Abstract
This paper presents a vertically positioned microfluidic system made of poly(dimethylsiloxane) (PDMS) and glass, which can be applied as a microbubble column (μBC) for biotechnological screening in suspension. In this μBC, microbubbles are produced in a cultivation chamber through an integrated nozzle structure. Thus, homogeneous suspension of biomass is achieved in the cultivation chamber without requiring additional mixing elements. Moreover, blockage due to produced carbon dioxide by the microorganisms—a problem predominant in common, horizontally positioned microbioreactors (MBRs)—is avoided, as the gas bubbles are released by buoyancy at the upper part of the microsystem. The patterned PDMS layer is based on an optimized two-lithographic process. Since the naturally hydrophobic PDMS causes problems for the sufficient production of microbubbles, a method based on polyelectrolyte multilayers is applied in order to allow continuous hydrophilization of the already bonded PDMS-glass-system. The μBC comprises various microelements, including stabilization of temperature, control of continuous bubble formation, and two optical configurations for measurement of optical density with two different sensitivities. In addition, the simple and robust application and handling of the μBC is achieved via a custom-made modular plug-in adapter. To validate the scalability from laboratory scale to microscale, and thus to demonstrate the successful application of the μBC as a screening instrument, a batch cultivation of Saccharomyces cerevisiae is performed in the μBC and compared to shake flask cultivation. Monitoring of the biomass growth in the μBC with the integrated online analytics resulted in a specific growth rate of 0.32 h−1, which is almost identical to the one achieved in the shake flask cultivation (0.31 h−1). Therefore, the validity of the μBC as an alternative screening tool compared to other conventional laboratory scale systems in bioprocess development is proven. In addition, vertically positioned microbioreactors show high potential in comparison to conventional screening tools, since they allow for high density of integrated online analytics and therefore minimize time and cost for screening and guarantee improved control and analysis of cultivation parameters.
INTRODUCTION
It is assumed that the percentage of microorganisms and mammalian cell lines that are yet to be discovered is around 95% to 97%, not including the significant number of knock-out mutants and recombinant strains, that could contribute to new biological product development in the food, pharmaceutical, energy, and environmental industry. Considering this together with the rapid progress of genomics, the development of cultivation platforms aiming towards reliable high-throughput (HTP) screening for bioprocess development remains a challenge. One of the major goals of screening is to perform parallel, automated and rapid readout that entails the estimation of the optimal process parameters for high yield bioproduction. Common screening methods are limited because the procedural expenses (e.g., sterilization, assembly, cleaning and calibration of sensors) escalate with an increasing quantity of microorganisms.1 The integration of online analytics and the continuous cultivation mode in conventional screening instruments, such as microwell plates, is very limited or even not possible. For this reason, innovative microenvironments are indispensable tools and their development has steadily increased over the last few years. For the study of microorganisms and cell lines, small volume cultures offer the advantageous combination of a global reduction in experimental costs and time with simultaneous process enhancement. This is related to the fact that microenvironments allow precise and fast control of procedural conditions together with an increased flexibility of parameter screening. During screening, continuous online monitoring and control of different physical, chemical, or biological parameters in these microenvironments are essential. This is due to the limitations of elaborate offline analytics, which results from the small available sample volumes in rapidly changing microcultures.2
The idea of miniaturization of existing macroscale reactor principles (e.g., stirred tank reactor or bubble column) instead of using microtiter plates goes along with the fact that conditions in a miniaturized system will be closer to those of the upscaled reactor. The first miniature stirred tank reactor was presented by Kostov et al.,3 who modified a 2 ml cuvette by implementing a miniaturized stirrer for active mixing and a small polymer gas distributor for sufficient aeration. Other examples where miniaturized bioreactors are mechanically agitated were reported by Harms et al.4 and Weuster-Botz et al.5 Instead of implementing stirrers, Reis et al. achieved mixing of the biomass via oscillating pump strokes.6 The recent developments in the field of miniaturized stirred tank bioreactors for applications in HTP bioprocess development were reviewed by Hortsch and Weuster-Botz.7 With regard to miniaturized bubble columns Doig et al.8 fabricated a 2 ml glass bubble column in which bubble formation was achieved via a porous polyethylene foil embedded in the bottom of the reaction chamber. Another vertical miniaturized bioreactor (volume of 1.5 to 2.5 ml) based on two communicating columns was developed by Diao et al.9 for cultivation of animal cell lines, in which mixing was achieved by periodical pumping of the culture medium via an air valve. For the cultivation of stem cells, Luni et al. presented a modified, standard 96-well plate in which stirring was provided by buoyancy-driven thermoconvection.10
The common reactor volume in miniaturized stirred tank reactors and bubble columns lies in the milliliter range, which may be considered as too large for some applications. Microfluidics represents the tackling of this issue. In addition, with microtechnology, innovative screening tools can be fabricated that simultaneously allow high density integration of fluidic structures and transducers for culture control and monitoring.11 This type of microdevice is generally known as a lab-on-chip (LoC) and provides the highest available performance from small detection volumes. Different integrable functional structures allow for online monitoring of various physical, chemical, and biological process parameters during cultivation. In addition, through the use of softlithographic techniques in combination with poly(dimethylsiloxane) (PDMS), inexpensive and disposable microchips can be produced with transparent and biocompatible characteristics.12 Up to date, several horizontally positioned microbioreactors (MBRs) operating in batch, fed-batch, and continuous modes have been reported. Schäpper et al.2 recently reviewed the current MBR platforms used in cultivation process development with volumes in the μl-range. Some of the MBRs feature online elements for different parameters such as dissolved oxygen (DO), pH, and optical density (OD). In most of the presented MBRs, active mixing for isotropic conditions was achieved with the use of commercially available miniature stir bars.13, 14, 15, 16 The basic materials of the chips included PDMS and/or polymethylmethacrylate. Another MBR was presented by Lee et al.17 and included micro-integrated peristaltic PDMS chambers that were used for mixing.
Although meaning a huge step forward, the most significant disadvantage of the horizontally positioned MBR is that microbubbles occuring frequently pose blockage or clogging problems in these microfluidic devices.18, 19 Microbubbles for example can appear in MBRs due to a change of medium during the experiment (in cultivation procedure, e.g., when the reactor device is inoculated with cells) or due to evaporation phenomenon (e.g., in batch cultivation). In long-term bioprocesses, bubble formation is inevitable because of the cells’ metabolism, which results in the conversion of oxygen to carbon dioxide. These uncontrolled, undesirable gas bubbles spoil the stable laminar flow and cause total channel clogging or strong divergence of the characteristic microfluidic flow followed by decreasing cell viability. Therefore, it is not trivial to completely eliminate bubbles in microfluidic passages. In this respect, a couple of methods for bubble removal in horizontally positioned microsystems have recently been proposed by different research groups. Kang et al.20 presented a pressure-driven bubble elimination method based on the blockage of all outlets, a constant inlet flow and the subsequent air permeation through the PDMS layer. However, this tedious method is not applicable for continuous cultivation procedures in MBRs. Another more complex method includes integrated active bubble traps presented by Skelley et al.21
An alternative for MBRs would be to have the flow in vertical direction and take advantage of buoyancy and hydrostatic forces to remove the bubbles. Surprisingly, this configuration is rather atypical for microfluidic systems and only a few vertically positioned LoCs have been presented: a microchip for polymerase chain reaction,22 a microflow cytometer based on gravity and electric forces,23 a microdevice for particle separation,24 and a microchip for the gravity-driven generation of microdisperse nanoliter particles.25
The work presented here aims to address this issue by implementing a novel microbubble column (μBC) that tackles the blockage and clogging due to produced bubbles, since the MBR is vertically positioned and bubbles are inherently released by buoyancy. Moreover, submerged aerobic cultivation is induced in the μBC via an additional gaseous (air or oxygen) phase in form of rising microbubbles that are produced through a microtechnologically patterned nozzle. A vertically positioned triphasic μBC possesses several advantages in comparison to the so far published MBRs (based on mechanical or peristaltic active mixing) such as simple construction, no mechanical moving parts, enhanced mass transfer properties, and low construction and operation costs (as the gas phase in the bioreactor can serve the dual functions of aeration and agitation). In order to control the physical parameters such as bubble formation and OD, different photonic elements have been implemented in the μBC. To validate the down-scalability from laboratory scale to microscale, a batch-cultivation was performed in the μBC and compared to a shake flask cultivation by referring to the OD over the cultivation time in both devices.
DESIGN OF THE μBC MICROCHIP
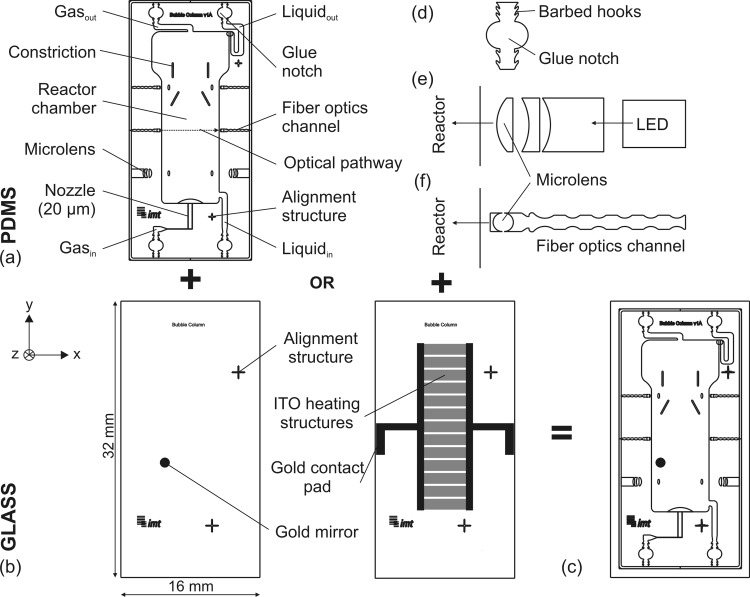
Figure 1a illustrates a schematic of the μBC microdevice (16 × 32 mm2), which contains two separate inlets for the gas and liquid phases, the reactor chamber, and the gas and liquid outlets. A heterogeneous circulation flow results from a constant gas throughput: cell suspension rises in the middle of the reactor chamber where the microbubbles are introduced and settles near the walls. The core of the μBC is the integrated gas nozzle (consisting of two parallel microchannels of 35 × 20 μm2 each) that is placed on the bottom of the reactor and permits the generation of microbubbles. Double photolithographic processing is necessary in order to attain a nozzle height smaller than the reactor height.
Figure 1.
Schematics of the μBC: (a) patterned PDMS layer, (b) microstructured glass bottoms with gold mirror for optical detection or with microheater, (c) PDMS-glass-microchip, (d) zoom in of implemented fluidic interface, (e) zoom in of the out-of-plane lens design that is patterned in PDMS to optically monitor bubble formation, (f) zoom in of implemented optical elements for OD monitoring in x-axis (in-plane).
The glass chip (Figure 1b), featuring functional elements for online analytics (such as gold mirror for optical measurements or microheater), overlaps in its dimensions the PDMS layer (Figure 1c) for two purposes: (1) providing stable support for the fluidic interface needles and (2) necessary space for gold structured pads and their electronic connection. Since the fluidic interface of the microchip is realized through commercially available needles, the challenge is to ensure a leakage-free interface that covers minimal space. This is achieved through integrated passive structures (Figure 1d) in the PDMS inlet and outlet channels featuring a combination of integrated glue notches and self-sealing barbed hooks. The semi-circle glue reservoirs are located at a distance of 500 μm from the edge of the PDMS chip. The barbed hooks—located both in front and behind of the reservoir—prevent glue from entering in the microchannel in order to avoid blockage of the channel. This structure offers a very reliable, space saving, and clean assembling method, as gluing is conducted within the microsystem and not outside of the device. Prior experimental tests have shown that these structures withstand pressures up to 1 bar.
Moreover, several functional elements for online measurements and analysis of physical and biological parameters are integrated within the μBC microchip. These elements are either integrated in the PDMS layer or microstructured on the glass bottom. The physical parameters addressed here include temperature stability and bubble formation monitoring. In addition, via integrated optical elements, the biological parameter of cell growth measured in OD can be analyzed in the μBC. In the following, the design and setup of the different elements for online analytics are explained.
Electrical microheater for temperature stability
One important requirement during cultivation is a defined constant temperature of the culture medium. Schäpper et al.2 reviewed the different engineering solutions for temperature stabilization and control within MBRs. The majority of presented heating systems are integrated on the exterior of the reactor and function via a temperature controlled incubator26 or a thermostated water bath. A few integrated microheaters have been presented that were microtechnologically fabricated and directly implemented in the MBR chip.27, 28 However, having an integrated heater in the MBR setup is by far the most preferable method of temperature control during cultivation, as it is simple, cheap, non-bulky and allows for parallel operation at different temperatures. Furthermore, it provides for quick response times and can also be used for quenching at high temperatures in order to rapidly deactivate biological material. Integrated heating structures made of indium tin oxide (ITO) are implemented in the μBC to allow homogeneous temperature stabilization. The microtechnologically fabricated microheater consists of resistors based on a parallel arrangement (Figure 1b). Via a subsequent chromium-gold layer, electrical pads for contacting the periphery are structured. The used ITO material features resistivities, which are too high to allow sufficient heating power when using supply voltages below 1 V. Therefore, a reliable isolation layer made of silicon nitride is required to prevent the water from electrolytic decomposition and thus the heating structures from erosion.
Optical measurement of bubble formation
Since constant and robust bubble formation and rising is important for a stable cultivation process (e.g., for continuous mixing), it should be monitored during the cultivation in the μBC. Bubble formation is dependent on several factors: (1) construction parameters (nozzle geometry, surface characteristics of the material), (2) process parameters (e.g., gas rates and applied gas pressure), and (3) material properties of the fluids (viscosity, density, surface tension). Moreover, monitoring of stopped bubble formation is always important, when for example OD has to be measured via the in-plane configuration (see next Sec. 2C). For this, it has to be assured that bubble rising has stopped before performing the optical OD measurement. To this effect, a simple and inexpensive out-of-plane optical setup was developed where light incoming from an LED is divergence-corrected by ways of a microlens patterned in the PDMS (Figure 1e) and coupled to a silicon photodiode that is located perpendicular to the optical path (in z-axis). The design of the microlens resembles a composite lens with a width of 1000 μm, which takes into account the different refractive indices of PDMS and air and thus imitates a classical collecting lens.29
Optical measurement of OD
Measuring the OD during the cultivation time gives information about the biomass growth and is thus a very crucial parameter for evaluating the performance of a bioprocess. Generally, the higher the biomass concentration, the higher the OD, but also the smaller the signal that reaches the readout. To this effect, two complementary configurations for measuring the OD have been implemented in the μBC.
In order to avoid any intervention in the running system (e.g., by switching off the bubble formation), a detection configuration was developed that allows for out-of-plane measurements of the OD (along the z-axis of the reactor and perpendicular to the PDMS cover). This configuration permits for implementation at positions void of bubbles and only requires a maximal area of 1 mm2 (Figure 1b). Light that is coupled via optical fibers located perpendicular to the bioreactor chamber is reflected by a gold mirror (which is structured on the glass substrate shown in Figure 1b) and coupled to a second fiber optics, which is in turn connected to the readout. Both fiber optics with a diameter of 230 μm each are closely placed next to each other in the optical bridge connector. Considering the numerical aperture of the fiber of 0.22 and the system thickness, this configuration allows to capture the light reflected from the gold mirror. The resulting optical path for performing the analysis of the biomass growth is twice the μBC depth (length in z-axis).
An additional configuration included in the design allows measuring with a higher sensitivity. To this effect, OD measurements are done in-plane (along the x-axis). Optical fiber channels are defined at both sides of the μBC that allow for stable and robust clamping of the fiber optics. Biconvex microlenses30 correct the numerical aperture of the fiber optics (Figure 1f). Using this configuration, the optical path length is 6.7 mm, resulting in a much longer interaction with the media to be analyzed than in the out-of-plane OD measurement.
MATERIALS AND METHODS
In Secs. 3A, 3B, 3C, 3D, the detailed processes for fabricating the μBC are explained. First, the patterned PDMS layer was produced using UV-depth lithography and softlithography. The PDMS was bonded to a previously structured glass bottom and the microfluidic interface was established. Subsequently, the microchip was surface modified in order to achieve hydrophilic surface characteristics. The details of each step are described below.
Fabrication of patterned PDMS layer
The fabrication of the patterned PDMS layer includes UV-depth lithography by using a negative photoresist (SU-8) and recursive molding by using softlithography and PDMS. A double photolithographic process was developed to structure the negative master. This process started with the sputtering of a chromium-gold layer on a 700 μm thick soda-lime glass substrate (Borofloat®33, Schott AG, Germany). The metallic layer was photolithographically structured with ma-P 1215 (micro resist technology GmbH, Germany). Afterwards, the first resist layer (SU-8 5) (MicroChem Corporation, MA, USA) was spun on the top side of the glass substrate at 2500 rpm for 30 s and dried for 10 min at 95 °C. This layer—acting both as a seed layer and an adhesion promoter—is flood exposed to UV-light and baked at 95 °C for 10 min. Before spin-coating of the subsequent structure layers, the seed layer was activated in oxygen plasma. The first thin layer of SU-8 25 (resulting to be 20 μm in height) that was patterned with the nozzle design, was spun-on with 2500 rpm and dried for 1.5 h at 95 °C. This structure layer was exposed to UV-light at 140 mJ/cm2 after aligning the mask to the alignment structures. A post exposure bake (PEB) for 20 min at 95 °C was followed. As the second layer features a total height of 500 μm with SU-8 50, it resulted to be better to first develop the first layer before spinning the thick layer. This procedure guaranteed a precise development of small structures in the first layer despite the thick layer. Therefore, unpolymerized regions of the SU-8 25 were removed in propylene glycol methyl ether acetate (PGMEA) (MicroChem Corporation, MA, USA). Prior to spin-coating of the second structure layer the first structured layer was baked again for 1 h at 95 °C. An intermediate layer of SU-8 25 on top of the developed first layer resulted to be favorable in order to better apply the thicker SU-8 50 on the master. The process parameters for this intermediate layer included a spin-coating at 1500 rpm with a subsequent baking for 1 h at 95 °C. Afterwards, SU-8 50 was spun at 700 rpm, leveled and dried at 95 °C for 2 h. The same process step (SU-8 50 at 700 rpm) was repeated, including additionally 4 h of drying. Thus, the total layer thickness of the second layer resulted to be 500 μm. The structure layer was exposed to UV-light at 700 mJ/cm2. After the PEB of 45 min at 95 °C, the layer was developed in PGMEA. Replicas of this double structured master were patterned by the use of PDMS (Sylgard 184 elastomer kit, Dow Corning, MI, USA). For that, the silicon elastomer and the curing agent were mixed in the standard ratio of 10:1, respectively, poured on the master and heated at 80 °C for 30 min. After polymerization the PDMS elements were peeled off the master, which can be reused afterwards. Since each μBC on the master is surrounded by a patterned SU-8 molding frame (0.25 mm of thickness), the molding procedure results to be simple and elaborate cutting can be avoided.
Fabrication of the microstructured glass bottom
A 700 μm thick soda-lime glass bottom (Borofloat®33, Schott AG, Germany) was structured with the ITO microheater as follows. The parallel heating structures were made of ITO (90 wt. % indium oxide and 10 wt. % tin oxide from FHR Anlagenbau GmbH, Germany), which was sputtered for 240 s in order to achieve a layer thickness of 80 nm. After spin-coating of ma-P 1215 resist, its exposure to UV light for 10 s (with the parallel heating structures) and development, the ITO conductors were etched in an acidic HCl 37%-water 1:10 solution. A chromium-gold layer was deposited by sputtering and photolithographically structured (using ma-P 1215) to form the pads for contacting the microheater to the periphery, the alignment structures and the gold mirror. Subsequently, silicon nitride obtained by plasma enhanced chemical vapor deposition (PECVD) at 350 °C was deposited and resulted in isolation layers of approximately 150 nm in thickness. After protection of the heating structures, the silicon nitride layer above the gold contact pads was finally plasma-etched in a tetrafluoromethane-oxygen atmosphere (60 ccm O2 and 200 ccm CF4 at 150 W for 30 min) in a barrel etcher (Typ 308 PC, Surface Technology Systems, Germany).
Bonding and procedure for fluidic interface
Before bonding, the reservoirs (resulting glue notches) of the fluidic interfaces were punched with a dispenser tip (ø 0.5 mm, Nordson, Germany). Then both elements—the patterned PDMS layer and the microstructured glass bottom—were irreversibly bonded to each other after surface activation in air plasma (plasma activate flecto 10 USB, Plasma Technology, Germany) at 30 W and 0.2 mbar for 90 s. In order to align both elements to each other, the surface activation was prolonged by surface wetting with ethanol. After bonding, previously cut needles (Sterican, ø 0.8 mm, B. Braun, Germany) were inserted in the fluidic inlet and outlet structures. Afterwards silicone glue (RS Components, Germany) was applied with a high pressure dispenser (Ultimus, EFD, UK) surrounding the needle and sealing the fluidic interface after curing.
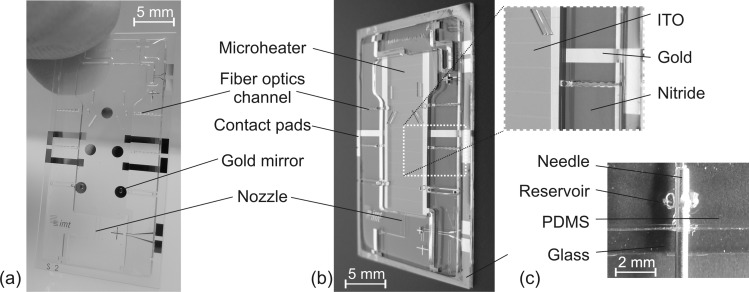
Figure 2 depicts the fabricated μBC with a reactor volume of 70 μl and its integrated microelements and fluidic interface.
Figure 2.
Image of the fabricated μBC: (a) with gold mirrors, (b) with microheater. (c) Photograph of one of the fluidic interfaces.
Hydrophilization procedure of the μbc
PDMS hydrophilization is indispensable for reliable bubble generation in the μBC. A hydrophilization procedure was developed within our research group that allows for stable hydrophilization characteristics of already closed microfluidic systems. The procedure is based on polyelectrolyte multilayers (PEMs) that can be assembled via continuous flow.31
The μBC chip was first cleaned with ethanol and ultrapure water. Before the layer-by-layer coating with PEMs, the chip was treated overnight with diluted HCl (0.1 M) in order to provide charged anchoring groups on the PDMS surface. Afterwards, the system was thoroughly rinsed with ultrapure water. The pretreated μBC was then exposed alternately to two polyelectrolyte solutions of poly(diallyldimethylammonium chloride) (PDADMAC) (containing 0.1 mol/l sodium chloride) and poly(acrylic acid) (PAA) (adjusted to pH 3.5) by automated injection via the fluid inlet. Each adsorption step took 30 s at a flow rate of 14 ml/min after which the μBC was rinsed with ultrapure water for 60 s at the same flow rate. After the end of the adsorption procedure, which took about 21 min in the whole, the μBC’s surface featured seven polyelectrolyte double layers, starting with the positively charged PDADMAC and ending with PAA as the outermost layer.
Preparation of inoculum cultures for cultivation
The feasibility of the μBC as a screening instrument compared to conventional shake flasks was analyzed by cultivation of the model microorganism Saccharomyces cerevisiae CCOS 538 (ATCC 32167) from the Culture Collection of Switzerland AG. Cells were cultivated in a modified Verduyn mineral medium with 20 g/l glucose as presented in Ref. 32. For all inoculum cultures carried out either in the μBC or in the baffled shake flask (Schott, Germany), cells (inoculated from agar plates) were grown in shake flasks (500 ml autoclaved flask with 100 ml of Verduyn medium at a pH of 4.5) at 120 rpm and at a constant temperature of 30 °C for 10 h. For cultivation experiments, the inoculum was diluted to an OD600nm of 0.4 (Spectrophotometer, SmartSpec 3000, Bio-Rad Laboratories GmbH, Germany). 100 ml was inoculated in a 500 ml shake flask and 2 ml was used for the μBC (see chap. V).
CHARACTERIZATION
Experimental setup
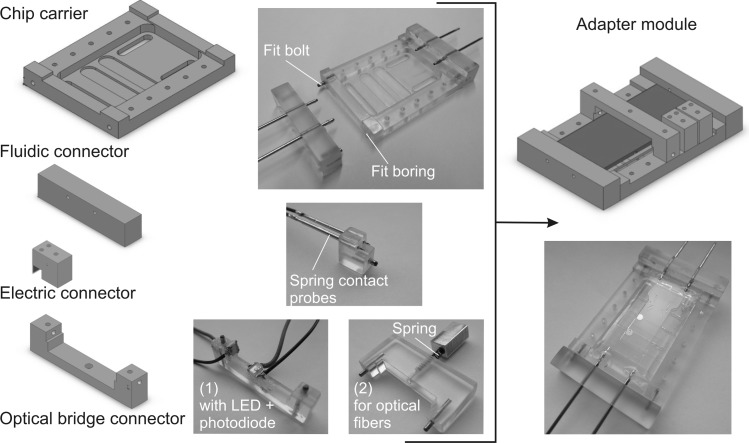
To perform a robust characterization of the proposed μBC, a custom-made adapter (Figure 3) was developed that fulfills the following requirements: (1) an adapter based on a plug‘n’play concept for easy replacement of single μBC modules and (2) integrated fluid, electronic, and optical connectors for stable micro-to-macro interface. The adapter module made of polycarbonate consists of a chip carrier and fluidic/electrical/optical plug-in connectors. The μBC microchip was placed on the chip carrier, which features through-holes for the plug-in connectors. Subsequently, the fluidic connector containing the needles was plugged into the chip carrier via the fit borings and fit bolts. One of the main advantages of the fluidic connector—apart from being attractive in use for disposable microsystems—is the provided stability of the integrated fluidic needles.
Figure 3.
Schematics and photographs of the adapter module made of polycarbonate including the chip carrier for the μBC microchip and the fluidic (including fit borings and fit bolts), electrical, and optical connectors. The two different optical bridge connectors for out-of-plane OD monitoring comprise either (1) an LED and photodetector (used for bubble formation monitoring) or (2) two optical fibers.
The requirements of the electrical and optical connectors address aspects such as universal applicability, reusability, reliability, and easy handling. The electrical plug offers an easy way of connecting microchips featuring microstructured contact pads to the system periphery (here, e.g., for contacting the microheater). Spring contact probes (INGUN Prüfmittelbau GmbH, Germany) are integrated into the electrical connector to obtain user-friendly and reliable contact between the microstructured gold-pads and the periphery. In addition, irreversible and elaborate soldering can be avoided. The basic optical connector consists of a plug-in bridge that spans the LoC-system along the z-axis (out-of-plane configuration). Two different optical connectors were designed and comprise the following: (1) an LED (Everlight 67–21 UWC/S400-XX/TR8) and a photodiode (Kingbright/KPS-3227 (2.7 × 3.2 × 1.1 mm3)) (used for bubble formation monitoring), (2) two multimode optical fibers (M24L05, Thorlabs GmbH, Germany) that are coupled via a spring in order to couple light emitted from a red LED to the μBC, as well as to carry the light emerging from the μBC to the microspectrometer (USB2000 + XR1-ES, Ocean Optics Germany GmbH). For the in-plane measurements of the OD, two multimode optical fibers were inserted in the microstructured fiber optics channels. The light was emitted by a halogen lamp (HL-2000-HP-FHSA, 20 W output, Ocean Optics Germany GmbH) and coupled via the output fiber optics to the microspectrometer.
Integrated ITO-heater for temperature stabilization
Thermal distribution patterns and thus the heating performance of the fabricated microheater structures were examined by monitoring heat images that were taken with a thermo infrared camera (THV550, Flir Thermovision, Germany). A linear regression (with an accuracy of 99.5%) between the temperature T of ca. 50 μl of water as a function of the required heating power P was obtained: T = 30.571·P + 23.018 Here, for achieving a constant temperature of 30 °C—as commonly used for many cultivation processes—a heating power of 0.2 W is therefore necessary. To determine the temperature distribution of the ITO electrodes within the μBC filled with water, thermal images were taken via an IR Thermal Radiometric Camera (MobIR® M3 InfraTec GmbH, Germany) connected to a triple power supply (HM 7042-5 Navair, USA). The distance between the chip and the camera was 5.5 cm. After setting the adequate voltage, it took 2 min until the temperature distribution reached a steady state. At this stage, the temperature fluctuation was less than ±0.5 °C and the image was acquired.33
Optical measurement of bubble formation
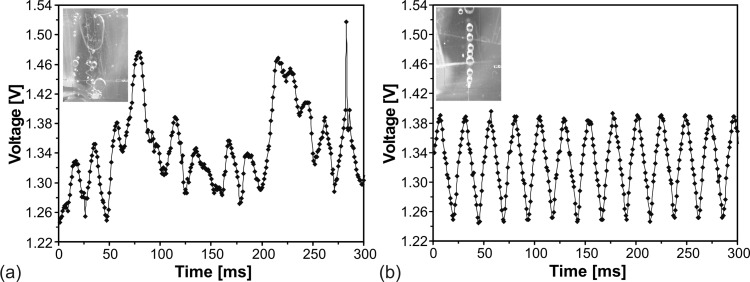
The functionality of the out-of-plane LED-photodiode configuration for bubble monitoring was examined for bubble formations (instable and stable) within a system of ethanol and air. The signal recording was done with the software “tracer daq” (Meilhaus Electronic GmbH, Germany). Figure 4 illustrates the differences in obtained signal from the photodetector for unstable (a) and stable bubble generation (b) that results in scattered and homogeneous profile, respectively. For the stable bubble formation, every voltage minimum correlates to one bubble that passes the measuring light path. In the unstable case, the typical waveform of the signal is missing and according to each bubble size a variation of peak height and width occurs. As the functionality of the inexpensive optical sensor setup was proven, it can also be employed to monitor other parameters such as foam production during cultivation.
Figure 4.
Voltage signal of the photodetector versus time demonstrating (a) unstable and (b) stable bubble formation.
Optical measurements of the OD
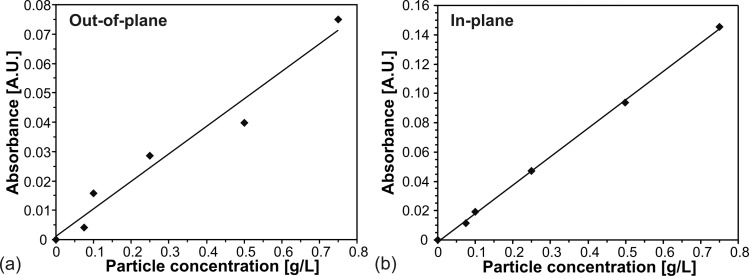
In order to compare the two designs for OD measurement (out-of-plane and in-plane), their optical performance was characterized using PMMA loaded deionized water suspensions (BB01N/2242, average particle size of 6.5 μm, Bangs Laboratories Inc., IN, USA) of different concentrations (0.075, 0.1, 0.25, 0.5, 0.75 g/l). These PMMA particles were chosen to mimic the cell suspension of S. cerevisiae, since they resemble their average cell size and density. The procedure for the absorbance measurements can be described with the following steps. First, a reference of the reactor chamber filled with water was taken. Subsequently, dilutions of progressively higher concentrations of PMMA particles were injected. For each concentration, a minimum of 10 consecutive scans was acquired to minimize the experimental error. Once the highest concentration had been analyzed, a water reference solution was injected and measured again to confirm that no drift of the base signal compared to the initial value had been produced during the characterization procedure.
Figure 5 shows the resulting calibration curves for the measurement in out-of-plane (a) and in-plane (b). The absorbance was seen to follow the Beer-Lambert law in both cases with an accuracy of 96.3% for out-of-plane and 99.9% for in-plane setup. With the values obtained from the linear fit, the limit of detection (LOD) of both optical elements could be deduced according to the 3-sigma IUPAC definition.34 In doing so, the LOD and the sensitivity for the out-of-plane optical system was obtained to be (0.11 ± 0.01) g/l and (0.094 ± 0.009) A.U./(g/l), respectively, whereas for the in-plane configuration these figures were (0.0147 ± 0.0002) g/l and (0.194 ± 0.002) A.U./(g/l). These results are due to the almost seven fold higher optical path of the online element in x-axis (6.7 mm) when compared to the path in z-axis (1 mm).
Figure 5.
Absorbance versus PMMA particle concentration of the OD measurement (a) out-of-plane and (b) in-plane.
Therefore, the here proposed μBC has demonstrated to be suitable to work in two different configurations (in- and out-of-plane) depending on the needed accuracy and sensitivity of the optical analysis. In general, when accuracy results to be sufficient, the online measurement in z-axis (out-of-plane) is more suited for measurements where a continuous flow is required. Conversely, if the flow can be stopped, the in-plane configuration—because of its sensitivity—is preferred.
CULTIVATION IN μBC VS. SHAKE FLASK
In order to prove the functionality and feasibility of the μBC in comparison to classical screening instruments, a batch cultivation of S. cerevisiae was simultaneously performed in both the μBC and a shake flask. For all developed microfluidic elements, it is paramount to assure a reliable prediction of process outcome on the microscale. Obtained data must therefore always be compared to results achieved on the macroscale, in so-called cross validations. This will validate that screening on the microscale is of sufficient and representative quality. If this is the case, microscreening tools will be of high economical interest.
First of all, a cultivation procedure had to be developed to operate the cultivation in the μBC. To evaluate the cultivation performance in both tools, the biomass was analyzed over the cultivation time via online and offline analysis in the μBC and the shake flask, respectively. The other successfully proven online analytic elements (microheater, bubble control, out-of-plane OD) were not applied for this first feasibility study in order to better focus on the cultivation procedure at microscale itself.
Cultivation procedure
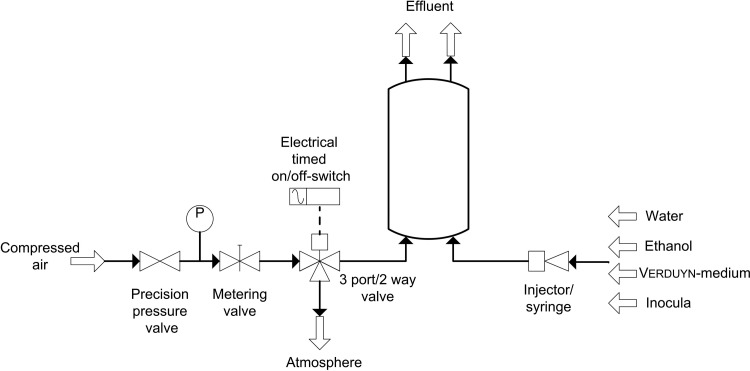
Prior to cultivation in the μBC, a suitable cultivation setup (Figure 6) and protocol were established.
Figure 6.
Schematic setup of the cultivation procedure in the μBC.
The peripheral setup included both a precision pressure valve (LRP-1/4-0,7, Festo AG & Co.KG, Germany) and a metering valve (M3A-H0L-V-SS-TC, Parker Hannifin Corporation, Germany) in order to set a defined flow rate for the introduced compressed air and to allow for formation of microbubbles within the μBC. For this measurement principle, the bubble formation had to be stopped in defined intervals, which was automatically performed via an electrical timed on/off-switch located behind the metering valve. When the control switch is active, the 3/2 way valve (cetoni GmbH, Germany) is directed in the way that air flows out to the atmosphere. During the preparation and inoculation procedure of the μBC, the injection of the different media was carried out with syringes. First, the μBC was flushed with 70% ethanol during 20 min for disinfection, then rinsed with 5 ml water and finally filled with Verduyn-medium. Then, the optical reference value was recorded with the microspectrometer. The reference cultivation was performed in a baffled shake flask (Schott, Germany) filled with 180 ml of preliminarily sterilized Verduyn-medium. The initial OD600nm in the reference cultivation shake flask was set to 0.4 using the pre-culture. Subsequently, 2 ml of the already inoculated cultivation media were taken from the reference shake flask and flushed in the μBC through the fluid inlet channel in order to guarantee identical initial conditions in both the μBC and the shake flask. The initial cultivation OD was monitored in the microchip and the gassing was readjusted. A constant and adequate bubble formation could be observed at a pressure of 115 mbar and a flow rate between 5 ml/h and 20 ml/h. Since the microheater was not used in this feasibility study, the shake flask was incubated at 120 rpm and a temperature of 24 °C. The OD in the shake flask cultivation was analyzed offline every 1 to 2 h in the spectrophotometer (SmartSpec 3000, Bio-Rad Laboratories GmbH, Germany), whereas the online monitoring of the OD in the μBC was automatically performed via the supplied software Spectrasuite (Ocean Optics Germany GmbH) once per hour.
Results
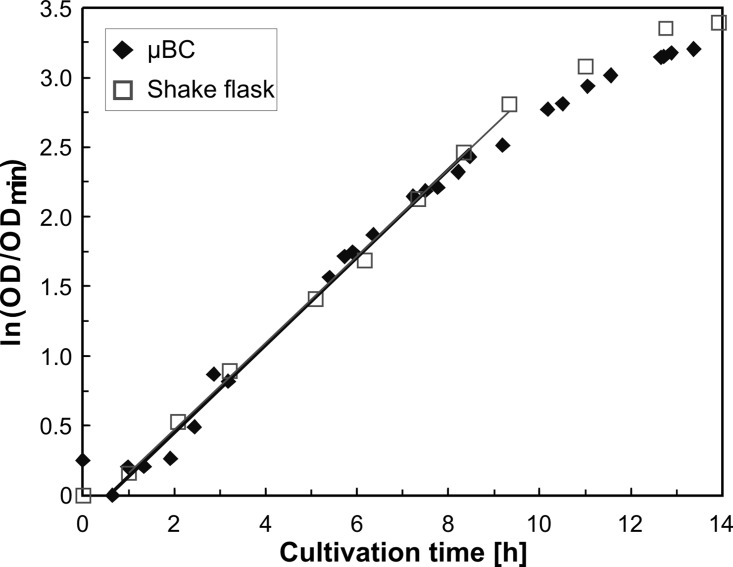
Within the surface modified μBC, a stable constant mixing via formation of microbubbles through constant gassing could be achieved for entire observation time (14 h). Figure 7 illustrates the obtained OD results over cultivation time for both the μBC and the shake flask cultivation. The values feature the average of two measurements per two samples taken out of the shake flask at a wavelength of 600 nm and the average of three measurements (with 1000 scans to average) taken online in the μBC in a time span of 5 min at the wavelength of 607 nm. The deviation of these three measurements was below 1.5% of the middle value showing how homogeneously the reactor was mixed when referring to the longest possible optical pathway in horizontal position. The expected batch curve including lag, exponential, and stationary phases could be depicted for both cultivations. The exponential growth in the μBC could be observed from 0.65 h until 8.5 h with an estimated specific growth rate (μ) of 0.32 h−1, whereas the cultivation in the shake flask showed exponential growth from 1 to 9.33 h with μ = 0.31 h−1. The cross-correlation of both the in-plane OD measurement in the μBC and the OD measurement with the photometer was evaluated for comparison and validation purposes by measuring different concentrations of S. cerevisiae.33 Considering these results, the validity of the μBC as an alternative screening tool compared to other laboratory scale systems in bioprocess development has been demonstrated. The μBC features two major advantages when compared to conventional cultivation tools such as shake flasks or microwellplates: (1) The possibility of fed-batch and continuous cultivations and (2) the high amount of online analytic elements that can be integrated in the microdevice via microtechnological procedures. The high density of integrated online analytics minimizes time and cost for screening and in addition guarantees improved control and analysis of cultivation parameters. In the future, these aspects will be investigated.
Figure 7.
OD versus cultivation time in the μBC and the shake flask.
CONCLUSION AND OUTLOOK
This paper presents for the first time a μBC based on microtechnological fabrication that could be successfully proven for its purpose as a new screening tool in biotechnological applications. With this reactor design one of the main risks—the blockage or clogging due to produced carbon dioxide bubbles within horizontally positioned MBRs—could be overcome. By vertical positioning of the microsystem, generated microbubbles, which induce the homogeneous mixing in the bioreactor, are released by buoyancy at the upper part of the chamber due to hydrostatic forces. The core of the disposable LoC-system—made of a patterend PDMS layer bonded to a glass bottom—is the nozzle structure for bubble formation. A two-lithographic process was optimized in order to achieve an SU-8 master with two structured layers (20 μm for the nozzle height and 500 μm for the overall chamber height). As microbubbles can only be generated when hydrophilic surface characteristics are existent, a surface modification based on PEMs was applied in the already bonded microsystem. Furthermore, the integration and functionality of various online analytics could be successfully proven. Among others, a microstructured microheater allowed for temperature stability of 30 °C—as it is commonly used for many cultivation processes—with an applied heating power of 0.2 W. Via integrated optical microelements, bubble formation and OD based on absorbance measurements could be reliably guaranteed. Two different settings for monitoring the OD were developed: out-of-plane (that does not require any intervention in the bubble rising) and in-plane (which only provides reliable results when bubble formation is shortly stopped). Since the in-plane method revealed with an LOD of (0.0147 ± 0.0002) g/l and a sensitivity of (0.194 ± 0.002) a.u./(g/l) better performance—which is due to the longer optical path—this optical principle was chosen for the cultivation feasibility study. To validate the scalability from laboratory scale to microscale, and thus to demonstrate the successful application of the μBC as a screening instrument, a batch cultivation of S. cerevisiae was conducted in the μBC and compared to shake flask cultivation. Monitoring of the biomass growth in the μBC resulted in a specific growth rate of 0.32 h−1, which was almost identical to the one achieved in the shake flask cultivation (0.31 h−1) and thus proves the validity of the microdevice as an alternative screening tool.
ACKNOWLEDGMENTS
The authors would like to thank the German Research Foundation (DFG) for support of this research in the framework of the Collaborative Research Group mikroPART FOR856 (Microsystems for particulate life-science products). One of the authors (S.B.) gratefully acknowledges the financial support of the Volkswagen Foundation. The research leading to these results has also received funding from the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement No. 209243.
References
- Zanzotto A., Szita N., Boccazzi R., Lessard P., Sinskey A. J., and Jensen K. F., Biotechnol. Bioeng. 87(2), 243 (2004). 10.1002/bit.20140 [DOI] [PubMed] [Google Scholar]
- Schäpper D., Alam M. N. H. Z., Scita N., Lantz A. E., and Gernaey K. V., Anal. Bioanal. Chem. 395, 679 (2009). 10.1007/s00216-009-2955-x [DOI] [PubMed] [Google Scholar]
- Kostov Y., Harms P., Randers-Eichhorn L., and Rao G., Biotechnol. Bioeng. 72(3), 346 (2001). [DOI] [PubMed] [Google Scholar]
- Harms P., Kostov Y., French J. A., Soliman M., Anjanappa M., Ram A., and Rao G., Biotechnol. Bioeng. 93(1), 6 (2006). 10.1002/bit.20742 [DOI] [PubMed] [Google Scholar]
- Kusterer A., Krause C., Kaufmann K., Arnold M., and Weuster-Botz D., Bioprocess. Biosyst. Eng. 31(3), 207 (2008). 10.1007/s00449-007-0195-z [DOI] [PubMed] [Google Scholar]
- Reis N., Gonçalves C. N., Vicente A. A., and Teixeira J. A., Biotechnol. Bioeng. 95(4), 744 (2006). 10.1002/bit.21035 [DOI] [PubMed] [Google Scholar]
- Hortsch R. and Weuster-Botz D., Adv. Appl. Microbiol. 73, 61 (2010). 10.1016/S0065-2164(10)73003-3 [DOI] [PubMed] [Google Scholar]
- Doig S. D., Diep A., and Baganz F., Biochem. Eng. J. 23, 97 (2005). 10.1016/j.bej.2004.10.014 [DOI] [Google Scholar]
- Diao J., Young L., Zhou P., and Shuler M. L., Biotechnol. Bioeng. 100(1), 72 (2008). 10.1002/bit.21751 [DOI] [PubMed] [Google Scholar]
- Rao G., Moreira A., and Brorson K., Biotechnol. Bioeng. 102(2), 348 (2009). 10.1002/bit.22192 [DOI] [PubMed] [Google Scholar]
- Luni C., Feldman H. C., Pozzobon M., De Coppi P., Meinhart C. D., and Elvassore N., Biomicrofluidics 4, 034105 (2010). 10.1063/1.3380627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides G. M., Ostuni E., Takayama S., Jiang X., and Ingber D. E., Annu. Rev. Biomed. Eng. 3, 335 (2001). 10.1146/annurev.bioeng.3.1.335 [DOI] [PubMed] [Google Scholar]
- Szita N., Boccazzi P., Zhang Z., Boyle P., Sinskey A. J., and Jensen K. F., Lab Chip 5, 819 (2005). 10.1039/b504243g [DOI] [PubMed] [Google Scholar]
- Boccazzi P., Zhang Z., Kurosawa K., Szita N., Bhattacharya S., Jensen K. F., and Sinskey A. J., Biotechnol. Prog. 22, 710 (2006). 10.1021/bp0504288 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Perozziello G., Boccazzi P., Sinskey A. J., Geschke O., and Jensen K. F., J. Assoc. Lab. Autom. 12, 143 (2007). 10.1016/j.jala.2006.10.017 [DOI] [Google Scholar]
- Schäpper D., Stocks S. M., Szita N., Lantz A. E., and Gernaey K. V., Chem. Eng. J. 160(3), 891 (2010). 10.1016/j.cej.2010.02.038 [DOI] [Google Scholar]
- Lee K. S., Boccazzi P., Sinskey A. J., and Ram R. J., Lab Chip 11, 1730 (2011). 10.1039/c1lc20019d [DOI] [PubMed] [Google Scholar]
- Jensen M. J., Goranovic G., and Bruus H., J. Micromech. Microeng. 14, 876 (2004). 10.1088/0960-1317/14/7/006 [DOI] [Google Scholar]
- Demming S., Vila-Planas J., Aliasghar Zadeh S., Edlich A., Franco-Lara E., Radespiel R., Büttgenbach S., and Llobera A., Electrophoresis 32(3–4), 431 (2011). 10.1002/elps.201000482 [DOI] [PubMed] [Google Scholar]
- Kang J. H., Kim Y. C., and Park J.-K., Lab Chip 8, 176 (2008). 10.1039/b712672g [DOI] [PubMed] [Google Scholar]
- Kelley A. M. and Voldman J., in Proceedings of International Conference on Miniaturized Systems for Chemistry and Life Sciences (2008), Vol. 12, p. 1360.
- Liu R. H., Yang J., Lenigk R., Bonanno J., and Grodzinski P., Anal. Chem. 76, 1824 (2004). 10.1021/ac0353029 [DOI] [PubMed] [Google Scholar]
- Yao B., Luo G., Feng X., Wang W., Chen L. X., and Wang Y.-M., Lab Chip 4, 603 (2004). 10.1039/b408422e [DOI] [PubMed] [Google Scholar]
- Huh D., Bahng J. H., Ling Y., Wei H.-H., Kripfgans O. D., Fowlkes B., Grothberg J. B., and Takayama S., Anal. Chem. 79, 1369 (2007). 10.1021/ac061542n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lin J.-M., and Knopp D., J. Micromech. Microeng. 18, 095014 (2008). 10.1088/0960-1317/18/9/095014 [DOI] [Google Scholar]
- van Leeuwen M., Heijnen J. J., Gardeniers H., Oudshoorn A., Noorman H., Visser J., van der Wielen L. A. M., and van Gulik W. M., Chem. Eng. Sci. 64, 455 (2009). 10.1016/j.ces.2008.09.023 [DOI] [Google Scholar]
- Yamamoto T., Nojimab T., and Fujii T., Lab Chip 6, 197 (2002). 10.1039/b205010b [DOI] [PubMed] [Google Scholar]
- Petronis S., Stangegaard M., Christensen C. B. V., and Dufva M., Biotechniques 40, 368 (2006). 10.2144/000112122 [DOI] [PubMed] [Google Scholar]
- Seo J. and Lee L. P., Sens. Actuators, B 99(2–3), 615 (2004). 10.1016/j.snb.2003.11.014 [DOI] [Google Scholar]
- Vila-Planas J., Fernández-Rosas E., Ibarlucea B., Demming S., Nogués C., Plaza J. A., Domínguez C., Büttgenbach S., and Llobera A., Nat. Protoc. 6, 1642 (2011). 10.1038/nprot.2011.383 [DOI] [PubMed] [Google Scholar]
- Schmolke H., Demming S., Edlich A., Magdanz V., Büttgenbach S., Krull R., Klages C.-P., and Franco-Lara E., Biomicrofluidics 4, 044113 (2010). 10.1063/1.3523059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich A., Magdanz V., Rasch D., Demming S., Zadeh S. A., Segura R., Kähler C., Radespiel R., Büttgenbach S., Franco-Lara E., and Krull R., Biotechnol. Prog. 26(5), 1259 (2010). 10.1002/btpr.449 [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4738587 for an IR image showing the temperature distribution of the ITO electrodes within the μBC filled with water (without gassing) and for experimental measurements using different concentrations of S. cerevisiae in both optical systems.
- Mocak J., Bond A. M., Mitchell S., and Scollary G., Pure Appl. Chem. 69(2), 297 (1997). 10.1351/pac199769020297 [DOI] [Google Scholar]