Abstract
Background
Bariatric surgery is one of the few effective treatments for morbid obesity but the weight loss and other health related outcomes for this procedure in large, diverse adolescent patient populations are not well characterized.
Objective
To analyze the prospective Bariatric Outcomes Longitudinal Database (BOLD) to determine the weight loss and health related outcomes in adolescents.
Setting
BOLD data is collected from 423 surgeons at 360 facilities in the United States.
Methods
Main outcome measures included anthropometric and comorbidity status at baseline (n=890) and at 3 (n=786), 6 (n=541), and 12 (n=259) months after surgery. Adolescents (75% female; 68% non-Hispanic white, 14% Hispanic, 11% non-Hispanic black, and 6% other) age 11-to-19 years were included in the analyses.
Results
The overall one year mean weight loss for those who underwent gastric bypass surgery was more than twice that of those who underwent adjustable gastric band surgery (48.6 kg versus 20 kg, P<0.001). Similar results were found for all other anthropometric changes and comparisons over one year between surgery types (P<0.001). In general, gastric bypass patients reported more improvement versus adjustable gastric band patients in comorbidities one year after surgery. There were a total of 45 readmissions among gastric bypass patients and 10 among adjustable gastric band patients with 29 and 8 reoperations required, respectively.
Conclusions
Weight loss at 3-, 6-, and 12-months after surgery is approximately double in adolescent males and females who underwent gastric bypass surgery versus those who underwent adjustable gastric band surgery. Bariatric surgery can safely and substantially reduce weight and related comorbidities in morbidly obese adolescents for at least 1 year.
Keywords: bariatric surgery, adolescents, cardiovascular outcomes, weight outcomes, morbid obesity
INTRODUCTION
The global epidemic of obesity is a worldwide public and clinical health issue.(1) According to the World Health Organization, obesity is rising by 30-million cases per year.(1) In the United States, life expectancy is projected to decrease as a consequence of obesity alone, prompting the investigation of more treatment options.(2) Moreover, the distribution of body-mass index (BMI, kilograms [kg] of weight divided by height in meters squared or kg/m2) has become skewed so that the heaviest have become even heavier.(3)
Specifically, the US Centers for Disease Control and Prevention recently began estimating the proportion of morbidly obese (BMI ≥ 97th percentile for age and sex) children and adolescents.(3) In 2007–2008, an estimated 12% of all US children between 2 and 19-years old were morbidly obese. Among 12-through-19-year-olds, the estimates were 11% for non-Hispanic whites, 15% for Mexican Americans and other Hispanics, and 19% for non-Hispanic blacks.(3)
Childhood-onset obesity has several health-related consequences that until recently were documented only in adulthood, including hypertension, insulin resistance, glucose intolerance, and dyslipidemia.(4,5) In turn, these conditions are risk factors for type 2 diabetes and cardiovascular disease in both childhood and adulthood.(6,7) Additionally, childhood obesity has also been associated with orthopedic problems,(8) polycystic ovarian syndrome,(9) non-alcoholic fatty liver disease,(10) anxiety,(11) and depression.(11) The majority of these health issues also track consistently into adulthood.(4)
Weight-loss surgery among both adults and adolescents has become increasingly recognized as effective treatment for these comorbidities, and it is considered to be a reasonable option when non-surgical methods of weight loss fail.(11,12) Current studies suggest that neither pharmacologic nor dietary treatment can maintain weight loss in obese adolescents as effectively as can weight-loss surgery.(12–14)
Although bariatric surgery is accepted as the treatment of choice for recalcitrant morbid obesity among adults, acceptance of the surgery for adolescents has not been universal. Adolescents account for a small percentage of the cases performed, but this percentage is expected to rise.(15) The long-term safety and efficacy of bariatric surgery in adolescents have not yet been determined, particularly in large geographically and ethnically diverse samples.(15,16) Specific concerns include the ability to obtain appropriate consent, risks of major surgery, long-term adherence to dietary recommendations, unknown long-term effects, and the probabilities of long-term weight maintenance and resolution of related comorbidities.(16–18) Although the literature on bariatric outcomes in adolescents has increased exponentially, most studies are small and usually from individual surgical practices.(17)
Accordingly, we analyzed data from the Bariatric Outcomes Longitudinal Database (BOLD), a large database that tracks outcomes in patients from a wide geographical area who have undergone bariatric surgery. We report here the effects of bariatric surgery on weight loss, comorbidities, and complications in a large multi-ethnic cohort of morbidly obese adolescents 1-year after surgery.
METHODS
The BOLD Database
Data for the BOLD database are collected prospectively from participants in the Bariatric Surgery Center of Excellence (BSCOE) program sponsored by the American Society for Metabolic and Bariatric Surgery (ASMBS).(19) Participating centers enter data collected on all bariatric surgery patients during preoperative visits, the hospital stay, and all postoperative visits. These data are used to monitor adherence to the requirements of the BSCOE program and to support quality improvement for the surgical treatment of obesity and its associated conditions.
Data Quality Assurance Procedures
All BSCOE programs undergo a site inspection before approval and recertification every 3-years. During the site inspection, the accuracy of BOLD data is verified in an impartially selected sample of 10% of medical records. All data on complications and readmissions are also reviewed for accuracy. Specifically, all surgeries reported in BOLD are compared with a hospital-generated surgery list while all complications and readmissions occurring within 30-days of surgery are verified. In addition, a 10% random chart review is performed. Any unreported reoperations, readmissions, deaths, transfers or revisions found during chart review trigger a 100% chart review. Inconsistencies noted during site inspections are reported to the Bariatric Surgery Review Committee (BSRC) who recommends whether the applicant should receive or maintain BSCOE designation status.
The BOLD software has built in numerous data validation and verification rules that are intended to prevent the entry of invalid, out-of-range and inconsistent data. The software will not accept out-of-range values (e.g., out-of-range values for height, weight, age) and data entry personnel are asked to confirm entries that within suspect ranges. Within BOLD is a data validation report that lists patients with questionable data that must be addressed. Sites are generally given 14-days to correct their data in BOLD. For those sites that do not use an electronic medical record to transfer data to BOLD, SRC encourages centers to use patient encounter forms to collect BOLD data during the patient encounter in the same format as the software.
The Surgical Review Corporation
The Surgical Review Corporation (SRC) was established in 2003 as an independent, non-profit organization dedicated to advancing the safety, efficacy, and efficiency of bariatric and metabolic surgical care worldwide. With the ASMBS, the SRC developed the BSCOE program and administers it on behalf of the Society. The primary function of the BSCOE program is to collect and analyze data to improve bariatric surgical care. Surgeons and hospitals qualify for BSCOE designation by passing a rigorous evaluation process verifying that they have a comprehensive, multidisciplinary bariatric program that meets or exceeds the approved clinical practice guidelines for bariatric surgery.(19)
Research Data
The Copernicus Group Independent Review Board (CGIRB) approved the use of BOLD data for research with a Waiver of Informed Consent. The BOLD study has been registered with the National Institutes of Health (NCT01002352). CGIRB determined that the BOLD study poses minimal risk to patients and that SRC has adequate safeguards in place to ensure confidentiality of the protected health information described in the study protocol. Patients (or their guardians if under age 18) are presented with a CGIRB-approved Patient Information Sheet or local IRB-approved document during their initial visit. Patients or their guardians informed the bariatric surgeon or his/her staff if they did not wish to participate in the study prior to their surgery. All consent process data was included in the BOLD database.
About 65% (169,000) of patients treated by surgeons participating in the BSCOE program have allowed their data to be analyzed for research purposes. Analyses (not presented here) showed that the demographic, preoperative characteristics (body mass index, prevalence of comorbidities) and 30-day safety outcomes (rates of mortality, serious complications, readmissions and reoperations) across all procedures do not differ substantially between those who are and are not included in the database. Data are currently entered into the database by more than 1000 surgeons from more than 600 facilities in the US (all states are represented with the exception of Vermont and New Mexico), representing approximately 85% of all facilities nationwide performing at least 10 bariatric procedures per year.
Patient Selection
We analyzed data from all patients 11-to-19-years old who had undergone bariatric surgery between April 2004 and October 2010 and who allowed their data to be used for research purposes. The data analyzed here came from 423 surgeons and 360 facilities participating in the BSCOE program.
Data Collection
At BSCOE, primary BOLD data is generally collected in medical charts (paper or electronic) by a health care provider. SRC encourages the use of BOLD patient encounter forms to streamline this effort. Several third party electronic medical record systems interface with BOLD to prevent duplicate data entry. Each surgical practice must appoint a BOLD Administrator who manages the administrative aspects of BOLD and is responsible for ensuring high quality BOLD data entry by the practice. The assignment of BOLD data entry responsibility varies across bariatric programs. Each BSCOE participant practice determines who within their program is most appropriate to enter data and is responsible for their participation in the training opportunities offered by SRC. BOLD data entry may be managed entirely by the surgical practice or may be shared with the hospital. The training and support provided to all data entry personnel by SRC includes on-demand BOLD data entry webinars, data entry guidelines, weekly ASK SRC teleconferences and general support through SRC’s helpdesk.
Variables Used for Analysis
We collected data on age, sex, race, weight, BMI, weight loss (difference between baseline weight [kg] and weight at each respective time point), surgery type, and the status of several cardiometabolic, psychosocial, and general comorbidities, including diabetes, hypertension, hyperlipidemia, asthma, gastroesophageal reflux disease, and depression.
Comorbidity severity is scored on a system based on the Assessment of Obesity Related Comorbidities scoring system,(20) the National Institutes of Health Longitudinal Assessment of Bariatric Surgery (LABS) protocol,(21) and an extensive review of the literature and was developed by BSCOE to report uniform outcomes. Severity is scored from zero to 5, with zero being no symptoms or evidence of disease, 1 being symptomatic but requiring no medication, 2 having a diagnosis but not requiring medication, 3 having a diagnosis and requiring medication, 4 being on more than one medication as a result of severe medical complications, and 5 having the most severe form of the comorbidity (e.g., poorly controlled by medications, organ damage and dysfunction). For example, ‘Depression’ was scored as follows (0) no symptoms of depression; (1) mild and episodic depression not requiring treatment; (2) moderate depression accompanied by some impairment, may require treatment; (3) moderate depression with significant impairment, treatment indicated; (4) severe depression definitely requiring intensive treatment; and (5) severe depression requiring hospitalization. Severity is assessed for each comorbidity at each postoperative visit by the surgeon.
Guidelines, including definitions,(19) were provided to all BSCOEs to document an adverse event/complication as 1) a death; 2) a complication that prolongs hospitalization (>18-hours beyond the expected discharge date), requires readmission to the emergency room or hospital (i.e., any hospital or facility stay that lasts for at least 24-hours) or requires treatment outside of standard postoperative care (e.g., a therapeutic surgical, endoscopic or radiological intervention, regardless of where the intervention is performed or a pharmacological treatment with the exception of over-the-counter drugs); (3) Within 30-days of surgery: All adverse events/complications, readmissions and reoperations are entered into BOLD, regardless of whether they appear to be related to the surgery; and (4) After 30-days post-surgery: All adverse events/complications that appear on the list of complications in BOLD must be entered.
All hospital readmissions and/or reoperations occurring as a result of a complication listed in BOLD must also be entered. Adverse events/complications unrelated to the surgery that do not appear on the list of complications in BOLD are entered. BSCOE participants are expected to report all complications in their patients that meet the criteria outlined above, even if these complications were managed by another health care provider. At routine post-discharge visits, BSCOE participants are instructed to review with patients any complications they may have had that were managed by another program. SRC site inspectors note the inclusion of documents from other health care providers within patient charts.
Intraoperative data used in the present study consisted of the procedure (gastric bypass or adjustable gastric band) and the date of surgery. The primary outcomes were weight loss, change in comorbidity severity, and surgical complications. Data were assessed before surgery and at 3-, 6-, and 12-months after surgery. Because not all patients have their follow-up appointment exactly at these times, the 3-month data collection point included data collected from 0-to-3-months after surgery, the 6-month time point consisted of data collected from 3-to-9 months after surgery, and the 12-month time point consisted of data collected 9-to-15 months after surgery.
Statistical Methods
Baseline differences in BMI Z scores(22) for all demographic variables were evaluated with analysis of variance. To assess changes over time for individual surgery types and between surgery types in weight and BMI, separate repeated-measures, linear mixed-models were fit using the MIXED procedure in SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina). An unstructured variance-covariance matrix was selected for each model to account for the correlation of within-patient repeated observations. Age at surgery, sex, ethnicity, surgery type (gastric bypass or adjustable gastric band), and time of data collection were the fixed covariates considered for potential inclusion in each model; patients were considered to be random. In a mixed model, the particular levels of fixed effects are of interest, and inferences are made for those specific levels; random effects are considered to be random samples from the population, and inferences are not made to a specific sample but to the entire population. The interaction between time and sex was also assessed. Contrasts were used to test for differences between groups at each time for mean values of weight, BMI, and the presence of comorbidities. The same mixed-model approach described above was also used to test for any selection bias between the whole sample (n=890) and a sub-sample (n=226) of patients for which data from all four time points were available by including an indicator variable for complete or incomplete data.23 Fisher exact test was applied to assess difference in comorbidities between the two types of surgery. Alpha was set at 0.05.
RESULTS
Of the 890 eligible adolescents, about 75% were females (mean age, 18.5-years), 68% were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, and 6% were “other” (Table 1). A total of 51% of the sample had gastric bypass surgery (99% were laparoscopic) and 49% had adjustable gastric band surgery. At baseline, males were significantly heavier than females. Those undergoing gastric bypass surgery were significantly heavier than those undergoing adjustable gastric band surgery. Non-Hispanic whites were significantly lighter than their ethnic group counterparts (Table 1). Because of these significant baseline differences in sex and ethnic group, all subsequent analyses are adjusted for these two variables in addition to age.
Table 1.
Demographic Characteristics of 890 Adolescents who Underwent Bariatric Surgery between 2004 and 2010
| Characteristic | Baseline BMI Z score, mean (SD) | p | |
|---|---|---|---|
| Age at surgery, mean (SD), years | 18.5 (0.1) | ||
|
| |||
| Sex, n (%) | <0.001 | ||
|
| |||
| Males | 225 (25.3) | 3.14 (0.2) | |
|
| |||
| Females | 665 (74.7) | 2.41 (0.2) | |
|
| |||
| Type of Surgery, n (%) | <0.001 | ||
|
| |||
| Gastric Bypass | 454 (51.0) | 2.67 (0.4) | |
|
| |||
| Adjustable Gastric Band | 436 (49.0) | 2.53 (0.4) | |
|
| |||
| Race, n (%) | |||
|
| |||
| Non-Hispanic White | 606 (68.1) | 2.57 (0.4)a,b | |
|
| |||
| Hispanic | 129 (14.5) | 2.68 (0.4) a | |
|
| |||
| Non-Hispanic Black | 98 (11.0) | 2.67 (0.3)b | |
|
| |||
| Other | 57 (6.4) | 2.60 (0.4) | |
Non-Hispanic white versus Hispanic, p=0.004
Non-Hispanic white versus non-Hispanic black, p=0.02
Patients may not have been eligible for follow-up analysis if they had surgery shortly before analysis. At 3-months, 88% of eligible patients were seen in follow-up (786 of 890), at 6-months, 66% of eligible patients were seen (541 of 821), and at 12-months 37% were seen (259 of 692).
When comparing surgery types, the overall one year mean weight loss for those who underwent gastric bypass surgery was more than twice that of those who underwent adjustable gastric band surgery (48.6 kg versus 20 kg, P<0.001; Table 2). Similar results were found for all other anthropometric changes and comparisons over one year between surgery types (P<0.001; Table 2).
Table 2.
Mean Anthropometric Measures after Bariatric Surgery (2004 – 2010) in Morbidly Obese Adolescents, by Type of Surgerya
| Gastric Bypass | ||||
|---|---|---|---|---|
| Pre-Surgery (n=454) | 3 Months after Surgery (n=402) | 6 Months After Surgery (n=258) | 12 Months After Surgery (n=108) | |
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimateb,c (95% CI) | |
| BMI (kg/m2) | 50.69(0.39) | 45.42(0.37) | 38.00(0.39) | 33.62(0.43) |
| BMI %ile | 99.42(0.03) | 99.07(0.04) | 96.38(0.29) | 91.48(0.92) |
| BMI Z score | 2.67(0.01) | 2.50(0.01) | 2.08(0.03) | 1.71(0.05) |
| BMI % changed | −0.36(0.05) | −3.04(0.28) | −7.85(0.88) | |
| Weight (kilograms) | 145.23(1.19) | 130.11(1.15) | 109.03(1.15) | 96.51(1.29) |
| Weight %ile | 99.67(0.03) | 99.35(0.05) | 96.58(0.36) | 91.90(1.06) |
| Weight Z score | 2.95(0.01) | 2.73(0.02) | 2.25(0.03) | 1.86(0.05) |
| WLe (kilograms) | 15.05(0.41) | 36.09(0.64) | 48.60(1.02) | |
| WL (%) | 10.35(0.28) | 25.38(0.45) | 33.69(0.61) | |
| Adjustable Gastric Band | ||||
| Pre-Surgery (n=436) | 3 Months after Surgery (n=384) | 6 Months After Surgery (n=283) | 12 Months After Surgery (n=151) | |
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimateb,c (95% CI) | |
| BMI (kg/m2) | 46.01(0.32) | 43.38(0.31) | 41.03(0.33) | 39.09(0.41) |
| BMI %ile | 99.20(0.03) | 98.91(0.04) | 98.17(0.20) | 97.24(0.33) |
| BMI Z score | 2.54(0.01) | 2.43(0.01) | 2.29(0.02) | 2.14(0.03) |
| BMI % change | −0.28(0.02) | −1.02(0.2) | −1.95(0.32) | |
| Weight (kilograms) | 130.43(0.96) | 122.97(0.94) | 116.35(0.97) | 110.66(1.18) |
| Weight %ile | 99.48(0.02) | 99.25(0.04) | 98.78(0.09) | 97.72(0.35) |
| Weight Z score | 2.77(0.01) | 2.64(0.02) | 2.51(0.02) | 2.36(0.03) |
| WL (kilograms) | 7.46(0.23) | 14.13(0.47) | 19.81(0.91) | |
| WL (%) | 5.70(0.16) | 10.73(0.34) | 14.89(0.67) | |
model adjusted by age, gender, and race.
P<0.001for change across all time points, for all anthropometric measures, for both surgery types. P<0.01 for changes between each time point, for all anthropometric measures, for both surgery types.
P<0.001for change across all time points, for all anthropometric measures between surgery types.
BMI % change = the difference between baseline BMI %ile and BMI %ile at each respective time point.
WL = estimated weight loss or the difference between baseline weight [kilograms] and weight at each respective time point. While % Excess Weight Loss ([%EWL], calculated by using the middle of the 1983 Metropolitan Life Insurance tables for median frame + % Weight Lost (%WL) + % Excess BMI Lost (%EBMIL) with excess > 25 kg/m2) is the standard measure used in the adult population, it is not appropriate for adolescents because the Metropolitan Life Insurance tables were designed for adults aged 25 to 59 years only.
Specifically, mean weight loss at 1-year for those who underwent gastric bypass surgery was 48.6 kg (P<0.001; Table 2). Similarly, mean BMI significantly decreased by 17.1 kg/m2 over the same period (P<0.001). Mean weight loss at 1 year for those who underwent adjustable gastric band surgery was 19.8 kg (P<0.001; Table 2). Similarly, mean BMI significantly decreased by 6.9 kg/m2 over the same period (P<0.001). Weight decreased the most between 3- and 6-months after surgery for those who underwent gastric bypass surgery (21 kg) and in the first 3-months among those who underwent adjustable band surgery (6.67 kg). Decreases in weight and BMI Z scores showed similar patterns (Table 2). For those undergoing gastric bypass surgery, the overall mean BMI percentile decreased from 99.4th percentile (obese) to the 91.5th percentile (overweight) 1-year after surgery. Gastric bypass surgery resulted in a BMI percentile decrease approximately 4 times that of adjustable gastric band surgery.
All anthropometric measurements significantly decreased over the study period for both males and females (Tables 3 and 4). Adolescent males undergoing gastric bypass surgery lost an average of approximately 58 kg while adolescent females undergoing gastric bypass surgery lost an average of 45.7 kg. Adolescent males undergoing adjustable gastric band surgery lost an average of 25.2 kg while adolescent females lost an average of 18.1 kg. Gastric bypass surgery resulted in a BMI percentile decrease approximately twice that of adjustable gastric band surgery among boys and over four times that of adjustable gastric band surgery in girls.
Table 3.
Mean Anthropometric Measures from Morbidly Obese Adolescent Males 1 Year after Bariatric Surgery Performed between 2004 and 2010, by Type of Surgerya
| Gastric Bypass | ||||
|---|---|---|---|---|
| Pre-Surgery (n=125) | 3 Months after Surgery (n=110) | 6 Months After Surgery (n=68) | 12 Months After Surgery (n=23) | |
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE)b | |
| BMI (kg/m2) | 53.60(0.79) | 47.46(0.75) | 40.40(0.78) | 35.30(0.95) |
| BMI %ile | 99.92(0.02) | 99.80(0.03) | 98.61(0.37) | 96.85(0.89) |
| BMI Z score | 3.21(0.02) | 2.99(0.03) | 2.58(0.06) | 2.18(0.11) |
| BMI % changec | −0.10(0.05) | −1.34(0.37) | −3.10(0.87) | |
| Weight (kilograms) | 167.58(2.36) | 148.32(2.30) | 126.39(2.39) | 110.14(3.02) |
| Weight %ile | 99.96(0.02) | 99.79(0.06) | 98.68(0.38) | 96.76(0.99) |
| Weight Z score | 3.61(0.04) | 3.27(0.05) | 2.74(0.07) | 2.26(0.12) |
| WLd (kilograms) | 19.40(0.97) | 41.42(1.32) | 57.97(2.31) | |
| WL (%) | 11.47(0.53) | 24.49(0.78) | 33.66(1.35) | |
| Adjustable Gastric Band | ||||
| Pre-Surgery (n=100) | 3 Months after Surgery (n=92) | 6 Months After Surgery (n=67) | 12 Months After Surgery (n=38) | |
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE)b | |
| BMI (kg/m2) | 49.14(0.77) | 46.18(0.78) | 43.40(0.82) | 41.31(0.85) |
| BMI %ile | 99.86(0.01) | 99.74(0.03) | 99.39(0.10) | 98.37(0.82) |
| BMI Z score | 3.07(0.03) | 2.94(0.03) | 2.75(0.05) | 2.57(0.07) |
| BMI % change | −0.12(0.02) | −0.50(0.10) | −1.48(0.80) | |
| Weight (kilograms) | 155.66(2.34) | 146.25(2.38) | 137.31(2.45) | 130.54(2.58) |
| Weight %ile | 99.93(0.01) | 99.85(0.02) | 99.54(0.11) | 98.63(0.65) |
| Weight Z score | 3.41(0.04) | 3.22(0.04) | 3.00(0.06) | 2.82(0.08) |
| WL (kilograms) | 9.46(0.59) | 18.41(1.15) | 25.18(1.84) | |
| WL (%) | 6.16(0.37) | 11.93(0.75) | 16.07(1.20) | |
model adjusted by age and race.
P<0.05for change across all time points, for all anthropometric measures, for both surgery types, with the exception of BMI percentile for adjustable gastric band. P<0.01 for changes between each time point, with the exception of BMI and weight percentile for adjustable gastric band between 6 and 12 months after surgery.
BMI % change = the difference between baseline BMI %ile and BMI %ile at each respective time point.
WL = estimated weight loss or the difference between baseline weight [kilograms] and weight at each respective time point. While % Excess Weight Loss ([%EWL], calculated by using the middle of the 1983 Metropolitan Life Insurance tables for median frame + % Weight Lost (%WL) + % Excess BMI Lost (%EBMIL) with excess > 25 kg/m2) is the standard measure used in the adult population, it is not appropriate for adolescents because the Metropolitan Life Insurance tables were designed for adults aged 25 to 59 years only.
Table 4.
Mean Anthropometric Measures from Morbidly Obese Adolescent Females 1 Year after Bariatric Surgery Performed between 2004 and 2010, by Type of Surgerya
| Gastric Bypass | ||||
|---|---|---|---|---|
| Pre-Surgery (n=329) | 3 Months after Surgery (n=292) | 6 Months After Surgery (n=190) | 12 Months After Surgery (n=85) | |
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE)b | |
| BMI (kg/m2) | 49.63(0.44) | 44.68(0.43) | 37.11(0.44) | 32.93(0.47) |
| BMI %ile | 99.25(0.03) | 98.81(0.05) | 95.59(0.37) | 89.96(1.12) |
| BMI Z score | 2.47(0.01) | 2.32(0.01) | 1.90(0.03) | 1.53(0.05) |
| BMI % changec | −0.43(0.05) | −3.64(0.35) | −9.18(1.07) | |
| Weight (kilograms) | 137.03(1.37) | 123.47(1.32) | 102.65(1.31) | 90.96(1.38) |
| Weight %ile | 99.57(0.04) | 99.20(0.06) | 95.84(0.47) | 90.52(1.30) |
| Weight Z score | 2.71(0.01) | 2.54(0.02) | 2.07(0.04) | 1.70(0.06) |
| WLd (kilograms) | 13.53(0.43) | 32.22(0.73) | 45.66(1.10) | |
| WL (%) | 9.94(0.33) | 25.67(0.54) | 33.65(0.67) | |
| Adjustable Gastric Band | ||||
| Pre-Surgery (n=336) | 3 Months after Surgery (n=292) | 6 Months After Surgery (n=216) | 12 Months After Surgery (n=113) | |
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE)b | |
| BMI (kg/m2) | 45.04(0.34) | 42.51(0.32) | 40.30(0.35) | 38.42(0.47) |
| BMI %ile | 98.99(0.03) | 98.65(0.05) | 97.81(0.26) | 96.88(0.35) |
| BMI Z score | 2.37(0.01) | 2.27(0.01) | 2.14(0.02) | 2.01(0.03) |
| BMI % change | −0.32(0.02) | −1.19(0.25) | −2.10(0.33) | |
| Weight (kilograms) | 122.57(1.03) | 115.71(0.98) | 109.83(1.01) | 104.57(1.31) |
| Weight %ile | 99.34(0.03) | 99.06(0.05) | 98.55(0.12) | 97.43(0.41) |
| Weight Z score | 2.57(0.01) | 2.47(0.02) | 2.35(0.02) | 2.22(0.04) |
| WL (kilograms) | 6.83(0.23) | 12.76(0.50) | 18.08(1.04) | |
| WL (%) | 5.56(0.18) | 10.36(0.39) | 14.52(0.79) | |
model adjusted by age, gender, and race.
P<0.001for change across all time points. P<0.01 for change between each time point, for all anthropometric measures, for both surgery types.
BMI % change = the difference between baseline BMI %ile and BMI %ile at each respective time point.
WL = estimated weight loss or the difference between baseline weight [kilograms] and weight at each respective time point. While % Excess Weight Loss ([%EWL], calculated by using the middle of the 1983 Metropolitan Life Insurance tables for median frame + % Weight Lost (%WL) + % Excess BMI Lost (%EBMIL) with excess > 25 kg/m2) is the standard measure used in the adult population, it is not appropriate for adolescents because the Metropolitan Life Insurance tables were designed for adults aged 25 to 59 years only.
The most prevalent preoperative comorbidities for both gastric bypass and adjustable gastric band patients were back pain (36%, 26%, respectively) and gastroesophageal reflux disease (28%, 25%, respectively). Among gastric bypass patients, these two most prevalent comorbidities were followed by hypertension and obstructive sleep apnea (26%), and depression (25%). Among adjustable gastric band patients, they were followed by depression (21%), musculoskeletal disorder (21%), and asthma (19%) (Table 5). Before surgery, gastric bypass patients were significantly more likely to have hypertension, liver disease, stress urinary incontinence, back pain, musculoskeletal disorders, psychosocial impairment, obstructive sleep apnea, and menstrual irregularities versus adjustable gastric band patients (P<0.05). One year after surgery, gastric bypass patients were significantly more likely to have improved lipid levels versus adjustable gastric band patients (P<0.05). In general, gastric bypass patients reported more improvement versus adjustable gastric band patients in comorbidities one year after surgery.
Table 5.
Percent Change in Comorbidities Among Morbidly Obese Adolescents After Treatment with Bariatric Surgery, by Surgery Type, 2004–2010
| Morbidity | Before Surgery N (%) | 12 Months after Surgery | ||||||
|---|---|---|---|---|---|---|---|---|
| Unchanged % | Improved % | Worsened % | ||||||
| Gastric Bypass | Adjustable Gastric Band | Gastric Bypass | Adjustable Gastric Band | Gastric Bypass | Adjustable Gastric Band | Gastric Bypass | Adjustable Gastric Band | |
| Cardiovascular Disease-Related | ||||||||
| Swelling in the Legs | 53(11.67) | 37(8.49) | 46.2 | 50.0 | 46.2 | 44.4 | 7.7 | 5.6 |
| Hypertension | 118(25.99)* | 80(18.35)* | 32.1 | 42.9 | 60.7 | 54.3 | 7.1 | 2.9 |
| Gastrointestinal-System-Related | ||||||||
| Gastroesophageal Reflux Disease | 127(27.97) | 109(25.00) | 32.4 | 47.6 | 61.8 | 45.2 | 5.9 | 7.1 |
| Liver Disease | 25(5.51)* | 11(2.52)* | 33.3 | 50.0 | 55.6 | 33.3 | 11.1 | 16.7 |
| General | ||||||||
| Abdominal Skin Irritation | 17(3.74) | 22(5.05) | 37.5 | 80.0 | 37.5 | 20.0 | 25.0 | 0.0 |
| Stress Urinary Incontinence | 31(6.83)* | 12(2.75)* | 22.2 | 50.0 | 66.7 | 50.0 | 11.1 | 0.0 |
| Metabolic-Related | ||||||||
| Diabetes | 67(14.76) | 65(14.91) | 14.3 | 40.9 | 78.6 | 59.1 | 7.1 | 0.0 |
| Hyperlipidemia | 65(14.32) | 61(13.99) | 29.4* | 76.7* | 58.8* | 23.3* | 11.8 | 0.0 |
| Musculoskeletal System-Related | ||||||||
| Back Pain | 162(35.68)* | 113(25.92)* | 48.8 | 45.0 | 51.2 | 50.0 | 0.0 | 5.0 |
| Musculoskeletal Disorder | 127(27.97)* | 90(20.64)* | 29.2 | 55.9 | 70.8 | 44.1 | 0.0 | 0.0 |
| Psychosocial/Behavioral-Related | ||||||||
| Alcohol Use | 43(9.47) | 37(8.49) | 58.3 | 77.8 | 25.0 | 11.1 | 16.7 | 11.1 |
| Depression | 115(25.33) | 93(21.33) | 60.9 | 47.2 | 34.8 | 41.7 | 4.3 | 11.1 |
| Psychosocial Impairment | 52(11.45)* | 32(7.34)* | 66.7 | 81.8 | 25.0 | 0.0 | 8.3 | 18.2 |
| Tobacco Use | 25(5.51) | 20(4.59) | 50.0 | 100.0 | 37.5 | 0.0 | 12.5 | 0.0 |
| Pulmonary System-Related | ||||||||
| Asthma | 94(20.70) | 84(19.27) | 60.0 | 71.0 | 40.0 | 22.6 | 0.0 | 6.5 |
| Obstructive Sleep Apnea | 117(25.77)* | 80(18.35)* | 43.3 | 46.2 | 56.7 | 46.2 | 0.0 | 7.7 |
| Pulmonary Hypertension | 17(3.74) | 8(1.83) | 16.7 | 60.0 | 66.7 | 40.0 | 16.7 | 0.0 |
| Reproductive System-Related | ||||||||
| Menstrual Irregularity | 85(18.72)* | 50(11.47)* | 53.6 | 62.5 | 32.1 | 37.5 | 14.3 | 0.0 |
| Polycystic Ovarian Syndrome | 41(9.03) | 45(10.32) | 58.3 | 68.8 | 33.3 | 31.3 | 8.3 | 0.0 |
p-value < 0.05
Because of low baseline prevalence rates, the following comorbidities were not included (and not analyzed further at follow-up): abdominal hernia (n=11), angina (n=12), congestive heart failure (n=2), ischemic heart disease (n=8), peripheral vascular disease (n=2), gout (n=7), decreased ambulatory functional status (n=2), fibromyalgia (n=4), pseudotumor cerebri (n=23), substance abuse (n=3), and obesity hypoventilation syndrome (n=11).
The 120 total medical complications within one year after surgery included one death from cardiac failure 5 months after a gastric bypass surgery. The most common complication for both surgery types was gastrointestinal (n=29 for gastric bypass patients, n=9 for adjustable gastric band patients) followed by nutritional deficiencies among those undergoing gastric bypass (n=24) and device-related issues among those undergoing adjustable gastric band procedure (n=5) (Table 6). There were a total of 45 readmissions among gastric bypass patients and 10 among adjustable gastric band patients with 29 and 8 reoperations required, respectively.
Table 6.
Complications Within One Year After Surgery, by Surgery Type among 890 Morbidly Obese Adolescents Undergoing Bariatric Surgery between 2002 and 2010
| Gastric Bypass (N=454) | Adjustable Gastric Band (N=436) | |
|---|---|---|
| Complication | N(%) | N(%) |
| Gastrointestinal Systema | 29(29.6) | 9(41) |
| Nutritional Deficiencyb | 24(24.5) | 2(9.1) |
| Surgical (bleeding/hemorrhage, intra-abdominal) | 3(3.1) | 1(4.5) |
| General (obstruction, abscess, internal hernia) | 16(16.3) | 2(9.1) |
| Band (port revision, removal, replacement)c | 0(0.0) | 3(13.6) |
| Pulmonary System (pneumonia, pulmonary embolism) | 7(7.1) | 0(0.0) |
| Device-Related | 8(8.2) | 5(22.7) |
| Skin or Soft Tissue | 3(3.1) | 0(0.0) |
| Death (by cardiac failure 6 months after surgery) | 1(1) | 0(0.0) |
| Endocrine/Metabolic System | 3(3.1) | 0(0.0) |
| Infection (at the surgical site) | 1(1) | 0(0.0) |
| Renal or Genitourinary System | 1(1) | 0(0.0) |
| Arrhythmia | 2(2) | 0(0.0) |
| TOTALd | 98(21.6) | 22(5) |
| Readmissions Required | 45(9.9) | 10(2.3) |
| Reoperation Required | 29(6.4) | 8(1.8) |
| Reason of Reoperation | ||
| Cholecystectomy | 6(20.8) | 2(25) |
| Othere | 7(24.1) | 0(0.0) |
| EGD with dilitation | 6(20.8) | 0(0.0) |
| Hernia repair, internal | 5(17.2) | 0(0.0) |
| Band replacement | 0(0.0) | 2(25) |
| Band, port revision | 0(0.0) | 2(25) |
| Band, removal | 0(0.0) | 2(25) |
| Small bowel obstruction, repair | 2(6.9) | 0(0.0) |
| Gastric tube, placement | 1(3.4) | 0(0.0) |
| Hernia repair, umbilical | 1(3.4) | 0(0.0) |
| Wound, debridement | 1(3.4) | 0(0.0) |
| TOTAL | 29(6.4) | 8(1.8) |
Nausea or vomiting, intestinal bleeding, diarrhea, gallstones
Vitamins A, B12, D, folate, iron, magnesium, zinc, electrolytes
Slippage (N =10,7.1%), stricture (N=6, 4.3%) (categories are not mutually exclusive)
Adverse event rate is significantly different between the gastric bypass and adjustable gastric band, 20.33% vs. 5.10% respectively.
Abdominal pain, internal hernia, repair of lapband tubing, diagnostic lap, LOA, parenteral hernia repair, gastroparesis internal hernia, repair of lapband tubing
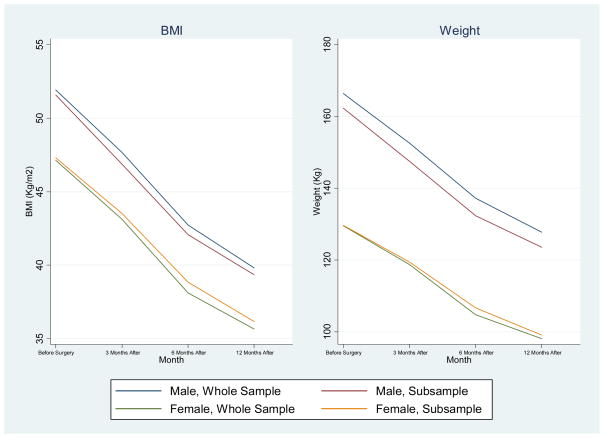
Analysis using the mixed-model approach described above to test for any selection bias between the whole sample (n=890) and a sub-sample (n=226) of patients for which data from all four time points were available by including an indicator variable for complete or incomplete data23 showed that the two samples did not differ on BMI or weight outcomes or between the proportion of males and females. (Figures 1 and 2).
Figure 1.
Change in Mean Body Mass Index and Weight for The Entire Sample (n=890) and The Sub-Sample With Complete Data (n=226) for Morbidly Obese Adolescents after Bariatric Surgery That Was Performed between 2004 and 2010*
*The difference between the two groups was not statistically significant for either BMI or weight.
Figure 2.
Change in Mean BMI and Weight for The Entire Sample (n=890) and The Sub-Sample with Complete Data (n=226) for Morbidly Obese Adolescents Over 1 Year after Bariatric Surgery That Was Performed between 2004 and 2010, by Gender.*
*All pairwise group differences were not significant.
DISCUSSION
In a large and diverse sample of multiethnic, morbidly obese adolescents, we found substantial decreases in weight and comorbidities 1-year after bariatric surgery. Surgery resulted in an average weight loss of more than 30 kg per person, a loss that far exceeds those reported in non-surgical weight-management programs.(24–26) The overall one year mean weight loss for those who underwent gastric bypass surgery was more than twice that of those who underwent adjustable gastric band surgery (48.6 kg versus 20 kg). Similar results were found for all other anthropometric changes and comparisons over one year between surgery types. Surgery also reduced weight and BMI among both males and females. Additionally, several physical and mental health comorbidities resolved or improved substantially 1-year after surgery, suggesting an improvement in quality of life. This substantial improvement in comorbidities 1-year after bariatric surgery has not been documented for other treatment options, nor with this number of adolescent patients.(28) Approximately 10 percent of gastric bypass patients and 2 percent of adjustable gastric band patients required readmission for a variety of causes. Of these readmissions, 6 percent of those who underwent gastric bypass and less than 2 percent of those undergoing adjustable gastric band required reoperation (also for a variety of reasons). Our findings indicate that bariatric surgery can be a safe and effective treatment option for morbid obesity and its ensuing comorbidities in adolescents.(29)
Although the childhood obesity epidemic continues unabated in most developed countries, non-surgical approaches to the long-term (1-year or more) management and decrease of overweight in childhood have had limited success.(23) Despite standardized criteria for qualifying adolescents for bariatric surgery,(29) obese children are not simply younger versions of obese adults; they are still developing and growing, both physically and psychologically. Extreme obesity should be treated sooner rather than later,(30) particularly in adolescents who may have not yet developed full-blown, related comorbidities, such as diabetes or heart disease. However, the optimal time in adolescence for bariatric surgery is as yet unknown.(31) Our findings support those of others(32,33) reporting that bariatric surgery before adulthood can result in substantial weight loss and resolution of comorbidities(34) and thus improve overall quality of life. Moreover, earlier treatment of obesity may prevent later costs. For example, children and adolescents with a primary or secondary diagnosis of overweight, obesity, or morbid obesity require longer hospital stays than do children without these diagnoses.(35)
Even as the emerging data on bariatric surgery, including those from randomized-controlled trials,(32) continue to show important long-term weight loss and improvement in most obesity-related comorbidities, many pediatric specialists are hesitant to refer patients for surgery. A survey of pediatricians in the US showed that although they believed pediatric obesity to be a major problem, less than half would be somewhat or very likely to refer an adolescent for surgery.(30) The medical community must strive to provide further data on long-term results after bariatric surgery, both in adults and adolescents.
Our study has some limitations. The data entered into BOLD by BSCOE participants are reported by participating surgeons and surgical practices on all post-discharge complications, even if another healthcare provider manages these complications. Thus, complications are potentially under-represented in the database. Variations in practice management among BSCOE participants may delay data entry, potentially resulting in incomplete follow-up data.
Another limitation is the lack of sensitivity of the Assessment of Obesity Related Comorbidities severity scoring system, which most likely reduced the accuracy of assessing the severity of comorbidities in our analysis. Specifically, the current scoring system indicates improvement in some comorbidities only when there is a major change in the type of treatment. For example, patients with diabetes may decrease the number of oral medications they take from two to one, with a marked improvement in hemoglobin A1C, without improving their stratification score. Additionally, pre-surgical information on nutritional deficiencies is not available to determine whether surgery is the cause of the deficiency or whether deficiencies existed before surgery.
Additionally, our findings and conclusions are limited by missing follow-up data, a common problem reported in the bariatric literature. Because of potential selection bias caused by losses to follow-up, we tested the differences between the entire sample and the sub-sample with follow-up data at all time points and found no differences. This finding supports the external validity of our overall conclusions. It should also be noted that unless the surgeon has obtained the follow-up data from the patient’s pediatrician and it is included with medical charts at the surgeon’s practice, SRC does not independently verify additional follow-up occurring outside the office. Moreover, older adolescents in particular are difficult to follow because they may leave the geographic area for education or employment. More age-relevant tracking procedures, such as those based on social media or handheld or cellular telephone devices, may be able to decrease losses to follow-up. Recognizing this challenge, the ASMBS Pediatric Committee has formed a subcommittee to explore the development of a Pediatric/Adolescent Center of Excellence program that will include a pediatrician component and a more flexible mechanism for collecting long-term data on adolescent patients. Nevertheless, this initial observational study of adolescents treated at BSCOEs provides a valuable snapshot of the adolescent bariatric surgery outcomes in a diverse population.
Additionally, it should be noted that of the 10% of charts that were audited at each BSCOE site every 3-years, it is possible that adolescents were not represented, as not all sites perform surgery on adolescent patients. Patients identified from the hospital surgery list that fall outside of NIH criteria or that are high risk are given greater consideration for inclusion in the 10% of charts identified for review but it is possible that not all adolescent charts were audited.
Finally, BOLD does not include dietary, exercise and/or lifestyle behavior modification which were or were not implemented as part of the patient’s treatment during the pre/postoperative period so it is not possible to analyze how these factors contribute to outcomes.
Conclusions
Weight loss at 3-, 6-, and 12-months after surgery is approximately double in adolescent males and females who underwent gastric bypass surgery versus those who underwent adjustable gastric band surgery. Our results support the conclusion that bariatric surgery can safely and substantially reduce weight and related comorbidities in morbidly obese, multiethnic adolescents for at least 1-year. The BOLD database and those like it are important in studying the long-term effects of bariatric surgery in this population. Multi-institutional, prospective data and studies are necessary to determine the outcomes of surgical responses to the obesity epidemic. With this longitudinal data, the questions of which patient groups, which surgical procedures, and when should surgery be performed can be answered.
Acknowledgments
We thank all the surgeons and centers of excellence that have contributed their data to BOLD. We also thank ASMBS for its continued efforts to support BOLD. Finally, we are grateful to all the ba riatric patients who have agreed to share their data for the current analyses.
Role of The Funding Source: The funding source (National Institutes of Health grant K01 DA 026993 [SEM]) had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Funding Source: National Institutes of Health grant K01 DA 026993 (SEM)
Footnotes
The corresponding author had full access to all data and had final responsibility to submit this report for publication after this manuscript was approved by the Surgical Review Corporation and coauthors.
Contributors: SEM, DW, KR, MM, and NDLC were responsible for the concept and design of the study. DW and BS provided study materials and patients. All authors analyzed and interpreted the data, wrote the report, and approved the final version of the report.
Conflict of Interest Statement: Dr. de la Cruz-Munoz is a consultant and proctor for Ethicon EndoSurgery, which manufactures equipment for performing bariatric surgery. No conflicts of interest to report for all other authors.
The corresponding author had full access to all data and had final responsibility to submit this report for publication after this manuscript was approved by the Surgical Review Corporation and coauthors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization World Health Organization. Obesity and Overweight Fact Sheet N_311. Geneva, Switzerland: WHO; 2006. [Accessed April 21, 2011]. http://www.who.int/mediacentre/factsheetsfs311/en/index.html. [Google Scholar]
- 2.Olshansky SJ. Projecting the future of U.S. health and longevity. Health Aff (Millwood) 2005;24(Suppl 2):W5R86–9. doi: 10.1377/hlthaff.w5.r86. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Chaoyang L. Defining the MS in children and adolescents: will the real definition please stand up? J Pediatr. 2008;152:160–4. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Messiah SE, Arheart K, Hlaing W, Natale R, Lipshultz SE, Miller TL. Body mass index, waist circumference, and cardiovascular disease risk factors among preschool-age children in the United States. Obesity. 2011 doi: 10.1038/oby.2011.353. doi:10L1038/oby.2011.353. [DOI] [PubMed] [Google Scholar]
- 6.Sun SS, Liang R, Huang TT, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult MS and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Must A, Anderson SE. Effects of obesity on morbidity in children and adolescents. Nutr Clin Care. 2003;6:4–12. [PubMed] [Google Scholar]
- 9.Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a position statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Miller TL, Messiah SE, editors. Pediatric metabolic syndrome: Comprehensive clinical review and related health issues. London, United Kingdom: Springer; 2012. pp. 1–400. [Google Scholar]
- 11.Davin SA, Taylor NM. Comprehensive review of obesity and psychological considerations for treatment. Psychol Health Med. 2009;14:716–25. doi: 10.1080/13548500903431501. [DOI] [PubMed] [Google Scholar]
- 12.Sugerman HJ, DeMaria EJ, Kellum JM, Sugerman EL, Meador JG, Wolfe LG. Effects of bariatric surgery in older patients. Ann Surg. 2004;240:243–7. doi: 10.1097/01.sla.0000133361.68436.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inge TH, Jenkins TM, Zeller M, et al. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010;156:103–8.e1. doi: 10.1016/j.jpeds.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De La Cruz-Munoz N, Messiah SE, Cabrera J, Torres C, Lopez-Mitnik G, Arheart KL. Four-year weight outcomes of laparoscopic gastric bypass surgery and adjustable gastric banding among multiethnic adolescents. Surg Obes Rel Dis. 2010;6:542–7. doi: 10.1016/j.soard.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Yermilov I, McGory ML, Shekelle PW, Ko CY, Maggard MA. Appropriateness criteria for bariatric surgery: beyond the NIH guidelines. Obesity. 2009;17:1521–7. doi: 10.1038/oby.2009.78. [DOI] [PubMed] [Google Scholar]
- 16.Hollenbeak CS, Rogers AM, Barrus B, Wadiwala I, Cooney RN. Surgical volume impacts bariatric surgery mortality: a case for centers of excellence. Surgery. 2008;144:736–43. doi: 10.1016/j.surg.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM. American Heart Association Childhood Obesity Research Summit: executive summary. Circulation. 2009;119:2114–23. doi: 10.1161/CIRCULATIONAHA.109.192215. [DOI] [PubMed] [Google Scholar]
- 18.Xanthakos SA, Daniels SR, Inge TH. Bariatric surgery in adolescents: an update. Adolesc Med Clin. 2006;17:589–612. doi: 10.1016/j.admecli.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 19.DeMaria EJ, Pate V, Warthen M, Winegar D. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2010;6:347–55. doi: 10.1016/j.soard.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Ali M, Maguire MB, Wolfe B. Assessment of obesity-related comorbidities: a novel scheme for evaluating bariatric surgical patients. J Am Coll Surg. 2006;202:70–77. doi: 10.1016/j.jamcollsurg.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, Pories WJ, Wolfe BM, Yanovski SZ. Longitudinal Assessment of Bariatric Surgery Consortium Writing Group. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. [accessed March 22, 2011];BMI - Body Mass Index: BMI for Children and Teens. 2011 Available at: http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-for-age.htm.
- 23.Chakraborty H, Gu H. RTI Press publication No MR-0009-0903. Research Triangle Park, NC: RTI International; 2009. A Mixed Model Approach for Intent-to-Treat Analysis in Longitudinal Clinical Trials with Missing Values. Retrieved May 20, 2011 from http://www.rti.org/rtipress. [PubMed] [Google Scholar]
- 24.Oude L, Luttikhuis H, Baur L, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–68. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomized controlled trial. Lancet. 2011;11:1344–5. doi: 10.1016/S0140-6736(11)61344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treadwell JR, Sun F, Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg. 2008;248:763–76. doi: 10.1097/SLA.0b013e31818702f4. [DOI] [PubMed] [Google Scholar]
- 28.Messiah SE, Carrillo-Iregui A, Garibay-Nieto G, Cossio S, Arheart K. Within-ethnic group comparison of metabolic syndrome components among morbidly obese adolescents. J Clin Hyperten. 2010;12:645–52. doi: 10.1111/j.1751-7176.2010.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring) 2009;17:901–10. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iqbal CW, Kumar S, Iqbal AD, Ishitani MB. Perspectives on pediatric bariatric surgery: identifying barriers to referral. Surg Obes Relat Dis. 2009;5:88–93. doi: 10.1016/j.soard.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Keidar A, Hecht L, Weiss R. Bariatric surgery in obese adolescents. Curr Opin Clin Nutr Metab Care. 2011;14:286–90. doi: 10.1097/MCO.0b013e3283453635. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien PE, Sawyer SM, Laurie D, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA. 2010;303:519–26. doi: 10.1001/jama.2010.81. [DOI] [PubMed] [Google Scholar]
- 33.Inge TH, Miyano G, Bean J, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123:214–22. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 34.De La Cruz-Munoz N, Messiah SE, Lopez-Mitnik G, Arheart KL, Lipshultz SE, Livingstone A. Laparoscopic gastric bypass surgery and adjustable gastric banding significantly decrease the prevalence of type 2 diabetes mellitus and pre-diabetes among morbidly obese multiethnic adults: long-term outcome results. J Amer Coll Surg. 2011;212:505–11. doi: 10.1016/j.jamcollsurg.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Hlaing WM, Messiah SE, Lipshultz SE, Ludwig DA. Obesity and length of hospital stay in children: a retrospective review of Florida agency for health care administration data. Prog Ped Cardiol. 2011;31:66–72. [Google Scholar]