Abstract
Objectives
We hypothesized that human atrial fibrillation (AF) may be sustained by localized sources (electrical rotors and focal impulses), whose elimination (Focal Impulse and Rotor Modulation, FIRM) may improve outcome from AF ablation.
Background
Catheter ablation for AF is a promising therapy, whose success is limited in part by uncertainty in the mechanisms that sustain AF. We developed a computational approach to map whether AF is sustained by several meandering waves (the prevailing hypothesis) or localized sources, then prospectively tested whether targeting patient-specific mechanisms revealed by mapping would improve AF ablation outcome.
Methods
We recruited 92 individuals during 107 consecutive ablation procedures for paroxysmal or persistent (72%) AF. Cases were prospectively treated, in a 2-arm 1:2 design, by ablation at sources (FIRM-Guided) followed by conventional ablation (n=36), or conventional ablation alone (n=71; FIRM-Blinded).
Results
Localized rotors or focal impulses were detected in 98 (97%) of 101 cases with sustained AF, each exhibiting 2.1±1.0 sources. The acute endpoint (AF termination or consistent slowing) was achieved in 86% of FIRM-guided versus 20% of FIRM-Blinded cases (p<0.001). FIRM ablation alone at the primary source terminated AF in 2.5 minutes (median; IQR 1.0–3.1). Total ablation time did not differ between groups (57.8±22.8 versus 52.1±17.8 minutes, p=0.16). During 273 days (median; IQR 132–681 days) after a single procedure, FIRM-Guided cases had higher freedom from AF (82.4% versus 44.9%; p<0.001) after a single procedure than FIRM-blinded cases with rigorous, often implanted, ECG monitoring. Adverse events did not differ between groups.
CONCLUSIONS
Localized electrical rotors and focal impulse sources are prevalent sustaining-mechanisms for human AF. FIRM ablation at patient-specific sources acutely terminated or slowed AF, and improved outcome. These results offer a novel mechanistic framework and treatment paradigm for AF. (ClinicalTrials.gov number, NCT01008722)
Keywords: Atrial Fibrillation, Ablation, Therapy, Electrical Rotors, Focal Beats, Multiwavelet reentry
Atrial fibrillation (AF) is the most common heart rhythm disturbance in the world and a leading cause of hospitalization and death (1). Unfortunately, its therapy remains suboptimal. Catheter ablation is a non-pharmacological therapy that aims to restore sinus rhythm by eliminating tissue causing AF (2,3), and is more effective than medications (4–6). Nevertheless, rigorous monitoring reveals that many patients experience ‘silent’ AF post ablation (7). Accordingly, the 1-year success for AF ablation off-medications is 40–60% for one procedure (3,8,9) with a ‘70–80% ceiling’ for 3 or more procedures (3,5,6,10). Thus, many patients require multiple, lengthy and costly procedures that confer at least modest risk (3).
Contemporary AF ablation is likely limited by 2 main factors. First, current tools may not create durable lesions, evidenced by pulmonary vein reconnection (11,12) and gaps in linear lesions (10,13) in patients with recurrent AF post ablation. However, an important second major limitation of AF ablation is that the mechanisms that perpetuate AF are not identified (3,14,15), in contrast to all other arrhythmias in which the perpetuating mechanism is the primary target for ablation.
Seminal observations by Haïssaguerre (2) revealed that ectopic beats from the pulmonary veins (PVs) may trigger AF, establishing the field of AF ablation with PV isolation as its cornerstone (3). However, the mechanisms that perpetuate paroxysmal or persistent AF, once triggered, are not defined (14,16). There are 2 prevailing hypotheses. The multiwavelet hypothesis proposes that continuously meandering electrical waves cause AF (15). However, this hypothesis does not readily explain spatial non-uniformities in AF (17,18), the fact that AF may terminate early in a procedure often before meandering wavelets are substantially constrained (3,10), yet the fact that ablation based on this hypothesis often has little acute periprocedural impact (3,19). Alternatively, the localized source hypothesis is based on experimental models in which organized reentrant circuits (rotors) (16,20) or focal impulses (18) disorganize into AF. However, there has been little (21,22) or no (15) evidence to support localized sources in human AF.
We hypothesized that human atrial fibrillation (AF), even with a wide range of presentations, is sustained by localized sources whose targeted elimination may improve outcome following AF ablation. We tested this hypothesis by developing a novel computational mapping approach to detect localized sources, then tested whether ablation of patient-specific AF sources (Focal Impulse and Rotor Modulation, FIRM) acutely modulates AF (by terminating or consistently slowing AF), and improves the long-term success of conventional ablation in the CONFIRM trial (CONventional ablation with or without FIRM).
Methods
Study Design and Enrollment
We enrolled 92 subjects with symptomatic AF undergoing 107 consecutive ablation procedures for standard indications (3) under specific IRB-approved informed consent. Subjects were ≥21 years of age, with AF despite one or more class I or III anti-arrhythmic drugs, and diverse phenotypes including paroxysmal AF (self-limiting episodes), persistent AF (requiring drugs or electrical shock to terminate), longstanding persistent AF (continuous AF for over 1 year) (3), and AF despite prior conventional ablation. The only exclusion was an inability or refusal to provide written informed consent for this study. Table 1 summarizes the patient characteristics.
Table I.
Characteristics of Clinical Cases
| Characteristic | Conventional Ablation (n=71) |
FIRM-Guided Ablation (n=36) |
P | |
|---|---|---|---|---|
| AF Presentation | 0.12 | |||
| Paroxysmal | 24 (34%) | 7 (19%) | ||
| Persistent | 47 (66%) | 29 (81%) | ||
| Age (years) | 61±8 | 63±9 | 0.34 | |
| Gender (Male/Female) | 68 / 3 | 34 / 2 | 1.00 | |
| Non-white race | 9 (13%) | 6 (17%) | 0.57 | |
| History of AF (months) | 45 (18–79) | 52 (38–110) | 0.04 | |
| Left Atrial diameter (mm) | 43±6 | 48±7 | 0.001 | |
| LVEF (%) | 55±12 | 53±15 | 0.59 | |
| CHADS2 score | ||||
| 0 or 1 | 38 (54%) | 13 (36%) | 0.09 | |
| 2 or more | 33 (46%) | 23 (64%) | ||
| NYHA Class | 0.47 | |||
| 0–I | 61 (86%) | 29 (81%) | ||
| II–III | 10 (14%) | 7 (19%) | ||
| Comorbid Conditions | ||||
| Hypertension | 50 (70%) | 31 (86%) | 0.07 | |
| Diabetes | 22 (31%) | 12 (33%) | 0.81 | |
| Prior Stroke/TIA | 12 (17%) | 6 (17%) | 0.95 | |
| Coronary Disease | 20 (28%) | 18 (50%) | 0.03 | |
| Hypercholesterolemia | 48 (68%) | 30 (86%) | 0.05 | |
| Prior Conventional Ablation | 18 (25%) | 15 (42%) | 0.08 | |
| Previously Failed >1 Anti-Arrhythmic Drug | 16 (23%) | 16 (44%) | <0.05 | |
| Class I | 16 (23%) | 9 (25%) | 0.78 | |
| Sotalol | 30 (42%) | 17 (47%) | 0.63 | |
| Dofetilide | 9 (13%) | 8 (22%) | 0.20 | |
| Amiodarone | 27 (38%) | 22 (61%) | 0.02 | |
| Days since amiodarone discontinued | 150 (60–365) | 365 (69–730) | 0.08 | |
| Concomitant Drug Therapy | ||||
| ACEI/ARB | 45 (63%) | 21 (58%) | 0.61 | |
| Beta Adrenoceptor Antagonists | 48 (68%) | 24 (67%) | 0.92 | |
| Calcium Channel Blockers | 21 (30%) | 11 (31%) | 0.92 | |
| Statins | 42 (59%) | 19 (53%) | 0.53 | |
Values are number (%), mean±SD, or median (interquartile range).
All patients were recruited prospectively under specific Institutional Review Board approval. We performed detailed AF recordings and used computational mapping to reveal localized sources (23) as detailed in the Supplemental Methods. Processing initially took days and could not be used to guide ablation. However, once computational efficiency made it possible to map sources intraprocedurally, we registered the CONFIRM trial (ClinicalTrials.gov number NCT01008722). Consecutive cases were thus prospectively enrolled in a 2-arm 1:2 case cohort design into the FIRM-Guided group whenever intraprocedural mapping was available, or the FIRM-Blinded group, blinded to any clinical factors. The FIRM-Guided group received targeted ablation of sources followed by conventional ablation (by SMN or DEK at 2 centers), while the FIRM-Blinded group received conventional ablation alone (by SMN, DEK or KS at 3 centers).
Electrophysiology Study
Electrophysiology study was performed after discontinuing anti-arrhythmic medications for 5 half lives, or >60 days for amiodarone (median 230 days, table 1). Catheters were advanced from the femoral veins to the right atrium, coronary sinus and trans-septally to the left atrium. A 64-pole basket catheter (Constellation, Boston Scientific, Natick, MA) was advanced through an 8.5Fr SL1 sheath (Daig Medical, Minnetonka, MN) to map the left atrium with a wide field of view in all patients. In n=73 patients (including all FIRM-guided cases), basket mapping was also performed in the right atrium. Basket insertion (<1 minute) was then followed by careful positioning (<5 minutes). Digital electroanatomic atrial shells were created for clinical guidance of conventional ablation (not FIRM) using NavX (St Jude, Minneapolis, MN) or Carto (Biosense-Webster, Diamond Bar, CA) systems. Intravenous heparin was infused to achieve an activated clotting time > 350 seconds in all cases. Figure 1 shows AF (figure 1A) mapped with 1 basket in the left atrium across a transseptal puncture and another in the right atrium (figure 1B). Unipolar and bipolar electrograms were filtered at 0.05 – 500 Hz and recorded at 1kHz sampling frequency for export from our physiological recorder (Bard, Lowell, MA).
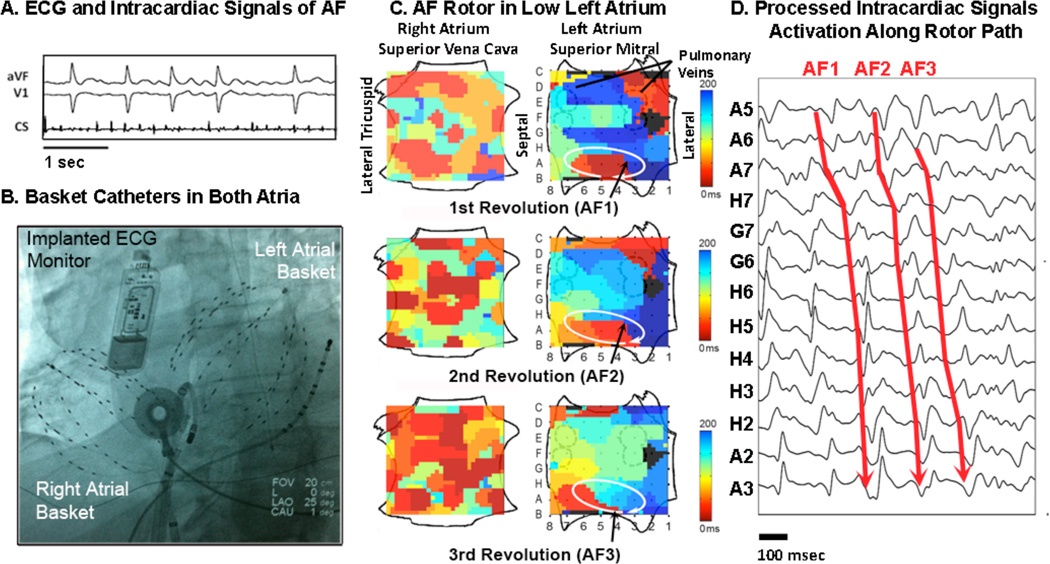
Figure 1. Computational Mapping of ‘Electrical Rotor’ During Atrial Fibrillation.
(A) Electrocardiogram and intracardiac signals in an 85 year old man during paroxysmal AF. (B) Fluoroscopy shows a 64 pole catheter in each atrium, an implanted continuous ECG monitor, diagnostic catheters in the coronary sinus and left atrium and an esophageal temperature probe at the inferior left atrium. (C) Left Atrial Rotor During AF, showing clockwise revolution (coded red to blue based on activation time scale) around a precessing center for 3 cycles (AF1–AF3; see Supplemental Movie 1). The right atrium depicts the superior and inferior vena cavae above and below, and lateral and medial tricuspid annuli at left and right. The left atrium depicts superior and inferior mitral annuli above and below, and pulmonary vein pairs. Electrode are labeled A–H and 1–8, respectively. (D) Computationally Processed and Filtered intracardiac signals show sequential activation over the rotor path for cycles AF1–AF3 (arrowed). FIRM ablation at this rotor terminated AF to sinus rhythm in < 1 minute (Supplemental movies 2–3).
In any patient presenting in sinus rhythm (n=28), AF was induced by pacing at cycle length (CL) 500 ms (120 beats/min), reducing in 50 ms steps to 300 ms (200 beats/min), then in 10 ms steps to AF. In two cases, isoproterenol was required to initiate AF, and was maintained throughout the procedure. Induced AF was mapped after > 15 minutes, and typically after 1–2 hours since induction was performed early in the case, based on preliminary data that spatial maps of induced and spontaneous AF converge within this time.
Sustained AF was seen, and FIRM-maps created, in 101 cases (including all FIRM-guided cases). The remaining 6 FIRM-blinded cases underwent conventional ablation in sinus rhythm.
Computational Mapping of Patient-Specific AF Mechanisms
The physiological rationale, algorithms and approach we have developed for AF mapping have recently been described (23) and are detailed in Supplemental Methods. Briefly, AF electrograms are analyzed in the context of rate-dependent repolarization (24,25), that indicate the shortest physiological time between successive activations during AF, and rate-dependent conduction slowing (26), used to identify mapped propagation paths that were physiologically possible. The resulting computational maps depict the propagation of electrical activity, color coded from early (in red) to late (in blue), in each atrium (figure 1C).
Computational AF maps were generated intra-procedurally in the FIRM-Guided group, and post-procedure in the FIRM-Blinded group using a novel system (RhythmView™, Topera Medical, Lexington, Massachussetts). FIRM maps of AF revealed electrical rotors (figure 1C, 2A) defined as sequential clockwise or counterclockwise activation contours (isochrones) around a center of rotation emanating outwards to control local AF activation, or focal impulses (figure 2C) defined by centrifugal activation contours (isochrones) from an origin. Rotors and focal impulses showed limited spatial precession (see below) and were considered AF sources only if consistent in multiple recordings over > 10 minutes (equating to thousands of cycles) to eliminate transient AF patterns of unclear functional significance.
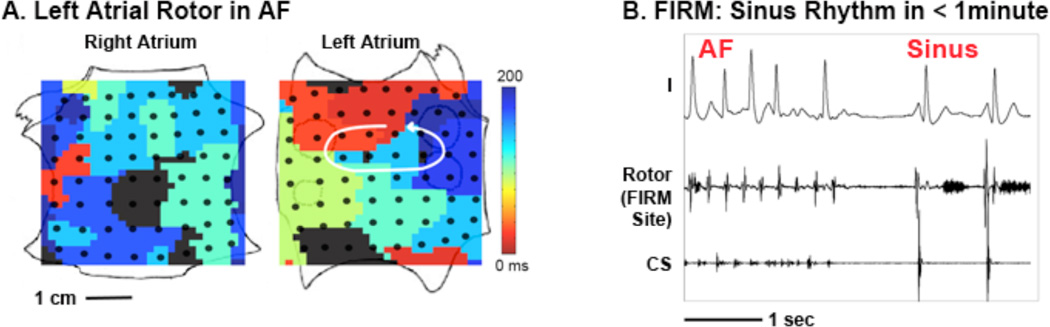
Figure 2. Acute Termination of Atrial Fibrillation to Sinus Rhythm By Focal Impulse and Rotor Modulation (FIRM) Ablation.
(A) Left atrial rotor with counterclockwise activation (red to blue) and disorganized right atrium during AF in a 60 year old man. (B) FIRM ablation at left atrial rotor terminated AF to sinus rhythm in < 1 minute, with ablation artifact recorded at rotor center. The patient is AF-free on implanted cardiac monitor at > 1 year. Scale bars 1 cm, 1 second; CS: coronary sinus electrogram. Atrial orientations as in figure 1.
Ablation Procedure
In FIRM-Guided subjects, ablation commenced with FIRM to eliminate sources. Radiofrequency energy was delivered using a 3.5 mm tip irrigated catheter (Thermocool, Biosense-Webster, Diamond Bar, CA) at 25–35 W or, in heart failure subjects, an 8 mm tip non-irrigated catheter (Blazer, Boston Scientific, Natick, MA) at 40–50 W , target 52°C. The catheter was maneuvered to the basket electrode overlying each source, using fluoroscopy (or digital atrial mapping), and radiofrequency energy was applied for 15–30 seconds. The catheter was moved within the area indicated by FIRM maps to represent the center of rotation or focal impulse or igin until AF terminated or ablation time at that source reached ≤ 10 minutes, whichever came first (typically <5 minutes/source). If AF terminated, attempts were made to reinitiate AF using the protocol described for AF initiation. If AF was successfully reinitiated, maps were recomputed and FIRM ablation was repeated for ≤ 3 sources (≤30 minutes permitted by protocol). Conventional ablation was then performed.
Conventional ablation (3), performed after FIRM ablation in the FIRM-Guided group, and as sole therapy in the FIRM-Blinded group, comprised wide area circumferential ablation to isolate the left and right pulmonary veins in pairs, with verification of pulmonary vein isolation using a circular mapping catheter (Lasso™, Biosense-Webster, Diamond Bar, CA). Ablation power, temperatures and duration were as noted for the FIRM-guided group. In persistent AF we also used a left atrial roof line; atrial tachycardia or flutter (n=7 cases) were ablated appropriately. No other ablation was performed. If AF persisted after completion of the ablation protocol in each group, cardioversion was performed. An esophageal temperature probe was maintained in proximity to the catheter during ablation, and energy was discontinued if a 1°C rise in temperature was noted.
Post-Procedure Clinical Management
Followup for recurrent arrhythmias met or exceeded guidelines (3). Within a 3 month blanking period post-ablation, anti-arrhythmic medications were continued and arrhythmias were managed with cardioversion if indicated. However, repeat ablation was not permitted. Subjects were then evaluated at 3,6,9,12,18 and 24 months. We detected recurrent arrhythmias using implanted continuous ECG monitors whenever possible, using Reveal XT™ (Medtronic, Minneapolis, MN) after its U.S. approval in 2009 (figure 1B), or clinically indicated pacemaker/defibrillators with AF detection algorithms. Continuous monitors were interrogated at scheduled visits and at interim visits for symptoms by nurses, then over-read by a physician, both blinded to the ablation approach. Implanted ECG monitors provide more rigorous monitoring (7,27) than in most prior AF treatment trials. Remaining subjects received 7-day patient-activated event recorders or 24-hour ambulatory ECGs at each visit.
Study Endpoints
The prespecified acute efficacy endpoint was AF termination or ≥10% AF slowing (≈15–20 ms prolongation of AF cycle length), selected as a rigorous marker of AF modulation (prior studies used AF slowing by ablation of 6 ms ≈3–4%) (28). AF cycle length was measured as the average in multiple samples over ≈ 5 minutes. The prespecified primary long-term efficacy endpoint was defined as freedom from A F for up to 2 years after a single procedure (median 273 days, IQR 132–681), defined as <1% burden using continuous implanted ECG monitors, or AF <30 seconds on intermittent monitors (3). Secondary efficacy measures included freedom from AF in patients undergoing their first ablation, and freedom from all atrial arrhythmias. The safety endpoint was a comparison of adverse events between groups.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Normality was evaluated using the Kolmogorov-Smirnov test. Comparisons between 2 groups were made with Student’s t-tests and summarized with means and standard deviations for independent samples if normally distributed or, if not normally distributed, evaluated with the Mann-Whitney U test and summarized with medians and quartiles. Nominal values are expressed as n (%) and compared with chi-square tests or the Fisher exact test for comparisons when expected cell frequency was < 5. Associations between continuous variables were evaluated with Spearman’s correlation. Raw event rates were compared with chi-square tests and event-free survival plots were made by the Kaplan-Meier method and compared with log-rank tests. A probability of < 0.05 was considered statistically significant throughout. Analysis was by intention-to-treat, and crossovers were not permitted.
Results
Table 1 summarizes the characteristics of our study population. Persistent AF was present in 81% of the FIRM-guided group, and 66% of the FIRM-blinded group.
Prevalence and Characteristics of Localized Sources for Human AF
Electrical rotors and focal impulses were present in 98/101 cases with sustained AF (97%), each subject demonstrating 2.1±1.0 sources (median 2, IQR 1–3) of w hich 70% were rotors and 30% focal impulses. For the AF rotor in figure 1C, figure 1D shows the corresponding electrical circuit (arrows) in noise-reduced AF signals, that precessed within an area of 1–2 cm2 in the low left atrium (see Supplemental movies 1–3).
AF sources were conserved for at least tens of minutes during mapping (FIRM-guided cases), lay in widespread locations in the left atrium (76%) including sites outside the pulmonary veins, posterior, inferior, roof and anterior regions, and in right atrium (24%) including the inferolateral, posterior and septal regions. When sources were present, their number was higher for persistent than paroxysmal AF (2.2±1.0 vs 1.7±0.9; median 2.0 vs 1.0; p=0.03) and for spontaneous versus induced (typically paroxysmal) AF (2.1±1.1 vs 1. 6±0.9; median 2.0 vs 1.0; p=0.01), but was unrelated to age (r=0.13, p=0.20), historical duration of AF (r=0.14, p=0.17), or whether subjects were undergoing first ablation or had had prior conventional ablation (2.1±1.1 vs 2.0±0.8; median 2.0 vs 2.0; p=0.71).
Acute Results of FIRM Ablation
In figures 2 and 3, FIRM ablation alone before conventional ablation terminated AF to sinus rhythm with <1 minute FIRM ablation at a left atrial rotor (figure 2), and with 5.5 minutes FIRM ablation at a right atrial rotor (figure 3).
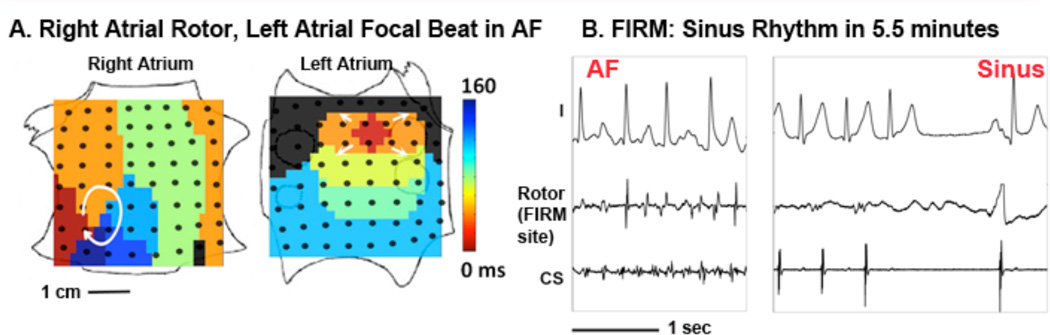
Figure 3. Acute Termination of AF (two sources) to Sinus Rhythm By FIRM Ablation.
(A) Right atrial rotor (clockwise) and simultaneous left atrial focal impulse (arrowed) during persistent AF in a 47 year old man. (B) FIRM ablation at right atrial rotor terminated AF to sinus rhythm in 5.5 minutes (see Supplemental movies 4–5). Note the slowing of AF rate during ablation. The left atrial focal impulse source was also treated by FIRM ablation. The patient is AF-free on implanted cardiac monitor at > 1 year. Scale bars 1 cm, 1 second; CS: coronary sinus electrogram. Atrial orientations as in figure 1.
By intention-to-treat analysis, FIRM ablation alone achieved the acute endpoint in 31/36 (86%) of patients. AF terminated in 20/36 cases (56%) with 4.3±6.3 minutes of FIRM ablation at the primary source (median 2.5 minutes, IQR 1.0–3.1; table 2). In the 11/36 cases in whom AF did not terminate, AF slowed by 33±12 ms (19±8%). Cases in whom FIRM ablation slowed rather than terminated AF had larger LA diameters (53±8 vs 46±6 mm; p<0.01) and more patients with LA diameter >55 mm (poor coverage because the LA was too large for the largest basket; 8/11 vs 1/20; p<0.001; Fisher exact test). FIRM ablation could not be completed in 4/36 cases in whom sources lay near the phrenic nerve, an atrial pacing lead, the compact AV node and esophagus, and FIRM ablation was not performed in 1 case without identified sources. Total FIRM ablation time (at all targeted sources) was 16.1±9.8 minutes (median 18.5, IQR 7.9–24.5; table 2).
Table 2.
Acute Results in all Cases or those with Sustained AF During Their Procedure
| Characteristic | Conventional Ablation |
FIRM Guided Ablation |
P | |
|---|---|---|---|---|
| Cases with Intraprocedural Sustained AF | 65/71 (92%) | 36/36 (100%) | 0.10 | |
| Subjects with AF Sources | 63/65 (97%) | 35/36 (97%) | 1.00 | |
| Acute Endpoint Achieved | 13/65 (20%) | 31/36 (86%) | <0.001 | |
| AF Termination Endpoint | 6/65 (9%) | 20/36 (56%) | <0.001 | |
| Ablation Time (min) at Primary source | – | 2.5 (1.0–3.1) | ||
| To Sinus Rhythm/Atrial Tachycardia | 3/3 | 16/4 | 0.29 | |
| AF Slowing (CL Prolongation) Endpoint, n (%) | 7/65 (11%) | 11/36 (31%) | 0.01 | |
| Extent of AF CL prolongation, ms (%) | 28±8 (18±6%) | 33±12(19±8%) | 0.38 | |
| Ablation time for Acute Endpoint (min) | 31.8 (22.1–71.5) | 18.5 (7.9–24.5) | <0.001 | |
| Total Procedural Ablation (all cases), min | 52.1±17.8 | 57.8±22.8 | 0.16 | |
| Complications (all cases) | 6 (8%) | 2 (6%) | 0.72 | |
| Cardiac Tamponade | 2 | 1 | ||
| Groin Bleed Requiring Transfusion | 3 | 1 | ||
| Vascular Injury Requiring Surgical Repair | 0 | 0 | ||
| Permanent Diaphragmatic Paralysis | 0 | 0 | ||
| Symptomatic Pulmonary Vein Stenosis | 1† | 0 | ||
| Stroke/TIA | 0 | 0 | ||
| Atrioesophageal Fistula | 0 | 0 | ||
| Death | 0 | 0 | ||
required stent
By comparison, in the FIRM-blinded group the acute endpoint was achieved in 13/65 cases with sustained AF (20%) after 43.4±28.0 (median 31.8, IQR 22.1–71.5) minutes ablation (p<0.001 for both comparisons against FIRM-guided limb).
Long term Efficacy
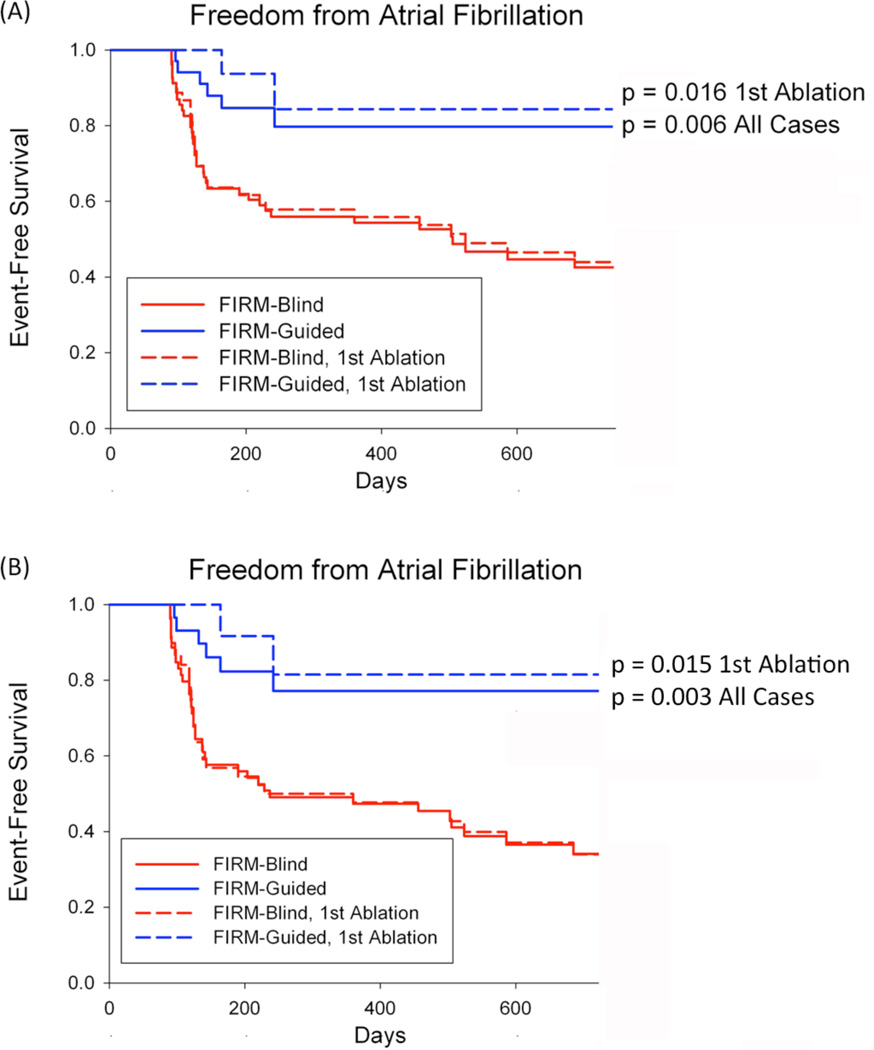
Two subjects in each group were lost to follow-up. By intention to treat analysis, single-procedure freedom from AF was higher for FIRM-guided than FIRM-blinded cases (82.4%, 28/34 versus 44.9%, 31/69; p<0.001) after 273 days (median; IQR 132–681). FIRM-guided therapy maintained its treatment benefit over FIRM-blinded therapy for first-time ablation cases (p<0.001). No FIRM-guided case recurred after ≈7 months as determined using mostly implanted ECG monitoring. Results were similar excluding subjects without AF who remained on anti-arrhythmic medications due to referring physician preference (79.3%, 23/29 FIRM-guided versus 35.6%, 21/59 FIRM-blinded; p<0.001). Freedom from any atrial tachyarrhythmia after a single procedure was also higher in FIRM-guided than blinded cases (24/34, 70.6% vs 27/69, 39.1%; p=0.003).
Kaplan-Meier survival plots are illustrated in figure 4. Followup was more rigorous in the FIRM-guided than FIRM-blinded groups (implantable ECG monitors in 30/34, 88.2% versus 18/69, 26.1%; p<0.001). Supplemental figures S1–S2 present examples of continuous and intermittent ECG monitoring for recurrent AF. Neither the total duration of ablation, the aggregate number nor the type of adverse events differed between groups (table 2).
Figure 4. Cumulative freedom from the Primary End Point (atrial fibrillation), in all cases and those at first ablation.
(A) For All cases; (B) Patients off Anti-Arrhythmic Medications. Intention-to-Treat Analysis, and p-values reflect the complete followup period.
Discussion
The CONFIRM trial demonstrates for the first time that human atrial fibrillation may be maintained by localized sources in the form of electrical rotors and focal impulses. Brief ablation (Focal Impulse and Rotor Modulation, FIRM) at patient-specific AF-sustaining sources was able to terminate or consistently slow persistent or paroxysmal AF prior to any conventional ablation in 86% of patients, and substantially increase long-term AF elimination using very rigorous monitoring compared to conventional AF ablation alone.
Localized Sources for Human AF
Electrical rotors in human AF were revealed using a novel computational mapping approach that analyses electrograms in a wide atrial field of view in the context of physiologically plausible activation rates and conduction dynamics (23–26). No prior trial has identified or successfully targeted localized human AF sources for acute AF termination and elimination on follow-up. However, a number of elegant reports have characterized organized reentry prior to AF (29), transient rotors in AF (21,22), and sites of rapid (17,18,30,31) or disorganized (32) AF.
The mechanistic role of rotors and focal sources in perpetuating AF is demonstrated by acute AF termination by brief FIRM ablation alone as we recently illustrated in a video case report (33). Patients in whom FIRM ablation slowed rather than terminated AF had sources that could not be eliminated, for safety considerations or protocol-imposed time limits, or had atria larger than current baskets (as illustrated in reference (23)) and may have had residual sources in unmapped regions. Rotors and focal sources were clinically relevant long-term AF perpetuators based on improved AF elimination using FIRM-guided versus conventional ablation.
Human AF rotors and focal impulses were fewer in number, longer-lived and more conserved in this study than suggested (20,21). This fact alters our conceptual framework for human AF, and enabled FIRM ablation to be practical and effective. Future work should study if structural (34) or electrical (14) remodeling, altered innervation (35) or other processes explain these differences in AF. The similar number of sources for patients with and without prior ablation suggests, on one hand, that prior ablation did not create sources and, on the other, that prior ablation may have been unsuccessful because it did not eliminate these AF sources. Both hypotheses require further testing.
Efficacy of FIRM-guided ablation
FIRM-guided ablation was more effective than conventional ablation in patients undergoing their first procedure (figure 4) as well as those with prior conventional ablation, who were included to compare the mechanisms of AF across a wide range of presentations. Moreover, FIRM-guided ablation showed substantial efficacy benefit despite the use of highly sensitive implanted ECG monitors in 86 % of cases. To the best of our knowledge, this is the highest usage of implanted ECG monitors in any AF treatment trial. The use of symptoms or intermittent ECG monitoring in prior AF therapy trials (7,27) likely underestimated the full burden of AF recurrence. Since only 23% of FIRM-blinded cases received implanted monitors, studies on the comparative efficacy of AF monitoring strategies (7,27) suggests that CONFIRM may actually underestimate the relative benefit of FIRM-guided over conventional ablation by ≈10 %.
FIRM Guided and Conventional Ablation
AF sources in this study are consistent with, and may explain, the results of conventional AF ablation. First, most sources lay in the left atrium, supporting current guidelines that primarily advocate left atrial ablation (3). Interestingly, the presence of right atrial sources in one-quarter of patients may explain the 70–80% success ceiling of conventional predominantly left atrial ablation for paroxysmal (6) and persistent (9) AF. Notably, prior studies that included right atrial ablation (36) achieved higher success rates. Second, diverse source locations are consistent with reports that widespread ablation may be required in both atria (10). Third, the higher number of sources in persistent than paroxysmal AF is consistent with lower success and more difficult procedures in the former group.
In CONFIRM, FIRM-guided ablation was followed by pulmonary vein isolation, yet total ablation time did not differ between groups (table 2) because the brief duration of FIRM ablation fell within case-to-case variations of conventional ablation time. Accordingly, FIRM-guided therapy presents an opportunity to improve ablation outcomes while avoiding more extensive strategies that may result in serious sequelae (3).
Limitations
This study has the limitations of a non-randomized design. However, subjects were enrolled consecutively and treated prospectively for pre-specified endpoints. The FIRM-Guided group had more subjects with persistent AF, higher co-morbidity and more intense monitoring than FIRM-Blinded subjects, thus potentially underestimating the benefit of FIRM-guided ablation. Second, although the basket may have suboptimal resolution for a small focal origin or rotor singularity (whose dimension is theoretically zero (16)), an AF source will ‘control’ larger atrial areas for which the basket field of view is currently unmatched in clinical practice. The size of a single ablation lesion (≈5–7 mm diameter) also places a practical limit on the necessary resolution. On the other hand, the largest available diameter (≈55–60 mm when fully deployed) limits the largest mappable atrial size. Third, more extensive FIRM ablation at sources beyond the ≤3 protocol limit may have yielded higher rates of AF termination and long-term success, but was not performed due to the study design. Future studies will target all detected sources for FIRM ablation. Finally, this first trial of FIRM guided ablation needs validation in larger populations, with balanced gender representation, by many more investigators and in randomized fashion. Such validation is already underway.
Conclusions
Human atrial fibrillation is typically caused by very few localized sources, that cause disorganization in the remaining atria. Focal Impulse and Rotor Modulation (FIRM) ablation to eliminate these sources was able to abruptly terminate or consistently slow persistent and paroxysmal AF in the vast majority of cases, and substantially improve long-term AF elimination over conventional ablation alone in this prospective case cohort study. FIRM mapping may open the possibility for several patient-tailored therapies for AF in addition to ablation for this highly prevalent disease with major public health and societal impact.
Supplementary Material
Acknowledgements
This work was supported by grants to SMN from the Doris Duke Foundation and National Institutes of Health (K23 HL70529, R01 HL83359, R01 HL83359-S1, K24 HL103800) (2001-present). We thank Antonio Moyeda, RCVT, Kenneth Hopper, RCVT, Judy Hildreth, RN, Sherie Janes, RN, Stephanie Yoakum, RNP, Elizabeth Greer, RN, Donna Cooper, RN and Kathleen Mills, BA for helping to perform the clinical study and collecting followup data. We are indebted to Michel Haïssaguerre, MD, Pierre Jaïs, MD and the Bordeaux group for their extremely helpful comments and suggestions during this project.
Footnotes
Disclosures: Drs. Narayan and Rappel are authors of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Dr. Narayan holds equity in Topera, and reports having received honoraria from Medtronic, St. Jude Medical, and Biotronik. Dr. Miller reports having received honoraria from Medtronic, St. Jude Medical, Biotronik, Biosense-Webster, Boston Scientific and is a scientific advisor to Topera. Dr. Shivkumar is a scientific advisor to Topera. Dr. Krummen and Mr. Clopton report no conflicts.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257. doi: 10.1007/s10840-012-9672-7. [DOI] [PubMed] [Google Scholar]
- 4.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-line Treatment of Symptomatic Atrial Fibrillation: A Randomized Trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 5.Oral H, Pappone C, Chugh A, et al. Circumferential Pulmonary-Vein A blation for Chronic Atrial Fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 6.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 7.Verma A, et al. Discerning the Incidence of Symptomatic and Asymptomatic Episodes of Atrial Fibrillation Pre-and Post-Radiofrequency Ablation (DISCERN-AF): A Prospective, Multicenter Study (Late Breaking Clinical Trial Abstract) Heart Rhythm. 2011;8 [Google Scholar]
- 8.Cheema A, Vasamreddy CR, Dalal D, et al. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;15:145–155. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 9.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 10.Haissaguerre M, Sanders P, Hocini M, et al. Catheter Ablation of Long-Lasting Persistent Atrial Fibrillation: Critical Structures for Termination. Journal of Cardiovascular Electrophysiology. 2005a;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang F, Antz M, Ernst S, et al. Recovered Pulmonary Vein Conduction as a Dominant Factor for Recurrent Atrial Tachyarrhythmias After Complete Circular Isolation of the Pulmonary Veins: Lessons From Double Lasso Technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Kilicaslan F, Pisano E, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–635. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 13.Sawhney N, Anand K, Robertson CE, Wurdeman T, Anousheh R, Feld GK. Recovery of Mitral Isthmus Conduction Leads to the Development of Macro-Reentrant Tachycardia after Left Atrial Linear Ablation for Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2011 doi: 10.1161/CIRCEP.111.964817. [DOI] [PubMed] [Google Scholar]
- 14.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 15.Allessie MA, de Groot NM, Houben RP, et al. The ElectroPathological Substrate of Longstanding Persistent Atrial Fibrillation in Patients with Structural Heart Disease: Longitudinal Dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 16.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of Left-to-Right Atrial Frequency Gradient in Paroxysmal but Not Persistent Atrial Fibrillation in Humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 18.Sahadevan J, Ryu K, Peltz L, et al. Epicardial Mapping of Chronic Atrial Fibrillation in Patients: Preliminary Observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 19.Beukema WP, Sie HT, Misier AR, Delnoy PP, Wellens HJ, Elvan A. Predictive factors of sustained sinus rhythm and recurrent atrial fibrillation after a radiofrequency modified Maze procedure. Eur J Cardiothorac Surg. 2008;34:771–775. doi: 10.1016/j.ejcts.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal Periodicity During Atrial Fibrillation in the Isolated Sheep Heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 21.Cuculich PS, Wang Y, Lindsay BD, et al. Noninvasive Characterization of Epicardial Activation in Humans With Diverse Atrial Fibrillation Patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atienza F, Calvo D, Almendral J, et al. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081–1092. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayan SM, Krummen DE, Rappel W-J. Clinical Mapping Approach To Diagnose Electrical Rotors and Focal Impulse Sources for Human Atrial Fibrillation. J Cardiovasc Electrophysiol. 2012a;23 doi: 10.1111/j.1540-8167.2012.02332.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and Activation Restitution Near Human Pulmonary Veins and Atrial Fibrillation Initiation: A Mechanism for the Initiation of Atrial Fibrillation by Premature Beats. J Am Coll Cardiol. 2008c;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization Alternans Reveals Vulnerability to Human Atrial Fibrillation. Circulation. 2011b;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalani G, Schricker A, Gibson M, Rostamanian A, Krummen DE, Narayan SM. Dynamic Conduction Slowing Precedes Human Atrial Fibrillation Initiation: Insights from Bi-Atrial Basket Mapping On Transitions to Atrial Fibrillation. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler P, Koehler J, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445–1452. doi: 10.1016/j.hrthm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi Y, O'Neill MD, Hocini M, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008;51:1003–1010. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y-J, Tai C-T, Kao T, et al. Electrophysiological Characteristics and Catheter Ablation in Patients With Paroxysmal Right Atrial Fibrillation. Circulation. 2005;112:1692–1700. doi: 10.1161/CIRCULATIONAHA.104.512731. [DOI] [PubMed] [Google Scholar]
- 30.Wu T-J, Doshi RN, Huang H-LA, et al. Simultaneous Biatrial Computerized Mapping During Permanent Atrial Fibrillation in Patients with Organic Heart Disease. J. Cardiovasc. Electrophysiol. 2002;13:571–577. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 31.Sanders P, Berenfeld O, Hocini M, et al. Spectral Analysis Identifies Sites of High-Frequency Activity Maintaining Atrial Fibrillation in Humans. Circulation. 2005a;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 32.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004a;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 33.Narayan SM, Patel J, Mulpuru S, Krummen DE. Focal Impulse and Rotor Modulation (FIRM) Ablation of Sustaining Rotors Abruptly Terminates Persistent Atrial Fibrillation To Sinus Rhythm With Elimination On Followup. Heart Rhythm. 2012b;9 doi: 10.1016/j.hrthm.2012.03.055. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng X, Scherlag BJ, Yu L, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 2011;57:563–571. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Hocini M, Nault I, Wright M, et al. Disparate Evolution of Right and Left Atrial Rate During Ablation of Long-Lasting Persistent Atrial Fibrillation. J Am Coll Cardiol. 2010;55:1007–1016. doi: 10.1016/j.jacc.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.