Abstract
The enzymatic preparation of biodiesel has been hampered by the lack of suitable solvents with desirable properties such as high lipase compatibility, low cost, low viscosity, high biodegradability, and ease of product separation. Recent interest in using ionic liquids (ILs) as advanced reaction media has led to fast reaction rates and high yields in the enzymatic synthesis of biodiesel. However, conventional (i.e., cation–anion paired) ILs based on imidazolium and other quaternary ammonium salts remain too expensive for wide application at industrial scales. In this study, we report on newly-synthesized eutectic ILs derived from choline acetate or choline chloride coupled with biocompatible hydrogen-bond donors, such as glycerol. These eutectic solvents have favorable properties including low viscosity, high biodegradability, and excellent compatibility with Novozym® 435, a commercial immobilized Candida antarctica lipase B. Furthermore, in a model biodiesel synthesis system, we demonstrate high reaction rates for the enzymatic transesterification of Miglyol® oil 812 with methanol, catalyzed by Novozym® 435 in choline acetate/glycerol (1 : 1.5 molar ratio). The high conversion (97%) of the triglyceride obtained within 3 h, under optimal conditions, suggests that these novel eutectic solvents warrant further exploration as potential media in the enzymatic production of biodiesel.
Introduction
Ionic liquids (ILs) are semi-organic fluids consisting wholly of ions which remain liquid at temperatures below 100 °C. As alternatives to conventional volatile organic solvents, ILs are enjoying an exponential growth in exploration as reaction media for biocatalysis,1–3 mainly due to their attractive features such as low volatility and tunable property sets. However, the most widely-known ILs derive from rather expensive cations (e.g., imidazoliums, pyridiniums, pyrrolidiniums, piperidiniums), preventing these promising solvents from being utilized at commercial scales. On the other hand, there is an increasing demand for inexpensive ILs readily available in large quantities for the industrial production of affordable commodities such as cellulosic ethanol4,5 and biodiesel.6–9
Recently, the Abbott group10–12 has demonstrated that the mixture of a solid organic salt and a suitable complexing agent can sometimes liquify at temperatures below 100 °C, a socalled ‘deep eutectic IL’. The mechanism put forth is that the complexing agent (typically a hydrogen-bond donor) interacts with the anion, thereby increasing its effective size and shielding its interaction with the cation, in turn inducing a depression in the melting point (Tm) of the mixture. A superb example is the mixture of choline chloride (Tm = 302 °C, 2-hydroxyethyl-trimethylammonium chloride, Scheme 1a) and urea (Tm = 133 °C) in a 1 : 2 molar ratio, resulting in a room-temperature deep eutectic IL (Tf = 12 °C).10 A major advantage of this approach is that inexpensive, bulk commodity, and non-toxic chemicals can frequently be employed, and the liquid properties can be fine-tuned by combining various organic salts and complexing agents in differentratios. Considering that many inexpensive quaternary ammonium salts are available and there exists a wide variety of amides, amines, carboxylic acids and alcohols to serve as complexing agents, the possibilities for forming new and inexpensive eutectic ILs are enormous. In particular, choline chloride, so-called vitamin B4, is produced on the Mtonne (million metric ton) scale annually as an additive for chicken feed, among other applications. In addition to its low cost, choline chloride is considered to be non-toxic and biodegradable.13 In fact, choline chloride is an essential micronutrient and human nutrient.14 Meanwhile, eutectic ILs can dissolve a variety of metal salts, acids (aromatic, amino, citric, benzoic), and polyols such as glucose or glycerol.10,11,15–17 Therefore, eutectic ILs derived from cholinium cations are suitable for numerous large-scale commercial applications.
Scheme 1.

Structures of choline chloride (1a), choline acetate (1b), and four hydrophobic ILs: [BMIM][Tf2N] (5), [Me(OEt)3-Et3N][Tf2N] (6), [Me(OEt)3-Et-Im][Tf2N] (7), and [Me(OEt)3-Me-Et-Im][Tf2N] (8).
Recently, Gorke et al.18 reported that several hydrolases (Candida antarctica lipase B (CALB), Candida antarctica lipase A (CALA), and epoxide hydrolase) maintained high activity in eutectic ILs based on choline chloride or ethylammonium chloride (hydrogen-bond donors included acetamide, ethylene glycol, glycerol, urea, and malonic acid). When used as cosolvents, eutectic ILs were also capable of improving the reaction rates and/or conversion efficiencies of hydrolases. The hydrogenbond (H-bond) network in eutectic ILs was rationalized as being responsible for the reduction in chemical potential of components of the ionic solvents, making them less reactive. Another recent study19 indicated that choline chloride/glycerol (1 : 2, molar ratio) was capable of preserving the integrity and viability of bacteria embedded in eutectic ILs prepared via a freeze-drying process, with implications for carrying out whole-cell biocatalysis in eutectic ILs.
One potential drawback of current eutectic ILs derived from choline halides and urea stems from their relatively high viscosities. For example, the choline chloride/urea (1 : 2 molar ratio) mixture has a fairly high viscosity of around 1200 mPa s at room temperature (RT) and 169 mPa s at 40 °C.12 These viscosities are considerably higher than many of the more fluid conventional ILs, introducing barriers to commercial adoption, such as masstransfer limitations, operability issues, and requirements for agitation. For instance, at RT, the viscosity of [BMIM][Tf2N] is roughly 49 mPa s.20 Indeed, with the exception of an IL bearing a glycol side-chain, the RT viscosity for 15 common ILs fell in the 49–272 mPa s range.20 Conversely, it is known that acetate- and formate-based ILs have lower melting points and viscosities than their halide analogues.21,22 Moreover, a recent study23 suggested that cholinium alkanoates, including the acetates, represent environmentally benign and biodegradable choices, with reduced toxicity compared to their corresponding sodium salts, allowing for the elaboration of ‘bio-ILs’.24 Therefore, our current study aims to modify the anion of the widely-used choline salt from chloride to acetate, coupled with the use of glycerol (Tm = 18.1 °C)25 as H-bond donor, in the hopes of achieving highly-fluid and environmentally-responsible eutectic ILs. In the work detailed here, we study CALB compatibility with our new eutectic solvents, with a focus on their suitability for enzyme-catalyzed biodiesel synthesis.
Biodiesel, comprising monoalkyl fatty esters, has become an attractive, renewable, and biodegradable fuel for diesel engines and heating systems.26 Recent studies have shown that lipase-compatible ILs are promising solvents for the enzymatic transes-terification of triglycerides during biodiesel synthesis.6,7,9,27,28 However, most of these ILs are based on expensive imidazolium salts, and the use of these solvents at tonnage-scales for the industrial production of biodiesel remains out of the realm of practicality, given the current costs associated with alkylimidazolium salts. Therefore, it will prove extremely valuable to develop lipase-compatible eutectic ILs from inexpensive and biodegradable cholinium salts. This study represents our preliminary results illustrating the promise and feasibility of such an approach.
Experimental
General
The following materials were obtained from Sigma–Aldrich: choline chloride, glycerol, ethylene glycol (EG), urea, Novozym® 435 (CALB immobilized on polyacrylate beads, product #L4777, Lot #067K3522; activity >10 U mg−1), Amberlyst® A26 hydroxide (OH) form, ethyl sorbate, methyl octanoate, and methyl decanoate. Miglyol® oil 812 NF/USP from Sasol was supplied by Fisher Scientific.
IL preparations
1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMIM][Tf2N], 5), triethyl (2-(2-methoxyethoxy)ethoxy)-ethylammonium bis(trifluoromethylsulfonyl)imide ([Me(OEt)3-Et3N][Tf2N], 6), 1-ethyl-3-(2-(2-methoxyethoxy)ethoxy)ethyl-imidazolium bis(trifluoromethylsulfonyl)imide ([Me(OEt)3-Et-Im][Tf2N], 7), and 1-ethyl-3-(2-(2-methoxyethoxy)ethoxy)ethyl-2-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Me-(OEt)3-Me-Et-Im][Tf2N], 8) were prepared and characterized as detailed in our earlier studies21,29 (see structures in Scheme 1).
Choline acetate (ChOAc) or other carboxylate analogues can be prepared by several common methods: (i) reaction of choline bicarbonate with acetic acid (or corresponding carboxylic acids);23 (ii) reaction of choline hydroxide with acetic acid (or corresponding carboxylic acids);24,30 (iii) metathesis on a column using an anion-exchange resin.31 In this study, we followed a column anionexchange approach to prepare choline acetate with high purity.31 To accomplish this, a chromatography column was packed with ca. 150 g of Amberlyst® A26 hydroxide (OH) form. The anionexchange resin was flushed with sodium hydroxide solution to ensure the complete loading of hydroxide anions on the column. The OH-form resin was then thoroughly washed with distilled water to remove superfluous sodium hydroxide residues. Next, the OH-form resin was exchanged to the acetate form by washing the column with concentrated ammonium acetate solutions (Caution: although effective for anion exchange, washing with acetic acid proved excessively exothermic). The efficiency of anion exchange was examined by silver nitrate testing of the eluate exiting the column. After rinsing out the residual ammonium acetate with distilled water, 15.8 g of choline chloride in 200 mL of water was passed through the acetate-exchanged column dropwise, and the choline acetate eluate was collected and dried in an oven at 100 °C for 24 h. The product weighed 14.0 g (76% yield). The Tm of choline acetate was reported as 51 °C,24 however, it is evident that this IL is highly hygroscopic and able to absorb moisture quickly when exposed to laboratory air. 1H NMR (300 MHz, D2O): δ (ppm) = 1.91 (s, 3H, CH3CO2-), 3.20 (s, 9H, (CH3)3N), 3.52 (t, 2H, CH2N, J = 4.8 Hz), and 4.06 (m, 2H, CH2OH). 13C NMR(D2O) δ (ppm) = 26.1 (s, CH3CO2-), 56.6 (t, (CH3)3N, J = 14.3 Hz), 58.3 (s, CH2OH), 70.2 (t, CH2N, J = 11.5 Hz), and 183.9 (s, CH3CO2-).
Eutectic ILs can be easily prepared by either thermally treating the admixed precursors10,12 or by freeze-drying a solution.19,32 Following the former method, we combined choline acetate (or choline chloride) and a given H-bond donor (e.g., glycerol) at an appropriate molar ratio at 60 °C under rigorous agitation for 30 min (or, in the case of choline chloride, 80 °C). The resulting clear liquid was dried over P2O5 at 45 °C for at least 2 weeks. The water contents for all eutectic ILs were determined in triplicate by Karl Fischer (KF) titration (Mettler Toledo C20X compact Coulometric; detection limit of 1 ppm water) at 20 °C, and were typically between 0.5 and 1.0 wt%. The same titrator was used in determining water contents of all solvents for the following reactions. Hydranal® Coulomat AG was used as analyte for the titration.
Thermal analysis of deep eutectic ILs
Thermogravimetric analysis (TGA) measurements to assess thermal stabilities were conducted on a TA Instruments 2950 with the DES samples (35–50 mg) micropipetted into open platinum pans and then heated from room temperature up to 500 °C at a heating rate of 10 °C min−1 under a protective gas atmosphere (nitrogen, flow rate: 50 cm3 min−1). The temperature of decomposition (Tdcp) was taken as the onset temperature at which 10% of the initial mass had been lost during the TGA scanning experiment. Differential scanning calorimetry (DSC) was carried out under a nitrogen atmosphere using a TA Instruments DSC Q100 fitted with a liquid nitrogen cooling system. Samples weighing 7–11 mg were hermetically sealed in aluminium pans and heated to 70 °C followed by cooling to −90 °C at a rate of 5 °C min−1 and finally heated back to 100 °C again at a rate of 5 °C min−1. This cooling–heating program was repeated for a second cycle. The appropriate thermal transitions (i.e., glass-transition temperatures, Tg; crystallization temperatures, Tc; melting points, Tm) were determined, when they occurred, from analysis of the second programmed reheating cycle (see Fig S1 in the ESI†).
Moisture absorption by eutectic mixtures
1.0 mL of IL was placed in a glass vial and incubated in an oil bath at 50 °C with gentle agitation. The vial was open to the air (with a relative humidity of 64% at 20 °C) for 24 h to achieve the water-absorbing equilibrium. The initial and final water contents were determined by the Karl Fischer titrator at 20 °C.
Enzymatic transesterification of ethyl sorbate with 1-propanol
All solvents and enzymes were dried over P2O5 prior to use. In a typical experiment, 50 μL of ethyl sorbate-containing 1propanol were added to 1.0 mL of IL within a capped glass vial. The final concentrations of ethyl sorbate and 1-propanol were 5 mM and 0.67 M, respectively. A typical reaction contained 1.0 vol% water. After adding 20 mg Novozym® 435, the reaction mixture was gently stirred in an oil bath at 50 °C. Periodically, a sample of the reaction mixture (50 μL) was withdrawn and diluted with 100 μL methanol. After centrifugation to spin out possible enzyme particles, the clear supernatant was subjected to HPLC analysis following methods detailed below. The concentration of propyl sorbate was calculated from its integrated area against the standard curve of sorbic acid.22 As Novozym 435 can potentially leach trace amounts of sorbic acid and sorbate ester,22 control experiments were conducted without the addition of ethyl sorbate. Thus, all activities reported are the net activities after subtraction of control rates. All experiments were run in duplicate. A representative HPLC chromatogram of the reaction is provided in Fig S2 of the ESI.†
CALB stability in eutectic IL
Novozym® 435 (20 mg, dried over P2O5) was incubated in 1.0 mL of eutectic IL under gentle agitation at 50 °C. After a certain incubation time, the enzyme-in-IL mixture was chilled on an ice bath before injection of 50 μL of 1-propanol containing dissolved ethyl sorbate, in order to minimize the enzymatic reaction for initial substrate concentration determination. The nominal initial substrate concentrations of ethyl sorbate and 1-propanol were 5 mM and 0.67 M, respectively. The reaction was maintained at 50 °C, and product concentrations were determined periodically by HPLC; the reaction rate was typically linear over the first hour.
Enzymatic transesterification of Miglyol® oil 812
Miglyol® oil 812 (0.11 g, ~100 μL; MgSO4-dried) was added into a micro-reaction vessel (5 mL total volume), containing a 1.0 mL mixture of IL and methanol. The oil was mostly dissolved in the IL/MeOH mixture after gentle agitation at 50 °C. A typical reaction contained 1.0 vol% water. Novozym® 435 (30 mg) was added to this mixture to initiate the reaction. The reaction mixture was sealed and incubated at 50 °C in an oil bath. After a certain reaction time, the reaction vessel was lifted from the oil bath and chilled briefly in an ice bath to condense the volatiles. Methanol (4.0 mL) was added into the reaction mixture to fully dissolve the triglycerides and fatty acid methyl esters. After centrifugation, the clear supernatant was injected into the HPLC and the method discussed below deployed. The integration of all triglyceride peaks at time t was compared with their initial peak areas at t0 to calculate their conversions. The product peaks were quantified through comparison with those of standard solutions of methyl octanoate and methyl decanoate. All experiments were run in duplicate, at least. The percent errors were less than 5%. A representative HPLC chromatogram of the reaction is shown in Fig S3 (see ESI†).
To isolate and purify the product for 1H NMR analysis, 30 mL of methanol was added into the reaction mixture. The enzyme was removed by filtration, and methanol was evaporated in vacuo. Distilled water (50 mL) was then mixed with the residue to dissolve IL and glycerol, and 50 mL of ethyl acetate was added to extract the fatty acid monoesters. The ethyl acetate layer was further washed with 50 mL of water to remove traces of IL and glycerol. After drying the organic layer with sodium sulfate and filtering off sodium sulfate, the product was collected by removal of ethyl acetate on a Schlenk line. The product was then dissolved in CDCl3 for NMR analysis (JEOL ECX-300 MHz).
Miglyol® oil 812
1H NMR (300 MHz, CDCl3) δ (ppm) = 0.85 (9 H, t, CH3 J = 6.5 Hz), 1.24 (29 H, m, aliphatic CH2), 1.58 (6 H, m, O2CCH2CH2), 2.30 (6 H, t, O2CCH2, J = 7.5 Hz), 4.09–4.30 (4 H, d,d,d, RCO2CH2CH(O2CR)CH2O2CR), 5.24 (1 H, m, RCO2CH2CH(O2CR)CH2O2CR).
Methyl octanoate and methyl decanoate produced from Miglyol® oil 812
1H NMR (300 MHz, CDCl3) δ (ppm) = 0.86 (9 H, t, CH3, J = 6.9 Hz), 1.25 (30 H, m, aliphatic CH2), 1.61 (6 H, m, O2CCH2CH2), 2.30 (6 H, m, O2CCH2), 3.65 (3H, s, CH3O2CR).
HPLC analysis of reaction mixtures
LC-20AT Shimadzu HPLC equipped with a SPD-20A UV–visible dual-wavelength detector was used in the analysis of reaction mixtures. The injection-loop volume was 20 μL. The column employed was a Phenomenex® Kinetex C18 column (100 mm × 4.6 mm, particle size 2.6 μm). The flow rate was 1.0 mL min−1. For the transesterification of ethyl sorbate with 1-propanol, the isocratic eluent consisted of 60 vol% methanol and 40 vol% water containing 1 vol% acetic acid; the UV detection wavelength was 258 nm. For the transesterification of Miglyol® oil with methanol, the isocratic eluent consisted of 95 vol% MeOH and 5 vol% water containing 1 vol% acetic acid, and the UV detection wavelength of 210 nm was used.
Results and discussion
Physical properties of new eutectic ILs
At first, we prepared choline acetate (1b, Scheme 1) from choline chloride through an anion-exchange column method.31 We then synthesized new eutectic ILs by mixing choline acetate and H-bond donors (e.g., urea, glycerol, or ethylene glycol) at 60 °C. The thermal stabilities of different IL mixtures were quantified by the decomposition temperature (Tdcp), which denotes the temperature at which 10% of the initial mass had been lost during the TGA scanning experiment. As summarized in Table 1, various molar ratios of choline acetate/glycerol showed little impact on the thermal stability of mixtures, whereas different combinations of the cholinium salts with various H-bond donors exerted greater influence on Tdcp. Most of the eutectic mixtures are stable to 200 °C or so, except for the cases of 1 : 2 choline chloride/ethylene glycol (EG) and 1 : 2 choline acetate/urea which showed distinctly lower Tdcp values of 121 and 182 °C, respectively.
Table 1.
Thermal decomposition temperatures and melting points of ionic mixtures
| Ionic mixture | Tdcp (°C) | Tm (°C) |
|---|---|---|
| Choline acetate/glycerol (1 : 1) | 204 | 20 |
| Choline acetate/glycerol (1 : 1.25) | 216 | 14 |
| Choline acetate/glycerol (1 : 1.5) | 212 | 13 |
| Choline acetate/glycerol (1 : 2) | 205 | 23 |
| Choline acetate/glycerol (1 : 3) | 205 | 20 |
| Choline acetate/EG (1 : 2) | 121 | 23 |
| Choline acetate/urea (1 : 2) | 182 | 18 |
| Choline chloride/urea (1 : 2) | 211 | 13 |
| Choline chloride/glycerol (1 : 2) | 216 | 23 |
Note: EG = ethylene glycol.
The melting points (Tm) of ionic mixtures were determined based on analysis of two consecutive DSC cooling–heating cycles; raw DSC data are provided within Fig. S1 in the ESI.† Based on the Tm results compiled in Table 1, the deep eutectic composition of choline acetate/glycerol is close to the 1 : 1.5 molar ratio (i.e., at the lowest observed Tm of 13 °C). The melting points of other eutectic ILs based on cholinium salts range from 13–23 °C. However, we observe that these eutectic mixtures remain viscous liquids at temperatures as low as −20 °C. The DSC scans gave no evidence for a freezing exotherm on the cooling segment, suggesting that these eutectics exist as stable supercooled liquids at sub-ambient temperatures. It has been shown that many ILs (including imidazolium, ammonium, and pyridinium salts) exhibit substantial supercooling, with freezing points typically 40–60 °C lower than the corresponding melting points.33–35 The supercooling phenomenon makes the precise and reliable determination of IL freezing and/or melting points a particular challenge.36 Currently, most studies of eutectic ILs are based on the direct observation of their freezing points (Tf),10–12,17 and relatively few make use of DSC to measure Tm or Tf.16,37–39 Based upon the visual onset of freezing, the eutectic composition of both choline chloride/urea (Tf = 12 °C)10 and choline chloride/glycerol (Tf near −40 °C)17,40 is 1 : 2. Relying on the DSC measurements, Morrison et al.37 observed that the choline chloride/urea system also forms a deep eutectic at the same 1 : 2 molar ratio, which showed a Tm of 17 °C. Our DSC results (Fig S1,† panel a) indicate this ionic mixture has a Tm of 13 °C.
Viscosity is another important property of eutectic ILs. As shown in Table 2, choline chloride/urea (1 : 2) has a high viscosity of 120 mPa s at 50 °C and choline acetate/glycerol (1 : 1.5) has a lower viscosity of 93 mPa s, whereas both choline chloride/glycerol (1 : 2) and choline acetate/glycerol (1 : 2) have considerably lower viscosities (~80 mPa s at 50 °C). These viscosities are roughly half that of glycerol (152 mPa s at 50 °C),25 although they are still higher than the viscosity of [BMIM][Tf2N] (49 mPa s at 25 °C),20 for example. Clearly, choice of H-bond donor is crucial to achieving a low solvent viscosity. Fig. 1 illustrates the influence of choline acetate/glycerol molar ratio on the viscosity of the resulting ionic mixture. The lowest viscosity (80 mPa s at 50 °C) was observed for a 1 : 2 molar ratio, whereas the eutectic composition was determined to be 1 : 1.5. This is consistent with literature observations pointing to the fact that the lowest viscosity often occurs in the vicinity of eutectic composition, but not necessarily precisely at the eutectic point.17 The advantages of using glycerol as the H-bond donor in forming eutectic ILs include its low toxicity, biodegradability, and rich availability (glycerol is a by-product of soap-making and biodiesel synthesis). Therefore, eutectic systems such as choline chloride/glycerol and choline acetate/glycerol will be inexpensive at large scales, renewable, and biodegradable. These are key considerations when seeking to scale up enzymatic processes, such as biodiesel production, in commercial settings.
Table 2.
Viscosities of eutectic ILs 50 °C in comparison with other solvents
| Solvent | Viscosity at 50 °C (mPa s) | Ref. |
|---|---|---|
| Glycerol | 152 | 25 |
| [BMIM][Tf2N] | 49 at 25 °C | 20 |
| Choline chloride/urea (1 : 2) | 120 | a |
| Choline chloride/glycerol (1 : 2) | 79 | a |
| Choline acetate/glycerol (1 : 1.5) | 93 | a |
| Choline acetate/glycerol (1 : 2) | 80 | a |
determined in this study using the Cannon-Fenske Routine (CFR) viscometer at 50 °C, with benzyl alcohol as reference (2.76 mPa s at 50 °C).25
Fig. 1.

Effect of component molar ratio on the viscosity of the choline acetate/glycerol mixture at 50 °C.
Evaluating lipase activity and selectivity in eutectic ILs
The model reaction selected to evaluate lipase activity in ILs involves the transesterification of ethyl sorbate with 1-propanol catalyzed by Novozym® 435. There are several reasons behind choosing this particular model reaction:22 (1) sorbate esters and sorbic acid have high extinction coefficients, and thus are both detectable at low concentrations by HPLC using UV detection (258 nm; Fig. S2†); this allows the substrate ethyl sorbate to be used in low concentrations; (2) this reaction is moderately fast and is suitable for collecting reaction rates; (3) our recent studies suggest that Novozym® 435 is capable of releasing traces of sorbic acid and sorbate ester into organic solvents and ILs;22 the impact of such migration on the reaction rate can be easily accounted for in our model system using appropriate controls. On the other hand, this enzymatic reaction is very sensitive to the water content. As shown in Fig. S4 of the ESI,† a small amount of water (as little as 0.4 vol%) causes an appreciable amount of hydrolysis of ethyl sorbate into sorbic acid in t-butanol, and the hydrolysis rate increases considerably with increased water content to 1.0 vol% and beyond. Therefore, the water content in this model transesterification reaction is a critical factor in controlling the reaction selectivity. This is firmly supported by data summarized in Table 3. Certainly, CALB shows lower selectivities toward the synthetic product (propyl sorbate) in ILs 5–8 at the higher water content (1 vol%). Additionally, eutectic ILs based on cholinium salts are far more hygroscopic than conventional ‘hydrophobic’ ILs. As illustrated by Fig. 2, the equilibrium water contents resulting from exposure to humid laboratory air for 24 h at 50 °C range from 3.5–6.0 wt% for the eutectics whilst two common [Tf2N-]-based ILs yield far lower water contents (≤0.20 wt%). In addition, the 1 : 2 choline acetate/urea system is considerably more hygroscopic than the other eutectic mixtures. Both the type of cholinium salt and the H-bond donor appear to influence the hygroscopic nature of these ionic mixtures. Fortunately, choline acetate/glycerol (1 : 2 or 1 : 1.5) is less hygroscopic compared with the two urea-based eutectic mixtures. Through extensive drying over P2O5, we were able to control the water content in these eutectic ILs to within ±1 vol%.
Table 3.
Activity and selectivity of Novozym® 435 in different solvents (1.0 v% water, except where otherwise noted)a
| Solvent | Initial activity (μmol min−1 g−1) |
Selectivity | |
|---|---|---|---|
| 2 | t-butanol | 0.57 | >99% |
| t-butanol (0.28 vol% water) | 0.74 | >99% | |
| 3 | 1-propanol | 0.10 | >99% |
| 4 | Glycerol | 0.71 | 38% |
| 5 | [BMIM][Tf2N] | 0.47 | 91% |
| [BMIM][Tf2N] (0.07 vol% water) | 0.84 | >99% | |
| 6 | [Me(OEt)3-Et3N][Tf2N] | 0.76 | 84% |
| [Me(OEt)3-Et3N][Tf2N] (0.43 vol% water) |
0.53 | >99% | |
| 7 | [Me(OEt)3-Et-Im][Tf2N] | 0.49 | 83% |
| [Me(OEt)3-Et-Im][Tf2N] (0.18 vol% water) |
0.75 | 92% | |
| 8 | [Me(OEt)3-Me-Et-Im][Tf2N] | 0.63 | 61% |
| [Me(OEt)3-Me-Et-Im][Tf2N] (0.42 vol% water) |
1.08 | 89% | |
| 9 | Choline chloride/urea (1 : 2) | 1.00 | >99% |
| 10 | Choline chloride/glycerol (1 : 2) | 1.12 | 45% |
| 11 | Choline acetate/urea (1 : 2) | 0.21 | 40% |
| 12 | Choline acetate/EG (1 : 2)b | 0.07 | 12% |
| 13 | Choline acetate/glycerol (1 : 1.5) | 1.02 | 99% |
| 14 | Choline acetate/1-propanol (1 : 1.5) |
0.040 | >99% |
The reaction assay involved enzymatic transesterification of ethyl sorbate and 1-propanol.
EG = ethylene glycol.
Fig. 2.

Water levels (wt%) present within the various IL systems following equilibration for 24 h at 50 °C in a humid environment (relative humidity of 64% at 20 °C) under gentle agitation; water contents were determined via Karl Fischer coulometric titration at 20 °C.
The model reaction involves the competitive attack on the rapidly-formed acyl-enzyme intermediate by different nucleophiles: (i) exclusive reaction with 1-propanol (present in 134-fold molar excess over ethyl sorbate) leads to the formation of propyl sorbate as the desired transesterification product; (ii) ethyl sorbate hydrolysis by water molecules results in the formation of sorbic acid; and (iii) nucleophilic attack by other alcohols (such as glycerol or ethylene glycol) present in eutectic ILs may form other sorbate esters as by-products. For this reaction, we define the lipase selectivity by dividing the yield of propyl sorbate by the conversion of ethyl sorbate. Since CALB (in particular, Novozym® 435) is known to be active in anhydrous organic solvents, we intentionally dried all solvents and enzymes over P2O5 to minimize the thermodynamic water activity (aw). The water content was typically controlled to within 1 vol%. Meanwhile, Gorke et al.18 have suggested ethylene glycol to be roughly an order of magnitude less reactive than 1-butanol, and glycerol to be over 600-fold less reactive than 1-butanol during the CALB-catalyzed transesterification of butyl valerate with 1-butanol in eutectic ILs prepared from choline chloride/ethylene glycol (EG) or choline chloride/glycerol. Therefore, the main competition in the transesterification of ethyl sorbate with 1-propanol is likely to be simply the hydrolysis of ethyl sorbate.
On the basis of this model transesterification reaction, we examined the activity and selectivity of Novozym® 435 (see Table 3) in some organic solvents (2–4) as well as conventional hydrophobic ILs (5–8, see Scheme 1), each containing a small known fraction of water. Based on previous work from our group and others,21,22,29,41–44 we know these ILs to be highly compatible with hydrolase activity. In the absence of CALB, no appreciable extent of hydrolysis nor transesterification occurred in any solvent. Careful examination of the entries in Table 3 reveals that CALB exhibited moderately high initial rates (0.47–0.76 μmol min−1 g−1) in t-butanol (2), glycerol (4) and ILs 5–8, each containing 1.0 vol% water. However, Novozym® 435 was poorly active in 1-propanol (3) (0.10 μmol min−1 g−1), suggestive of substrate inhibition in this system. Meanwhile, CALB showed high selectivities of 83–99% in most of these solvents studied (i.e., 2, 3, 5–7) at a water content of 1.0 vol% or below, indicating minimal impact of side reactions. Notably, however, in glycerol (4) containing 1 vol% water, the fairly high activity was accompanied by a poor selectivity of only 38%.
Alongside these results, Table 3 also includes activity and selectivity assessments for Novozym® 435 in several eutectic ILs. We find that lipase exhibits poor activity (0.07–0.21 μmol min−1 g−1) paired with low selectivity (12–40%) in choline acetate/urea (1 : 2) (11), and choline acetate/ethylene glycol (1 : 2) (12). On the contrary, high initial rates (1.00 to 1.12 μmol min−1 g−1) were observed in choline chloride/urea (1 : 2) (9), choline chloride/glycerol (1 : 2) (10), and choline acetate/glycerol (1 : 1.5) (13). These activities were considerably higher than those obtained in t-butanol (2), glycerol (4), and ILs 5–8 at the same water loading. It is, however, remarkable how widely the selectivity varies in the different eutectic systems. Whereas a selectivity of over 99% was achieved in the cases of eutectic ILs 9 and 13, it was quite low (12–45%) for the remaining eutectics. Notably, the initial rate in choline acetate/1-propanol (1 : 1.5) (14) was only 0.040 μmol min−1 g−1. As discussed earlier, the lipase is likely inhibited by high concentrations of the substrate 1-propanol (see entry 3 in Table 3). In any event, this direct comparison accentuates the potential attraction of employing glycerol in eutectic formulations applied to biocatalysis. Meanwhile, it is important to appreciate the fact that the hydroxyl group in the cholinium cation can hypothetically participate in enzymatic transesterification by providing a competing nucleophile. Along these lines, Falcioni et al.45 observed that hydroxyl groups in diethanolammonium chloride were reactive during the subtilisin-catalyzed transesterification of N-acetyl-L-phenylalanine methyl ester and 1-propanol. Our GC-MS analyses (Fig. S5 in ESI†), however, rule out cholinium propyl ester or glycerol propyl ester as significant by-products.
Overall, the best combination of high activity with maintenance of good selectivity for CALB happens in the choline acetate/glycerol (1 : 1.5) eutectic system (13). However, it is instructive to examine the lipase activity at additional stoichiometries of this mixture, in light of the fact that glycerol is a by-product during biodiesel synthesis. As illustrated in Fig. 3, the initial activity increases with the relative amount of glycerol in the mixture, although a 1 : 1.5 molar ratio yields the highest overall selectivity. A probable explanation is that higher proportions of glycerol reduce the molar concentration of acetate in the eutectic mixture, an ion known to cause enzyme destabilization when present at high levels.21
Fig. 3.

Effect of the molar ratio of choline acetate to glycerol on lipase activity and selectivity (conditions: 1.0 mL IL, 5 mM ethyl sorbate, 0.67 M 1-propanol, 20 mg Novozym® 435, 1.0 vol% water, 50 °C). Samples were periodically withdrawn for HPLC analysis during the initial hour of reaction.
A further stability study (see Experimental for procedures) indicated that Novozym® 435 maintained 92% of its activity after 48 h of pre-incubation in choline acetate/glycerol (1 : 1.5), and maintained 50% of its activity after 168 h of pre-incubation. Although preliminary, these results clearly suggest that eutectic ILs of this sort are minimally destructive to protein structure.
Fluorescence and CD spectroscopic methods were previously employed to interpret conformational changes of α-chymotrypsin46,47 and CALB47,48 dissolved in hydrophobic ILs (e.g., [EMIM][Tf2N] and [BuMe3N][Tf2N]), as well as lysozyme,49 chymotrypsin and subtilisin45 in hydrophilic alkylammonium ILs. However, our fluorescence measurements conducted in choline acetate/glycerol (1 : 1, 1 : 1.5, and 1 : 2) indicated complete quenching of intrinsic tryptophan (Trp) residues in CALB (data not shown). Similar observations have been recently reported by our group (CALB in ILs)44 and others (proteases in [MMIM][Me2PO450] and cellulase in ILs51). Although this occurrence might easily be misinterpreted as being due to denaturation, various amines and quaternary ammoniums are, in fact, well known as quenching agents.52–54 Indeed, alkylpyridinium ions find frequent use as quenchers to assess micellar aggregation numbers.55 Thus, while the exact mechanism remains unclear, this observation is not altogether surprising. Possible scenarios involve photoinduced electron transfer from tryptophan to the IL cation,51 exciplex formation, and inner filter effects arising from overlapping IL optical absorption and Trp emission.56,57
Biodiesel synthesis from model triglycerides in eutectic ILs
Taken together, the attractive properties (i.e., low cost, renewability, good fluidity, biodegradability, biocompatibility) of these novel eutectic systems based on combining choline acetate with glycerol emerge as particularly promising solvents for performing biocatalysis. To examine the utility of this eutectic IL in a more targeted application, we examined the enzymatic synthesis of biodiesel from the model lipid Miglyol® oil 812, a mixture of triglycerides of caprylic acid (C8) and capric acid (C10). This selection was made due to the fast reaction rate of triglycerides composed of shortchain fatty acids, and for its well-known composition, allowing for a straightforward analysis. The Miglyol® oil 812 is composed of four major triglycerides with the following distribution: C8C8C8 (24.0%), C8C8C10 (43.3%), C8C10C10 (26.9%), and C10C10C10 (5.8%) based upon HPLC analysis.9 The enzymatic transesterification of Miglyol® oil with methanol catalyzed by Novozym® 435 involves the competition between transesterification synthesis of biodiesel and enzymatic hydrolysis of triglycerides into fatty acids in the presence of water.
In order to begin evaluating the choline acetate/glycerol eutectic IL in biodiesel conversion, we first examined the impact of the initial molar ratio of choline acetate to glycerol on the Novozym®-catalyzed conversion of Miglyol® oil into the corresponding methyl alkanoates. As shown in Fig. 4, 1 : 1.5 choline acetate/glycerol gave the highest conversion of 82% after 1 h. In comparison, the 1 : 1 and 1 : 2 mixtures showed lower total conversions of 19% and 67%, respectively. Our rationalization of this behavior is as follows. Within 1 : 1 choline acetate/glycerol, acetate anions are able to strongly engage in H-bonding with the enzyme surface, inducing lipase inactivation. The increase in relative glycerol content alleviates these negative interactions to some extent, however, when glycerol is present in a larger excess, as in the 1 : 2 choline acetate/glycerol system, the reaction equilibrium shifts unfavorably for the reason that glycerol is a by-product of biodiesel synthesis. In keeping with this viewpoint, the transesterification reaction of Miglyol oil conducted in pure glycerol as the solvent saw a further lowered yield to 55% after 1 h of reaction (Fig. 4). A similar conversion efficiency was observed in the 1 : 2 choline chloride/glycerol eutectic. Based upon this preliminary screening, subsequent studies focused on the use of 1 : 1.5 choline acetate/glycerol as the eutectic solvent system for testing biodiesel synthesis from Miglyol® oil.
Fig. 4.

Effect of initial molar ratio of eutectic ILs on the conversion of Miglyol® oil (reaction conditions: 1.0 mL eutectic IL, 0.11 g Miglyol® oil, 200 μL methanol, 30 mg dried Novozym® 435, 1.0 vol% water, 50 °C, 1 h). ChOAc denotes choline acetate.
Having selected the 1 : 1.5 choline acetate/glycerol system for more detailed analysis, we studied the effects of methanol concentration and water content on the transesterification of Miglyol® oil. Fig. 5 reveals that the optimum methanol concentration is about 20 vol% methanol in 1 : 1.5 choline acetate/glycerol, achieving a conversion of 82% in 1 h. As expected, higher methanol concentrations led to inactivation of Novozym® 435; in particular, the reaction in pure methanol resulted in a mere 20% conversion into the monoesters. The overall conversion in this case is a convolution of several offsetting factors: (1) higher concentrations of methanol induce additional lipase inactivation, (2) a higher concentration of methanol lowers the acetate concentration, which is beneficial to lipase stabilization, and (3) a higher methanol concentration also decreases the medium viscosity, which may reduce hindrance in substrate transport. Therefore, the optimal methanol concentration must balance these factors. Since CALB is known to be active in dried conditions,58–60 the addition of water to the transesterification reaction showed a slightly deteriorative effect on the Miglyol® conversion (82% at 1.0 vol% water compared to 68% at 2.1 vol% water). Therefore, no additional water beyond 1.0 vol% was employed in subsequent reactions.
Fig. 5.
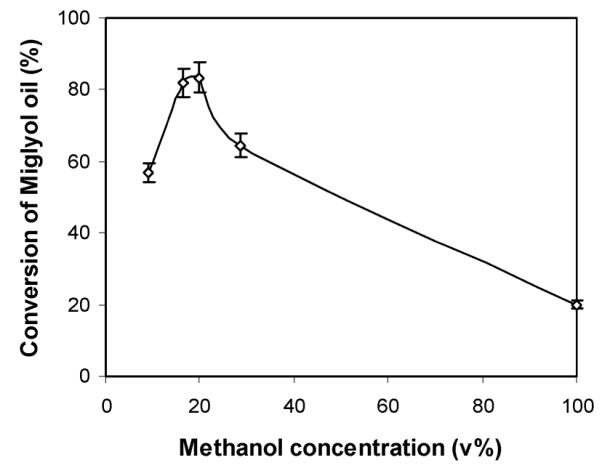
Effect of methanol concentration on the conversion of Miglyol® oil (reaction conditions: 1.0 mL choline acetate/glycerol (1 : 1.5), 0.11 g Miglyol® oil, various amounts of methanol, 30 mg dried Novozym® 435, 1.0 vol% water, 50 °C, 1 h).
Based on the optimal reaction conditions established (choline acetate/glycerol 1 : 1.5, 20 vol% methanol, 1.0 vol% water, and Novozym® 435), we examined the reaction progress of enzymatic transesterification of Miglyol® oil and methanol (Fig. 6). The reaction was very rapid in the eutectic IL, with 97% conversion achieved after 3 h. The slight decrease in conversion beyond 3 h is tentatively attributed to possible enzymatic glycerolysis in the presence of excess glycerol during the extended reaction period. In contrast, the same reaction in conventional ILs requires 24–96 h to reach completion (up to 70% in 1 h). The fast reaction rate in eutectic ILs is attributed to the high compatibility of these solvents with the lipase enzyme. An additional benefit is derived from the fact that biodiesel is only partially soluble in eutectic ILs, simplifying product separation.
Fig. 6.

Time course for the enzymatic transesterification of Miglyol® oil under these optimized conditions: 1.0 mL choline acetate/glycerol (1 : 1.5), 0.11 g Miglyol® oil, 200 μL methanol, 30 mg dried Novozym® 435, 1.0 vol% water, and 50 °C.
Conclusions
We report on the synthesis and exploration of novel, inexpensive, and biodegradable eutectic mixtures based upon choline acetate coupled with glycerol as hydrogen-bond donor species. These emergent ionic solvents are considerably less viscous than those derived from choline chloride and urea, and are capable of maintaining high biocatalytic properties of CALB. We find these new eutectic ILs to be suitable solvents for the enzymatic transesterification of triglycerides with methanol. Under optimal conditions, high conversions (82–97%) of Miglyol® oil were achieved within 1–3 h using Novozym® 435 in choline acetate/glycerol (1 : 1.5). These highly encouraging results suggest that eutectic ILs based on cholinium salts and glycerol merit further consideration as ‘green’ solvents for the enzymatic preparation of biodiesel, among other areas. Future studies in our groups will explore the enzymatic conversion of long-chain fatty acid triglycerides, such as vegetable oils and algae biomass, using these promising eutectic fluids.
Supplementary Material
Acknowledgements
G.A.B. acknowledges support via a Presidential Early Career Award for Scientists and Engineers (PECASE). H.Z. acknowledges the support from the NIH RIMI grant (5P20MD003941).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c0ob01011a
Notes and references
- 1.van Rantwijk F, Sheldon RA. Chem. Rev. 2007;107:2757–2785. doi: 10.1021/cr050946x. [DOI] [PubMed] [Google Scholar]
- 2.Moniruzzaman M, Nakashima K, Kamiya N, Goto M. Biochem. Eng. J. 2010;48:295–314. [Google Scholar]
- 3.Zhao H. J. Chem. Technol. Biotechnol. 2010;85:891–907. [Google Scholar]
- 4.Dadi AP, Varanasi S, Schall CA. Biotechnol. Bioeng. 2006;95:904–910. doi: 10.1002/bit.21047. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Jones CL, Baker GA, Xia S, Song Z, Olubajo O, Person VN. J. Biotechnol. 2009;139:47–54. doi: 10.1016/j.jbiotec.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Sunitha S, Kanjilal S, Reddy PS, Prasad RBN. Biotechnol. Lett. 2007;29:1881–1885. doi: 10.1007/s10529-007-9471-x. [DOI] [PubMed] [Google Scholar]
- 7.Gamba M, Lapis AAM, Dupont J. Adv. Synth. Catal. 2008;350:160–164. [Google Scholar]
- 8.Earle MJ, Plechkova NV, Seddon KR. Pure Appl. Chem. 2009;81:2045–2057. [Google Scholar]
- 9.Zhao H, Song Z, Olubajo O, Cowins JV. Appl. Biochem. Biotechnol. 2010;162:13–23. doi: 10.1007/s12010-009-8717-6. [DOI] [PubMed] [Google Scholar]
- 10.Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Chem. Commun. 2003:70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- 11.Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. J. Am. Chem. Soc. 2004;126:9142–9147. doi: 10.1021/ja048266j. [DOI] [PubMed] [Google Scholar]
- 12.Abbott AP, Capper G, Gray S. ChemPhysChem. 2006;7:803–806. doi: 10.1002/cphc.200500489. [DOI] [PubMed] [Google Scholar]
- 13.Boethling RS, Sommer E, DiFiore D. Chem. Rev. 2007;107:2207–2227. doi: 10.1021/cr050952t. [DOI] [PubMed] [Google Scholar]
- 14.Blusztajn JK. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- 15.Abbott AP, Capper G, Swain BG, Wheeler DA. Trans. Inst. Metal Finishing. 2005;83:51–53. [Google Scholar]
- 16.Hou Y, Gu Y, Zhang S, Yang F, Ding H, Shan Y. J. Mol. Liq. 2008;143:154–159. [Google Scholar]
- 17.Abbott AP, Cullis PM, Gibson MJ, Harris RC, Raven E. Green Chem. 2007;9:868–872. [Google Scholar]
- 18.Gorke JT, Srienc F, Kazlauskas RJ. Chem. Commun. 2008:1235–1237. doi: 10.1039/b716317g. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez MC, Ferrer ML, Yuste L, Rojo F, del Monte F. Angew. Chem., Int. Ed. 2010;49:2158–2162. doi: 10.1002/anie.200905212. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, O’Hare B, Dong J, Arzhantsev S, Baker GA, Wishart JF, Benesi AJ, Maroncelli M. J. Phys. Chem. B. 2008;112:81–92. doi: 10.1021/jp076462h. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D. Green Chem. 2008;10:696–705. [Google Scholar]
- 22.Zhao H, Song Z. Biochem. Eng. J. 2010;49:113–118. [Google Scholar]
- 23.Petkovic M, Ferguson JL, Gunaratne HQN, Ferreira R, Leitão MC, Seddon KR, Rebelo LPN, Pereira CS. Green Chem. 2010;12:643–649. [Google Scholar]
- 24.Fukaya Y, Iizuka Y, Sekikawa K, Ohno H. Green Chem. 2007;9:1155–1157. [Google Scholar]
- 25.Lide DR. CRC Handbook of Chemistry and Physics. 84th ed CRC Press Inc.; New York: 2003. [Google Scholar]
- 26.Fukuda H, Kondo A, Noda H. J. Biosci. Bioeng. 2001;92:405–416. doi: 10.1263/jbb.92.405. [DOI] [PubMed] [Google Scholar]
- 27.Ha SH, Lan MN, Lee SH, Hwang SM, Koo Y-M. Enzyme Microb. Technol. 2007;41:480–483. [Google Scholar]
- 28.Yang Z, Zhang K-P, Huang Y, Wang Z. J. Mol. Catal. B: Enzym. 2010;63:23–30. [Google Scholar]
- 29.Zhao H, Song Z, Olubajo O. Biotechnol. Lett. 2010;32:1109–1116. doi: 10.1007/s10529-010-0262-4. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y, Lu X, Zhou Q, Dong K, Yao H, Zhang S. Chem.–Eur. J. 2008;14:11174–11182. doi: 10.1002/chem.200800620. [DOI] [PubMed] [Google Scholar]
- 31.Dinarès I, de Miguel CG, Ibáñez A, Mesquida N, Alcalde E. Green Chem. 2009;11:1507–1510. [Google Scholar]
- 32.Gutierrez MC, Ferrer ML, Mateo CR, del Monte F. Langmuir. 2009;25:5509–5515. doi: 10.1021/la900552b. [DOI] [PubMed] [Google Scholar]
- 33.Ngo HL, LeCompte K, Hargens L, McEwen AB. Thermochim. Acta. 2000;357–358:97–102. [Google Scholar]
- 34.Zhou Z-B, Takeda M, Ue M. J. Fluorine Chem. 2004;125:471–476. [Google Scholar]
- 35.Duan Z, Gu Y, Zhang J, Zhu L, Deng Y. J. Mol. Catal. A: Chem. 2006;250:163–168. [Google Scholar]
- 36.Wilkes JS. J. Mol. Catal. A: Chem. 2004;214:11–17. [Google Scholar]
- 37.Morrison HG, Sun CC, Neervannan S. Int. J. Pharm. 2009;378:136–139. doi: 10.1016/j.ijpharm.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 38.Shamsuri AA, Abdullah DK. J. Phys. Sci. 2010;21:15–28. [Google Scholar]
- 39.Smiglak M, Bridges NJ, Dilip M, Rogers RD. Chem.–Eur. J. 2008;14:11314–11319. doi: 10.1002/chem.200801811. [DOI] [PubMed] [Google Scholar]
- 40.Abbott AP, Harris RC, Ryder KS, D’Agostino C, Gladden LF, Mantle MD. Green Chem. 2011;13:82–90. [Google Scholar]
- 41.Laszlo JA, Compton DL. Biotechnol. Bioeng. 2001;75:181–186. doi: 10.1002/bit.1177. [DOI] [PubMed] [Google Scholar]
- 42.Lozano P, de Diego T, Guegan J-P, Vaultier M, Iborra JL. Biotechnol. Bioeng. 2001;75:563–569. doi: 10.1002/bit.10089. [DOI] [PubMed] [Google Scholar]
- 43.Lozano P, De Diego T, Carrié D, Vaultier M, Iborra JL. Biotechnol. Lett. 2001;23:1529–1533. [Google Scholar]
- 44.Zhao H, Jones CL, Cowins JV. Green Chem. 2009;11:1128–1138. [Google Scholar]
- 45.Falcioni F, Housden HR, Ling Z, Shimizu S, Walker AJ, Bruce NC. Chem. Commun. 2010;46:749–751. doi: 10.1039/b916497a. [DOI] [PubMed] [Google Scholar]
- 46.De Diego T, Lozano P, Gmouh S, Vaultier M, Iborra JL. Biotechnol. Bioeng. 2004;88:916–924. doi: 10.1002/bit.20330. [DOI] [PubMed] [Google Scholar]
- 47.Lozano P, De Diego T, Gmouh S, Vaultier M, Iborra JL. Biocatal. Biotransform. 2005;23:169–176. [Google Scholar]
- 48.De Diego T, Lozano P, Gmouh S, Vaultier M, Iborra JL. Biomacromolecules. 2005;6:1457–1464. doi: 10.1021/bm049259q. [DOI] [PubMed] [Google Scholar]
- 49.Mann JP, McCluskey A, Atkin R. Green Chem. 2009;11:785–792. [Google Scholar]
- 50.Wehofsky N, Wespe C, Cerovsky V, Pech A, Hoess E, Rudolph R, Bordusa F. ChemBioChem. 2008;9:1493–1499. doi: 10.1002/cbic.200800025. [DOI] [PubMed] [Google Scholar]
- 51.Bose S, Armstrong DW, Petrich JW. J. Phys. Chem. B. 2010;114:8221–8227. doi: 10.1021/jp9120518. [DOI] [PubMed] [Google Scholar]
- 52.Hamity M, Lema RH, Suchetti CA. J. Photochem. Photobiol., A. 1998;115:163–168. [Google Scholar]
- 53.Almgren M, Medhage B, Mukhtar E. J. Photochem. Photobiol., A. 1991;59:323–334. [Google Scholar]
- 54.Chen M, Grätzel M, Thomas JK. Chem. Phys. Lett. 1974;24:65–68. [Google Scholar]
- 55.Hansson P, Jönsson B, Ström C, Söderman O. J. Phys. Chem. B. 2000;104:3496–3506. [Google Scholar]
- 56.Page TA, Kraut ND, Page PM, Baker GA, Bright FV. J. Phys. Chem. B. 2009;113:12825–12830. doi: 10.1021/jp904475v. [DOI] [PubMed] [Google Scholar]
- 57.Baker SN, McCleskey TM, Pandey S, Baker GA. Chem. Commun. 2004:940–941. doi: 10.1039/b401304m. [DOI] [PubMed] [Google Scholar]
- 58.Réjasse B, Lamare S, Legoy M-D, Besson T. Org. Biomol. Chem. 2004;2:1086–1089. doi: 10.1039/b401145g. [DOI] [PubMed] [Google Scholar]
- 59.Réjasse B, Besson T, Legoy M-D, Lamare S. Org. Biomol. Chem. 2006;4:3703–3707. doi: 10.1039/b610265d. [DOI] [PubMed] [Google Scholar]
- 60.Zhao H, Baker GA, Song Z, Olubajo O, Zanders L, Campbell SM. J. Mol. Catal. B: Enzym. 2009;57:149–157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


