, Nohynek L, Oksman-Caldentey KM, Saarela M. Weakening of salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J Agric Food Chem. 2007;55:3905–3912. doi: 10.1021/jf070190y. [DOI] [PubMed] [Google Scholar]
- Damier-Piolle L, Magnet S, Bremont S, Lambert T, Courvalin P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother. 2008;52:557–562. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage PB. Multidrug-resistant bacteria: overcoming antibiotic permeability barriers of gram-negative bacteria. Ann Med. 2001;33:167–171. doi: 10.3109/07853890109002073. [DOI] [PubMed] [Google Scholar]