Abstract
Background and Objectives
Studies investigating the association between glutathione S-transferase M1 (GSTM1) gene polymorphism and laryngeal cancer risk have reported conflicting results. The aim of the present study was to conduct a meta-analysis assessing the possible associations of GSTM1 gene polymorphism with laryngeal cancer risk.
Methods
The relevant studies were identified through a search of PubMed, Embase, ISI Web of Knowledge and Chinese National Knowledge Infrastructure until May 2011 and selected on the basis of the established inclusion criteria for publications, then a meta-analysis was performed to quantitatively summarize association of GSTM1 polymorphism with laryngeal cancer susceptibility.
Results
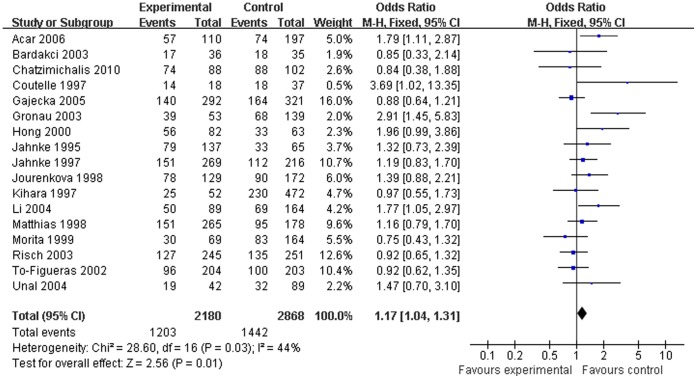
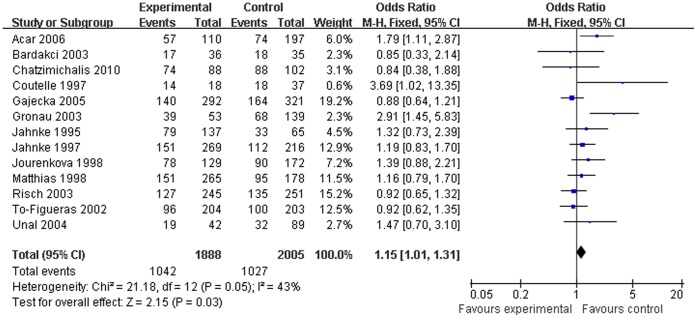
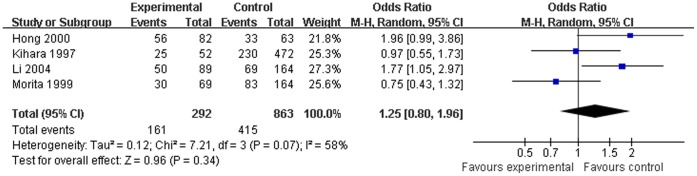
Seventeen studies were included in the present meta-analysis (2,180 cases and 2,868 controls). The combined results based on all studies showed that GSTM1 null genotype was associated with increased laryngeal cancer risk (OR = 1.17, 95% CI = 1.04∼1.31). When stratifying for race, GSTM1 null genotype exhibited increased laryngeal cancer risk in Caucasians (OR = 1.15, 95% CI = 1.01∼1.31), while no significant association was detected in Asians (OR = 1.25, 95% CI = 0.80∼1.96). In the subgroup analysis based on source of controls, significant associations were observed in the population-based studies (OR = 1.15, 95% CI = 1.01∼1.31) yet not in the hospital-based studies (OR = 1.25, 95% CI = 0.93∼1.67). Furthermore, in the subgroup analysis based on sample size, significant associations were also found in studies with at least 50 cases and 50 controls (OR = 1.15, 95% CI = 1.02∼1.30) but not in studies with fewer than 50 cases or 50 controls (OR = 1.46, 95% CI = 0.87∼2.46).
Conclusions
This meta-analysis supported that the GSTM1 gene polymorphism was associated with laryngeal cancer, particularly in Caucasians, and these associations varied in different subgroup, which indicated that population-based study with larger sample size was more appropriate in design of future study.
Introduction
Squamous cell carcinoma (SCC) of larynx is the most frequent malignancy in the head and neck region, the risk of which results from complex interactions between many genetic and environmental factors [1]. Numerous epidemiological studies indicate that tobacco smoking and alcohol consumption play a critical role in the development of the disease [2]. Despite many individuals have been exposed to these exogenous risk factors, laryngeal SCC does not develop in all exposed people, which is suggested that susceptibility to cancer might be due to genetic polymorphisms in certain genes that cause differences in the metabolism of carcinogens [3]. Accumulating evidence indicates that genetic polymorphisms have also been extensively investigated to identify inherited genetic risk for laryngeal SCC [4].
Human glutathione S-transferases (GSTs), which can be divided into classes of alpha, mu, pi, sigma and theta on the basis of chromosomal location and sequence homology, are a family of cytosolic enzymes that play an important role in the detoxification of potential carcinogens [5]. Among GSTs gene family, GSTM1 gene located on the chromosome 1p13.3, participates in the deactivation of carcinogenic intermediates of polycyclic aromatic hydrocarbons present in tobacco [6], which has been found polymorphic in the population, that is, “present” or “null” genotype may characterize an individual. Interindividual variation in the GSTM1 genotypes has been investigated in relation to various types of cancers [7].
Many studies have evaluated the potential role of GSTM1 null genotype in the development of laryngeal SCC. The first study conducted by Jahnke et al. suggested that the GSTM1 null genotype was not associated with a risk to develop laryngeal cancer [8]. Conversely, the study conducted by Coutelle et al. demonstrated that the risk of laryngeal SCC might be associated with the GSTM1 null genotype [9]. A series of related studies were carried out later, however, results were generally inconsistent and inconclusive. Concerning the GSTM1 null genotype in laryngeal cancer, two meta-analyses have been published before 2009, nevertheless, they had not reached unanimity in their conclusions [10], [11]. The earlier meta-analysis by Hashibe et al. did not point to any association of GSTM1 gene polymorphisms with laryngeal cancer susceptibility [10], whereas the most recent study by Zhuo et al. supported that GSTM1 deficiency was associated with laryngeal cancer risk [11]. Careful examination of the meta-analysis by Zhuo et al. revealed that three otherwise eligible case-control studies had not been taken into account, on the other hand, only one subgroup analysis on ethnicity was performed, without concerning others such as source of controls and sample size. Therefore, we conducted this updated meta-analysis that might increase statistical power to address the possible associations of GSTM1 gene polymorphism with laryngeal cancer risk.
Materials and Methods
Literature Search Strategy
The meta-analysis process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12] (Checklist S1). Eligible studies published up to May 2011, were identified by a search of PubMed, Embase, ISI Web of Knowledge, and Chinese National Knowledge Infrastructure, with a combination of the following keywords: “Glutathione S-transferase M1,” “GSTM1,” “polymorphism,” “larynx,” and “cancer.” The search was done without restriction on language, and was conducted on human subject. All searched papers were retrieved, and their references were checked as well for other relevant publications. Review articles were also searched to find additional eligible studies. For papers on the same population or with overlapping data, only the most recent or the ones with the largest group of subject data-set were included in this analysis. To identify potentially eligible papers, the title and abstract of each paper identified by the literature search were assessed independently by two authors. Disagreements were resolved by discussion.
Inclusion and Exclusion Criteria
All papers involving studies that investigated GSTM1 polymorphism and laryngeal cancer risk were included. The following inclusion criteria were used for the paper selection: 1) The association of laryngeal cancer with GSTM1 polymorphisms was clearly evaluated; 2) Only the case-control studies were considered; 3) The paper described the diagnoses of laryngeal cancer and the sources of cases and controls; 4) The polymorphism was determined by polymerase chain reaction (PCR)-based method, using the peripheral blood sample; 5) Enough information about the number of laryngeal cancer cases and controls studied with the different GSTM1 genotypes were offered. Accordingly, the following exclusion criteria were also used: 1) Study design other than case-control method; 2) Not offering the source of cases and controls and other essential information; 3) Reviews and repeated literature were also excluded.
Data Extraction
From each of the eligible papers, the following data were extracted: first author’s surname, publication year, country of origin, ethnicity, source of controls, genotyping methods, and numbers of different genotypes of cases and controls, respectively. Data were extracted independently by the same authors previously mentioned, and consensuses were reached on all items. Discrepancies were resolved by discussion.
Statistical Analysis
The strength of the associations between laryngeal cancer and the GSTM1 polymorphism was estimated by odds ratio (OR) and 95% confidence interval (CI), based on the genotype frequencies in cases and controls. To calculate the pooled OR, the fixed-effect model and the random-effect model were used. The significance of the pooled OR was determined by the Z-test and P<0.05 was considered as statistically significant. Heterogeneity was tested by the I2 statistic [13]. The analyses were also conducted on the subgroups of studies based on ethnicity (Caucasian versus Asian), source of controls (population versus hospital based) and sample size (number of cases and controls ≥50 versus number of cases or controls <50). Visual inspection of asymmetry in funnel plots was used to estimate the potential publication bias [14]. In order to supplement the funnel plot, Begg’s funnel plots [15] and Egger’s regression method [16] were performed (P<0.05 was considered representative of statistically significant publication bias). Sensitivity analysis was conducted by removing each individual study in turn from the total and re-analyzing the remainder. Statistical analysis was done using the program Review Manager 5.1.2 (2011, The Cochrane Collaboration) and the statistical software Stata10.1 (Stata Corporation, College Station, TX).
Results
Study Characteristics
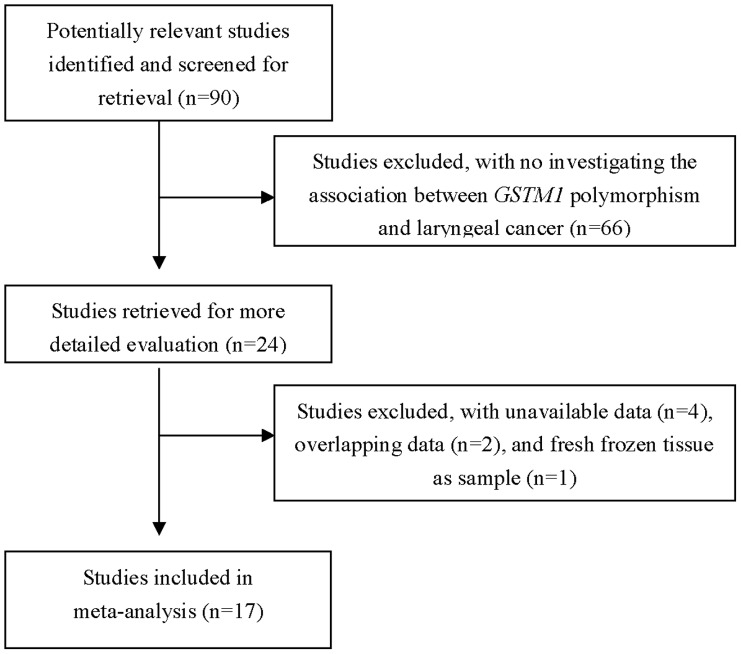
The literature search identified 90 related articles through PubMed, Embase, ISI Web of Knowledge, and Chinese National Knowledge Infrastructure. With the step of screening the title or abstract, 24 eligible studies were selected (Figure 1). Of the 24 articles selected, 4 studies were excluded because of the lack of data about GSTM1 status of laryngeal cancer [4], [17], [18], [19], 2 studies [20], [21] were excluded by reason of their data overlapped with that of other two studies respectively [22], [23], study by Hanna et al. [24]was excluded because polymorphism was determined in fresh frozen tissue specimens. Finally, 17 studies including 2,180 laryngeal SCC cases and 2,868 controls [8], [9], [22], [23], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], were included in this meta-analysis based on inclusion and exclusion criteria (Table 1). Thirteen of the studies were conducted in Caucasians, and four in Asians. Population-based controls were used in 15 studies and hospital-based controls were used in 2 studies. There were 14 studies with larger sample size (number of cases and controls ≥50) and 3 studies with small sample size (number of cases or controls <50). Almost all of the cases were pathologically confirmed.
Figure 1. Flow chart of study selection.
Table 1. Characteristics of all studies included in the meta-analysis.
| Author (Year) | Ethnicity (Country) | Control source | Genotyping method | Cases | Controls | ||
| Null | Present | Null | Present | ||||
| Acar 2006 | Caucasian (Turkey) | PB | multiplex PCR | 57 | 53 | 74 | 123 |
| Bardakci 2003 | Caucasian (Turkey) | PB | multiplex PCR | 17 | 19 | 18 | 17 |
| Chatzimichalis 2010 | Caucasian (Greece) | PB | multiplex PCR | 74 | 14 | 88 | 14 |
| Coutelle 1997 | Caucasian (France) | PB | PCR | 14 | 4 | 18 | 19 |
| Gajecka 2005 | Caucasian (Poland) | PB | multiplex PCR | 140 | 152 | 164 | 157 |
| Gronau 2003 | Caucasian (Germany) | PB | multiplex PCR | 39 | 14 | 68 | 71 |
| Hong 2000 | Asian (Korea) | PB | multiplex PCR | 56 | 26 | 33 | 30 |
| Jahnke 1995 | Caucasian (Germany) | PB | PCR | 79 | 58 | 33 | 32 |
| Jahnke 1997 | Caucasian (Germany) | PB | PCR | 151 | 118 | 112 | 104 |
| Jourenkova 1998 | Caucasian (France) | HB | multiplex PCR | 78 | 51 | 90 | 82 |
| Kihara 1997 | Asian (Japan) | PB | multiplex PCR | 25 | 27 | 230 | 242 |
| Li 2004 | Asian (China) | PB | multiplex PCR | 50 | 39 | 69 | 95 |
| Matthias 1998 | Caucasian (Germany) | HB | multiplex PCR | 151 | 114 | 95 | 83 |
| Morita 1999 | Asian (Japan) | PB | PCR | 30 | 39 | 83 | 81 |
| Risch 2003 | Caucasian (Germany) | PB | multiplex PCR | 127 | 118 | 135 | 116 |
| To-Figueras 2002 | Caucasian (Spain) | PB | multiplex PCR | 96 | 108 | 100 | 103 |
| Unal 2004 | Caucasian (Turkey) | PB | PCR | 19 | 23 | 32 | 57 |
Abbreviations: PB, population-based study; HB, hospital-based study; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism.
Test of Heterogeneity
No heterogeneity was observed in the analysis of hospital-based study (I2 = 0). Moderate heterogeneity was detected in the analysis of Caucasian population (I2 = 43%), population-based study (I2 = 50%), the studies stratified by sample size (large-sample study, I2 = 47%; small-sample study, I2 = 40%) and the overall studies (I2 = 44%). High heterogeneity was found in the analysis of Asian population (I2 = 58%). A random-effect model was used in the analysis of Asian population, and fixed-effect models were selected in other analyses.
Quantitative Data Synthesis
Both the overall analysis and the subgroup analysis based on ethnicity, source of controls, and sample size were performed (Table 2). The combined results based on all studies showed that the GSTM1 null genotype was associated with increased laryngeal cancer risk (OR = 1.17, 95% CI = 1.04∼1.31, Figure 2). When stratifying for race, the pooled ORs for GSTM1 null genotype were 1.15 (95% CI = 1.01∼1.31, Figure 3) in Caucasians and 1.25 (95% CI = 0.80∼1.96, Figure 4) in Asians, which indicated that the significant association confined to Caucasians but not Asians. In the subgroup analysis based on source of controls, significant associations were observed in the population-based studies (OR = 1.15, 95% CI = 1.01∼1.31) yet not in the hospital-based studies (OR = 1.25, 95% CI = 0.93∼1.67). Furthermore, significant associations were also found in studies with large sample size (OR = 1.15, 95% CI = 1.02∼1.30) but not in studies with small sample size (OR = 1.46, 95% CI = 0.87∼2.46).
Table 2. Overall and subgroup analysis of GSTM1 and laryngeal cancer risk.
| Meta-analysis groups | Number of studies | Number of Cases/controls | I 2(%) | OR (95% CI ) | Z test Pvalue | Begg testP value | Egger test P value |
| Overall analysis | 17 | 2180/2868 | 44 | 1.17(1.04, 1.31) | 0.01 | 0.11 | 0.06 |
| Ethnicity | |||||||
| Caucasian | 13 | 1888/2005 | 43 | 1.15(1.01, 1.31) | 0.03 | 0.08 | 0.07 |
| Asian | 4 | 292/863 | 58 | 1.25(0.80, 1.96) | 0.34 | 1.00 | 0.81 |
| Source of controls | |||||||
| Population | 15 | 1786/2518 | 50 | 1.15 (1.01, 1.31) | 0.03 | 0.11 | 0.07 |
| Hospital | 2 | 394/350 | 0 | 1.25(0.93, 1.67) | 0.14 | 1.00 | – |
| Sample size | |||||||
| ≥50 cases and ≥50 controls | 14 | 2084/2707 | 47 | 1.15 (1.02, 1.30) | 0.02 | 0.10 | 0.09 |
| <50 cases or <50 controls | 3 | 96/161 | 40 | 1.46 (0.87, 2.46) | 0.15 | 1.00 | 0.61 |
Figure 2. Overall association between GSTM1 null genotype and laryngeal cancer risk for all subjects (fixed effects).
Figure 3. Association between GSTM1 null genotype and laryngeal cancer risk for Caucasian subjects (fixed-effects).
Figure 4. Association between GSTM1 null genotype and laryngeal cancer risk for Asian subjects (random-effects).
Sensitivity Analysis and Publication Bias
A single study involved in the meta-analysis was deleted each time to reflect the influence of the individual data-set to the pooled ORs, and the corresponding pooled ORs were not materially altered (data not shown), indicating that our results were statistically robust. In order to compare the difference and evaluate the sensitivity of the meta-analyses, we also reported the results of the random effects model, the combined OR was 1.21 (95% CI = 1.03∼1.44), similar to the results of the fixed effects model.
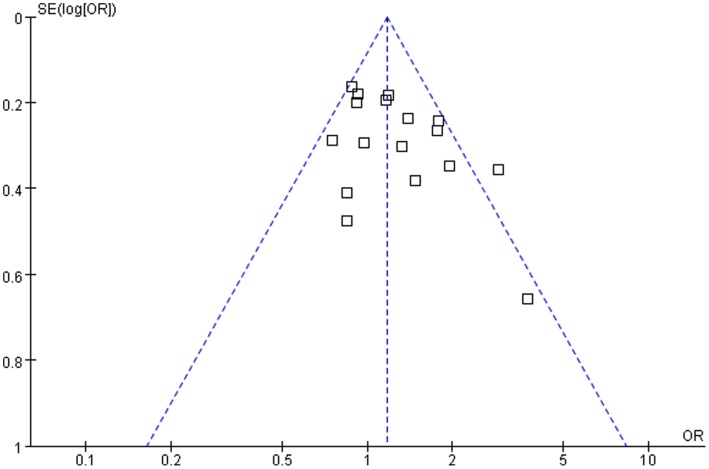
Funnel plots were created to assess the possible publication biases, the shape of which did not reveal any evidence of obvious asymmetry (Figure 5). Begg’s funnel plot indicated no evidence for funnel plot asymmetry in either overall analysis (P = 0.11) or subgroup analyses. Egger’s test also suggested the absence of publication bias in either overall analysis (P = 0.06) or subgroup analyses.
Figure 5. Funnel plot of association between GSTM1 polymorphism and laryngeal cancer risk.
Discussion
A series of studies have indicated that GSTM1 gene polymorphism may contribute to risk of laryngeal SCC. However, the results of these studies assessing the association between laryngeal SCC susceptibility and GSTM1 genotype have been contradictory. Hence, we undertook this meta-analysis, which supported that GSTM1 gene polymorphism was associated with laryngeal cancer and suggested that GSTM1 null genotype had an effect on the risk of developing laryngeal cancer among Caucasians.
As one of the most important phase II enzymes, GSTM1 was known to abolish enzyme activities and therefore has been linked with an increase in a number of cancers, most likely due to increased susceptibilities to environmental toxins and carcinogens. Previous meta-analyses suggest that GSTM1 null genotypes might have a correlation with increased susceptibilities to breast cancer [38] and lung cancer in Chinese people [7], [39]. In contrast, a number of meta-analyses indicate no marked associations of GSTM1 gene polymorphism with hepatocellular carcinoma [40], gastric cancer [41], esophageal cancer [42] and prostate cancer [43]. The present meta-analysis, which included 2,180 cases of laryngeal SCC and 2,868 controls, maintained that there was association between GSTM1 gene polymorphism and laryngeal SCC susceptibility. The combined results based on all studies showed that GSTM1 null genotype was associated with increased laryngeal cancer risk (OR = 1.17, 95% CI = 1.04∼1.31). Considering the variation of ethnic populations, we further utilized subgroup analysis with respect to ethnicity. When stratifying for race, GSTM1 null genotype exhibited increased laryngeal cancer risk in Caucasian populations (OR = 1.15, 95% CI = 1.01∼1.31), lack of significant association was detected in Asian populations (OR = 1.25, 95% CI = 0.80∼1.96), suggesting a possible role of ethnic differences in genetic backgrounds and the environment they live in. On the other hand, it is likely that the observed ethnic differences may be due to chance because small number of studies among Asians may lead to insufficient statistical power to detect a slight effect.
The subgroup analysis based on source of controls also suggested that the GSTM1 null genotype was associated with laryngeal cancer risk in the population-based studies, which supported the association between GSTM1 polymorphism and laryngeal cancer risk. However, in the hospital-based studies, no significant association was detected. Since it is conceivable that such controls may just represent a sample of ill-defined reference population, and may not be representative of the general population very well, particularly when the genotypes under investigation were associated with the disease conditions that the hospital-based controls may have. In the subgroup analysis by sample size, the pooled OR in studies with larger sample size (number of cases and controls ≥50) was approximately the same in all the studies, which indicated that larger sample size with adequate power was one of the important factors in the design of case-control studies. The use of population-based studies with larger sample size is, therefore, more appropriate in such genetic association studies. It was worth mentioning that power was an issue as limited numbers were in the Asian, small study, and hospital-based groups, therefore, the case-control data were not enough to strongly support our meta-analysis result in these subgroups, which must be interpreted with caution.
Several limitations of this study should be addressed. First, the sample size was still relatively small for some stratified analyses. Second, only published studies were included in the meta-analysis, therefore, publication bias may have occurred, even though the use of a statistical test did not show it. Third, we were unable to obtain information from most studies on the presence or absence of a history of smoking, because of lack of the investigation of gene-environment interactions. Finally, our meta-analysis was based on unadjusted estimates, whereas a more precise analysis could be performed if individual data were available and would allow for an adjustment estimate. Despite the limitation, our meta-analysis significantly increased the statistical power of the analysis based on substantial number of cases and controls from different studies.
In conclusion, this meta-analysis supported that the GSTM1 gene polymorphism was associated with laryngeal cancer, particularly in Caucasians. As studies among other ethnic populations are currently limited, it is of great essentiality to conduct large-sample studies with wider spectrum of subjects to investigate the relationship between GSTM1 gene polymorphism and laryngeal SCC risk, which would greatly help summarize the results from published papers.
Supporting Information
(DOC)
Acknowledgments
We are indebted to the authors of the primary studies.
Funding Statement
The authors have no support or funding to report.
References
- 1. Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, et al. (2007) Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in central europe. American Journal of Epidemiology 165: 814–820. [DOI] [PubMed] [Google Scholar]
- 2. Koskinen WJ, Brondbo K, Dahlstrand HM, Luostarinen T, Hakulinen T, et al. (2007) Alcohol, smoking and human papillomavirus in laryngeal carcinoma: a Nordic prospective multicenter study. Journal of Cancer Research and Clinical Oncology 133: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frank SA (2004) Genetic predisposition to cancer - Insights from population genetics. Nature Reviews Genetics 5: 764–772. [DOI] [PubMed] [Google Scholar]
- 4. Boccia S, Cadoni G, Sayed-Tabatabaei FA, Volante M, Arzani D, et al. (2008) CYP1A1, CYP2E1, GSTM1, GSTT1, EPHX1 exons 3 and 4, and NAT2 polymorphisms, smoking, consumption of alcohol and fruit and vegetables and risk of head and neck cancer. Journal of Cancer Research and Clinical Oncology 134: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cotton SC, Sharp L, Little J, Brockton N (2000) Glutathione S-transferase polymorphisms and colorectal cancer: A HuGE review. American Journal of Epidemiology 151: 7–32. [DOI] [PubMed] [Google Scholar]
- 6. Bolt HM, Thier R (2006) Relevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicology. Current drug metabolism 7: 613–628. [DOI] [PubMed] [Google Scholar]
- 7. Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JPT (2008) Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: A literature-based systematic HuGE review and meta-analysis. American Journal of Epidemiology 167: 759–774. [DOI] [PubMed] [Google Scholar]
- 8. Jahnke V (1995) Studies on glutathione S-transferase GSTM1 and GSTT1 genotypes and susceptibility to laryngeal cancer. Laryngo-, Rhino-, Otologie 74: 691–694. [DOI] [PubMed] [Google Scholar]
- 9. Coutelle C, Ward PJ, Fleury B, Quattrocchi P, Chambrin H, et al. (1997) Laryngeal and oropharyngeal cancer, and alcohol dehydrogenase 3 and glutathione S-transferase M1 polymorphisms. Human Genetics 99: 319–325. [DOI] [PubMed] [Google Scholar]
- 10. Hashibe M, Brennan P, Strange RC, Bhisey R, Cascorbi I, et al. (2003) Meta- and pooled analyses of GSTM1, GSTT1, GSTP1, anti CYP1A1 genotypes and risk of head and neck cancer. Cancer Epidemiology Biomarkers & Prevention 12: 1509–1517. [PubMed] [Google Scholar]
- 11. Zhuo W-L, Wang Y, Zhuo X-L, Zhu B, Zhu Y, et al. (2009) Polymorphisms of CYP1A1 and GSTM1 and laryngeal cancer risk: evidence-based meta-analyses. Journal of Cancer Research and Clinical Oncology 135: 1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS medicine 6. [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. British Medical Journal 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennett DA, Latham NK, Stretton C, Anderson CS (2004) Capture-recapture is a potentially useful method for assessing publication bias. Journal of Clinical Epidemiology 57: 349–357. [DOI] [PubMed] [Google Scholar]
- 15. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 16. Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peters ES, McClean MD, Marsit CJ, Luckett B, Kelsey KT (2006) Glutathione S-transferase polymorphisms and the synergy of alcohol and tobacco in oral, pharyngeal, and laryngeal carcinoma. Cancer Epidemiology Biomarkers & Prevention 15: 2196–2202. [DOI] [PubMed] [Google Scholar]
- 18. Cabelguenne A, Loriot MA, Stucker I, Blons H, Koum-Besson E, et al. (2001) Glutathione-associated enzymes in head and neck squamous cell carcinoma and response to cisplatin-based neoadjuvant chemotherapy. International Journal of Cancer 93: 725–730. [DOI] [PubMed] [Google Scholar]
- 19. Olshan AF, Weissler MC, Watson MA, Bell DA (2000) GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiology Biomarkers & Prevention 9: 185–191. [PubMed] [Google Scholar]
- 20. Jahnke V, Matthias C, Fryer A, Strange R (1996) Glutathione S-transferase and cytochrome P-450 polymorphism as risk factors for squamous cell carcinoma of the larynx. American Journal of Surgery 172: 671–673. [DOI] [PubMed] [Google Scholar]
- 21. Becher H, Ramroth H, Ahrens W, Risch A, Schmezer P, et al. (2005) Occupation, exposure to polycyclic aromatic hydrocarbons and laryngeal cancer risk. International Journal of Cancer 116: 451–457. [DOI] [PubMed] [Google Scholar]
- 22. Jahnke V, Strange R, Matthias C, Fryer A (1997) Glutathione S-transferase and cytochrome P450 genotypes as risk factors for laryngeal carcinoma. European Archives of Oto-Rhino-Laryngology 254: S147–S149. [DOI] [PubMed] [Google Scholar]
- 23. Risch A, Ramroth H, Raedts V, Rajaee-Behbahani N, Schmezer P, et al. (2003) Laryngeal cancer risk in Caucasians is associated with alcohol and tobacco consumption but not modified by genetic polymorphisms in class I alcohol dehydrogenases ADH1B and ADH1C, and glutathione-S-transferases GSTM1 and GSTT1. Pharmacogenetics 13: 225–230. [DOI] [PubMed] [Google Scholar]
- 24. Hanna E, MacLeod S, Vural E, Lang N (2001) Genetic deletions of glutathione-S-transferase as a risk factor in squamous cell carcinoma of the larynx: A preliminary report. American Journal of Otolaryngology 22: 121–123. [DOI] [PubMed] [Google Scholar]
- 25. Gajecka M, Rydzanicz M, Jaskula-Sztul R, Kujawski M, Szyfter W, et al. (2005) CYP1A1, CYP2D6, CYP2E1, NAT2, GSTM1 and GSTT1 polymorphisms or their combinations are associated with the increased risk of the laryngeal squamous cell carcinoma. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis 574: 112–123. [DOI] [PubMed] [Google Scholar]
- 26. Gronau S, Koenig-Greger D, Jerg M, Riechelmann H (2003) Gene polymorphisms in detoxification enzymes as susceptibility factor for head and neck cancer? Otolaryngology-Head and Neck Surgery 128: 674–680. [DOI] [PubMed] [Google Scholar]
- 27. Morita S, Yano M, Tsujinaka T, Akiyama Y, Taniguchi M, et al. (1999) Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to head-and-neck squamous-cell carcinoma. International Journal of Cancer 80: 685–688. [DOI] [PubMed] [Google Scholar]
- 28. Unal M, Tamer L, Ates NA, Akbas Y, Pata YS, et al. (2004) Glutathione S-transferase M1, T1, and P1 gene polymorphism in laryngeal squamous cell carcinoma. American Journal of Otolaryngology 25: 318–322. [DOI] [PubMed] [Google Scholar]
- 29. Kihara M, Kubota A, Furukawa M, Kimura H (1997) GSTM1 gene polymorphism as a possible marker for susceptibility to head and neck cancers among Japanese smokers. Cancer Letters 112: 257–262. [DOI] [PubMed] [Google Scholar]
- 30. Chatzimichalis M, Xenellis J, Tzagaroulakis A, Sarof P, Banis K, et al. (2010) GSTT1, GSTM1, GSTM3 and NAT2 polymorphisms in laryngeal squamous cell carcinoma in a Greek population. Journal of Laryngology and Otology 124: 318–323. [DOI] [PubMed] [Google Scholar]
- 31. Hong YJ, Lee JK, Lee GH, Hong SI (2000) Influence of glutathione S-transferase M1 and T1 genotypes on larynx cancer risk among Korean smokers. Clinical Chemistry and Laboratory Medicine 38: 917–919. [DOI] [PubMed] [Google Scholar]
- 32. Jourenkova N, Reinikainen M, Bouchardy C, Dayer P, Benhamou S, et al. (1998) Larynx cancer risk in relation to glutathione S-transferase M1 and T1 genotypes and tobacco smoking. Cancer Epidemiology Biomarkers & Prevention 7: 19–23. [PubMed] [Google Scholar]
- 33. To-Figueras J, Gene M, Gomez-Catalan J, Pique E, Borrego N, et al. (2002) Microsomal epoxide hydrolase and glutathione S-transferase polymorphisms in relation to laryngeal carcinoma risk. Cancer Letters 187: 95–101. [DOI] [PubMed] [Google Scholar]
- 34. Matthias C, Bockmuhl U, Jahnke V, Jones PW, Hayes JD, et al. (1998) Polymorphism in cytochrome P450 CYP2D6, CYP1A1, CYP2E1 and glutathione S-transferase, GSTM1, GSTM3, GSTT1 and susceptibility to tobacco-related cancers: studies in upper aerodigestive tract cancers. Pharmacogenetics 8: 91–100. [PubMed] [Google Scholar]
- 35. Acar H, Ozturk K, Muslumanoglu MH, Yildirim MS, Cora T, et al. (2006) Relation of glutathione S-transferase genotypes (GSTM1 and GSTT1) to laryngeal squamous cell carcinoma risk. Cancer Genetics and Cytogenetics 169: 89–93. [DOI] [PubMed] [Google Scholar]
- 36. Li L, Lin P, Deng Y-f, Zhu Z-l, Lu H-h (2004) Relationship between susceptibility and prognosis of laryngeal cancer and genetic polymorphisms in CYP1A1 and GSTM1. Zhonghua Er Bi Yan Hou Ke Za Zhi 39: 2–7. [PubMed] [Google Scholar]
- 37. Bardakci F, Canbay E, Degerli N, Coban L (2003) Relationship of tobacco smoking with GSTM1 gene polymorphism in laringeal cancer. Journal of Cellular and Molecular Medicine 7: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sull JW, Ohrr H, Kang DR, Nam CM (2004) Glutathione S-transferase M1 status and breast cancer risk: A meta-analysis. Yonsei Medical Journal 45: 683–689. [DOI] [PubMed] [Google Scholar]
- 39. Shi XQ, Zhou SH, Wang ZX, Zhou ZC, Wang ZZ (2008) CYP1A1 and GSTM1 polymorphisms and lung cancer risk in Chinese populations: A meta-analysis. Lung Cancer 59: 155–163. [DOI] [PubMed] [Google Scholar]
- 40. White DL, Li D, Nurgalieva Z, El-Serag HB (2008) Genetic variants of glutathione S-transferase as possible risk factors for hepatocellular carcinoma: A HuGE systematic review and meta-analysis. American Journal of Epidemiology 167: 377–389. [DOI] [PubMed] [Google Scholar]
- 41. La Torre G, Boccia S, Ricciardi G (2005) Glutathione S-transferase M1 status and gastric cancer risk: a meta-analysis. Cancer Letters 217: 53–60. [DOI] [PubMed] [Google Scholar]
- 42. Yang C-X, Matsuo K, Wang Z-M, Tajima K (2005) Phase I/II enzyme gene polymorphisms and esophageal cancer risk: a meta-analysis of the literature. World J Gastroenterol 11: 2531–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ntais C, Polycarpou A, Ioannidis JPA (2005) Association of GSTM1, GSTT1, and GSTP1 gene polymorphisms with the risk of prostate cancer: A meta-analysis. Cancer Epidemiology Biomarkers & Prevention 14: 176–181. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)