Abstract
Background
In the aldehyde dehydrogenase 2 (ALDH2) gene, the ALDH2*2 allele, prevalent in East Asian populations, encodes an enzyme with severely reduced activity, thereby disrupting the normal metabolism of alcohol. Possession of the ALDH2*2 allele has been repeatedly shown to be associated with lower risk for alcohol dependence, and reduced alcohol use. However, relatively few studies have considered whether the magnitude of the effect of ALDH2 polymorphism upon drinking is related to developmental stage, or varies by environmental context.
Methods
In a longitudinally assessed sample of 356 adopted adolescents and young adults of East Asian descent, we examined the progression over time of the relationship between ALDH2 genotype and multiple measures of drinking behavior. We also sought to determine whether the environmental influences of non-biological parent and elder sibling alcohol use and misuse, as well as deviant peer behavior, moderated the effect of ALDH2 genotype upon alcohol use.
Results
Across all measures of alcohol use, the association between ALDH2*2 allele possession and reduced drinking went from negligible to moderate between mid-adolescence and early adulthood. A combined index of adoptive parent alcohol use and misuse consistently moderated the protective effect of the ALDH2*2 allele across measures of quantity and frequency of alcohol use, and symptomology, such that high parental alcohol use and misuse reduced the protective effect of the ALDH2*2 allele, while low parental alcohol use and misuse enhanced the effect of the allele. Neither a combined index of elder sibling alcohol use and misuse, nor deviant peer behavior were consistently related to the effect of ALDH2 genotype.
Conclusions
The protective effect of the ALDH2*2 allele increases over the course of adolescence and young adulthood and is modified by the environmental influence of parental alcohol use and misuse. As such, ALDH2 provides a model system for exploring the nature of gene-environment interplay across development.
Keywords: Gene-environment Interplay, Aldehyde Dehydrogenase, ALDH2, Adoption, Asian-Americans
Introduction
Although developmental patterns of alcohol use vary between individuals (Brown et al., 2008), on average, alcohol use increases in accelerating fashion over the course of adolescence (Walden, et al., 2007), typically reaching a maximum during early adulthood (Sher, Grekin, & Williams, 2005), and declining thereafter (Moore et al., 2005). Twin studies, and studies using other genetically informative family designs, indicate that genetic factors account for a substantial proportion of variance in alcohol use (McGue, 1999), but the magnitude of genetic effects upon alcohol use may change over the course of development. Some studies have shown that shared environmental influences are important to the initiation and establishment of alcohol use during adolescence, but give way to increasing genetic influences upon alcohol use phenotypes through adolescence and into adulthood (Kendler, et al., 2008; Rose and Dick, 2005). The pattern of increasing genetic relationship to alcohol use-related phenotypes with age has also been observed in association with measured genetic polymorphisms in candidate genes. For example, the strength of the association observed between a polymorphism in the GABA-A receptor alpha 2 subunit gene GABRA2 and alcohol dependence was shown to increase with age (Dick et al., 2006).
Twin, adoption, and other genetically informative studies designed to disentangle genetic factors from those of the social environment, suggest that heritable effects upon adolescent alcohol use may be both magnified and attenuated by environmental factors (Dick, 2011; van der Zwaluw, 2009). Generally, in less constrained (Dick, 2011), or more adverse environments, such as when the alcohol use of family members and peers is high (Hicks, et al., 2009), variance attributable to genetic influences upon adolescent alcohol use and other externalizing behaviors increases. There is also evidence that environmental moderation may modulate the magnitude of the influence of specific measured genetic polymorphisms upon alcohol use. For instance, one study found a polymorphism in the dopamine transporter gene DAT1 to be associated with serious alcohol problems only among young men who also had alcoholic fathers (Vaske, et al., 2009).
The enzyme aldehyde dehydrogenase 2 (ALDH2) performs an essential step in the normal function of the pathway of ethanol metabolism, by mediating the oxidation of acetaldehyde to acetate in the mitochondria of the liver and other tissues (Bosron and Li, 1986). While very rare in other populations (Li et al., 2009), among East Asian populations a substantial proportion of the populace carries an ALDH2 gene variant rs671 resulting in a Glu504Lys amino acid change (Yoshida, et al., 1984; Li et al., 2009), which codes for an enzyme with greatly diminished oxidative efficacy (Crabb, et al., 2004). Reduced enzyme activity causes build-up of acetaldehyde in tissues after consumption of alcohol (Wall et al., 1997), which produces a number of dysphoric symptoms, including facial flushing, nausea, and headache (Eriksson, 2001). Consequently, it has been repeatedly observed that, relative to those homozygous for the allele producing a fully active enzyme (ALDH2*1), individuals carrying the reduced activity ALDH2 variant (ALDH2*2) drink less (Hendershot et al., 2009), and are less likely to become alcohol dependent (Luczak, et al., 2006).
The relationship between ALDH2*2 possession and lower alcohol use may increase during late adolescence and early adulthood. Doran, et al. (2007) assessed a sample of Asian American college students during the first year of college, and again in the second year. They found that the frequency of heavy drinking episodes was not related to ALDH2 genotype during the freshman year, but was during the sophomore year. Possession of the ALDH2*2 allele, the authors concluded, protected against progression towards heavy drinking from freshman to sophomore year, and made desistance from heavy drinking during this period more likely. Similarly, the association between ALDH2 genotype and alcohol use outcomes in a sample of Korean college students in Korea increased between an initial freshman year assessment and follow-up 6 years later (Kim, et al., 2010).
Because ALDH2 specifically affects alcohol use via a known mechanism of altered alcohol metabolism, it is not likely to contribute to the same largely genetic latent factor that accounts for a large proportion of the variance in both alcohol use and other externalizing behaviors (Krueger et al., 2002). Therefore, environmental moderators of genetic influences upon alcohol use shown in twin and other family-based designs, as well as in candidate gene studies involving polymorphisms in genes active within the nervous system, may not similarly moderate the effects of ALDH2 polymorphism. However, indirect evidence indicates that the strength of the effect of ALDH2 upon drinking may be influenced by the environment. Higuchi et al. (1994) noted that between 1979 and 1992, a progressively greater proportion of alcoholics in Japan possessed the ALDH2*2 allele. This change is presumed to be the result of sociocultural shifts in Japan during the interim years, resulting in increased prevalence of alcohol use (Higuchi, 2007). These results imply, albeit indirectly, that the protection against maladaptive alcohol use afforded by possession of the ALDH2*2 allele may be attenuated by environments where drinking is more common, or social pressures to drink are high.
In conducting the present study, we expected that possession of the ALDH2*2 allele would be associated with lower levels of alcohol use, and that this association would become stronger over the course of adolescence and into early adulthood. We predicted, further, that the environmental effects of parent and elder sibling alcohol use, and level of deviant peer behavior, would each moderate the effect of ALDH2 genotype, and that these interactive effects would also increase with the age of the target participants. By studying adopted individuals, we were able to assess the environmental effect of parent and sibling drinking upon ALDH2 effect, unconfounded by shared genetic influences.
Materials and Methods
The Sibling Interaction and Behavior Study (SIBS) is a prospective longitudinal study of sibling pairs, including both adopted and non-adopted adolescents, and their parents (McGue, et al., 2007). Sibling participants and their parents attended an intake (IN) assessment when the siblings were in mid-adolescence, and the siblings were re-assessed at approximately 3.5 year intervals, in late adolescence (FU1), and again in early adulthood (FU2). Participants visited the research facility for IN and FU1 assessments, but the FU2 assessment was conducted over the phone.
Sample
Because the SNP variant (rs671) resulting in reduced ALDH2 enzymatic activity is almost exclusively found in East Asian populations, we genotyped only those SIBS participants of Korean descent, amounting to 356 participants. All participants had been adopted, with an average (SD) age at placement of .39 (.55) years. Phenotypic data were available for all 356 participants at IN and FU1 assessments, and 266 participants at FU2, because the second follow-up assessment is still ongoing. Age at IN was M(SD) = 14.81(1.81), at FU1: M(SD) = 18.13(1.99), and at FU2: M(SD) = 22.25(1.79). Of the genotyped participants, 59% were female.
Among the SIBS families from whom at least one child was genotyped for the ALDH2 SNP rs671, all adoptive parents were white except for two step-parents of Asian descent, and one step-parent of mixed ethnicity. Alcohol use data were available for the elder non-biological siblings of 165 genotyped participants at IN, 160 elder siblings at FU1, and 128 elder siblings at FU2. As the SIBS study includes only sibling pairs, each genotyped participant had the possibility of only one elder sibling. Elder siblings were .22 to 4.73 years older than target siblings (M(SD) = 2.33(.91) years). Of these elder siblings, 17 were white, 145 were of Korean descent, and 3 were of mixed ethnic origin. Of the 145 elder siblings of Korean descent, 126 were themselves genotyped participants, while the other 19 non-genotyped elder siblings of Korean descent did not have available DNA samples.
Genotyping
Participants provided samples of either peripheral blood (n = 191) or buccal swab (n=165). The ALDH2 Glu504Lys polymorphism (rs671) was genotyped using an Applied Biosystems TaqMan drug metabolism genotyping assay (Foster City, CA, USA). Additional details regarding the genotyping procedure have been previously published (Irons et al., 2007). Of 356 genotyped samples, 260 (73%) were homozygous ALDH2*1/*1, 87 (24.5%) were heterozygous ALDH2*1/*2, and 9 (2.5%) were homozygous ALDH2*2/*2. These genotype proportions did not deviate from Hardy Weinberg equilibrium, X2(1) = 0.28, p = 0.6. The frequency of the ALDH2*2 allele that we observed (14.8%) was approximately equivalent to previous studies of the polymorphism in samples of Korean descent (Li et al., 2009; Luczak, et al., 2006). ALDH2 genotype did not vary by sex, X2(1) = 1.87, p = .17.
Measures
All SIBS participants, both parents and children, underwent structured interviews including the assessment of DSM-IV diagnostic criteria, producing symptom counts and diagnostic status for alcohol abuse and alcohol dependence. Diagnostic data for parents (who were assessed at intake only), covered parental lifetime. Diagnostic data for sibling participants covered lifetime for assessment at intake, and covered the interval elapsing since their last assessment (typically over 3 years) at FU1 and FU2. To account for the possibility that symptoms may be forgotten when reporting on past disorder, participants were classified as alcohol dependent if they satisfied criteria for either definite (meeting all DSM-IV criteria) or probable (missing one symptom) alcohol dependence. Alcohol abuse, which requires only one symptom, was diagnosed only when full DSM-IV criteria were met.
We also measured the alcohol use of parents and sibling participants (including both target siblings and their co-siblings) aged 16 or older at the time of assessment using a modified version of the Substance Abuse Module (SAM; Robins, et al., 1987), which was originally developed to supplement the World Health Organization’s Composite International Diagnostic Interview (Robins et al., 1988). These measures were collected at all three assessment time points for sibling participants, but at intake assessment only for parents. To all sibling participants younger than 16 at the time of assessment, we administered a computerized substance use questionnaire, which included alcohol-use items re-scaled to be equivalent to those in the SAM. To index alcohol use for genotyped participants, older siblings, and parents, we used four items included on both the SAM and the computerized substance use questionnaire: frequency of drinking sessions over the year preceding the current assessment, average amount of alcohol consumed during drinking sessions over the year preceding the current assessment, maximum amount of alcohol consumed during a single drinking session over the participant’s lifetime until the current assessment, and frequency of intoxication over the participant’s lifetime until the current assessment. These items were combined, with equal weighting, to form a drinking index representing participants’ overall drinking behavior.
Peer behavior was assessed at all three assessments using a nine item questionnaire regarding delinquent, antisocial, or otherwise deviant peer behaviors, asking sibling participants to evaluate statements such as “My friends get into trouble with the police” or “My friends work hard to get good grades in school.” Only a single item on this questionnaire referred directly to peer alcohol use (“My friends drink alcohol or beer.”). Each item was scored on a four point scale for proportion of peers exhibiting the indicated behavior (“None of my friends”, “Just a few of my friends”, “Most of my friends”, or “All of my friends”), and item scores were summed.
Analyses
Because possession of a single ALDH2*2 allele is sufficient to produce substantially diminished enzyme activity (Crabb, et al., 1989), for analyses including ALDH2 genotype, individuals both homozygous and heterozygous for the ALDH2*2 allele were grouped together, and contrasted with individuals homozygous for the ALDH2*1 allele. Age was centered for analysis by subtracting the minimum observed age (10.73 years) from each participant’s age. Count data (not including age), considered both as predictors and outcomes, were log-transformed for analysis. Individuals who had never had a drink at the time of their latest assessment (n = 56) were excluded from analyses. Status as a lifetime never-drinker did not differ by possession of the ALDH2*2 allele at the latest assessment for which data was available for each individual (X2(1) = .48, p = .49) nor at IN (X2(1) = .06, p = .81), FU1 (X2(1) < .01, p = .96), or FU2 (X2(1) = .14, p = .70). In assessing the main effects of ALDH2 genotype, as well as effects of moderation by parental alcohol use and misuse, and peer deviance, all 300 genotyped participants who had ever had a drink by the time of their latest assessment were included in the analyses of all compared models. However, in assessing the potential moderating effect of elder sibling alcohol use and misuse, analyses included only the 165 genotyped participants for whom elder sibling data were available.
Both the drinking index and the sum of the alcohol abuse and alcohol dependence symptom counts were each averaged across mothers and fathers, so that parental alcohol use represents maternal and paternal alcohol use in combination. Because their distributions were non-normal, for all participants the drinking index and the sum of alcohol dependence and alcohol abuse symptom counts were each log-transformed. For analysis, for parents and elder siblings separately, the log-transformed drinking index and the log-transformed sum of alcohol abuse and alcohol dependence symptom counts were standardized and averaged to form a combined alcohol problem index, which was itself then standardized. All standardization was calculated relative to the variable values of the total number of available SIBS parents (N=1164) and both adopted and non-adopted offspring (N=1232), including both genotyped and nongenotyped SIBS participants.
To account for intra-individual correlation across longitudinal observations, for counted data outcomes we used mixed modeling as implemented in SAS (SAS Institute, Inc., Cary, North Carolina) PROC MIXED (Bryk and Raudenbush, 1992; Singer, 1998) and generalized estimating equations (Liang and Zeger, 1986) for binary outcomes. Within each model comparison set, likelihood ratio testing (LRT) was used to compare the least complex model to each of the successive models that appended one or more additional terms. We identified the best fitting model within each model set as the one that minimized the Akaike’s information criterion (AIC; Akaike, 1973) for quantitative outcomes, or the comparable quasi-likelihood function under the independence model criterion (QICC; Pan, 2001) for binary outcomes. For binary outcomes, we also considered Wald tests to determine the significance of added terms, as more complex models were compared.
For each outcome, we first fit a best-fitting baseline model including fixed effects for sex, age, and polynomials of age up to the cubic term (for analyses of count data outcomes) or squared term (for analyses of binary outcomes). The best-fitting baseline model included a sexby-age interaction for all continuous phenotypes, but not for the binary alcohol dependence or abuse phenotype. Preliminary model fitting did not support the inclusion of an interaction term between sex and ALDH2 genotype, nor any role for elder sibling sex, for any alcohol use outcomes, so these terms were excluded from the baseline model and subsequent model comparisons. To the baseline model, we fit a series of models adding terms first for the effect of ALDH2 genotype, then for the change in ALDH2 effect over time (that is, an ALDH2 by age interaction). Next, separately for the effects of parental alcohol use and misuse (ie: the parental combined alcohol problem index), elder sibling alcohol use and misuse (ie: the elder sibling combined alcohol problem index), and peer deviance, we fit a successive series of incrementing models including the change in the effect of each potential environmental moderator over time, the interaction of each with ALDH2 genotype, and the change in the interaction of each with ALDH2 genotype over time.
Results
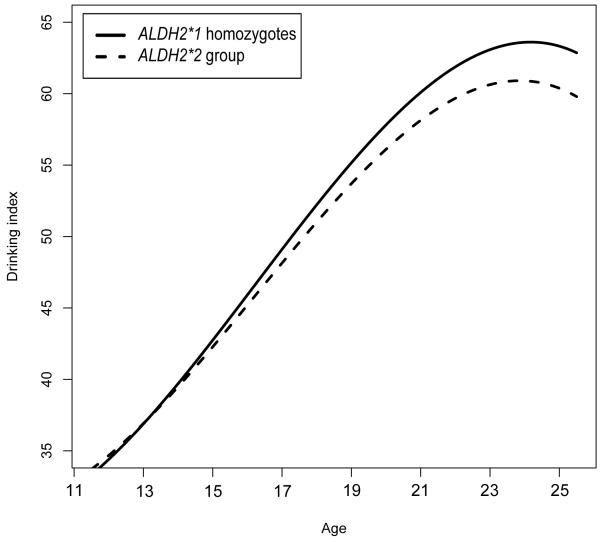
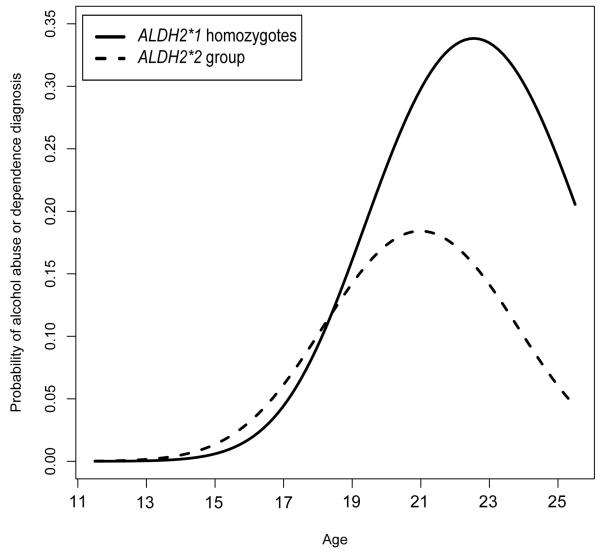
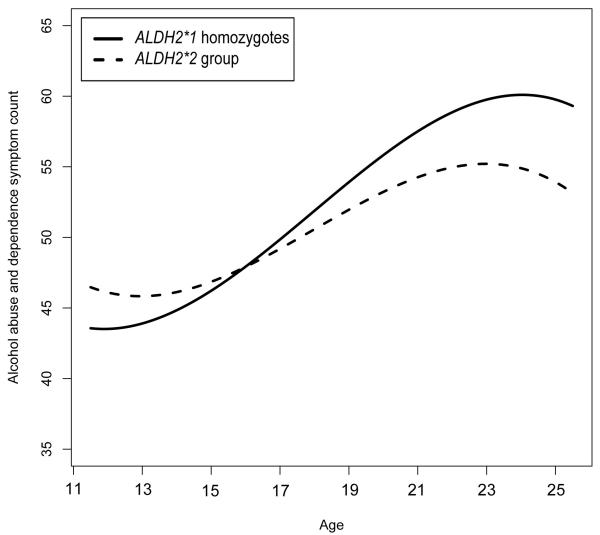
Table 1 displays the non-transformed observed values of the descriptive statistics for genotyped participant ages, as well as the primary alcohol use outcomes (drinking index, alcohol abuse and dependence symptom sum, and alcohol abuse or dependence diagnosis), at each assessment stage (IN, FU1, and FU2) by ALDH2 genotype. Model fit comparisons are given in Table 2. For all three outcomes, likelihood ratio tests indicated that including the ALDH2 main effect and the age by ALDH2 interaction effect significantly improved model fit relative to the baseline model. Likewise, the best-fitting model by minimized AIC or QICC included the main effect of ALDH2 as well as the interaction of ALDH2 with age.
Table 1.
Descriptive Statistics for Age and Alcohol Use Outcomes by Assessment Stage
| Stage | |||||
|---|---|---|---|---|---|
| Age | M (SD) | IN | FU1 | FU2 | |
| ALDH2*1 homozygotes | 14.8 (1.9) | 18.1 (2.1) | 22.3 (1.9) | ||
| ALDH2*2 group | 14.8 (1.6) | 18.1 (1.8) | 22.1 (1.6) | ||
| Drinking index | M (SD) | ||||
|
| |||||
| ALDH2*1 homozygotes | 1.30 (2.92) | 5.62 (5.32) | 10.02 (4.92) | ||
| ALDH2*2 group | 1.14 (2.86) | 4.53 (4.72) | 7.65 (4.77) | ||
| Alcohol abuse and dependence symptom sum | M (SD) | ||||
|
| |||||
| ALDH2*1 homozygotes | .11 (.76) | .51 (1.34) | .91 (1.56) | ||
| ALDH2*2 group | .11 (.62) | .41 (1.43) | .49 (1.27) | ||
| Alcohol abuse or dependence diagnosis | (%) | ALDH2*1 homozygotes | 2.3 | 14.6 | 32.1 |
|
| |||||
| ALDH2*2 group | 2.1 | 10.4 | 19.5 | ||
Table 2.
Model Comparisons Evaluating Change in Alcohol Use Outcomes as a Function of ALDH2 Genotype and Age
| Drinking index | ||||||
|---|---|---|---|---|---|---|
| Model | Predictors | −2LL change |
−2LL df |
LRT p-value |
AIC | |
| A | Sex, Sex*Age, Age, Age2, Age3 | 5458.5 | 5474.5 | |||
| B | A + ALDH2 | 5453.8 | −4.7 | 1 | .03 | 5471.8 |
| C | B + ALDH2*Age | 5450.6 | −7.9 | 2 | .02 | 5470.6 |
| Alcohol abuse and dependence symptom sum | ||||||
|---|---|---|---|---|---|---|
| Model | Predictors | −2LL change |
−2LL df |
LRT p-value |
AIC | |
| A | Sex, Sex*Age, Age, Age2, Age3 | 5269.6 | 5285.6 | |||
| B | A + ALDH2 | 5267.8 | −1.8 | 1 | .18 | 5285.8 |
| C | B + ALDH2*Age | 5259.6 | −10 | 2 | .01 | 5279.6 |
| Alcohol abuse or dependence diagnosis | |||
|---|---|---|---|
| Model | Predictors | Wald X2 p-value for added term(s) |
QICC |
| A | Sex, Age, Age2 | 473.9 | |
| B | A + ALDH2 | .29 | 473.7 |
| C | B + ALDH2*Age | .02 | 471.0 |
−2LL = −2 Log Likelihood; LRT = Likelihood Ratio Test. LRT p-values < 0.05 are in bold. Lowest AIC or QICC among each model comparison set, representing best-fitting model, is in bold.
To understand the nature of the ALDH2 by age interaction, table 3 provides best-fitting model estimated mean alcohol use phenotype values for each genotype group at three ages (15, 18, and 22), which correspond approximately to the sample mean age at each assessment. For every phenotype, alcohol use occurred with very low incidence at early ages, but increased over the course of adolescence and early adulthood before leveling off or decreasing near the end of early adulthood (figures 1-3). Effect sizes for continuous outcomes, and odds ratio for the discrete outcome, show that, as we expected, the protective effect of the ALDH2*2 allele against all forms of alcohol use also increased with participant age (this trend may also be observed for each of the outcomes displayed on figures 1-3). For example, for the drinking index, the effect size for the estimated standardized mean difference between the ALDH2 groups grew from d = −.07 at age 15, to .22 at age 18 and .40 at age 22. Likewise, the predicted probabilities of alcohol abuse or dependence diagnosis showed the protective effect of the ALDH2*2 allele increased with age, from OR = 2.29 at age 15, to 1.10 at age 18 and .42 at age 22. Although the OR might suggest that ALDH2*2 is associated with increased risk at age 15, it is important to recognize that rates of diagnosis are very low at this age (approximately 2% for both genotypes), in which case the estimated OR is statistically unstable.
Table 3.
Estimated Alcohol Use Outcome Values and Effect Sizes for ALDH2 Genotype Groups at Selected Ages
| Age | |||||
|---|---|---|---|---|---|
| Drinking index (log-transformed) | M (SD) | 15 | 18 | 22 | |
| ALDH2*1 homozygotes | 0.43 (.07) | 1.52 (.05) | 2.64 (.07) | ||
| ALDH2*2 group | 0.38 (.09) | 1.38 (.07) | 2.38 (.09) | ||
| Effect size (d) | 0.07 | 0.22 | 0.40 | ||
| (95% CI) | (-.19, .33) | (-.04, .40) | (.14, .66) | ||
| Age | |||||
|---|---|---|---|---|---|
| Abuse and dependence symptom sum (log-transformed) |
M (SD) | 15 | 18 | 22 | |
| ALDH2*1 homozygotes | 0.02 (.04) | 0.31 (.03) | 0.66 (.05) | ||
| ALDH2*2 group | 0.05 (.05) | 0.24 (.04) | 0.46 (.07) | ||
| Effect size (d) | -0.08 | 0.16 | 0.49 | ||
| (95% CI) | (-.34, .18) | (-.10, .42) | (.23, .75) | ||
| Age | |||||
|---|---|---|---|---|---|
| Probability of alcohol abuse or dependence diagnosis |
Probability (95% CI) |
15 | 18 | 22 | |
| ALDH2*1 homozygotes | 0.006 (.002, .016) |
0.09 (.06, .14) |
0.33 (.25, .43) |
||
| ALDH2*2 group | 0.014 (.005, .035) |
0.10 (.06, .17) |
0.17 (.09, .31) |
||
| ALDH2*2 Odds Ratio | 2.29 | 1.10 | 0.42 | ||
Effect size is in the form of Cohen’s d
Figure 1.
Best-fitting model estimated drinking index by age (t-standardized and log-transformed). Lines represent ALDH2 genotype groups.
Figure 3.
Best-fitting model estimated probability of alcohol abuse or dependence diagnosis by age. Lines represent ALDH2 genotype groups.
Results of the analysis of the effects of parental alcohol use and misuse are given in Table 4; comparable results for the effects of elder sibling alcohol use and misuse and peer deviance are given in Supplementary tables S1 and S2, respectively. The means of potential environmental influences did not differ between ALDH2 genotype groups (parent combined alcohol problem index: t(898) = .06, p = .96; elder sibling combined alcohol problem index t(363) = −.7, p = .48; peer deviance t(826) = .98, p = .33), indicating absence of gene-environment correlation. Birth order status (ie; being either a younger or elder sibling) also did not vary between ALDH2 genotype groups, X2(1) = 0.04, p = 0.84.
Table 4.
Model Comparisons Evaluating Change in Alcohol Use Outcomes as a Function of the Parental Combined Alcohol Problem Index Covariate
| Drinking index | ||||||
|---|---|---|---|---|---|---|
| Model | Predictors | −2LL | −2LL change |
df | LRT p-value |
AIC |
| C* | Model C including only participants with non-missing values for Parent combined alcohol problem index |
5450.6 | 5470.6 | |||
| D | C + Parent combined index | 5445.9 | 4.70 | 1 | .03 | 5467.9 |
| E | D + Parent combined index*Age | 5444.9 | 5.70 | 2 | .06 | 5468.9 |
| F | D + Parent combined index*ALDH2 | 5442.2 | 8.40 | 2 | .01 | 5466.2 |
| D + Parent combined index*Age | ||||||
| G | + Parent combined index *ALDH2 | 5441.2 | 9.40 | 3 | .02 | 5467.2 |
| H | G + Parent combined index*Age*ALDH2 | 5441.2 | 9.40 | 4 | .05 | 5469.2 |
| Alcohol abuse and dependence symptom sum | ||||||
|---|---|---|---|---|---|---|
| Model | Predictors | −2LL | −2LL change |
df | LRT p-value |
AIC |
| C* | Model C including only participants with non-missing values for Parent combined alcohol problem index |
5259.6 | 5279.6 | |||
| D | C + Parent combined index | 5258.9 | 0.70 | 1 | .41 | 5280.9 |
| E | D + Parent combined index *Age | 5258.6 | 1.00 | 2 | .61 | 5282.6 |
| F | D + Parent combined index *ALDH2 | 5255.5 | 4.10 | 2 | .13 | 5279.5 |
| D + Parent combined index *Age | ||||||
| G | + Parent combined index *ALDH2 | 5255.3 | 4.30 | 3 | .23 | 5281.3 |
| H | G + Parent combined index *Age*ALDH2 | 5255.1 | 4.50 | 4 | .48 | 5283.1 |
| Alcohol abuse or dependence diagnosis | |||
|---|---|---|---|
| Model | Predictors | Wald X2 p-value for added term(s) |
QICC |
| C* | Model C including only participants with non-missing values for Parent combined alcohol problem index |
471.0 | |
| D | C + Parent combined index | .39 | 472.6 |
| E | D + Parent combined index *Age | .84 | 474.8 |
| F | D + Parent combined index *ALDH2 | .07 | 472.2 |
| D + Parent combined index *Age | |||
| G | + Parent combined index *ALDH2 | .60, .08 | 474.8 |
| H | G + Parent combined index *Age*ALDH2 | .02 | 473.5 |
−2LL = −2 Log Likelihood; LRT = Likelihood Ratio Test. LRT and Wald X2 p-values < 0.05 are in bold. Lowest AIC or QICC among each model comparison set, representing best-fitting model, is in bold.
Our primary interest is in determining whether the ALDH2 effect on drinking outcomes was moderated by each environmental indicator (as indicated by inclusion of the two-way interaction term in model F in the tables) and further whether this effect was moderated by age (by inclusion of the three-way interaction effect in model H). Regarding the parental combined alcohol problem index, for the drinking index and alcohol abuse and dependence symptom count outcomes, but not the binary alcohol abuse or dependence diagnosis outcome, the AIC-determined best-fitting model included the hypothesized interaction between the parental combined alcohol problem index and ALDH2 polymorphism (table 4, Model F for each outcome). However, by the likelihood ratio test, for the alcohol abuse and dependence symptom count outcome, models including the influence of the parental combined alcohol problem index did not differ significantly from the best fitting model without the parental combined alcohol problem index (Model C*). In no case was there evidence for the three-way ALDH2 by Parent combined alcohol problem index by Age interaction effect.
Regarding the effect of the elder sibling combined alcohol problem index, for the drinking index measure, the AIC-determined best-fitting model included the hypothesized interaction between the elder sibling combined alcohol problem index and ALDH2 polymorphism (table S1, Model G). However, contrary to hypotheses, there was no evidence for moderation of ALDH2 effect by the elder sibling combined alcohol problem index upon the combined alcohol abuse and dependence symptom count, nor the diagnosis of alcohol abuse or dependence. Notably, for all three alcohol use outcomes, the magnitude of the positive relationship between the elder sibling combined alcohol problem index and the target sibling drinking index decreased over time. Similarly, we found no consistent evidence for interactions of ALDH2 with deviant peer behavior (table S2).
For the drinking index and alcohol abuse and dependence symptom count outcomes, estimation by best-fitting models generally reflects that, as expected, the magnitude of the protective effect afforded by ALDH2*2 against alcohol had a negative relationship to the level of the parental combined alcohol problem index (table 5). The same pattern is shown in estimates by the best-fitting model for the moderation of ALDH2 effect upon the drinking index outcome by the elder sibling combined alcohol problem index, and also upon alcohol abuse or dependence by peer deviance (table S3).
Table 5.
Estimated Alcohol Use Outcome Values and Effect Sizes for ALDH2 Genotype Groups at Selected Ages as a Function of High and Low Parental Combined Alcohol Problem Index
| Age | ||||
|---|---|---|---|---|
| Drinking index (log-transformed) | M (SD) | 15 | 18 | 22 |
| Parent combined alcohol problem index 1 SD above mean | ||||
| ALDH2*1 homozygotes | 0.45 (.07) | 1.55 (.06) | 2.67 (.08) | |
| ALDH2*2 group | 0.55 (.11) | 1.56 (.10) | 2.56 (.11) | |
| High parent combined alcohol problem index effect size (d) | −0.15 | −0.01 | 0.17 | |
| (95% CI) | (−.41, .11) | (−.27, .25) | (−.09, .43) | |
| Parent combined alcohol problem index 1 SD below mean | ||||
| ALDH2*1 homozygotes | 0.39 (.08) | 1.48 (.07) | 2.60 (.08) | |
| ALDH2*2 group | 0.20 (.11) | 1.20 (.09) | 2.21 (.11) | |
| Low parent drinking effect size (d) | 0.30 | 0.43 | 0.61 | |
| (95% CI) | (.04, .56) | (.17, .69) | (.35, .87) | |
| Age | ||||
|---|---|---|---|---|
| Alcohol abuse and dependence symptom count (log-transformed) |
M (SD) | 15 | 18 | 22 |
| Parent combined alcohol problem index 1 SD above mean | ||||
| ALDH2*1 homozygotes | 0.01 (.04) | 0.30 (.04) | 0.65 (.06) | |
| ALDH2*2 group | 0.13 (.07) | 0.32 (.06) | 0.55 (.08) | |
| High parent combined alcohol problem index effect size (d) | −0.30 | −0.05 | 0.27 | |
| (95% CI) | (−.56, −.04) | (−.31, .21) | (.01, .53) | |
| Parent combined alcohol problem index 1 SD below mean | ||||
| ALDH2*1 homozygotes | 0.02 (.05) | 0.31 (.04) | 0.66 (.06) | |
| ALDH2*2 group | −0.03 (.07) | 0.16 (.06) | 0.39 (.08) | |
| Low parent combined alcohol problem index effect size (d) | 0.12 | 0.36 | 0.69 | |
| (95% CI) | (−.14, .38) | (.10, .62) | (.42, .95) | |
Results are displayed only for comparisons where best-fitting model includes environmental covariate. Effect size is in the form of Cohen’s d
Discussion
Consistent with many previous studies, we found that adolescent and young adult participants who carried the ALDH2*2 allele exhibited reduced alcohol use compared to ALDH2*1 homozygotic participants. For every phenotypic outcome (drinking index, alcohol dependence and abuse symptom count, and alcohol dependence or abuse diagnosis), the best-fitting model included terms for the effect of ALDH2 genotype (table 2). Furthermore, best-fitting models for each of these primary phenotypes included an age by ALDH2 genotype interaction, such that the protective effect of ALDH2*2 increased over the course of adolescence and young adulthood,. This pattern is in accordance with earlier research showing that ALDH2 genotype is not related to the initiation of alcohol use, or to ever having been intoxicated (Wall, et al., 2001), and that evidence for association between the ALDH2*2 allele and diminished alcohol use may not be apparent in young samples (Hendershot, et al., 2005) but may increase during young adulthood (Doran et al., 2007; Kim et al., 2010).
The influence of ALDH2 polymorphism upon drinking behaviors has been previously shown to be partially mediated by cognitive factors (Hendershot et al., 2011). One possible mechanism by which the genetic effect of ALDH2*2 upon drinking behavior might increase with age is via learning processes, whereby alcohol cognitions develop in response to alcohol sensitivity experienced during past drinking sessions. In this framework, the effect of ALDH2*2 might be expected to increase over time, as individuals, following initial exposure to alcohol, learn to avoid ingesting larger amounts of alcohol, in order to avoid the adverse physiological effects that make up the alcohol sensitivity syndrome.
In the present study, we found that parental drinking (gauged by the parental combined alcohol problem index) was related to the strength of the effect of ALDH2 genotype upon both the drinking index, and the alcohol abuse and dependence symptom count, such that the protective effect of ALDH2*2 was stronger when parental drinking was lower, and weaker when parental drinking was higher (table 5). This finding is in accord with the previous observation that ALDH2*2 apparently afforded reduced protection against alcohol dependence as environmental exposure to alcohol use increased (Higuchi et al., 1994)—although in our sample, parental moderation of ALDH2 genotype effect did not extend to diagnosis of alcohol abuse and dependence per se.
Because genotyped participants in this study were adopted, mostly by white adoptive parents, into American families, our inference regarding the phenotypic effects of variation in ALDH2 is enhanced. Caucasian adoptive parents do not carry the ALDH2*2 allele themselves, but in non-adoptive families of ALDH2*2 carriers, at least one biological parent must also carry ALDH2*2, and thus is likely to experience the protection against alcohol use associated with the allele. Therefore, a passive gene-environment correlation may develop when ALDH2*2 carrying individuals are raised by their biological parents, whereby the influence of parental ALDH2 genotype results in a family environment characterized by fewer models of adult drinking, and, to the extent that offspring drinking is environmentally influenced by parent drinking, this environmental influence may compound upon, and be confounded with, the purely biological effect of the offspring’s own ALDH2 genotype. Consequently, in families where ALDH2*2 carrying offspring are raised by ALDH2*2 carrying biological parents, the protective effect associated with the ALDH2*2 allele is likely to be amplified, because it will also include the environmentally-mediated influence of parental ALDH2*2 genotypes (although it has not yet been demonstrated that this gene-environment correlation actually exists). In our sample, where white adoptive parents do not carry the ALDH2*2 allele, differences in alcohol use associated with participants’ ALDH2 genotype should be exclusively a function of that genotype, and will not be confounded with parental ALDH2 genotype. Similarly, although a large proportion of the elder siblings of genotyped target participants were themselves of Korean descent, they were not biological siblings to the target participants, so the environmental effect of elder sibling drinking was not confounded by a systematic relationship between target participant and elder sibling ALDH2 genotypes.
For analyses involving potential moderation of the effect of ALDH2 polymorphism by elder sibling alcohol use (gauged by the elder sibling combined alcohol problem index) and peer deviance (table S3), results were less consistent than those involving moderation by parental alcohol use. These inconsistencies may reflect limitations in the measurements available for elder sibling alcohol use and peer deviance. Notably, since not every participant had an older sibling, fewer observations were available for analyses assessing the effect of elder sibling drinking, reducing power to detect moderating effect upon the ALDH2 polymorphism. Moreover, although alcohol-related peer influences have previously been shown to moderate the heritability of alcohol use (Dick et al., 2007; Agrawal et al., 2010), it is possible that measures of adopted familial alcohol use may exert a more readily apparent moderating influence upon ALDH2 genotype effect than peer deviance, because they are specific measures of environmental exposure to alcohol use, whereas the scale of deviant peer behavior that we used reflected the proportion of peers engaging in a wider variety of externalizing behaviors. Because this ALDH2 polymorphism influences alcohol use via a known mechanism which specifically affects alcohol metabolism, ALDH2 would not be expected to contribute to variation in the largely genetic latent factor which likely influences alcohol use and other externalizing behaviors in common (Krueger et al., 2002). Peer deviance in general, although highly correlated with adolescent alcohol use (Sher et al., 2005), may be too indirectly related to environmental alcohol exposure to moderate the influence of ALDH2 genotype. SIBS study participants were residents of Minnesota, where alcohol use and binge drinking in adolescents and young adults are substantially above mean national levels (Substance Abuse and Mental Health Services Administration, 2010), so the omission of a sensitive and specific measure of peer alcohol use may be critical.
Genetic factors may influence the selection of peers, or may be otherwise correlated with peer identity or behavior. In fact, a longitudinal twin study found that genetic factors accounted for the entirety of the relationship between direct-report peer alcohol use and adolescent drinking (Hill, et al., 2008). However, in our sample, peer deviance did not vary by ALDH2 genotype, suggesting that it is appropriate to consider its influence as a potential environmental moderator of ALHD2 effect.
Meta-analyses of the association between alcohol dependence diagnosis and ALDH2 polymorphism suggest a rather large genetic effect (Luczak, et al., 2006; Zintzaras, et al., 2006), although these meta-analyses largely comprise case-control studies in fully adult samples, unlike our own. In contrast, for the alcohol use outcomes we examined, the magnitude of the protection associated with possession of the ALDH2*2 allele ranged from small to moderate (table 3). There are multiple possible reasons why possession of the ALDH2*2 allele might afford only a small-to-moderate level of protection in our sample. First, as described above, the estimated effect of possession of the ALDH2*2 allele in this sample of adopted participants should be free from inflation due to confounding introduced by the environmental influence of a biological parent or parents who also possesses the allele. Further, the maximum ALDH2*2 effect that we observed for each alcohol use phenotype occurred at the later ages included in the sample, so it is possible that the effect may continue to increase with age, beyond the ages at which our participants were assessed. Alternatively, though, rather than continuing to increase with age, the magnitude of the effect of ALDH2 polymorphism may peak when alcohol use is typically highest - that is, during young adulthood (Sher et al., 2005). In fact, in this sample, the effect of ALDH2*2 possession appears to level off or even diminish near the eldest observations. Although previous studies have noted increasing effect of ALDH2 polymorphism across periods of one or more years during young adulthood (Doran, et al., 2007; Kim et al., 2010), the development of the effect of ALDH2*2 possession upon alcohol use between young adulthood and later adulthood has yet to be studied.
To conclude, in this longitudinal sample of adopted participants of Asian descent assessed in adolescence and young adulthood, we re-confirmed the observation that possession of the ALDH2*2 allele, which results in deficient ALDH2 enzyme activity, was also associated with multiple measures of reduced alcohol use, and lower risk for alcohol-related psychopathology. We also observed that the reduction in alcohol use associated with ALDH2*2 genotypes became more pronounced over the course of adolescence and into young adulthood - a period during which alcohol use also typically increases. Finally, we noted that parent and elder sibling alcohol use, as well as peer deviancy, may moderate the protective effect of ALDH2*2 - although moderation by sibling alcohol use and peer deviancy was distinctly less certain than that by parent alcohol use, and none of the potential environmental moderators exhibited a strong and incontrovertible influence across all measures of target participant alcohol use.
While our observations support the notion that the effect of ALDH2 polymorphism develops longitudinally and may be environmentally moderated, we did not determine the mechanisms by which these processes occur. This problem may be addressed in future research by including longitudinally-assessed measures of alcohol-related motives and cognitions, as well as more detailed measures of familial and social alcohol exposure, for example, household alcohol availability, and peer alcohol use and alcohol-oriented attitudes.
Supplementary Material
Figure 2.
Best-fitting model estimated alcohol abuse and dependence symptom count by age (t-standardized and log-transformed). Lines represent ALDH2 genotype groups.
Acknowledgment
Supported in part by USPHS grants # AA11886 and MH066140 and NCRR/NIH grant # M01-RR00400
References
- Agrawal A, Balasubramanian S, Smith EK, Madden PA, Bucholz KK, Heath AC, Lynskey MT. Peer substance involvement modifies genetic influences on regular substance involvement in young women. Addiction. 2010;105:1844–53. doi: 10.1111/j.1360-0443.2010.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory. Akademiai Kiado; Budapest: 1973. [Google Scholar]
- Bosron WF, Li TK. Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology. 1986;6:502–10. doi: 10.1002/hep.1840060330. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk A, Raudenbush S. Hierarchical linear models for social and behavioral research: Applications and data analysis methods. Sage; Newbury Park, CA: 1992. [Google Scholar]
- Crabb DW, Edenberg HJ, Bosron WF, Li TK. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant. The Journal of clinical investigation. 1989;83:314–6. doi: 10.1172/JCI113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. The Proceedings of the Nutrition Society. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- DeLucia C, Belz A, Chassin L. Do adolescent symptomatology and family environment vary over time with fluctuations in paternal alcohol impairment? Developmental Psychology. 2001;37:207–16. doi: 10.1037/0012-1649.37.2.207. [DOI] [PubMed] [Google Scholar]
- Dick DM. Gene-environment interaction in psychological traits and disorders. Annual Review of Clinical Psychology. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr., Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics. 2006;36:577–90. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Changing environmental influences on substance use across development. Twin Research and Human Genetics: The Official Journal of the International Society for Twin Studies. 2007;10:315–26. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Myers MG, Luczak SE, Carr LG, Wall TL. Stability of heavy episodic drinking in Chinese- and Korean-American college students: effects of ALDH2 gene status and behavioral undercontrol. Journal of Studies on Alcohol and Drugs. 2007;68:789–97. doi: 10.15288/jsad.2007.68.789. [DOI] [PubMed] [Google Scholar]
- Eriksson CJ. The role of acetaldehyde in the actions of alcohol (update 2000) Vol. 25. Clinical and Experimental Research; Alcoholism: 2001. pp. 15S–32S. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological medicine. 1997;27:1381–96. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Collins SE, George WH, Wall TL, McCarthy DM, Liang T, Larimer ME. Associations of ALDH2 and ADH1B genotypes with alcohol-related phenotypes in Asian young adults. Alcoholism, Clinical and Experimental Research. 2009;33:839–47. doi: 10.1111/j.1530-0277.2009.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, MacPherson L, Myers MG, Carr LG, Wall TL. Psychosocial, cultural and genetic influences on alcohol use in Asian American youth. Journal of studies on alcohol. 2005;66:185–95. doi: 10.15288/jsa.2005.66.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Witkiewitz K, George WH, Wall TL, Otto JM, Liang T, Larimer ME. Evaluating a cognitive model of ALDH2 and drinking behavior. Alcoholism, Clinical and Experimental Research. 2011;35:91–8. doi: 10.1111/j.1530-0277.2010.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, South SC, Dirago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66:640–8. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Imazeki H, Kinoshita T, Takagi S, Kono H. Aldehyde dehydrogenase genotypes in Japanese alcoholics. Lancet. 1994;343:741–2. doi: 10.1016/s0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Maesato H, Osaki Y. Japan: alcohol today. Addiction. 2007;102:1849–62. doi: 10.1111/j.1360-0443.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- Hill J, Emery RE, Harden KP, Mendle J, Turkheimer E. Alcohol use in adolescent twins and affiliation with substance using peers. Journal of Abnormal Child Psychology. 2008;36:81–94. doi: 10.1007/s10802-007-9161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons DE, McGue M, Iacono WG, Oetting WS. Mendelian randomization: a novel test of the gateway hypothesis and models of gene-environment interplay. Development and Psychopathology. 2007;19:1181–95. doi: 10.1017/S0954579407000612. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Lee SI, Shin CJ, Son JW, Ju G. The genetic factors affecting drinking behaviors of korean young adults with variant aldehyde dehydrogenase 2 genotype. Psychiatry Investigation. 2010;7:270–7. doi: 10.4306/pi.2010.7.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–24. [PubMed] [Google Scholar]
- Li H, Borinskaya S, Yoshimura K, Kal’ina N, Marusin A, Stepanov VA, Qin Z, Khaliq S, Lee MY, Yang Y, Mohyuddin A, Gurwitz D, Mehdi SQ, Rogaev E, Jin L, Yankovsky NK, Kidd JR, Kidd KK. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Annals of Human Genetics. 2009;73:335–45. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73 [Google Scholar]
- Luczak SE, Glatt SJ, Wall TL. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychological Bulletin. 2006;132:607–21. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- McGue M. The behavioral genetics of alcoholism. Current Directions in Psychological Science. 1999;8:109–115. [Google Scholar]
- McGue M, Keyes M, Sharma A, Elkins I, Legrand L, Johnson W, Iacono WG. The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS) Behavior Genetics. 2007;37:449–462. doi: 10.1007/s10519-007-9142-7. [DOI] [PubMed] [Google Scholar]
- Moore AA, Gould R, Reuben DB, Greendale GA, Carter MK, Zhou K, Karlamangla A. Longitudinal patterns and predictors of alcohol consumption in the United States. American Journal of Public Health. 2005;95:458–65. doi: 10.2105/AJPH.2003.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Babor T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis; 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–77. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM. Gene-environment interplay in adolescent drinking behavior. Alcohol Reasearch & Health. 2005;28:222–229. [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24:323–355. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . State estimates of substance use from the 2007-2008 national surveys on drug use and health. Office of Applied Studies; Rockville, MD: 2010. [Google Scholar]
- van der Zwaluw CS, Engels RC. Gene-environment interactions and alcohol use and dependence: current status and future challenges. Addiction. 2009;104:907–14. doi: 10.1111/j.1360-0443.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- Vaske J, Beaver KM, Wright JP, Boisvert D, Schnupp R. An interaction between DAT1 and having an alcoholic father predicts serious alcohol problems in a sample of males. Drug and Alcohol Dependence. 2009;104:17–22. doi: 10.1016/j.drugalcdep.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Walden B, Iacono WG, McGue M. Trajectories of change in adolescent substance use and symptomatology: impact of paternal and maternal substance use disorders. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2007;21:35–43. doi: 10.1037/0893-164X.21.1.35. [DOI] [PubMed] [Google Scholar]
- Wall TL, Peterson CM, Peterson KP, Johnson ML, Thomasson HR, Cole M, Ehlers CL. Alcohol metabolism in Asian-American men with genetic polymorphisms of aldehyde dehydrogenase. Annals of Internal Medicine. 1997;127:376–9. doi: 10.7326/0003-4819-127-5-199709010-00007. [DOI] [PubMed] [Google Scholar]
- Wall TL, Shea SH, Chan KK, Carr LG. A genetic association with the development of alcohol and other substance use behavior in Asian Americans. Journal of abnormal psychology. 2001;110:173–8. doi: 10.1037//0021-843x.110.1.173. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:258–61. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zintzaras E, Stefanidis I, Santos M, Vidal F. Do alcohol-metabolizing enzyme gene polymorphisms increase the risk of alcoholism and alcoholic liver disease? Hepatology. 2006;43:352–61. doi: 10.1002/hep.21023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.