Abstract
Aims
The role of sonic hedgehog (SHH) in epithelial mesenchymal transition (EMT) of pancreatic cancer (PC) is known, however, its mechanism is unclear. Because SHH promotes tumor development predominantly through Gli1, we sought to understand its mechanism by identifying Gli1 targets in pancreatic cancer cells.
Methods
First, we investigated invasion, migration, and EMT in PC cells transfected with lentiviral Gli1 interference vectors or SHH over-expression vectors in vitro and in vivo. Next, we determined the target gene profiles of Gli1 in PC cells using cDNA microarray assays. Finally, the primary regulatory networks downstream of SHH-Gli1 signaling in PC cells were studied through functional analyses of these targets.
Results
Our results indicate there is decreased E-cadherin expression upon increased expression of SHH/Gli1. Migration of PC cells increased significantly in a dose-dependent manner within 24 hours of Gli1 expression (P<0.05). The ratio of liver metastasis and intrasplenic miniature metastasis increased markedly upon activation of SHH-Gli1 signals in nude mice. Using cDNA microarray, we identified 278 upregulated and 59 downregulated genes upon Gli1 expression in AsPC-1 cells. The data indicate that SHH-Gli1 signals promote EMT by mediating a complex signaling network including TGFβ, Ras, Wnt, growth factors, PI3K/AKT, integrins, transmembrane 4 superfamily (TM4SF), and S100A4.
Conclusion
Our results suggest that targeting the molecular connections established between SHH-Gli1 signaling and EMT could provide effective therapies for PC.
Introduction
Sonic hedgehog (SHH) is involved in embryonic organogenesis as a morphogen. Inappropriate activation of SHH signals during pancreas formation results in agenesis and several pancreatic diseases [1]. SHH is excluded from the developing pancreas as well as the mature organ, but is upregulated in chronic pancreatitis, early pancreatic intraepithelial neoplasia (PanIN) lesions, and invasive pancreatic cancer (PC) [2]. Aberrant SHH upregulation was reported in subtotal human PC cells and might be a primary critical mediator of PC development [3].
The Hedgehog (HH) signaling pathway is closely related to tumor metastasis and prognosis in clinical studies and is required for PC tumor metastasis in orthotopic mouse models [3], [4]. Recently, this pathway was thought to orchestrate the reprogramming of cancer cells via epithelial mesenchymal transition (EMT). Interestingly, recent evidence found that SHH was significantly upregulated in gemcitabine-resistant PC cells that simultaneously express cancer stem cell (CSCs) markers [5]. Because the SHH-induced target gene products could contribute to the self-renewal, survival, and migration of cancer progenitor cells and Gli1 may play a crucial role in the malignant behavior of PC cells [6], [7], identifying Gli1 targets is a logical step to understand its mechanism in PC cells.
The goal of this study was to provide a framework for the primary regulatory networks downstream of SHH-Gli1 signaling in PC cells. We also sought to determine if specific Gli1 target genes connect SHH-Gli1 signaling and EMT, thus providing a therapeutic strategy for PC.
Materials and methods
Cell culture
The PC cell lines (BxPC3, AsPC-1, and Panc-1 were all saved by the Chinese Academy of Sciences.) were cultured in RPMI-1640 supplemented with 10% fetal calf serum (FCS). All cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air.
Vector construction and cell infection
Lentiviral transfer vectors for human Gli1 shRNA or SHH cDNA were constructed by Genechem Co., Ltd, Shanghai, China. This system includes the lentiviral vector pLVTHM, the envelope plasmid pMD2G, and the packaging plasmids pRsv-REV and pMDlg-pRRE. The lentivirus-SHH (L-SHH) contains a 3.3-kb SHH coding sequence and the lentivirus-Gli1i (L-Gli1i) contains small hairpin Gli1 RNA to the targeting sequence of the shRNA, as previously described (5′-CTCCACAGGCATACAGGAT-3′) [8]. The lentivirus-control (L-C) did not include Gli1 interference sequences or SHH cDNA sequences and served as control. Lentiviral constructs were verified by DNA sequencing. Recombinant lentivirus was produced by transiently transfecting 293T cells following a standard protocol. When BxPC3, AsPC-1, and Panc-1 cells were approximately 50% confluent (in RPMI-1640 containing 2% FCS), they were infected with the lentiviral constructs at MOI of 5. Cells were harvested after 72 hours for further experiments. To identify functional L-SHH and L-Gli1i constructs, we routinely analyzed SHH and Gli1 expression by qRT-PCR.
RNA extraction and real time RT-PCR assays
Total RNA was extracted with Trizol reagent (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer's protocol. Total RNA (100 ng) was reverse transcribed in 20 μl volume and 2 μl cDNA was used for PCR, according to the manufacturer's instructions. (TaKaRa Biotechnology, Dalian, China). The primer sequences are shown in Table 1. CT (cycle threshold) values were standardized to CT values of GAPDH.
Table 1. The primer sequences for real time RT-PCR assays.
| Gene | Primer Sequences | Annealing Temperature (°C) | Size (bp) |
| Gli1 | F: 5′-TCTGCCCCCATTGCCCAC TTG-3′ | 56 | 480 |
| R: 5′-TACATAGCCCCCAGCCCATAC CTC-3′ | |||
| Shh | F: 5′-CGGAGCGAGGAAGGGA AAG-3′ | 56 | 262 |
| R: 5′-TTGGGGATAAACTGCTTGTA GGC-3′ | |||
| Patched1 | 5′-CGGCGTTCTCAATGGGCTGGT TTT-3′ | 54 | 376 |
| 5′-GTGGGGCTGCTGTTTCGGGT TCG-3′ | |||
| GAPDH | F: 5′-ACGGATTTGGTCGTATT GGG-3′ | 54 | 208 |
| R: 5′-TGGAAGATGGTGATGGG ATT-3′ | |||
| E-cadherin | F: 5′- CAATGCCGCCATCGCT TAC -3′ | 56 | 421 |
| R: 5′- CAAAATGCCATCGTTGTTC ACT -3′ |
Protein extraction and western blotting assays
Total protein was extracted with RIPA buffer according to standard methods and samples were normalized for protein content using a commercially available kit (Bio-Rad Laboratories Inc Philadelphia, PA USA). Protein samples were separated by 6% SDS-PAGE (for Gli1 protein) and 12% SDS-PAGE (for SHH, E-cadherin, and GAPDH). Proteins were transferred to PVDF membranes and membranes were incubated for 2 h in TBST buffer, followed by incubation overnight at 4°C with the primary antibodies [1∶1000 (v/v) for SHH, E-cadherin, or GAPDH and 1∶500 (v/v) for Gli1] in blocking solution and visualization using the ECL detection system (GE Healthcare Biosciences, Piscataway, NJ, USA).
Transwell assays
Cell invasion assays (24-well sample kits; Chemicon, Bedford, MA, USA) were used to study PC cell line invasion and migration. Briefly, PC cells (1×105) were separately seeded in serum-free media in Matrigel pre-coated transwell chambers (upper chamber), which contained polycarbonate membranes with 8-μm pores. Media containing 2% FCS was added into the bottom chamber. The transwell chambers were then placed on the 24-well plates. After incubation for 24 h, migration of PC cells was determined by photographing the membrane through the microscope. Counts were recorded from the 5 areas with the highest cell concentrations at high power magnification (×200). The mean value of the fields was considered the migration count of PC cells.
Cell growth assays
Cell growth was determined using MTT [3-(4, 5 dimethyl-2-thiazolyl)-2.5-diphenyl- 2H-tetrazolium bromide] assays. Briefly, PC cell lines were plated in 96-well plates. MTT assays were performed after 12, 24, 48, and 72 hours and optical densities were determined at a wavelength of 490 nm.
Liver metastases induction by splenic injection
Three groups of AsPC-1 cells (lentivirus-Gli1i, lentivirus-control, and lentivirus-SHH) were used to detect metastasis after intrasplenic inoculation into nude mice as previously described [9]. Briefly, mice were anesthetized with methoxyflurane, a minor abdominal left flank incision was made, and the spleen was exposed. AsPC-1 cells were injected into the spleen with a 30-gauge needle. The spleen was returned to the abdomen, and the wound was closed in one layer with wound clips. After 8 weeks, we harvested the liver and spleen and produced continuous frozen sections. We stained the sections with hematoxylin and eosin and counted spleen tumors, intrasplenic miniature metastases, and liver metastases under a fluorescence microscope and optical microscope. All animal experiment protocols used in this study were approved by the Animal Research Committee of Tongji University.
cDNA microarray analyses
AsPC-1 cells transduced with L-Gli1i and L-C were used in cDNA microarray assays with the Affymetrix Human Genome U133 Plus2.0 Array GeneChip. Three experiments were performed on a single total RNA preparation from the cells. Signal values are presented as the mean value of 3 replicate experiments. cDNA microarray assays and statistical analyses of the gene expression results were performed as described previously [10].
Statistical Analyses
For all statistical analyses, we used SPSS17.0 software (SPSS, Inc, Chicago, IL, USA). Continuous variables are expressed as the mean ± SE. Non-paired Student's t-tests were used for statistical evaluation. P<0.05 was considered statistically significant.
Results
Lentiviral-Gli1i and -SHH transduction efficiency and PC cell EMT is regulated by SHH-Gli1 signaling
We transfected three PC cell lines with the lentiviral Gli1 interference vector (L-Gli1i), SHH over-expression vector (L-SHH), and control vector (L-C). We verified alterations in activation of SHH-Gli1 signaling by evaluating the expression of SHH, Gli1, and Patched1 using real-time RT-PCR. The real-time RT-PCR data revealed that the Gli1 and Patched1 genes were significantly downregulated by L-Gli1i transduction, whereas Gli1 and Patched1 were upregulated by L-SHH transduction compared with L-C (P<0.01; Figure 1A). Gli1 and Patched1 were target genes in most cell types with SHH signaling activated, therefore, the results suggest the lentiviral vectors efficiently changed the activation of SHH-Gli1 signals. The E-cadherin mRNA levels were drastically reduced by increased SHH/Gli1-expression in PC cells. A similar trend was observed with the E-cadherin protein.
Figure 1. Lentiviral-Gli1i and -SHH transduction efficiency and PC cell EMT is regulated by SHH-Gli1 signaling.
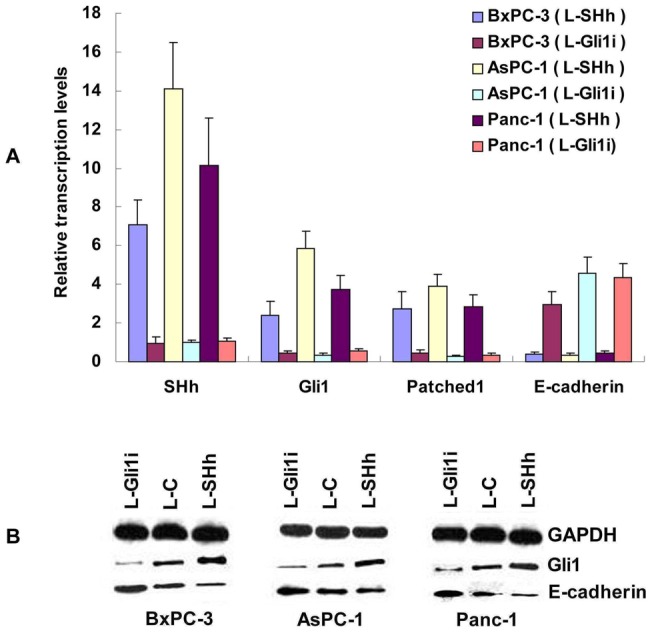
A: Expression of SHH, Gli1, Patched1, and E-cadherin mRNAs in the presence of L-Gli1i and SHH transduction. B: Western blot showing protein expression of Gli1, E-cadherin, and GAPDH in pancreatic cancer cell lines.
PC cell invasion and migration is regulated by SHH-Gli1 signaling
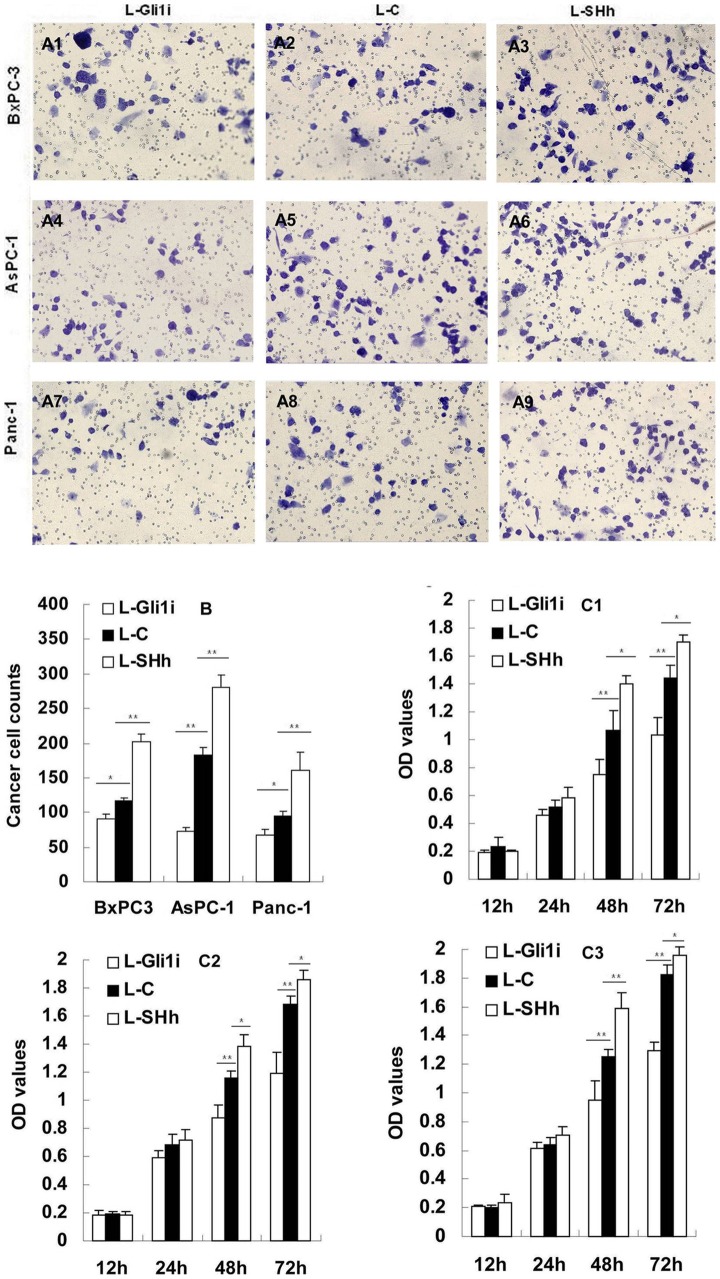
Data from the transwell assays showed that an increased number of cells from the PC cell lines invaded in a Gli1 dose-dependent manner through the Matrigel-coated filter within 24 hours (P<0.05; Figure 2A1–A9, B).
Figure 2. SHH-Gli1 signaling regulates PC cell invasion and migration.
A1-9: Crystal violet staining of PC cells through polycarbonate membrane pores (×200 magnification). B: Cell counts of migrating PC cells as analyzed by transwell assay. C1-3: Cell proliferation as determined by MTT (C1: BxPC-3 cells; C2: AsPC-1 cells; C3: Panc-1 cells). *P<0.05, **P<0.01.
The SHH-Gli1 signaling pathway regulates PC cell proliferation
Our MTT data showed that the L-Gli1i/SHH transduction did not significantly influence cell proliferation within 24 hours. However, after 48 hours, PC cell proliferation increased with viral transduction (Figure 2C1–C3).
Liver metastases after injection of AsPC-1 cells into nude mice is regulated by SHH-Gli1 signaling
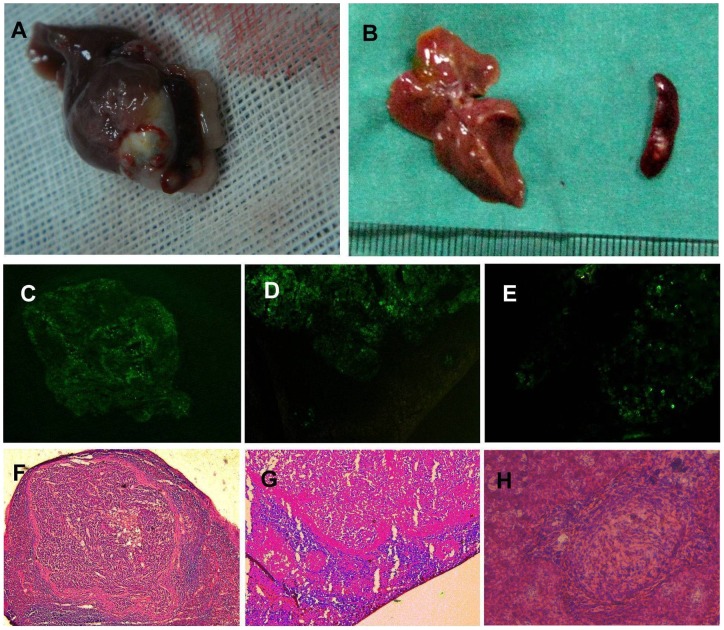
Our data from the nude mice model showed that 8 weeks after intrasplenic injection of AsPC-1 cells, there were spleen tumors in 8 of 10 mice in the L-Gli1i group, 8 of 9 mice in L-C group, and 9 of 9 animals in the L-SHH group. The average numbers of splenic miniature tumors were 2.6, 4.9, and 8.9, respectively. The incidence of liver metastases was 3 of 8 mice in the L-Gli1i group, 5 of 8 mice in the L-C group, and 8 of 9 animals in the L-SHH group. The average numbers of liver metastases were 2.7, 4.2, and 6.7, respectively (Figure 3, Table 2).
Figure 3. Experimental metastasis model of intrasplenic inoculation into nude mice.
A: Spleen tumors and liver metastases in a macroscopic specimen of the L-SHH group. B: Spleen tumors and liver metastases in a macroscopic specimen of the L-C group. C, D, and E: Fluorescence microscopy images. F, G, and H: Lightmicroscopy images. (C, F: Spleen tumors from the L-Gli1i group; D, G: Intrasplenic miniature metastases from the L-C group; E, H: Liver metastases from the L-SHH group). *P<0.05, **P<0.01.
Table 2. Intrasplenic and liver metastases induced by splenic injection in nude mice.
| Groups | Tumorigenicity | Metastases | Liver metastasis | |
| Incidence | Number | |||
| L-Gli1i | 8(10) | 2.6 | 3(8) | 2.7 |
| L-C | 8(9) | 4.9 | 5(8) | 4.2 |
| L-SHH | 9(9) | 8.9 | 9(9) | 6.7 |
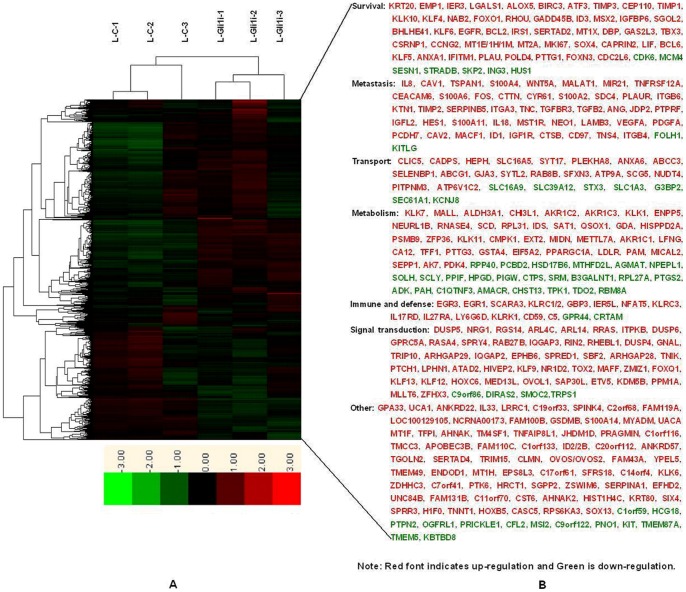
cDNA microarray analyses of Gli1 target genes in AsPC-1 cells
The Patched1 gene, a direct target of Gli1, was upregulated 1.71341-fold in this study. Therefore, we set 1.7-fold regulation as the target gene standard. Using this threshold, the target gene profile data showed that 278 genes were upregulated and 59 genes were downregulated upon Gli1 in AsPC-1 cells. (Table 3). The regulated genes were classified into different categories based on well-documented and established biological or pathological function. Genes regulated by Gli1 belong principally belong to the following categories: cell invasion/migration, angiogenesis, cell survival, transport, metabolism, signal transduction, and immune system defense (Figure 4). We then compared these target genes with previous data by searching the Medline database to screen for differentially expressed PC genes and SHH signaling pathway target genes. Utilizing this approach, we identified 58 upregulated genes (Table 4) and 1 downregulated gene upon Gli1 inhibition in our screen that were previously been found to be similarly regulated in PC. Using the same method, we found 22 upregulated genes upon Gli1 inhibition that were previously found to be correlated with SHH signaling (Table 4). Moreover, 15 of 22 genes that were reported to be overexpressed in PC were involved in cell metastasis, including ITGB4, ANG, VEGFA, S100A4, WNT5A, and TGFB2 as well as cell survival, such as BCL2, BIRC3, IGFBP6, KLF4, and PLAU. At least 8 genes (WNT5A, BCL2, IGFBP6, PTCH1, MSX2, TGFB2, HOXC6, and SOX13) were previously demonstrated to be direct targets of SHH signaling [10], [11].
Table 3. The target genes upon Gli1 in AsPC-1 cells.
| Public ID | Gene Symbol | Gene Title | Fold |
| AI732381 | KRT20 | keratin 20 | 7.74061 |
| NM_005046 | KLK7 | kallikrein-related peptidase 7 | 6.69379 |
| NM_001423 | EMP1 | epithelial membrane protein 1 | 5.70263 |
| NM_004430 | EGR3 | early growth response 3 | 5.49528 |
| U16996 | DUSP5 | dual specificity phosphatase 5 | 4.74988 |
| NM_005814 | GPA33 | glycoprotein A33 (transmembrane) | 4.53642 |
| AA702248 | UCA1 | urothelial cancer associated 1 | 4.4864 |
| NM_000584 | IL8 | interleukin 8 | 4.14464 |
| NM_001323 | CST6 | cystatin E/M | 4.04605 |
| NM_003897 | IER3 | immediate early response 3 | 4.01373 |
| AU147399 | CAV1 | caveolin 1, caveolae protein, 22 kDa | 3.9368 |
| NM_002305 | LGALS1 | lectin, galactoside-binding, soluble, 1 | 3.52748 |
| AF133425 | TSPAN1 | tetraspanin 1 | 3.42412 |
| NM_002961 | S100A4 | S100 calcium binding protein A4 | 3.42195 |
| L12260 | NRG1 | neuregulin 1 | 3.38084 |
| AL049313 | CLIC5 | chloride intracellular channel 5 | 3.19394 |
| NM_003392 | WNT5A | wingless-type MMTV integration site family, member 5A | 3.19181 |
| W80468 | MALAT1 | metastasis associated lung adenocarcinoma transcript 1 (non-protein coding) | 3.1871 |
| AI925518 | ANKRD22 | ankyrin repeat domain 22 | 3.16604 |
| BC003179 | MALL | mal, T-cell differentiation protein-like | 3.11744 |
| AI935123 | AHNAK2 | AHNAK nucleoprotein 2 | 3.09801 |
| AF037195 | RGS14 | regulator of G-protein signaling 14 | 3.09657 |
| NM_000698 | ALOX5 | arachidonate 5-lipoxygenase | 3.02439 |
| AV733950 | EGR1 | early growth response 1 | 2.95766 |
| BF674052 | MIR21 | microRNA 21 | 2.94012 |
| NM_000691 | ALDH3A1 | aldehyde dehydrogenase 3 family, memberA1 | 2.93342 |
| NM_016639 | TNFRSF12A | tumor necrosis factor receptor superfamily, member 12A | 2.93325 |
| M18728 | CEACAM6 | carcinoembryonic antigen-related cell adhesion molecule 6 | 2.86749 |
| NM_014624 | S100A6 | S100 calcium binding protein A6 | 2.83904 |
| AI912173 | CADPS | Ca++-dependent secretion activator | 2.81662 |
| BC004490 | FOS | v-fos FBJ murine osteosarcoma viral oncogene homolog | 2.7865 |
| AB024518 | IL33 | interleukin 33 | 2.70788 |
| U37546 | BIRC3 | baculoviral IAP repeat-containing 3 | 2.6776 |
| M80927 | CHI3L1 | chitinase 3-like 1 (cartilage glycoprotein-39) | 2.67293 |
| NM_003542 | HIST1H4C | histone cluster 1, H4c | 2.65858 |
| AI139629 | ATAD2 | ATPase family, AAA domain containing 2 | 2.6584 |
| NM_001674 | ATF3 | activating transcription factor 3 | 2.64602 |
| AB007830 | SCARA3 | scavenger receptor class A, member 3 | 2.63993 |
| BG475299 | CTTN | cortactin | 2.63957 |
| NM_001554 | CYR61 | cysteine-rich, angiogenic inducer, 61 | 2.61643 |
| NM_000362 | TIMP3 | TIMP metallopeptidase inhibitor 3 | 2.57069 |
| NM_005978 | S100A2 | S100 calcium binding protein A2 | 2.54785 |
| M33376 | AKR1C2 | aldo-keto reductase family 1, member C2 | 2.53992 |
| AB018580 | AKR1C3 | aldo-keto reductase family 1, member C3 | 2.50169 |
| NM_002999 | SDC4 | syndecan 4 | 2.47093 |
| BG435404 | ARL4C | ADP-ribosylation factor-like 4C | 2.4621 |
| AL023584 | HIVEP2 | human immunodeficiency virus type I enhancer binding protein 2 | 2.43299 |
| U08839 | PLAUR | plasminogen activator, urokinase receptor | 2.42304 |
| NM_014799 | HEPH | hephaestin | 2.39676 |
| AL162069 | KRT80 | keratin 80 | 2.39224 |
| AK026736 | ITGB6 | integrin, beta 6 | 2.37268 |
| AI554514 | SIX4 | SIX homeobox 4 | 2.34565 |
| NM_007018 | CEP110 | centrosomal protein 110 kDa | 2.32987 |
| NM_001206 | KLF9 | Kruppel-like factor 9 | 2.3297 |
| NM_025168 | LRRC1 | leucine rich repeat containing 1 | 2.32702 |
| BF589024 | KTN1 | kinectin 1 (kinesin receptor) | 2.32234 |
| NM_003254 | TIMP1 | TIMP metallopeptidase inhibitor 1 | 2.3168 |
| BF107565 | TIMP2 | TIMP metallopeptidase inhibitor 2 | 2.31548 |
| AF213678 | C19orf33 | chromosome 19 open reading frame 33 | 2.31041 |
| NM_005416 | SPRR3 | small proline-rich protein 3 | 2.31004 |
| NM_004695 | SLC16A5 | similar to MCT///solute carrier family 16, member 5 | 2.30392 |
| NM_025047 | ARL14 | ADP-ribosylation factor-like 14 | 2.28222 |
| AI582818 | SYT17 | Synaptotagmin XVII | 2.27965 |
| NM_002639 | SERPINB5 | serpin peptidase inhibitor, clade B (ovalbumin), member 5 | 2.25488 |
| L10038 | KLK1 | kallikrein 1 | 2.25193 |
| NM_014471 | SPINK4 | serine peptidase inhibitor, Kazal type 4 | 2.25172 |
| NM_002204 | ITGA3 | integrin, alpha 3 | 2.25128 |
| NM_006270 | RRAS | related RAS viral (r-ras) oncogene homolog | 2.24532 |
| AI761621 | NR1D2 | nuclear receptor subfamily 1, group D, member 2 | 2.23073 |
| AA211909 | TOX2 | TOX high mobility group box family member 2 | 2.23018 |
| BC002710 | KLK10 | kallikrein-related peptidase 10 | 2.21885 |
| AU147777 | C2orf68 | chromosome 2 open reading frame 68 | 2.21694 |
| NM_002160 | TNC | tenascin C | 2.20758 |
| AW193698 | TGFBR3 | transforming growth factor, beta receptor III | 2.20145 |
| NM_145280 | FAM119A | family with sequence similarity 119, member A | 2.19395 |
| AA609053 | ENPP5 | ectonucleotide pyrophosphatase/phosphodiesterase 5 (putative function) | 2.19289 |
| AU145950 | TGFB2 | transforming growth factor, beta 2 | 2.18613 |
| AW471181 | LOC100129105 | similar to hCG1821214 | 2.17711 |
| AK026748 | NEURL1B | neuralized homolog 1B (Drosophila) | 2.17305 |
| BF514079 | KLF4 | Kruppel-like factor 4 (gut) | 2.16858 |
| NM_002260 | KLRC1/2 | killer cell lectin-like receptor subfamily C, member ½ | 2.16641 |
| NM_002221 | ITPKB | inositol 1,4,5-trisphosphate 3-kinase B | 2.16 |
| NM_001145 | ANG | angiogenin, ribonuclease, RNase A family, 5 | 2.15958 |
| AI761728 | RNASE4 | ribonuclease, RNase A family, 4 | 2.14588 |
| AI821565 | NCRNA00173 | non-protein coding RNA 173 | 2.14337 |
| BC005047 | DUSP6 | dual specificity phosphatase 6 | 2.13885 |
| AB032261 | SCD | stearoyl-CoA desaturase (delta-9-desaturase) | 2.12989 |
| AI024869 | FAM100B | family with sequence similarity 100, member B | 2.12696 |
| H98994 | PLEKHA8 | Pleckstrin homology domain containing, family A (phosphoinositide binding specific) member 8 | 2.12631 |
| AA716425 | JDP2 | Jun dimerization protein 2 | 2.12282 |
| NM_003979 | GPRC5A | G protein-coupled receptor, family C, group 5, member A | 2.12099 |
| AL136680 | GBP3 | guanylate binding protein 3 | 2.11632 |
| BF337329 | NAB2 | NGFI-A binding protein 2 (EGR1 binding protein 2) | 2.10899 |
| AL021977 | MAFF | v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | 2.10067 |
| NM_018530 | GSDMB | gasdermin B | 2.10044 |
| NM_020672 | S100A14 | S100 calcium binding protein A14 | 2.09621 |
| AI348010 | RPL31 | ribosomal protein L31 | 2.08777 |
| BF110608 | IER5L | immediate early response 5-like | 2.08615 |
| AW117498 | FOXO1 | forkhead box O1 | 2.08564 |
| AF070622 | ZMIZ1 | zinc finger, MIZ-type containing 1 | 2.08135 |
| AV703259 | IDS | iduronate 2-sulfatase | 2.08022 |
| BE971383 | SAT1 | spermidine/spermine N1-acetyltransferase 1 | 2.07809 |
| BE908995 | MYADM | myeloid-associated differentiation marker | 2.0759 |
| AL096776 | RHOU | ras homolog gene family, member U | 2.073 |
| BC000145 | H1F0 | H1 histone family, member 0 | 2.0689 |
| NM_015675 | GADD45B | growth arrest and DNA-damage-inducible, beta | 2.05986 |
| NM_001155 | ANXA6 | annexin A6 | 2.05797 |
| BE301252 | QSOX1 | quiescin Q6 sulfhydryl oxidase 1 | 2.05632 |
| NM_020037 | ABCC3 | ATP-binding cassette, sub-family C (CFTR/MRP), member 3 | 2.0476 |
| NM_002167 | ID3 | inhibitor of DNA binding 3, dominant negative helix-loop-helix protein | 2.04538 |
| AJ005683 | NFAT5 | nuclear factor of activated T-cells 5, tonicity-responsive | 2.03963 |
| AF019638 | GDA | guanine deaminase | 2.03934 |
| AB011110 | RASA4 | RAS p21 protein activator 4 | 2.0382 |
| D31771 | MSX2 | msh homeobox 2 | 2.03765 |
| NM_002178 | IGFBP6 | insulin-like growth factor binding protein 6 | 2.0353 |
| NM_002840 | PTPRF | protein tyrosine phosphatase, receptor type, F | 2.02798 |
| W48843 | SPRY4 | sprouty homolog 4 (Drosophila) | 2.02513 |
| AF322916 | UACA | uveal autoantigen with coiled-coil domains and ankyrin repeats | 2.01803 |
| AF543190 | HISPPD2A | histidine acid phosphatase domain containing 2A | 2.01272 |
| NM_003944 | SELENBP1 | selenium binding protein 1 | 2.00877 |
| AI806131 | IGFL2 | IGF-like family member 2 | 2.00535 |
| NM_002800 | PSMB9 | proteasome (prosome, macropain) subunit, beta type, 9 | 2.00438 |
| AJ011712 | TNNT1 | troponin T type 1 (skeletal, slow) | 2.00126 |
| AW965339 | SGOL2 | shugoshin-like 2 (S. pombe) | 1.99085 |
| BE973687 | HES1 | hairy and enhancer of split 1, (Drosophila) | 1.98785 |
| NM_003407 | ZFP36 | zinc finger protein 36, C3H type, homolog (mouse) | 1.98751 |
| NM_006853 | KLK11 | kallikrein-related peptidase 11 | 1.98605 |
| BF246115 | MT1F | metallothionein 1F | 1.98245 |
| AI676059 | FOXQ1 | forkhead box Q1 | 1.98014 |
| AF021834 | TFPI | tissue factor pathway inhibitor (lipoprotein-associated coagulation inhibitor) | 1.97581 |
| AL390127 | KLF13 | Kruppel-like factor 13 | 1.97416 |
| NM_016308 | CMPK1 | cytidine monophosphate (UMP-CMP) kinase 1, cytosolic | 1.97344 |
| NM_000401 | EXT2 | exostoses (multiple) 2 | 1.96824 |
| BG287862 | AHNAK | AHNAK nucleoprotein | 1.9654 |
| NM_002261 | KLRC3 | killer cell lectin-like receptor subfamily C, member 3 | 1.96114 |
| AI189753 | TM4SF1 | transmembrane 4 L six family member 1 | 1.95878 |
| BF438386 | RAB27B | RAB27B, member RAS oncogene family | 1.95437 |
| AA020010 | KLF12 | Kruppel-like factor 12 | 1.95402 |
| AL512725 | MIDN | Midnolin | 1.94948 |
| BE857425 | BHLHE41 | basic helix-loop-helix family, member e41 | 1.94834 |
| BU683415 | KLF6 | Kruppel-like factor 6 | 1.94815 |
| BF338045 | TNFAIP8L1 | tumor necrosis factor, alpha-induced protein 8-like 1 | 1.94402 |
| NM_014033 | METTL7A | methyltransferase like 7A | 1.93619 |
| S68290 | AKR1C1 | aldo-keto reductase family 1, member C1 | 1.93607 |
| NM_004503 | HOXC6 | homeobox C6 | 1.93452 |
| BE217882 | JHDM1D | jumonji C domain containing histone demethylase 1 homolog D (S. cerevisiae) | 1.93221 |
| BF739767 | PRAGMIN | homolog of rat pragma of Rnd2 | 1.93063 |
| AW007080 | IL17RD | interleukin 17 receptor D | 1.92254 |
| NM_021039 | S100A11 | S100 calcium binding protein A11 | 1.92157 |
| AI983115 | IL27RA | interleukin 27 receptor, alpha | 1.92132 |
| NM_002147 | HOXB5 | homeobox B5 | 1.91937 |
| NM_021246 | LY6G6D | lymphocyte antigen 6 complex, locus G6D | 1.91452 |
| NM_001562 | IL18 | interleukin 18 (interferon-gamma-inducing factor) | 1.91426 |
| NM_002447 | MST1R | macrophage stimulating 1 receptor (c-met-related tyrosine kinase) | 1.9139 |
| AL355708 | NEO1 | neogenin homolog 1 (chicken) | 1.90936 |
| NM_024115 | C1orf116 | chromosome 1 open reading frame 116 | 1.90905 |
| AF439512 | KLRK1 | killer cell lectin-like receptor subfamily K, member 1 | 1.90656 |
| AW157070 | EGFR | epidermal growth factor receptor | 1.90531 |
| AW151924 | LFNG | LFNG O-fucosylpeptide 3-beta-N-acetylglucosaminyltransferase | 1.90039 |
| AA133277 | BCL2 | BCL2 | 1.90004 |
| AI474666 | IRS1 | insulin receptor substrate 1 | 1.8898 |
| BF752277 | CA12 | carbonic anhydrase XII | 1.884 |
| NM_004915 | ABCG1 | ATP-binding cassette, sub-family G (WHITE), member 1 | 1.88313 |
| N51717 | TMCC3 | transmembrane and coiled-coil domain family 3 | 1.88267 |
| NM_014755 | SERTAD2 | SERTA domain containing 2 | 1.88025 |
| NM_004900 | APOBEC3B | apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3B | 1.87712 |
| AL133033 | MED13L | mediator complex subunit 13-like | 1.87401 |
| NM_005952 | MT1X | metallothionein 1X | 1.87389 |
| L25541 | LAMB3 | laminin, beta 3 | 1.87302 |
| AF022375 | VEGFA | vascular endothelial growth factor A | 1.87138 |
| AA588400 | OVOL1 | ovo-like 1(Drosophila) | 1.86924 |
| BF726530 | GJA3 | gap junction protein, alpha 3, 46 kDa | 1.86225 |
| NM_144508 | CASC5 | cancer susceptibility candidate 5 | 1.85939 |
| U79283 | DBP | D site of albumin promoter (albumin D-box) binding protein | 1.85936 |
| AW271106 | IQGAP3 | IQ motif containing GTPase activating protein 3 | 1.85751 |
| N21426 | SYTL2 | Synaptotagmin-like 2 | 1.85066 |
| AI860012 | GAS2L3 | Growth arrest-specific 2 like 3 | 1.847 |
| NM_002607 | PDGFA | platelet-derived growth factor alpha polypeptide | 1.83526 |
| NM_003225 | TFF1 | trefoil factor 1 | 1.83446 |
| AL136924 | RIN2 | Ras and Rab interactor 2 | 1.83375 |
| AI674565 | FAM110C | family with sequence similarity 110, member C | 1.8328 |
| BF508679 | C1orf133 | chromosome 1 open reading frame 133 | 1.83104 |
| NM_021000 | PTTG3 | pituitary tumor-transforming 3 | 1.83022 |
| AI819238 | ID2 | inhibitor of DNA binding 2, dominant negative helix-loop-helix protein | 1.82891 |
| NM_001512 | GSTA4 | glutathione S-transferase alpha 4 | 1.82147 |
| BG498334 | RPS6KA3 | ribosomal protein S6 kinase, 90 kDa, polypeptide 3 | 1.82032 |
| NM_016569 | TBX3 | T-box 3 | 1.81904 |
| AL034550 | C20orf112 | chromosome 20 open reading frame 112 | 1.81828 |
| BE669553 | ANKRD57 | ankyrin repeat domain 57 | 1.81439 |
| AI862477 | SAP30L | SAP30-like | 1.81113 |
| AI091372 | CSRNP1 | cysteine-serine-rich nuclear protein 1 | 1.8103 |
| AI807023 | RAB8B | RAB8B, member RAS oncogene family | 1.80823 |
| AW134535 | CCNG2 | cyclin G2 | 1.80731 |
| BC028219 | TGOLN2 | trans-golgi network protein 2 | 1.8022 |
| BC014155 | RHEBL1 | Ras homolog enriched in brain like 1 | 1.80161 |
| NM_001394 | DUSP4 | dual specificity phosphatase 4 | 1.79908 |
| AV747725 | EIF5A2 | eukaryotic translation initiation factor 5A2 | 1.79722 |
| AU146709 | SERTAD4 | SERTA domain containing 4 | 1.79659 |
| AF220133 | TRIM15 | tripartite motif-containing 15 | 1.79617 |
| AL031602 | MT1E/1H/1M | metallothionein 1E/1H/1M | 1.79612 |
| AI082827 | GNAL | guanine nucleotide binding protein (G protein), alpha activating activity polypeptide, olfactory type | 1.79573 |
| NM_005953 | MT2A | metallothionein 2A | 1.79486 |
| NM_024734 | CLMN | calmin (calponin-like, transmembrane) | 1.7933 |
| AW594320 | OVOS/OVOS2 | similar to hCG38149///ovostatin///ovostatin 2 | 1.792 |
| AF116571 | SOX13 | SRY (sex determining region Y)-box 13 | 1.79158 |
| NM_013261 | PPARGC1A | peroxisome proliferator-activated receptor gamma, coactivator 1 alpha | 1.79113 |
| NM_004240 | TRIP10 | thyroid hormone receptor interactor 10 | 1.79072 |
| BE644809 | PCDH7 | protocadherin 7 | 1.78886 |
| NM_004454 | ETV5 | ets variant 5 | 1.78653 |
| BF001806 | MKI67 | antigen identified by monoclonal antibody Ki-67 | 1.78641 |
| BG528420 | SOX4 | SRY (sex determining region Y)-box 4 | 1.78459 |
| AW264102 | FAM43A | family with sequence similarity 43, member A | 1.78133 |
| NM_023925 | CAPRIN2 | caprin family member 2 | 1.78062 |
| NM_016061 | YPEL5 | yippee-like 5 (Drosophila) | 1.77979 |
| NM_002309 | LIF | leukemia inhibitory factor | 1.77963 |
| AL541655 | TMEM49 | transmembrane protein 49 | 1.77918 |
| AF131747 | ENDOD1 | endonuclease domain containing 1 | 1.77616 |
| BF197655 | CAV2 | caveolin 2 | 1.77602 |
| NM_000527 | LDLR | low density lipoprotein receptor | 1.77329 |
| NM_030971 | SFXN3 | sideroflexin 3 | 1.7732 |
| NM_004815 | ARHGAP29 | Rho GTPase activating protein 29 | 1.77236 |
| NM_005951 | MT1H | metallothionein 1H | 1.77077 |
| NM_024526 | EPS8L3 | EPS8-like 3 | 1.76872 |
| NM_006633 | IQGAP2 | IQ motif containing GTPase activating protein 2 | 1.76851 |
| AB014511 | ATP9A | ATPase, class II, type 9A | 1.76617 |
| BF975929 | C17orf61 | chromosome 17 open reading frame 61 | 1.76436 |
| NM_001706 | BCL6 | B-cell CLL/lymphoma 6 | 1.76343 |
| BE379006 | CD59 | CD59 molecule, complement regulatory protein | 1.76235 |
| AW081113 | SFRS18 | splicing factor, arginine/serine-rich 18 | 1.76215 |
| AI932310 | C14orf4 | chromosome 14 open reading frame 4 | 1.75504 |
| AB029290 | MACF1 | microtubule-actin crosslinking factor 1 | 1.75486 |
| NM_006618 | KDM5B | lysine (K)-specific demethylase 5B | 1.75327 |
| NM_004445 | EPHB6 | EPH receptor B6 | 1.75236 |
| BF342524 | SPRED1 | sprouty-related, EVH1 domain containing 1 | 1.75156 |
| AB030824 | KLF5 | Kruppel-like factor 5 (intestinal) | 1.75153 |
| BF038548 | PAM | peptidylglycine alpha-amidating monooxygenase | 1.74921 |
| D13889 | ID1 | inhibitor of DNA binding 1, dominant negative helix-loop-helix protein | 1.74845 |
| AW276572 | SBF2 | SET binding factor 2 | 1.74723 |
| AI935647 | ARHGAP28 | Rho GTPase activating protein 28 | 1.7444 |
| NM_002774 | KLK6 | kallikrein-related peptidase 6 | 1.74312 |
| BE965029 | MICAL2 | microtubule associated monoxygenase, calponin and LIM domain containing 2 | 1.73804 |
| NM_000700 | ANXA1 | annexin A1 | 1.73654 |
| R59093 | TNIK | TRAF2 and NCK interacting kinase | 1.73504 |
| BF111925 | ZDHHC3 | zinc finger, DHHC-type containing 3 | 1.73496 |
| NM_003020 | SCG5 | secretogranin V (7B2 protein) | 1.73476 |
| W73230 | C7orf41 | chromosome 7 open reading frame 41 | 1.73475 |
| NM_005410 | SEPP1 | selenoprotein P, plasma, 1 | 1.73357 |
| AL044092 | IGF1R | insulin-like growth factor 1 receptor | 1.73199 |
| AA749101 | IFITM1 | interferon induced transmembrane protein 1 (9–27) | 1.73188 |
| W47179 | CTSB | cathepsin B | 1.72986 |
| NM_005975 | PTK6 | PTK6 protein tyrosine kinase 6 | 1.7249 |
| AW511135 | NUDT4 | nudix (nucleoside diphosphate linked moiety X)-type motif 4 | 1.72406 |
| NM_001784 | CD97 | CD97 molecule | 1.72395 |
| K03226 | PLAU | plasminogen activator, urokinase | 1.72296 |
| NM_021173 | POLD4 | polymerase (DNA-directed), delta 4 | 1.7214 |
| AA886888 | PPM1A | protein phosphatase 1A (formerly 2C), magnesium-dependent, alpha isoform | 1.72092 |
| AA158731 | TNS4 | tensin 4 | 1.71933 |
| NM_152327 | AK7 | adenylate kinase 7 | 1.7149 |
| NM_004219 | PTTG1 | pituitary tumor-transforming 1 | 1.7148 |
| AI521254 | HRCT1 | histidine rich carboxyl terminus 1 | 1.7145 |
| AI800110 | SGPP2 | sphingosine-1-phosphate phosphotase 2 | 1.71439 |
| AV707102 | PDK4 | pyruvate dehydrogenase kinase, isozyme 4 | 1.71397 |
| BG054916 | PTCH1 | patched homolog 1 (Drosophila) | 1.71341 |
| AI692595 | ZSWIM6 | zinc finger, SWIM-type containing 6 | 1.71248 |
| AF119873 | SERPINA1 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 1 | 1.71135 |
| AW664179 | EFHD2 | EF-hand domain family, member D2 | 1.71043 |
| NM_000213 | ITGB4 | integrin, beta 4 | 1.70999 |
| NM_024679 | LPHN1 | latrophilin 1 | 1.70907 |
| BF063164 | PITPNM3 | PITPNM family member 3 | 1.70843 |
| NM_001735 | C5 | complement component 5 | 1.70584 |
| AL021707 | UNC84B | unc-84 homolog B (C. elegans) | 1.70583 |
| NM_014690 | FAM131B | family with sequence similarity 131, member B | 1.70547 |
| BG024886 | MLLT6 | myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila); translocated to, 6 | 1.70508 |
| AW051527 | FOXN3 | forkhead box N3 | 1.70455 |
| AB028951 | CDC2L6 | cell division cycle 2-like 6 (CDK8-like) | 1.70418 |
| BG402859 | ZFHX3 | zinc finger homeobox 3 | 1.70332 |
| BC006128 | C11orf70 | chromosome 11 open reading frame 70 | 1.70168 |
| NM_144583 | ATP6V1C2 | ATPase, H+ transporting, lysosomal 42 kDa, V1 subunit C2 | 1.70137 |
| NM_006638 | RPP40 | ribonuclease P/MRP 40 kDa subunit | 0.58782 |
| AL136721 | PCBD2 | pterin-4 alpha-carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1 alpha (TCF1) 2 | 0.58533 |
| U89281 | HSD17B6 | hydroxysteroid (17-beta) dehydrogenase 6 homolog (mouse) | 0.58343 |
| BF185922 | MTHFD2L | methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2-like | 0.58139 |
| BE502436 | C1orf59 | chromosome 1 open reading frame 59 | 0.57847 |
| AA430072 | HCG18 | HLA complex group 18 | 0.57488 |
| NM_002828 | PTPN2 | protein tyrosine phosphatase, non-receptor type 2 | 0.57432 |
| NM_024576 | OGFRL1 | opioid growth factor receptor-like 1 | 0.57366 |
| NM_004778 | GPR44 | G protein-coupled receptor 44 | 0.56901 |
| AA404269 | PRICKLE1 | prickle homolog 1 (Drosophila) | 0.56846 |
| BC005090 | AGMAT | agmatine ureohydrolase (agmatinase) | 0.56447 |
| NM_024718 | C9orf86 | chromosome 9 open reading frame 86 | 0.56282 |
| AV726166 | CFL2 | cofilin 2 (muscle) | 0.56207 |
| AW274756 | CDK6 | cyclin-dependent kinase 6 | 0.56119 |
| BG401568 | SLC16A9 | solute carrier family 16, member 9 (monocarboxylic acid transporter 9) | 0.56011 |
| AL577823 | NPEPL1 | Aminopeptidase-like 1 | 0.55752 |
| BF433759 | SOLH | small optic lobes homolog (Drosophila) | 0.55631 |
| AA911739 | SCLY | Selenocysteine lyase | 0.55584 |
| NM_005729 | PPIF | peptidylprolyl isomerase F | 0.55446 |
| BE000929 | MSI2 | musashi homolog 2 (Drosophila) | 0.55395 |
| AF264784 | TRPS1 | trichorhinophalangeal syndrome I | 0.55347 |
| BC041970 | C9orf122 | chromosome 9 open reading frame 122 | 0.54891 |
| AV733347 | PNO1 | partner of NOB1 homolog (S. cerevisiae) | 0.5455 |
| AL574184 | HPGD | hydroxyprostaglandin dehydrogenase 15-(NAD) | 0.54429 |
| NM_152725 | SLC39A12 | solute carrier family 39 (zinc transporter), member 12 | 0.54364 |
| AI936566 | MCM4 | minichromosome maintenance complex component 4 | 0.54165 |
| BF037819 | PIGW | phosphatidylinositol glycan anchor biosynthesis, class W | 0.54099 |
| AJ002077 | STX3 | syntaxin 3 | 0.53917 |
| NM_000222 | KIT | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | 0.52724 |
| NM_014454 | SESN1 | sestrin 1 | 0.527 |
| NM_001905 | CTPS | CTP synthase | 0.52427 |
| NM_004172 | SLC1A3 | solute carrier family 1 (glial high affinity glutamate transporter), member 3 | 0.52231 |
| NM_012297 | G3BP2 | GTPase activating protein (SH3 domain) binding protein 2 | 0.51989 |
| AB038950 | STRADB | STE20-related kinase adaptor beta | 0.51532 |
| AW168915 | FOLH1 | folate hydrolase (prostate-specific membrane antigen) 1 | 0.51296 |
| NM_003132 | SRM | spermidine synthase | 0.51065 |
| AB050855 | B3GALNT1 | beta-1,3-N-acetylgalactosaminyltransferase 1 (globoside blood group) | 0.50955 |
| AF346602 | SEC61A1 | Sec61 alpha 1 subunit (S. cerevisiae) | 0.50743 |
| BC005335 | TMEM87A | transmembrane protein 87A | 0.50511 |
| BF224146 | TMEM5 | transmembrane protein 5 | 0.50445 |
| BC001441 | SKP2 | S-phase kinase-associated protein 2 (p45) | 0.49395 |
| BC004284 | RPL27A | ribosomal protein L27a | 0.49291 |
| NM_000963 | PTGS2 | prostaglandin-endoperoxide synthase 2 | 0.49211 |
| NM_001123 | ADK | adenosine kinase | 0.48824 |
| NM_019604 | CRTAM | cytotoxic and regulatory T cell molecule | 0.48563 |
| NM_000277 | PAH | phenylalanine hydroxylase | 0.4723 |
| AF161419 | ING3 | inhibitor of growth family, member 3 | 0.46565 |
| NM_004507 | HUS1 | HUS1 checkpoint homolog (S. pombe) | 0.46307 |
| AA888589 | C1QTNF3 | C1q and tumor necrosis factor related protein 3 | 0.45976 |
| BF514158 | KCNJ8 | potassium inwardly-rectifying channel, subfamily J, member 8 | 0.40579 |
| NM_017594 | DIRAS2 | DIRAS family, GTP-binding RAS-like 2 | 0.40548 |
| AA669114 | KBTBD8 | kelch repeat and BTB (POZ) domain containing 8 | 0.38891 |
| AI796120 | AMACR/C1QTNF3 | alpha-methylacyl-CoA racemase///C1q and tumor necrosis factor related protein 3 | 0.38848 |
| AA677272 | CHST13 | carbohydrate (chondroitin 4) sulfotransferase 13 | 0.37567 |
| NM_022445 | TPK1 | thiamin pyrophosphokinase 1 | 0.37373 |
| NM_005651 | TDO2 | tryptophan 2,3-dioxygenase | 0.36276 |
| AB014737 | SMOC2 | SPARC related modular calcium binding 2 | 0.34035 |
| AF119835 | KITLG | KIT ligand | 0.32023 |
| BC017770 | RBM8A | RNA binding motif protein 8A | 0.22761 |
Figure 4. cDNA microarray analyses of Gli1 target genes in AsPC-1 cells.
A: cDNA microarray data cluster comparing L-C and L-Gli1i cells. B: Functional classification of differentially expressed genes. See also Table 2.
Table 4. PC-related genes and Hedgehog-related genes reported previously in the target genes profile data.
| Public ID | Gene Symbol | PC-related | Hedgehog-related |
| AA133277 | BCL2 | P | P* |
| NM_002178 | IGFBP6 | P | P* |
| BG054916 | PTCH1 | P | P* |
| NM_003392 | WNT5A | P | P* |
| D31771 | MSX2 | P | P* |
| U37546 | BIRC3 | P | P |
| BF514079 | KLF4 | P | P |
| K03226 | PLAU | P | P |
| AW157070 | EGFR | P | P |
| BE973687 | HES1 | P | P |
| NM_000584 | IL8 | P | P |
| NM_001145 | ANG | P | P |
| AF022375 | VEGFA | P | P |
| NM_002961 | S100A4 | P | P |
| W47179 | CTSB | P | P |
| NM_020037 | ABCC3 | P | |
| NM_004219 | PTTG1 | P | |
| AI732381 | KRT20 | P | |
| NM_002167 | ID3 | P | |
| AB030824 | KLF5 | P | |
| NM_000700 | ANXA1 | P | |
| BC002710 | KLK10 | P | |
| BU683415 | KLF6 | P | |
| BF001806 | MKI67 | P | |
| NM_000698 | ALOX5 | P | |
| NM_001323 | CST6 | P | |
| NM_006633 | IQGAP2 | P | |
| L12260 | NRG1 | P | |
| W48843 | SPRY4 | P | |
| AF021834 | TFPI | P | |
| NM_002774 | KLK6 | P | |
| AF119873 | SERPINA1 | P | |
| AL044092 | IGF1R | P | |
| U08839 | PLAUR | P | |
| BF589024 | KTN1 | P | |
| NM_002447 | MST1R | P | |
| AA158731 | TNS4 | P | |
| BF674052 | MIR21 | P | |
| L25541 | LAMB3 | P | |
| NM_001562 | IL18 | P | |
| NM_002607 | PDGFA | P | |
| NM_005978 | S100A2 | P | |
| NM_002999 | SDC4 | P | |
| M18728 | CEACAM6 | P | |
| NM_014624 | S100A6 | P | |
| NM_001512 | GSTA4 | P | |
| NM_005046 | KLK7 | P | |
| M80927 | CHI3L1 | P | |
| BE301252 | QSOX1 | P | |
| S68290 | AKR1C1 | P | |
| NM_003225 | TFF1 | P | |
| BE379006 | CD59 | P | |
| NM_001554 | CYR61 | P | |
| NM_002204 | ITGA3 | P | |
| AU147399 | CAV1 | P | |
| NM_002160 | TNC | P | |
| NM_021039 | S100A11 | P | |
| BC004490 | FOS | P | |
| AU145950 | TGFB2 | P* | |
| AF116571 | SOX13 | P* | |
| AI474666 | IRS1 | P | |
| NM_016569 | TBX3 | P | |
| NM_002309 | LIF | P | |
| NM_004503 | HOXC6 | P* | |
| AI091372 | CSRNP1 | P |
P positive correlation, *Direct target genes of Hedgehog signalings.
Discussion
In this study, cell survival target genes could be divided into several types: (1) proliferation-related genes, such as IGFBP6, IGF1R, IRS1, EGFR, and ALOX5, (2) apoptosis-related genes, such as BIRC3 and Bcl-2, (3) Cell cycle-related genes, such as CCNG2, CDC2L6, and CDK6, and (4) CSC orCSCs maintenance-related genes. The stem cell phenotype predominantly included EMT, anti-treatment, and stem cell markers. The IGF signaling pathway was a key proliferation-related pathway and the Bcl-2 family was an important classic apoptotic signaling pathway. It was reported that Gli1 directly regulates CCND transcription and our data suggests it may regulate CCNG2 in the same manner [7]. The ABCC3 gene encodes multidrug resistance-associated protein 3 (MRP3), which is involved in chemotherapy resistance of cancer cells [12]. Moreover, MTS upregulation and CTPS downregulation has also been reported to lead to chemotherapy resistance [13]. In addition, KLF4 is a stem cell marker that promotes cancer stem cell population maintenance and CD59 upregulation may be associated with tumor cell immune escape [14], [15]. Interestingly, HUS1 downregulation likely weakens the DNA damage repair mechanisms [16].
Angiogenesis is necessary for cancer metastasis as well as for CSCs microenvironment maintenance. Substantial evidence suggests that activated SHH signaling may be one angiogenesis-initiating signaling pathway during pancreas carcinogenesis, though its exact mechanism is not known [17]. In this study, we found that Gli1 significantly upregulated pro-angiogenic factors, including ANG, VEGFA, PDGFA, TNFRSF12A, and IL-8, suggesting it has an important regulatory role in PC angiogenesis. Moreover, in this study, VEGF and PDGF were upregulated at the same time, suggesting that the proangiogenesis mechanisms of the SHH pathway are not just involved in endothelial cells (ECs) tuberformation, but also vessel wall maturation.
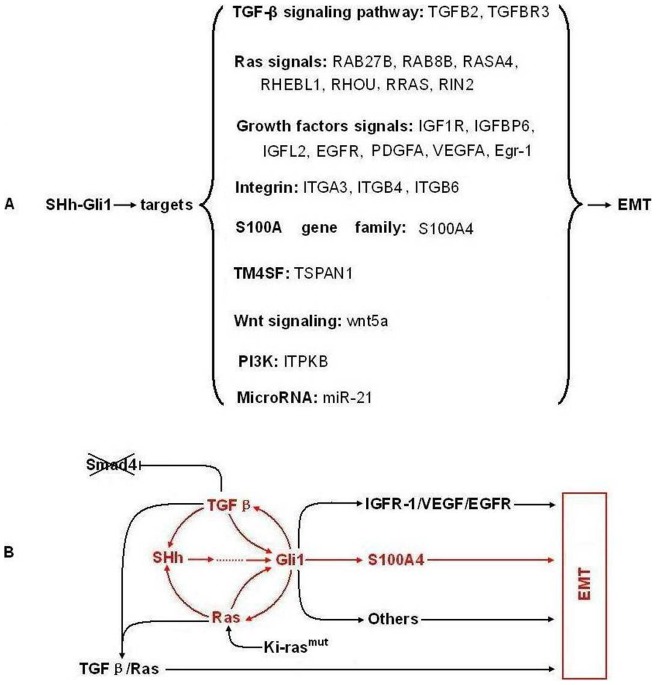
It was reported that SHH signaling pathway activation accompanied EMT, and EMT is required for migration of SHH-responsive cells during tissue morphogenesis. However, there was no evidence that Gli1 directly regulated Snail or Slug transcription. In the present study, target profile data showed that SHH signaling in EMT involved a complex crosstalk network (Figure 5A). The EMT-related target genes are summarized as follows: (1) TGF-β signaling pathway: TGFβ2 and TGFβR3. Previous studies showed that TGFβ signaling is significantly elevated in PC with Smad4 mutation, resulting in the loss of Smad4-dependent cell growth inhibition and increased Smad4 independent EMT [18]. (2) Ras signaling pathway: RAB27B, RAB8B, RASA4, RHEBL1, RHOU, RRAS, and RIN2. Data indicate the Ras/ERK1/2 pathways are involved in the mesenchymal transformation of PC cells [19]. (3) Wnt signaling pathway: wnt5a. Previous study indicate that wnt5a promotes EMT through a non-classical pathway [20]. (4) PI3K/AKT signaling pathway: ITPKB. PI3K was found to strengthen Snail nuclear colonization through PAK1 activation of the AKT signaling pathway in EMT [21]. AKT functions as a central point to transduce extracellular (growth factors including insulin, IGF-1, and EGF) and intracellular (such as mutated or activated receptor tyrosine kinases, PTEN, Ras, and Src) signals [22]. (5) Growth factor and receptor signaling pathways: IGF1R, IGFBP6, IGFL2, EGFR, PDGFA, and VEGFA. Previous studies have demonstrated that abnormal activation of these pathways promotes epithelial-derived tumor expansion and progression through promotion of EMT-like transitions. Regarding mechanisms, IGFR signaling induces expression of the transcription factors Snail and Zeb [23]. PDGF may induce EMT via activation of the Wnt signaling pathway [24]. VEGF and EGF can increase of Snail and Twist protein expression [25]. (6) Integrins: ITGA3, ITGB4, and ITGB6. It has been reported that the α3 and β4 subunits can make up laminin-binding integrins with other subunits, such as α3β1 or α6β4, and these subunits can be palmitoylated that may contribute to integrin-tetraspanin interactions [26]. The potential prometastatic functions of these integrin subunits, particularly β4, were reported previously and tyrosine phosphorylation of the β4 Shc-binding site results in disassembly of hemidesmosomes and mobilization of signaling-activated α6β4 integrin. Mobilized α6β4 switches from keratin to actin filament association and may mediate migration and invasion of laminin isoforms [26]. (7) TM4SF: TSPAN1. TSPAN1 gene over-expression was detected in liver cancer, prostate cancer, gastric cancer, cervical cancer, and colorectal cancer [27]. It has been proposed that TSPAN1 gene expression correlates with cell proliferation and cancer prognosis. Our data suggests that TSPAN1, as a member of TM4SF, may participate in the EMT process of PC cells. However, it remains to be determined how it interacts with integrins, growth factors, or other TM4S proteins. (8) MicroRNAs: miR-21. Studies have shown that miR-21 is associated with PC metastasis and prognosis and may play a role in TGF-β -induced EMT [28], [29]. (9) S100A gene family: S100A4. It has been reported that S100A4 and E-cadherin are inversely regulated in several cell systems and that S100A4 promotes the expression of the essential transcription factors, Twist and Snail, in the EMT process, as well as mesenchymal markers, including vimentin and MMPs [30], [31].
Figure 5. The EMT molecular network mediated by SHH-Gli1 signaling in PC cells.
A: Target genes and signaling involved in EMT regulated by Gli1 in PC cells; B: The putative crosstalk model within the EMT molecular network mediated by SHH-Gli1 signaling.
Interesting, our data suggests that the EMT molecular network mediated by SHH signaling may contain at least two important positive feedback loops in PC cells. The first is the positive feedback between SHH and TGFβ signaling. In vitro and in vivo evidence suggests the crosstalk between TGFβ and SHH results in reciprocal induction. TGFβ upregulated SHH and activated Gli1 during EMT induction; however, SHH signaling upregulated TGFβ2 and TGFBR3 as demonstrated in this and a previous study [32], [33]. The second positive feedback loop is between SHH and Ras signaling. Previously, studies showed that k-ras mutation was an essential mechanism of SHH and Gli1 upregulation in PC cells and in this study, we found that Gli1 upregulated several Ras-related genes to activate Ras signaling [34].
Based on previous studies and our data, we speculate that this molecular network might start with k-ras mutations, followed by SHH signaling activation, and finally, the TGFβ signal joins and a positive feedback loop forms between the three pathways. The SHH signal was continuously enhanced through this positive feedback and directly promotes EMT via regulation of EMT-related Gli1 target genes, such as IGFR1, VEGF, EGF, and S100A4. (Figure 5B). However, this molecular network model may be more complex with the participation of additional signaling proteins, such as integrins, PI3K/AKT, and WNT.
Acknowledgments
We thank all members of the Central Laboratory of the Tenth Hospital of Tongji University.
Funding Statement
This work was funded by grants from the National Natural Science Foundation of China (81072005 and 81172312) and Shanghai Science and Technology Committee (10ZR1423300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mfopou JK, De Groote V, Xu X, Heimberg H, Bouwens L (2007) Sonic hedgehog and other soluble factors from differentiating embryoid bodies inhibit pancreas development. Stem Cells 25: 1156–1165. [DOI] [PubMed] [Google Scholar]
- 2. Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, et al. (2010) Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 138: 1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai J, Ai K, Du Y, Chen G (2011) Sonic hedgehog expression correlates with distant metastasis in pancreatic adenocarcinoma. Pancreas 40: 233–236. [DOI] [PubMed] [Google Scholar]
- 4. Bailey JM, Mohr AM (2009) Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene 28: 3513–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, et al. (2009) Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology 137: 1102–1113. [DOI] [PubMed] [Google Scholar]
- 6. Seidel K, Ahn CP, Lyons D, Nee A, Ting K, et al. (2010) Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development 137: 3753–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katoh Y, Katoh M (2009) Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med 9: 873–886. [DOI] [PubMed] [Google Scholar]
- 8. Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, et al. (2004) Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci U S A 101: 12561–12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Partecke IL, Kaeding A, Sendler M, Albers N, Kühn JP, et al. (2011) In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. BMC Cancer 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu M, Gipp J, Yoon JW, Iannaccone P, Walterhouse D, et al. (2009) Sonic hedgehog-responsive genes in the fetal prostate. J Biol Chem 284: 5620–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feldmann G, Habbe N, Dhara S, Bisht S, Alvarez H, et al. (2008) Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut 57: 1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sasaki T, Hirota T, Ryokai Y, Kobayashi D, Kimura M, et al. (2011) Systematic screening of human ABCC3 polymorphisms and their effects on MRP3 expression and function. Drug Metab Pharmacokinet 26: 374–386. [DOI] [PubMed] [Google Scholar]
- 13. Nishio R, Tsuchiya H, Yasui T, Matsuura S, Kanki K, et al. Disrupted plasma membrane localization of equilibrative nucleoside transporter 2 in the chemoresistance of human pancreatic cells to gemcitabine (dFdCyd). Cancer Sci 102: 622–629. [DOI] [PubMed] [Google Scholar]
- 14. Walker E, Manias JL, Chang WY, Stanford WL (2011) PCL2 modulates gene regulatory networks controlling self-renewal and commitment in embryonic stem cells. Cell Cycle 10: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui W, Zhao Y, Shan C, Kong G, Hu N, et al. (2012) HBXIP upregulates CD46, CD55 and CD59 through ERK1/2/NF-κB signaling to protect breast cancer cells from complement attack. FEBS Lett 586: 766–771. [DOI] [PubMed] [Google Scholar]
- 16. Luncsford PJ, Chang DY, Shi G, Bernstein J, Madabushi A, et al. (2010) A structural hinge in eukaryotic MutY homologues mediates catalytic activity and Rad9-Rad1-Hus1 checkpoint complex interactions. J Mol Biol 403: 351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, et al. (2009) Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 30: 580–588. [DOI] [PubMed] [Google Scholar]
- 18. Levy L, Hill CS (2005) Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol 25: 8108–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Botta GP, Reginato MJ, Reichert M, Rustgi AK, Lelkes PI (2012) Constitutive K-RasG12D activation of ERK2 specifically regulates 3D invasion of human pancreatic cancer cells via MMP-1. Mol Cancer Res 10: 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ripka S, König A, Buchholz M, Wagner M, Sipos B, et al. (2007) WNT5A – target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis 28: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 21. Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, et al. (2010) The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit. Genes Cancer 1: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou WY, et al. (2012) Transforming growth factor-β1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol Biol Rep 39: 3549–3556. [DOI] [PubMed] [Google Scholar]
- 23. Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, et al. (2008) Insulinlike growth factor-I-dependent up-regulation of ZEB1 drives epithelial-tomesenchymal transition in human prostate cancer cells. Cancer Res 68: 2479–2488. [DOI] [PubMed] [Google Scholar]
- 24. Yang L, Lin C, Liu ZR (2006) P68 RNA helicase mediates PDGFinduced epithelial mesenchymal transition by displacing Axin from betacatenin. Cell 127: 139–155. [DOI] [PubMed] [Google Scholar]
- 25. Lee MY, Chou CY, Tang MJ, Shen MR (2008) Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res 14: 4743–4750. [DOI] [PubMed] [Google Scholar]
- 26. Stipp CS (2010) Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med 18 12: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen L, Zhu YY, Zhang XJ, Wang GL, Li XY, et al. (2009) TSPAN1 protein expression: a significant prognostic indicator for patients with colorectal adenocarcinoma. World J Gastroenterol 15: 2270–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu J, Li A, Hong SM, Hruban RH, Goggins M (2012) MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Re 18: 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zavadil J, Narasimhan M, Blumenberg M, Schneider RJ (2007) Transforming growth factor-beta and microRNA:mRNA regulatory networks in epithelial plasticity. Cells Tissues Organs 185: 157–161. [DOI] [PubMed] [Google Scholar]
- 30. Zhang HY, Zheng XZ, Wang XH, Xuan XY, Wang F, et al. (2012) S100A4 mediated cell invasion and metastasis of esophageal squamous cell carcinoma via the regulation of MMP-2 and E-cadherin activity. Mol Biol Rep 39: 199–208. [DOI] [PubMed] [Google Scholar]
- 31. Boye K, Mælandsmo GM (2010) S100A4 and Metastasis A Small Actor Playing Many Roles. The American Journal of Pathology 176: 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katoh Y, Katoh M (2009) Integrative genomic analyses on GLI1: positive regulation of GLI1 by Hedgehog-GLI, TGFbeta-Smads, and RTK-PI3K-AKT signals, and negative regulation of GLI1 by Notch-CSL-HES/HEY, and GPCR-Gs-PKA signals. Int J Oncol 35: 187–192. [DOI] [PubMed] [Google Scholar]
- 33. Fan Q, He M, Sheng T, Zhang X, Sinha M, et al. (2010) Requirement of TGFbeta signaling for SMO-mediated carcinogenesis. J Biol Chem 285: 36570–36576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lauth M, Bergström A, Shimokawa T, Tostar U, Jin Q, et al. (2010) DYRK1B-dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat Struct Mol Biol 17: 718–725. [DOI] [PubMed] [Google Scholar]