SUMMRY
Panaxadiol (PD) is a purified sapogenin of ginseng saponins, which exhibits anticancer activity. Epigallocatechin gallate (EGCG), a major catechin in green tea, is a strong botanical antioxidant. In this study, we investigated the possible synergistic anticancer effects of PD and EGCG on human colorectal cancer cells and explored the potential role of apoptosis in the synergistic activities. Effects of selected compounds on HCT-116 and SW-480 human colorectal cancer cells were evaluated by an MTS cell proliferation analysis. Cell cycle distribution and apoptotic effects were analyzed by flow cytometry after staining with PI/RNase or annexin V/PI. Cell growth was suppressed after treatment with PD (10 and 20 μM) for 48 h. When PD (10 and 20 μM) was combined with EGCG (10, 20 and 30 μM), significantly enhanced antiproliferative effects were observed in both cell lines. Combining 20 μM of PD with 20 μM and 30 μM of EGCG significantly decreased S-phase fractions of cells. In the apoptotic assay, the combination of PD and EGCG significantly increased the percentage of apoptotic cells compared with PD alone (P < 0.01). The synergistic apoptotic effects were also supported by docking analysis, which demonstrated that PD and EGCG bound in two different sites of the annexin V protein. Data from this study suggested that apoptosis might play an important role in the EGCG enhanced antiproliferative effects of PD on human colorectal cancer cells.
Keywords: Panaxadiol, Epigallocatechin gallate, Antiproliferation, Cell cycle, Apoptosis, Docking analysis, Human colorectal cancer
INTRODUCTION
Colorectal cancer is the third most common malignancy in the United States and the second most frequent cause of cancer-related death (HawkLevin, 2005; Jemal et al., 2010). Although curative surgical resection is possible in 70–80% of patients after diagnosis, almost half of them will die from recurrence of cancer (Yao et al., 2008). However, natural products have been a valuable source of new candidate compounds for adjuvant therapy (Cragg et al., 2009; Gullett et al., 2010).
We recently observed that a ginsenoside aglycon protopanaxadiol (PPD) showed antiproliferative effects on human colorectal cancer cells (Du et al., 2011). Panaxadiol (PD), a pseudoaglycone of diol-type triterpenoid with a dammarane skeleton (Fig. 1), is an active anticancer compound in steamed American ginseng (Qi et al., 2010). We also showed that steamed American ginseng extract increased reactive oxygen species (ROS) levels and induced mitochondrial damage in colorectal cancer cells (Li et al., 2010; Wang et al., 2009). We hypothesize that antioxidants may decrease the level of ROS and enhance PD-induced apoptosis in colorectal cancer cells, and this hypothesis was proven in part by our recent observation that the tumoricidal effect of PD was enhanced by a natural phenol compound epicatechin (Rodriguez et al., 2010).
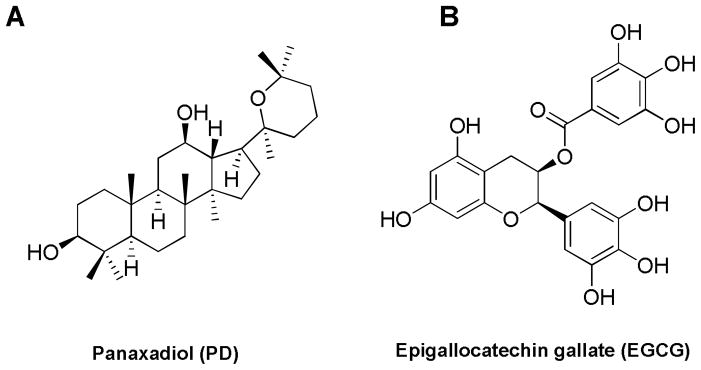
Fig. 1.
Chemical structures of panaxadiol (PD) and epigallocatechin gallate (EGCG).
Epigallocatechin gallate (EGCG), a well-known antioxidant, is believed to be the major biologically active compound in green tea (Kawai et al., 2005; Kim et al., 2009; Nakazato et al., 2005). A highly popular beverage globally, green tea contains a number of polyphenol compounds referred to as catechins, which have been demonstrated to possess strong ROS scavenging activity. Of these, EGCG showed the most potent antioxidant potential (LambertElias, 2010).
In this study, we investigated the possible synergistic antiproliferative effects of PD and EGCG in two human colorectal cancer cell lines, HCT-116 and SW-480. The effects of these compounds on cell cycle and apoptosis were also evaluated, and the docking model of these compounds to annexin V, a protein related to apoptosis, was simulated.
MATERIALS AND METHODS
Reagents and materials
All cell culture plasticware were obtained from Falcon Labware (Franklin Lakes, NJ) and Techno Plastic Products (Trasadingen, Switzerland). Trypsin, McCoy’s 5A and Leibovitz’s L-15 media, and phosphate buffered saline were obtained from Mediatech, Inc. (Herndon, VA). Penicillin G/streptomycin and EGCG was obtained from Sigma-Aldrich (St. Louis, MO). An MTS assay kit, CellTiter 96 Aqueous One Solution Cell Proliferation Assay, was obtained from Promega (Madison, WI). An annexin V-FITC apoptosis detection kit was obtained from BD Biosciences (Rockville, MD). PI/RNase staining buffer was obtained from BD Biosciences Pharmingen (San Diego, CA). PD was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Cell culture
The human colorectal cancer cell lines HCT-116 and SW-480 were obtained from American Type Culture Collection (ATCC). Cells were routinely grown in McCoy’s 5A medium (for HCT-116) or Leibovitz’s L-15 medium (for SW-480) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (50 units/ml). Cells were maintained in a tissue culture flask and kept in a humidified incubator (5% CO2 in air at 37°C). The medium was changed every 2–3 days. When the cells reached 70%–80% confluence, they were trypsinized, harvested, and seeded into a new tissue culture flask.
Cell proliferation assay
The effect of PD and EGCG on the proliferation of HCT-116 and SW-480 cell lines was determined using a modified trichrome stain (MTS) assay. Cancer cells were plated into a 96-well plate at a density of 1×104 cells/well. After seeding for 24 h, the cells were treated with PD, EGCG or both at various concentrations. All experiments were performed in triplicate. At the end of the sample exposure period, either 48 h or 72 h, the medium of each well was discarded and 100 μl of fresh medium and 20 μl of CellTiter 96 aqueous solution were added. The plate was returned to the incubator where it remained for 1–4 h in a humidified atmosphere at 37°C. Then 60 μl of medium from each well was transferred to an ELISA 96-well plate, and the absorbance of the formazan product was measured at 490 nm. The blank control was recorded by measuring the absorbance at 490 nm with wells containing medium mixed with CellTiter 96 aqueous solution but no cells. Results were expressed as a percentage of control (vehicle set at 100%).
Cell cycle analysis
The cell cycle profile was assayed by flow cytometry after staining with PI/RNase, and the assay data from PD and EGCG were compared. HCT-116 cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed, and cells were treated with PD, EGCG or both at different concentrations (10, 20, 30 μM). Cells were incubated for 72 h before harvesting. The cells were fixed gently with 80% ethanol before being placed in a freezer for 2 h. They were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 30 μl of phosphate buffered saline (PBS) containing 40 μg/ml propidium iodide and 0.1 mg/ml RNase. Cells were incubated in a dark room for 20 min at room temperature before cell cycle analysis with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and the FlowJo software (Ashland, OR). For each measurement, at least 10,000 cells were counted.
Apoptotic analysis
HCT-116 cells were seeded in 24-well tissue culture plates. After 24 h, the medium was changed and PD, EGCG or both drugs were added. After treatment for 48 h, cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then culture medium containing 10% FBS (and floating cells) was added to inactivate trypsin. When gentle pipetting was completed, the cells were centrifuged for 5 min at 1500 g. The supernatant was removed and cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer’s instructions. Untreated cells were used as control for double staining. Immediately after staining the cells were analyzed by a FACScan flow cytometer. For each measurement, at least 20,000 cells were counted.
Docking analysis
Annexin V protein (annexin 5A, PDB code: 2H0K) was selected for docking analysis. The Surflex-Dock program was used to determine the binding sites of PD and EGCG to annexin V. The binding affinities of these compounds to annexin was also determined, along with the locations of the hydrogen bonds formed.
Statistical analysis
The data are presented as mean ± standard error (SE). A one-way ANOVA and the Student’s t-test were used to determine whether the results had statistical significance. The level of statistical significance was set at P<0.05.
RESULTS
Antiproliferative effects of PD and EGCG on two human colon cancer cell lines
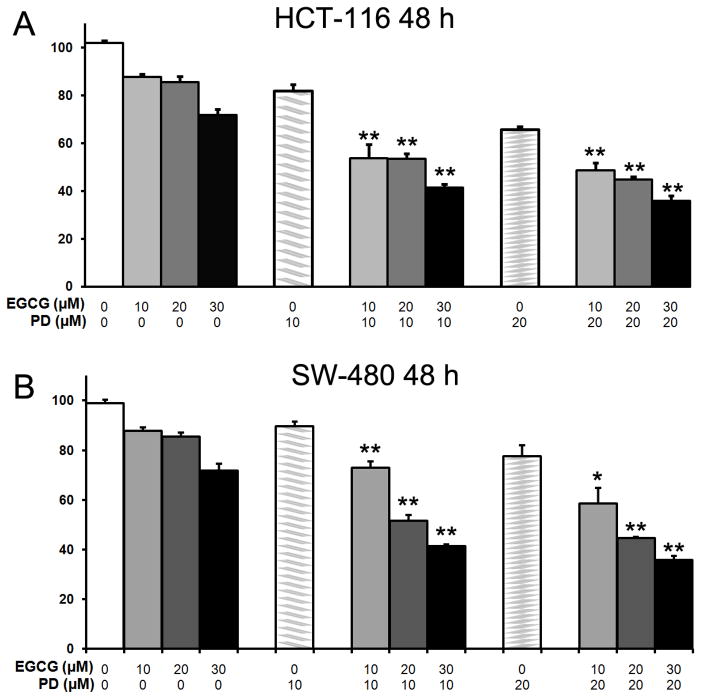
After treatment with PD, EGCG or both drugs for 48 h, the proliferation of HCT-116 and SW-480 cells was slightly suppressed dose dependently in each case. Concentrations of EGCG at 10, 20, or 30 μM and/or PD at 10 or 20 μM (Fig. 2) were tested. In HCT-116 cells, PD showed a 19.1±1.1% antiproliferative effect at 10 μM, and 34.3±2.2% at 20 μM. For the combination of PD at 10 μM plus EGCG at 10, 20, and 30 μM showed a 47.1±1.5%, 47.7±1.1% and 58.5±1.5% antiproliferative effect, respectively. At a PD concentration of 20 μM plus EGCG at 10, 20, and 30 μM, the antiproliferative effect is 52.4±2.1%, 56.5±1.1% and 65.7±1.5%. Compared to PD use only, EGCG significantly enhanced antiproliferation of PD (P<0.01, Figure 2A).
Fig. 2.
Antiproliferative effects of EGCG and PD on human colorectal cancer cells. HCT-116 and SW-480 cells were treated with PD (10 and 20 μM) and/or EGCG (10, 20, 30 μM) for 48 hours. Data are presented as mean ± standard error of mean. *P < 0.05, **P < 0.01, versus corresponding PD only.
In SW-480 cells, PD showed a 19.1±1.1% antiproliferative effect at 10 μM, and 22.3±2.3% at 20 μM.PD at 10 μM plus EGCG at 10, 20, and 30 μM showed a 27.1±1.5%, 48.4±1.1% and 58.5±1.5% antiproliferative effect. At a PD concentration of 20 μM plus EGCG at 10, 20, and 30 μM, the antiproliferative effect is 41.5±2.2%, 55.3±1.1% and 64.3±1.5%. EGCG significantly enhanced antiproliferative effects of PD use only (Figure 2B). The results suggest that in both the two human colorectal cancer cell lines, the antiproliferative effect of PD can be enhanced by EGCG.
Effects of PD and EGCG on cell cycle distribution in HCT-116 cells
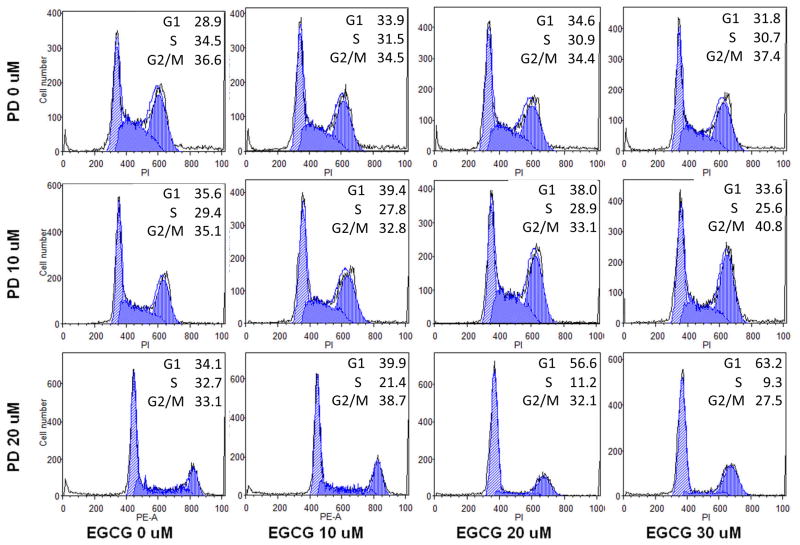
After staining with PI, the assay data from cells treated with PD, EGCG, and both drugs at various concentrations were compared. As shown in Fig. 3, compared with control (28.9% in G1 phase, 34.5% in S phase, and 36.6% in G2/M phase), treatment with 10–30 μM EGCG did not change the cell cycle profile of HCT-116 cells. At a PD concentration of 10 μM, the fractions of cells in different cell cycle phases were 35.6% (G1 phase), 29.4% (S phase) and 35.1% (G2/M phase); these data show that the G1 phase was slightly arrested. After treatment with 10–30 μM of EGCG plus PD at 10 μM, the cell cycle profile range was 33.9%, 34.6%, and 31.8% (G1 phase); 31.5%, 30.9%, and 30.7% (S phase) and 34.5%, 34.4%, and 37.4% (G2/M phase), respectively. At a PD concentration of 20 μM, the fractions of cells in different cell cycle phases were 34.1% (G1 phase), 32.7% (S phase) and 33.1% (G2/M phase); the G1 phase was slightly arrested. After treatment with 10–30 μM of EGCG plus PD at 20 μM, the cell cycle profile range was 39.9%, 56.6%, and 63.2% (G1 phase), 21.4%, 11.2%, and 9.3% (S phase) and 38.7%, 32.1%, and 27.5% (G2/M phase). Notably, the G1 phase was increased and the S phase was decreased. These data suggest that PD increases cell cycle arrest at the G1 phase, EGCG increases arrest at the G2/M phase, and that the combination of both significantly decreases time spent in S phase.
Fig. 3.
Effects of EGCG and PD on HCT-116 cell cycle. HCT-116 cells were treated with PD (10 μM) and/or EGCG (10, 20, 30 μM) for 72 h. Cell cycle profile was determined using flow cytometry after staining with PI/RNase. Percentage of cells in G1, S and G2/M phases are indicated.
Apoptotic effects of PD and EGCG on HCT-116 cells
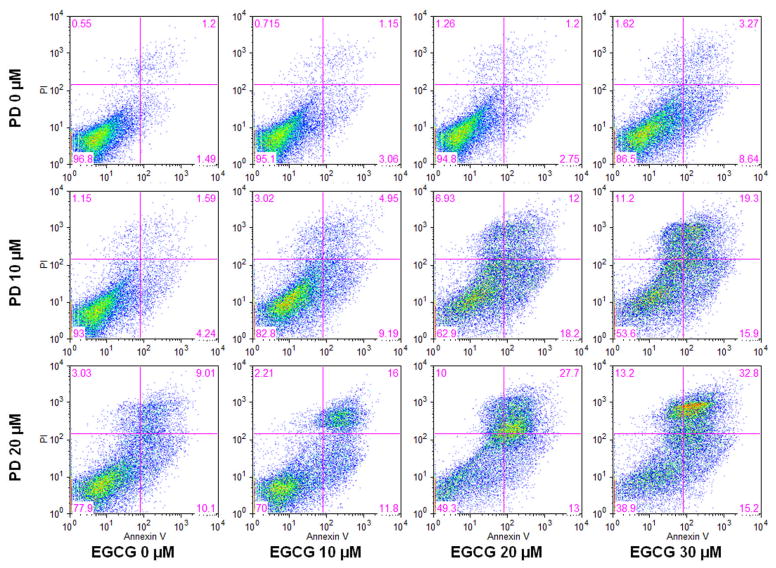
To further characterize the potential mechanism of the anticancer effect of these two medicines, we performed an apoptotic assay by flow cytometry after staining with annexin V and PI. Compared with the control, it was shown that PD induced apoptosis in HCT-116 cells. At concentrations of 10, 20 and 30 μM, EGCG did not induce obvious apoptosis. However, the combination of EGCG and PD was significantly more potent in inducing apoptosis than one would expect from simply combining their effects when acting alone. After treatment for 72 h, the percentage of apoptotic cells induced by PD at 10 and 20 μM was 5.7% and 19.0%, respectively. By EGCG at concentrations of 10, 20, and 30 μM the percentage was 4.2%, 3.95% and 11.9% respectively. By PD at 10 μM plus EGCG at 10, 20, and 30 μM, the percentage range was 14.1%, 30.2% and 35.2%, respectively. By PD at 20 μM plus EGCG at 10, 20, and 30 μM, the percentage range was 27.8%, 40.7% and 48.0% respectively, compared to control at 2.6% (Fig. 4). These results suggested that the antiproliferative effect of PD plus EGCG could be mediated by the induction of apoptosis.
Fig. 4.
Effects of EGCG and PD on HCT-116 cell apoptosis. HCT-116 cells were treated with PD (10, 20 μM) and/or EGCG (10, 20, 30 μM) for 72 h. Apoptosis was quantified using flow cytometry after staining with annexin V/PI.
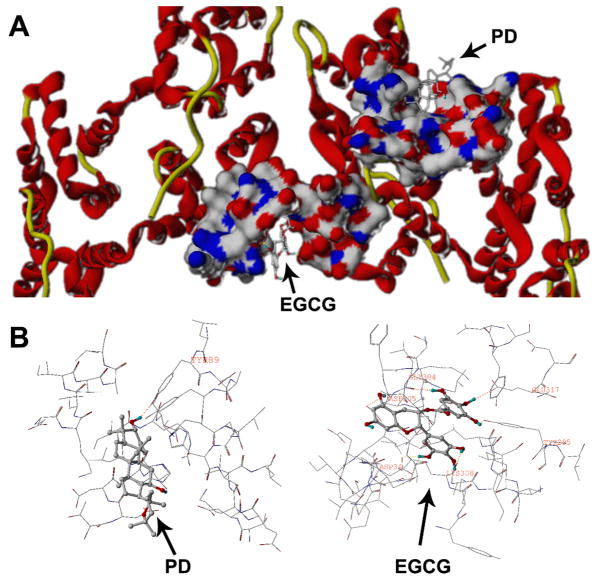
Molecular docking of PD and EGCG to annexin 5A protein
The combination of PD and EGCG displayed a significant pro-apoptotic effect. Annexin V (annexin A5) is an endogenous protein that binds with high affinity and specificity to phosphatidylserine, which is presented on the cell surface early on in the process of apoptosis (GerkeMoss, 2002). We selected annexin V as our target protein for docking analysis. Fig. 5 shows the interactions between PD, EGCG and annexin V at different binding sites. It can be seen that EGCG lies in the middle groove of the protein while PD lies in the right hemi chain. The docking results (Fig. 5) showed that EGCG formed hydrogen bonds with Gly 304, Asp 305, Asp 34, Lys 308, Tyr 295 and Glu 317, while PD formed a hydrogen bond with Tyr 89. These assays are in agreement with the results of our apoptosis study.
Fig. 5.
Docking analysis of EGCG and PD on the annexin V protein. EGCG and PD bind to distinct binding sites on the protein. A surface and ribbon view is shown above (A), while an insight ligand-site model is shown below (B).
DISCUSSION
Ginseng, including Asian ginseng and American ginseng, occupies a prominent position on the list of the best-selling natural products in the world. Ginseng has been reported to have a wide range of pharmacological effects, including anticancer activity (Choi, 2008; JangShin, 2010; Zhang et al., 2009). Compared to unsteamed white ginseng, steamed, or red, ginseng has significantly increased anticancer activity (Helms, 2004) Recently, we observed steamed ginseng could increase the level of ROS, which would activate the NF-κB pathway and contribute to the survival of colorectal cancer cells treated with anticancer compounds (Li et al., 2010). This observation suggested that decreasing ROS induced by steamed ginseng might further increase cancer cell death. Previous study has shown that at concentrations of 100–250 μg/ml, steamed ginseng extracts could significantly inhibit human colorectal cell proliferation (Sun et al., 2011; Wang et al., 2007). These studies showed that ginseng extracts and ginsenosides exhibited anticancer effects towards a panel of malignant cells. Green tea has been associated with health benefits and chemopreventive effects on cancer for a long time, and identified bioactive compounds in green tea is a group of polyphenols known as catechins (Singh et al., 2011; Yang et al., 2009). Considering that PD is one of the most active anticancer compounds found in ginseng (Qi et al., 2011; Wang et al., 2006), and EGCG is a strong natural antioxidant in green tea, in this study, we tested their combined anticancer effects (Cabrera et al., 2006).
Using two commonly used colorectal cancer cell lines HCT-116 and SW-480, we evaluated the interaction of PD and EGCG in inhibition of cancer cell activity. After co-treatment with 20μM PD plus 30μM EGCG for 48 h, the antiproliferative effect was 60.6% and 75.7%, respectively, much higher than that of either compound alone, or that predicted by addition of the effects of the two compounds. This suggests that EGCG significantly boosts the antiproliferative effect of PD on HCT-116 cells and may reduce the dose of PD needed to achieve the desired effects.
To explore the synergistic antiproliferative effect of PD and EGCG, we evaluated the effect of the two compounds on cancer cell cycle regulation. Using the HCT-116 cell line, the cell cycle profile was assayed by flow cytometry after staining with PI. If DNA is damaged, the cell cycle is halted at the transition from G1 to S phase, or at the transition from G2 to M phase (HartwellWeinert, 1989). We found that PD and EGCG significantly increased the fractions of cells in G1 and G2/M phases, but decreased the cell percentage in S phase, suggesting that the combination of PD and EGCG damaged cell DNA, and inhibited cancer cell growth.
To further characterize the potential mechanism of the anticancer effect of PD plus EGCG, we performed an apoptotic assay by flow cytometry after staining with annexin V and PI. Annexin V can be detected in both the early and late stages of apoptosis. PI enters the cell in late apoptosis or necrosis. Viable cells were negative for both annexin V and PI (lower left quadrant); early apoptotic cells were positive for annexin V and negative for PI (lower right quadrant); late apoptotic or necrotic cells displayed both positive annexin V and PI (upper right quadrant); non-viable cells which underwent necrosis were positive for PI and negative for annexin V (upper left quadrant). In the apoptotic analysis by flow cytometry, we found the percentage of apoptotic cells increased more than twice in the co-treatment group of PD 20 μM plus EGCG 30 μM compared with PD or EGCG only. This suggests a potential synergistic interaction of the two compounds in the induction of apoptosis.
It has been reported that binding of annexin V to the surface of apoptotic cells influences the progression of the cell death program. Annexin V is an endogenous protein that binds with high affinity and specificity to phosphatidylserine, which is presented on the cell surface early on in the process of apoptosis. Annexin V could delay activation of caspase 3 as well as accelerate the cell death program, after binding to the surface of cells treated with a cytostatic agent (Monceau et al., 2004). The effect of annexin binding on cell death depends on the type of cell and the apoptosis-inducing trigger. Inhibition of annexin V by antibody delayed programmed cell death (van Genderen et al., 2008). In our docking analysis, PD and EGCG were found to bind in two different sites of annexin V, while EGCG showed significant binding affinity with annexin V. The binding of both compounds to annexin V further provides a synergistic mechanism for their pro-apoptotic effect.
In summary, a synergistic anticancer effect of PD and EGCG is observed in human colorectal cancer cells. This is the first time that antiproliferative activity of a ginseng product was evaluated in combination with the major biologically active compound in green tea. Mechanistic observation and docking analysis suggested that this synergistic effect could be mediated through induction of apoptosis. Data from this study also suggest that the anticancer activity of the active ginseng compound PD could be enhanced by other antioxidants.
Acknowledgments
This work was supported in part by the NIH/NCCAM grants P01 AT004418 and K01 AT005362.
References
- Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–18. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–43. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- Du GJ, Dai Q, Williams S, Wang CZ, Yuan CS. Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities. Anticancer Drugs. 2011;22:35–45. doi: 10.1097/CAD.0b013e32833fde29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–71. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–81. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–34. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–91. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–74. [PubMed] [Google Scholar]
- Jang HI, Shin HM. Wild Panax ginseng (Panax ginseng C.A. Meyer) protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am J Chin Med. 2010;38:949–60. doi: 10.1142/S0192415X10008378. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Sasaki S, Watanabe T, Takahashi K, Nagawa H. Epigallocatechin gallate induces apoptosis of monocytes. J Allergy Clin Immunol. 2005;115:186–91. doi: 10.1016/j.jaci.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim HI, Chung J, Jeong TS, Park HR. (−)-Epigallocatechin-3-gallate (EGCG) increases the viability of serum-starved A549 cells through its effect on Akt. Am J Chin Med. 2009;37:723–34. doi: 10.1142/S0192415X09007193. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang CZ, He TC, Yuan CS, Du W. Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett. 2010;289:62–70. doi: 10.1016/j.canlet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monceau V, Belikova Y, Kratassiouk G, Charue D, Camors E, Communal C, Trouve P, Russo-Marie F, Charlemagne D. Externalization of endogenous annexin A5 participates in apoptosis of rat cardiomyocytes. Cardiovasc Res. 2004;64:496–506. doi: 10.1016/j.cardiores.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Nakazato T, Ito K, Ikeda Y, Kizaki M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin Cancer Res. 2005;11:6040–9. doi: 10.1158/1078-0432.CCR-04-2273. [DOI] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–54. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–95. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Du GJ, Wang CZ, Yuan CS. Letter to the editor: Panaxadiol’s anticancer activity is enhanced by epicatechin. Am J Chin Med. 2010;38:1233–5. doi: 10.1142/S0192415X10008597. [DOI] [PubMed] [Google Scholar]
- Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Qi LW, Du GJ, Mehendale SR, Wang CZ, Yuan CS. Red notoginseng Higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011;125:1299–305. doi: 10.1016/j.foodchem.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen HO, Kenis H, Hofstra L, Narula J, Reutelingsperger CP. Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim Biophys Acta. 2008;1783:953–63. doi: 10.1016/j.bbamcr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–74. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Li XL, Wang QF, Mehendale SR, Fishbein AB, Han AH, Sun S, Yuan CS. The mitochondrial pathway is involved in American ginseng-induced apoptosis of SW-480 colon cancer cells. Oncol Rep. 2009;21:577–84. doi: 10.3892/or_00000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, Aung HH, Xie JT, Tong R, He TC, Yuan CS. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–42. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zhao H, Sun Y, Lin F, Tang L, Chen P. Combined chemotherapy of hydroxycampothecin with oxaliplatin as an adjuvant treatment for human colorectal cancer. Tohoku J Exp Med. 2008;215:267–78. doi: 10.1620/tjem.215.267. [DOI] [PubMed] [Google Scholar]
- Zhang R, Jie J, Zhou Y, Cao Z, Li W. Long-term effects of Panax ginseng on disposition of fexofenadine in rats in vivo. Am J Chin Med. 2009;37:657–67. doi: 10.1142/S0192415X09007144. [DOI] [PubMed] [Google Scholar]