Abstract
Many amputees suffer from post-amputation pain, which can be extremely debilitating, decrease quality of life, increase the risk of depression, and negatively affect interpersonal relationships and the ability to work. Present methods of treatment, including medications, are often unsatisfactory in reducing post-amputation pain. Electrical stimulation of the nerve innervating the painful area could reduce the pain, but peripheral nerve stimulation is rarely used to treat post-amputation pain because present methods require invasive surgical access and precise placement of the leads in close proximity (≤ 2 mm) with the nerve. The present study investigated a novel approach to peripheral nerve stimulation in which a lead was placed percutaneously a remote distance (> 1 cm) away from the femoral nerve in a patient with severe residual limb pain 33 years following a below-knee amputation. Electrical stimulation generated ≥ 75% paresthesia coverage, reduced residual limb pain by > 60%, and improved quality of life outcomes as measured by the pain interference scale of the Brief Pain Inventory-Short Form (100% reduction in pain interference), Pain Disability Index (74% reduction in disability), and the Patient Global Impression of Change (Very Much Improved) during a 2-week home trial. There were no adverse events. The ability to generate significant paresthesia coverage and pain relief with a single lead inserted percutaneously and remotely from the target nerve holds promise for providing relief of post-amputation pain.
Keywords: Amputee, phantom limb pain, residual limb pain, electrical stimulation, peripheral nerve stimulation
Introduction
Amputation can lead to chronic pain in many patients, and up to 70%–80% of patients have significant pain.1–2 Following amputation, patients may have two types of chronic pain: phantom limb pain and/or residual limb pain. The pain can be extremely debilitating to amputees, significantly decrease their quality of life, increase their risk of depression, and negatively affect their inter-personal relationships and their ability to work.3–5 In amputees with moderate to severe pain, it is frequently the pain following amputation rather than the loss of a limb that most impacts the activities of daily living, prevents completion of simple tasks, and correlates most negatively with return to employment.6–8 Poorly treated residual limb pain can further impair function by preventing the use of prostheses. Present methods of treatment, which are primarily medications, are often unsatisfactory in reducing residual limb pain, and have the potential for unwanted side effects, addiction or misuse.9–13 The present study investigates the feasibility of reducing residual limb pain using a novel method of delivering electrical stimulation.
Electrical stimulation can reduce post-amputation pain when it is delivered via spinal cord stimulation (SCS)14–18 or peripheral nerve stimulation (PNS).19–25 SCS can provide pain relief in well-selected amputees, but the number of recent studies has been limited26 and the literature reports a variable success rate.14–18,26–28 PNS has the potential to be an effective therapy when the majority of the pain is confined to the distribution of one to two nerves29–30, such as with lower-extremity amputation when the post-amputation pain is limited to the distribution of the femoral and/ or sciatic nerve.20–21,23 Despite the potential for pain relief, clinical use of PNS has been limited in part because there is no large randomized-controlled clinical trial demonstrating its efficacy and safety, and most of the present PNS methods typically require invasive surgical access and precise placement of the lead(s) in close proximity (≤ 2 mm) with the nerve.25,30–34
Presently, precise placement of multiple electrode contacts in close proximity with the nerve is required to provide selective stimulation of the target sensory neurons (i.e. type Ia and Ib) that evoke the comfortable sensations (paresthesia) associated with pain relief and avoid activation of the non-target motor neurons (type alpha) and sensory neurons (type III and IV) that can generate unwanted muscle contractions and painful sensations, respectively20,23,35. It can be particularly challenging to achieve selective stimulation of target fibers in the large diameter nerve trunks of the lower extremity, such as the trunk of the femoral nerve. A review of case studies indicates PNS has a historically lower success rate in the lower extremity relative to the upper extremity21–22,30,35–37, possibly due to the challenge of selectively activating only the target sensory neurons located deep in the center of a larger diameter nerve trunk without activating the non-target motor neurons35,38, which can be further complicated by displacement of the electrode contacts relative to their initial precise placement along the nerve during weight bearing movements of the lower extremity.30–31,35,39–40
Present solutions to selective activation of target fibers in the lower extremity include surgical dissection of the nerve and placement of multi-contact electrodes along the nerve trunk30–31,35 or more distal placement of the electrodes on the smaller diameter nerve branches.34,41 As an alternative to present approaches, it was hypothesized that a single-contact electrode lead could be placed at the level of the nerve trunk to provide greater paresthesia coverage and that placement of the lead remote from the nerve trunk would enable selective stimulation of only the target sensory fibers. A method of PNS that does not require surgical access or precise placement of the lead in close proximity with the nerve would be beneficial because it could reduce the barriers to using PNS. We hypothesized that a lead could be inserted percutaneously at a significant distance (> 1 cm) from the femoral nerve in a lower-extremity amputee and still produce comfortable paresthesia coverage and reduce post-amputation pain without unwanted muscle contractions.
Case Report
The present case study was approved by the FDA under an Investigational Device Exemption (IDE), and Investigational Review Board approval was obtained. The research study followed standard clinical practice guidelines. The subject was a 49-year-old African-American male who reported severe residual limb pain (RLP) secondary to a below-the-knee amputation of his right leg following a motor vehicle accident 33 years prior to enrollment into the study. The subject reported that the RLP remained severe despite a history of using narcotic analgesics, anticonvulsants, non-steroidal anti-inflammatory drugs (NSAIDs), physical therapy, and nerve blocks. The subject perceived pain throughout his residual limb as indicated on the pain diagram (Figure 1) with the most intense pain located just above the level of amputation. The subject reported no phantom limb pain throughout the study with the exception of one diary entry (score of 2 on an 11-point numerical rating scale) on a single day during the baseline period. In addition to the amputation, pain, and related sequelae, the medical history of the subject included sickle cell anemia but did not include diabetes or peripheral vascular disease.
Figure 1.
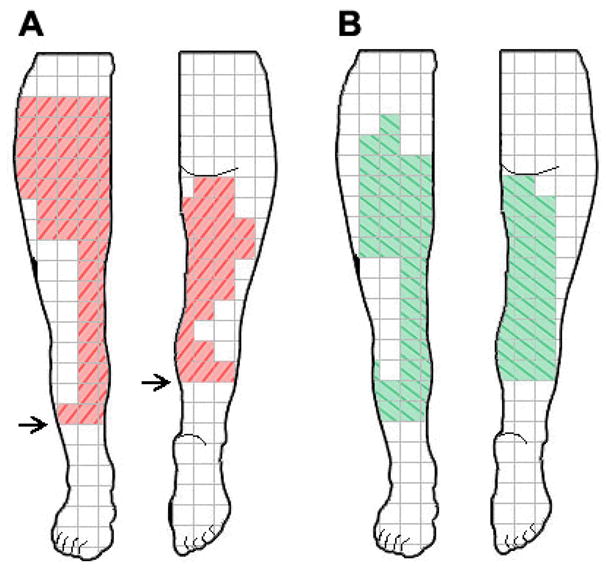
The subject indicated areas of pain (A) and areas of stimulation-evoked paresthesia coverage (B).Arrows indicate level of amputation on the front and back views of the leg, respectively.
The subject enrolled in the study after providing informed consent and meeting all eligibility requirements. Inclusion criteria included a well-healed unilateral lower extremity amputation, daily worst residual limb pain and/or phantom limb pain score ≥ 4 on an 11-point numerical rating scale on the Brief Pain Inventory-Short Form (BPI-SF) Question #3 (BPI3), Beck Depression Inventory (BDI-II) score of ≤ 20, and age ≥ 18 years. Exclusion criteria included the absence of sepsis, infection, diabetes mellitus type I and II, implanted electronic devices, anticoagulation therapy (aside from aspirin therapy), history of valvular heart disease, previous limb injections within the past six months, pregnancy and any previous allergy to skin contact materials and/ or anesthetic agents. The subject had no potential secondary gain issues at the time of enrollment.
After providing informed consent and medical history, the subject was sent home with a diary and asked to record medication usage and worst-pain levels every day for the duration of the 8-week study. Throughout the study, the subject reported taking the following medications daily: one multivitamin (1 time/day), ibuprofen (800mg, 3 times/day), and gabapentin (600 – 800mg, 3 times/day). At the end of the 2-week baseline period, the subject requested that his dose of gabapentin be increased from 600mg to 800mg in response to a recent back injury unrelated to the study. The subject continued to take the 800mg dose of gabapentin for the remaining 6 weeks of the study. The 3-day average of the BPI3 (daily worst pain) scores during baseline was 7.7.
After completing the 2-week baseline period, the subject returned to the clinic for lead placement and electrical stimulation testing. The lead was a fine-wire helical coil wound from a seven-strand, type 316L stainless steel wire with a single anchoring barb and electrode contact. The lead was insulated with perfluoroalkoxy and preloaded in a 20-gauge, insulated hypodermic needle introducer. The subject was placed in a supine position to allow access to the femoral nerve using an anterior approach. The insertion site was cleansed using aseptic technique and local anesthesia was administered. No sedation was used.
Prior to placing the fine-wire lead, a monopolar needle electrode (24-gauge, Jari Electrode Supply, Gilroy, CA) was inserted below the femoral crease and lateral to the femoral artery to within 0.5 – 1 cm of the femoral nerve under ultrasound guidance to deliver test stimulation. Test stimulation (40 μs, 1 mA, 50 Hz) was delivered with a regulated-current stimulator (Maxima II, Empi, Inc., St. Paul, MN) to confirm that the angle of insertion (40° from skin surface) and the length of needle under the skin (3.6 cm) evoked a comfortable paresthesia in the region of pain innervated by the femoral nerve. Once confirmed, the monopolar needle electrode was withdrawn and replaced with the fine-wire lead under ultrasound guidance using the same insertion site and the same approach except that the introducer was only inserted 2 cm under the skin, placing the lead remotely (> 1 cm away) from the nerve.
Correct lead placement was confirmed by evoking a comfortable paresthesia with stimulation (50 μs, 1 mA, 50 Hz) that covered ≥ 75% of the painful area without evoking muscle contractions, qualifying the subject to proceed to the 2-week home trial. The stimulator was replaced with a regulated-voltage stimulator (Rehabilicare NT2000, Empi, Inc., St. Paul, MN) that was approved for the home trial. Stimulation pulse width was set at 30 μs and amplitude was incrementally increased to evoke the maximum comfortable paresthesia coverage (≥ 75%). The lead was deployed by withdrawing the needle introducer while maintaining pressure at the skin surface. The lead was coiled outside the skin to create a strain-relief loop, and the exit site was bandaged with waterproof bandages (Tegaderm by 3M, St. Paul, MN). The subject was instructed regarding the use of the stimulator and care of the bandages before progressing to the first week of the home trial.
The subject returned as planned to the clinic after the first week of the home trial for bandage change, exit site inspection, and an increase in stimulus pulse width from 30 μs to 40 μs. The subject reported improved comfort in response to the change in pulse width, and the subject progressed to the second week of the home trial. The subject returned as planned after the second week of the home trial for lead removal and again for the 1-week and 4-week follow-up visits.
During lead placement and the subsequent 2-week home trial, the subject reported comfortable paresthesia coverage of ≥ 75% of the region of residual limb pan (RLP) (Figure 1), and no muscle contractions were observed in response to electrical stimulation.
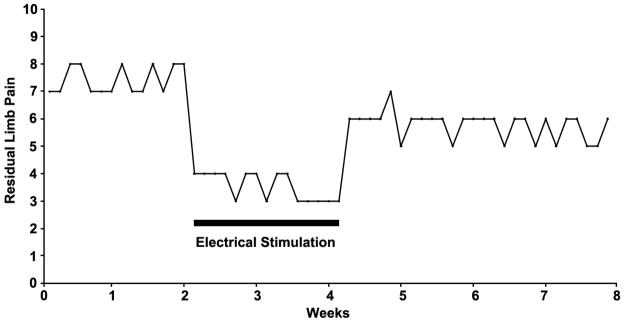
Electrical stimulation of the femoral nerve reduced the RLP by 60% from baseline by the end of the 2-week home trial (Figure 2). The mean pain interference score for the BPI-SF decreased from 6.3 at baseline to 1.7 (73% improvement) after the first week of stimulation and to 0 (100% improvement) after the second week of stimulation (Table 1). The mean Pain Disability Index (PDI) score was 7 at baseline and decreased to 3.8 (45% improvement) after the first week of stimulation and further decreased to 1.8 (74% improvement) after the second week of stimulation (Table 1). The sum of scores on the BDI-II was 0 at baseline and at the end of the 2-week stimulation home trial (Table 1). Relative to baseline, the subject reported on the Patient Global Impression of Change (PGIC) scale that he felt “Much Improved” after the first week of stimulation and “Very Much Improved” after the second week of stimulation. The lead was removed intact, and no adverse events were reported.
Figure 2.
Worst pain in the last 24 hours (BPI3) as reported by the subject in the daily diary.
Table 1.
Outcome measures collected during baseline, after the first week of stimulation, after the second week of stimulation, after the first week of follow-up (F/U), and after the fourth week of follow-up. Percent change from baseline is reported in parentheses (%) where applicable.
| Baseline | Stimulation Trial | 1 Wk F/U | 4 Wk F/U | ||
|---|---|---|---|---|---|
| Stimulation Off | Stimulation On | Stimulation On | Stimulation Off | Stimulation Off | |
| Wk 0 | Wk 3 | Wk 4 | Wk 8 | Wk 8 | |
| Brief Pain Inventory Short Form (BPI-SF) | |||||
| BPI3-Worst pain in last week | 8 | 4 (50%) | 3 (63%) | 6 (25%) | 6 (25%) |
| BPI9-Meanpain interference scores | 6.3 | 1.7(73%) | 0 (100%) | 1.9 (70%) | 1.9 (70%) |
| Pain Disability Index (PDI)-meanscores | 7.0 | 3.8(45%) | 1.8(74%) | 3.3 (52%) | 3.3 (52%) |
| BDI-II Beck Depression Inventory sum of scores | 0 | 1 (3%) | 0 (0%) | 1 (3%) | 1 (3%) |
| PGIC-Patient Global Impression of Change | n/a | Much Improved | Very Much Improved | Minimally Improved | Minimally Improved |
Discussion
This case report describes the first time peripheral nerve stimulation (PNS) has generated clinically significant relief of post-amputation pain using a lead placed percutaneously a remote distance away from the femoral nerve. During the 2-week home trial of stimulation, 60% improvement was observed in the BPI3 (worst daily pain), which translated into a reduction in pain classification from severe pain (score ≥ 7) to minor pain (score ≤ 3) and correlated with similar improvements in quality of life measures.
The 2-week home trial produced complete resolution (100%) of the interference of pain on daily activities and mood as measured by the BPI-SF, and it greatly reduced (74%) the impact of pain on physical functioning and activities of daily living as measured by the PDI. Emotional functioning was not impaired by pain at baseline, and it did not change significantly throughout the study as measured by the BDI-II. The subject reported that his overall quality of life (activity limitations, symptoms, and emotions) related to his pain was “Very much improved” (the maximum score possible) by the end of the 2-week home trial relative to baseline as measured by the PGIC.
The method of PNS used in the present case is distinct from peripheral nerve field stimulation or subcutaneous stimulation in which the lead is placed in the region of pain to activate nearby nerve branches and provide pain relief to the local surrounding area.42–44 In the present study, the lead was placed outside of the area of pain to activate the femoral nerve trunk and provide relief to distal areas of pain. From Figure 1, stimulation appeared to generate paresthesia coverage in the regions innervated by the femoral nerve and in regions of the posterior limb. It is unclear why the subject perceived paresthesia in the posterior limb, but it may have been due to repositioning of tissue and innervation from the anterior leg during the initial amputation and remodeling surgery as part of the reconstruction of the residual limb following the original traumatic injury, it may have been related to the plasticity and reorganization of the central nervous system following amputation, and it is also possible that stimulation may have activated a branch of the sciatic nerve, such as the posterior cutaneous nerve of the thigh.
Gate-control theory as proposed by Melzack and Wall may explain how activation of large myelinated nerve fibers by PNS can inhibit transmission of pain signals (and “close the gate”) from the spinal cord to higher centers in the central nervous system to decrease the perception of pain.45–47 Though other theories exist, gate theory is the most common explanation of the mechanism through which PNS achieves pain relief by inhibiting activity in central pain pathways, such as the spinothalamic tract.47–50
This case report complements previous studies of spinal cord stimulation14–18 and PNS19–25 that indicate electrical stimulation has the potential to provide significant pain relief when stimulation generates > 50% paresthesia coverage of the painful region.15 In the present study, the subject reported ≥ 75% paresthesia coverage and > 60% relief of post-amputation pain during the 2-week trial, suggesting that further investigation is warranted to determine if long-term relief can be provided by an implantable version of the present therapy.
PNS offers the potential to deliver therapeutic stimulation to the nerve innervating the region of pain and limit the distribution of paresthesia to the area in which it is needed.29 However, PNS is seldom used to treat post-amputation pain because there are no data from clinical trials and available PNS systems can be technically challenging to place in close proximity to the nerve.25,29–30,33,51 Traditionally, electrical stimulation of a large peripheral nerve trunk, such as the femoral nerve, has required surgical access and dissection to place a cuff-, paddle-, or plate-style lead in intimate contact with the nerve.19–25,30 However, recent studies have shown that cylindrical leads can be placed percutaneously in close proximity (≤ 2 mm) to the nerve under ultrasound guidance.32–34 The present study builds on this foundation by demonstrating that a lead can be placed percutaneously and remotely (> 1 cm away) from the nerve and still obtain significant paresthesia coverage and pain relief.
One limitation of the study was that the lead was too fine of a wire to be visualized with ultrasound once the needle introducer was withdrawn, preventing verification of the final distance between the nerve and the lead following deployment. Thus, given the technique used for placement, a nerve-to-lead distance of greater than 1 cm was likely but not certain. Other limitations of the present study included the short duration of the therapy (2 weeks) and follow-up (4 weeks), the lack of a placebo or other comparison, and the case-report study design.
Future studies are needed to confirm this result in additional patients, compare PNS to other treatments as has been done with spinal cord stimulation52–53, and determine if stimulation can be delivered to the sciatic nerve trunk with a single percutaneously-placed lead. Previous studies indicate that the individual branches of the sciatic nerve (e.g. the common peroneal and posterior tibial nerve branches) can be stimulated with leads placed percutaneously in close proximity to these relatively smaller-diameter nerve branches.34 If future studies can build on this technique and expand its potential clinical applicability by enabling a single lead to be placed percutaneously at the level of the larger trunk of the sciatic nerve and activate selectively only the target sensory neurons, it could provide therapeutic relief to the entire distribution of the sciatic nerve with a simpler, less invasive procedure. The ability to generate significant paresthesia coverage and pain relief with a single lead inserted percutaneously and remotely from the target nerve holds promise for providing relief of post-amputation pain.
Acknowledgments
This work was sponsored by NDI Medical, Cleveland, OH and supported in part by grant award number R43NS066523 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Disclosures: NDI Medical (the sponsor of this study) and SPR Therapeutics (a subsidiary of NDI Medical) have a commercial interest in the device presented in this case report. Richard Rauck, MD, Leonardo Kapural, MD, PhD, and Steven P. Cohen, MD are consultants to NDI Medical. Rosemary Zang, RN is an employee of SPR Therapeutics, and Joseph Boggs, PhD is an employee of NDI Medical.
Device status: An investigational percutaneous peripheral nerve stimulator system (NDI Medical) including a percutaneous intramuscular lead and commercially available external stimulators (Maxima® II and Rehabilicare® NT2000, Empi, Inc, St. Paul, MN) was used during this clinical study. The peripheral nerve stimulation systems were provided by NDI Medical.
Bibliography
- 1.Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, Robinson LR. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000 Aug;81(8):1039–44. doi: 10.1053/apmr.2000.7583. [DOI] [PubMed] [Google Scholar]
- 2.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005 Oct;86(10):1910–9. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 3.Kashani JH, Frank RG, Kashani SR, Wonderlich SA, Reid JC. Depression among amputees. J Clin Psychiatry. 1983 Jul;44(7):256–8.4. [PubMed] [Google Scholar]
- 4.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994 Jul;151(7):979–86. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 5.Cansever A, Uzun O, Yildiz C, Ates A, Atesalp AS. Depression in men with traumatic lower part amputation: a comparison to men with surgical lower part amputation. Mil Med. 2003 Feb;168(2):106–9. [PubMed] [Google Scholar]
- 6.Millstein S, Bain D, Hunter GA. A review of employment patterns of industrial amputees--factors influencing rehabilitation. Prosthet Orthot Int. 1985 Aug;9(2):69–78. doi: 10.3109/03093648509164708. [DOI] [PubMed] [Google Scholar]
- 7.Whyte AS, Carroll LJ. A preliminary examination of the relationship between employment, pain and disability in an amputee population. Disabil Rehabil. 2002 Jun 15;24(9):462–70. doi: 10.1080/09638280110105213. [DOI] [PubMed] [Google Scholar]
- 8.Rudy TE, Lieber SJ, Boston JR, Gourley LM, Baysal E. Psychosocial predictors of physical performance in disabled individuals with chronic pain. Clin J Pain. 2003 Jan-Feb;19(1):18–30. doi: 10.1097/00002508-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Sherman RA, Sherman CJ, Gall NG. A survey of current phantom limb pain treatment in the United States. Pain. 1980 Feb;8(1):85–99. doi: 10.1016/0304-3959(80)90092-5. [DOI] [PubMed] [Google Scholar]
- 10.Sherman RA, Sherman CJ. Prevalence and characteristics of chronic phantom limb pain among American veterans. Results of a trial survey. Am J Phys Med. 1983 Oct;62(5):227–38. [PubMed] [Google Scholar]
- 11.Sherman RA, Sherman CJ, Parker L. Chronic phantom and residual limb pain among American veterans: results of a survey. Pain. 1984 Jan;18(1):83–95. doi: 10.1016/0304-3959(84)90128-3. [DOI] [PubMed] [Google Scholar]
- 12.Jahangiri M, Jayatunga AP, Bradley JW, Dark CH. Prevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine and bupivacaine. Ann R Coll Surg Engl. 1994 Sep;76(5):324–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenquist RW, Haider N. Phantom limb pain. In: Benzon HT, Rathmell JP, Wu CL, Turk DC, Argoff CE, editors. Raj’s practical management of pain. Philadelphia: Mosby Elsevier; 2008. pp. 445–53. [Google Scholar]
- 14.Krainick JU, Thoden U, Riechert T. Spinal cord stimulation in post-amputation pain. Surg Neurol. 1975 Jul;4(1):167–70. [PubMed] [Google Scholar]
- 15.Krainick JU, Thoden U, Riechert T. Pain reduction in amputees by long-term spinal cord stimulation. Long-term follow-up study over 5 years. J Neurosurg. 1980 Mar;52(3):346–50. doi: 10.3171/jns.1980.52.3.0346. [DOI] [PubMed] [Google Scholar]
- 16.Nielson KD, Adams JE, Hosobuchi Y. Phantom limb pain. Treatment with dorsal column stimulation. J Neurosurg. 1975 Mar;42(3):301–7. doi: 10.3171/jns.1975.42.3.0301. [DOI] [PubMed] [Google Scholar]
- 17.Miles J, Lipton S. Phantom limb pain treated by electrical stimulation. Pain. 1978 Dec;5(4):373–82. doi: 10.1016/0304-3959(78)90006-4. [DOI] [PubMed] [Google Scholar]
- 18.Krainick JU, Thoden U. Spinal cord stimulation in postamputation pain. In: Siegfried J, Zimmermann M, editors. Phantom and residual limb pain. Berlin: Springer-Verlag; 1981. pp. 163–166. [Google Scholar]
- 19.Long DM. Electrical stimulation for relief of pain from chronic nerve injury. J Neurosurg. 1973 Dec;39(6):718–22. doi: 10.3171/jns.1973.39.6.0718. [DOI] [PubMed] [Google Scholar]
- 20.Nashold BS, Jr, Goldner JL. Electrical stimulation of peripheral nerves for relief of intractable chronic pain. Med Instrum. 1975 Sep-Oct;9(5):224–5. [PubMed] [Google Scholar]
- 21.Picaza JA, Cannon BW, Hunter SE, Boyd AS, Guma J, Maurer D. Pain suppression by peripheral nerve stimulation. Part II. Observations with implanted devices. Surg Neurol. 1975 Jul;4(1):115–26. [PubMed] [Google Scholar]
- 22.Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976 Dec;45(6):692–9. doi: 10.3171/jns.1976.45.6.0692. [DOI] [PubMed] [Google Scholar]
- 23.Nashold BS, Jr, Goldner JL, Mullen JB, Bright DS. Long-term pain control by direct peripheral-nerve stimulation. J Bone Joint Surg Am. 1982 Jan;64(1):1–10. [PubMed] [Google Scholar]
- 24.Gybels JM, Van Calenbergh F. The treatment of pain due to peripheral nerve injury by electrical stimulation of the injured nerve. In: Lipton S, Tunks E, Zoppi M, editors. Advances in pain research and therapy. Vol. 13. New York: Raven Press; 1990. pp. 217–222. [Google Scholar]
- 25.North RB. Spinal cord and peripheral nerve stimulation: technical aspects. In: Simpson BA, editor. Electrical stimulation and the relief of pain. New York: Elsevier; 2003. pp. 183–195. [Google Scholar]
- 26.Viswanathan A, Phan PC, Burton AW. Use of spinal cord stimulation in the treatment of phantom limb pain: case series and review of the literature. Pain Pract. 2010 Sep-Oct;10(5):479–84. doi: 10.1111/j.1533-2500.2010.00374.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain--some predictors of success. A 15-year experience. Surg Neurol. 1998 Aug;50(2):110–20. doi: 10.1016/s0090-3019(98)00012-3. discussion 120-1. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Ledesma MJ, García-March G, Diaz-Cascajo P, Gómez-Moreta J, Broseta J. Spinal cord stimulation in deafferentation pain. Stereotact Funct Neurosurg. 1989;53(1):40–5. doi: 10.1159/000099520. [DOI] [PubMed] [Google Scholar]
- 29.Gybels JM, Nuttin BJ. Peripheral nerve stimulation. In: Loeser JD, editor. Bonica’s management of pain. New York: Lippincott Williams & Wilkins; 2001. pp. 1851–1856. [Google Scholar]
- 30.Stanton-Hicks M. Peripheral nerve stimulation for pain peripheral neuralgia and complex regional pain syndrome. In: Krames ES, Peckham PH, Rezai AR, editors. Neuromodulation. Vol. 1. New York: Elsevier; 2009. pp. 397–407. [Google Scholar]
- 31.Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007 Mar;14(3):216–21. doi: 10.1016/j.jocn.2005.11.007. discussion 222-3. [DOI] [PubMed] [Google Scholar]
- 32.Huntoon MA, Huntoon EA, Obray JB, Lamer TJ. Feasibility of ultrasound-guided percutaneous placement of peripheral nerve stimulation electrodes in a cadaver model: part one, lower extremity. Reg Anesth Pain Med. 2008 Nov-Dec;33(6):551–7. doi: 10.1016/j.rapm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Huntoon MA, Hoelzer BC, Burgher AH, Hurdle MF, Huntoon EA. Feasibility of ultrasound-guided percutaneous placement of peripheral nerve stimulation electrodes and anchoring during simulated movement: part two, upper extremity. Reg Anesth Pain Med. 2008 Nov-Dec;33(6):558–65. doi: 10.1016/j.rapm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009 Nov;10(8):1369–77. doi: 10.1111/j.1526-4637.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 35.Stanton-Hicks M. Transcutaneous and peripheral nerve stimulation. In: Simpson BA, editor. Pain research and clinical management, Vol 15: Electrical stimulation and the relief of pain. New York: Elsevier; 2003. pp. 37–55. [Google Scholar]
- 36.Kirsch WM, Lewis JA, Simon RH. Experiences with electrical stimulation devices for the control of chronic pain. Med Instrum. 1975 Sep-Oct;9(5):217–20. [PubMed] [Google Scholar]
- 37.Sweet WH. Control of pain by direct electrical stimulation of peripheral nerves. Clin Neurosurg. 1976;23:103–11. doi: 10.1093/neurosurgery/23.cn_suppl_1.103. [DOI] [PubMed] [Google Scholar]
- 38.Goldner JL, Nashold BS, Jr, Hendrix PC. Peripheral nerve electrical stimulation. Clin Orthop Relat Res. 1982 Mar;163:33–41. [PubMed] [Google Scholar]
- 39.Waisbrod H, Panhans C, Hansen D, Gerbershagen HU. Direct nerve stimulation for painful peripheral neuropathies. J Bone Joint Surg Br. 1985 May;67(3):470–2. doi: 10.1302/0301-620X.67B3.2987272. [DOI] [PubMed] [Google Scholar]
- 40.Gybels J, Kupers R. Central and peripheral electrical stimulation of the nervous system in the treatment of chronic pain. Acta Neurochir Suppl (Wien) 1987;38:64–75. doi: 10.1007/978-3-7091-6975-9_10. [DOI] [PubMed] [Google Scholar]
- 41.Hassenbusch SJ, Stanton-Hicks M, Schoppa D, Walsh JG, Covington EC. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996 Mar;84(3):415–23. doi: 10.3171/jns.1996.84.3.0415. [DOI] [PubMed] [Google Scholar]
- 42.Goroszeniuk T, Kothari S, Hamann W. Subcutaneous neuromodulating implant targeted at the site of pain. Reg Anesth Pain Med. 2006 Mar-Apr;31(2):168–71. doi: 10.1016/j.rapm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral nerve field stimulation in chronic abdominal pain. Pain Physician. 2006 Jul;9(3):261–6. [PubMed] [Google Scholar]
- 44.Goroszeniuk T, Kothari S. Subcutaneous targeted stimulation. In: Krames E, Peckham P, Rezai A, editors. Neuromodulation. Boston: Elsevier; 2009. pp. 417–27. [Google Scholar]
- 45.Melzack R, Wall PD. On the nature of cutaneous sensory mechanisms. Brain. 1962 Jun;85:331–56. doi: 10.1093/brain/85.2.331. [DOI] [PubMed] [Google Scholar]
- 46.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(699):971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 47.Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967 Jan 6;155(758):108–9. doi: 10.1126/science.155.3758.108. [DOI] [PubMed] [Google Scholar]
- 48.Chung JM, Lee KH, Hori Y, Endo K, Willis WD. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain. 1984 Jul;19(3):277–93. doi: 10.1016/0304-3959(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 49.Keller T, Krames E. “On the Shoulders of Giants”: A History of the Understandings of Pain, Leading to the Understandings of Neuromodulation. Neuromodulation. 2009;12(2):77–84. doi: 10.1111/j.1525-1403.2009.00196.x. [DOI] [PubMed] [Google Scholar]
- 50.Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976 Dec;45(6):692–9. doi: 10.3171/jns.1976.45.6.0692. [DOI] [PubMed] [Google Scholar]
- 51.Strege DW, Cooney WP, Wood MB, Johnson SJ, Metcalf BJ. Chronic peripheral nerve pain treated with direct electrical nerve stimulation. J Hand Surg [Am] 1994 Nov;19(6):931–9. doi: 10.1016/0363-5023(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 52.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106. doi: 10.1227/01.neu.0000144839.65524.e0. discussion 106-7. [DOI] [PubMed] [Google Scholar]
- 53.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomized controlled trial in patients with failed back surgery syndrome. Pain. 2007;Nov132(1–2):179–88. doi: 10.1016/j.pain.2007.07.028. Epub 2007 Sep 12. [DOI] [PubMed] [Google Scholar]