Abstract
Background
Chemotherapy is frequently administered in repetitive cycles. Adolescents with cancer suffer from multiple symptoms related to chemotherapy but knowledge of symptom trajectories across a cycle is limited. Examining trajectories over a cycle may reveal key periods to manage symptoms.
Objectives
The aims of this pilot were to describe the trajectory of symptoms (pain, sleep, fatigue, appetite, nausea, fatigue) and biological and behavioral variables (anxiety, stress, hematologic function) across one cycle; and examine relationships between variables.
Interventions/Methods
Nine adolescents with cancer within six months of diagnosis participated. Data were collected by surveys, chart review, and biologic measures on days 1 and 2 of the cycle, one week later (nadir), and day 1 of the following cycle. To evaluate the trajectory, a simple random effects repeated measures analysis was computed.
Results
The significant trajectories were fatigue (P = 0.003), difficulty sleeping (P = 0.032), and nausea (P = 0.04). Most of the adolescents reported some anticipatory anxiety about receiving chemotherapy. Significant correlations between symptoms and biobehavioral variables included anticipatory anxiety and nausea (P = 0.86, P = 0.003), trait anxiety and fatigue (r = −0.82, P < 0.001), and stress and pain (r = 0.78, P = 0.039).
Conclusions
Multiple symptoms were experienced across the cycle. Three symptoms displayed significant trajectories indicating that patterns of symptoms may be anticipated.
Implications for Practice
Pilot findings suggest monitoring symptoms, stress and anxiety across a cycle is important, not only during chemotherapy administration, but also prior to being admitted for chemotherapy.
Adolescents with cancer frequently experience symptoms from their illness and treatments that are challenging for them to manage. Yet few studies have focused on improving symptom management in adolescents with cancer and of those retrieved not all have been effective.1,2 The adolescent’s readiness to attend to his illness and symptoms may explain why some interventions are not wholly effective. Adolescents in particular are not always ready and willing to learn how to manage their illness.3 A model that may be useful in identifying when interventions may be more timely for adolescents is the shifting perspectives model of chronic illness which suggests that individuals shift focus between illness and wellness, and will better receive information when their focus is on their illness.4 One factor that can trigger a shift from wellness to illness is physical suffering. For adolescents with cancer, symptom exacerbation is a form of physical suffering.
Chemotherapy has been identified as a major cause of symptom exacerbation in adolescents as opposed to the cancer itself.5,6 Chemotherapy is frequently delivered in repetitive cycles in many cancers of adolescence. Generally, a cycle includes the time of administration of chemotherapy, the time that the chemotherapy attacks the cells, and the time of recovery of normal cells from the chemotherapy effects. Examining the trajectory of symptoms across a cycle of chemotherapy may provide a distinct and useful depiction of physical suffering from that of across the entire course of treatment. In addition, a biobehavioral approach to investigating the symptom experience is critical to understanding the effects of biological and behavioral variables on symptoms in order to effectively intervene upon these variables and improve symptom outcomes. As defined by the National Institute of Nursing Research (NINR), “Biobehavioral research encompasses the interactions among biological, behavioral, and social factors and their effect on outcomes.” 7(p. 10) Evidence suggests that the symptom experience can be influenced by certain biological and behavioral variables such as anxiety, stress, and hematologic function, thus would be important to include when investigating symptoms. The purpose of this pilot study was to identify periods of physical suffering over a cycle of chemotherapy in adolescents with cancer who are early in treatment. Specifically, we aimed: (1) to describe the trajectory (change over time) of symptoms (pain, sleep, fatigue, appetite, nausea, fatigue) and biological and behavioral variables (anxiety, stress, hematologic function) across one cycle of chemotherapy; and (2) to examine the relationships between severity of symptoms and biological and behavioral variables.
Findings may help to begin to identify periods of physical suffering associated with cycles of chemotherapy. Ultimately, such information may be useful in determining more appropriate times to test symptom management interventions in this population. Clinically, because cycles are repeated, a more thorough understanding of the symptom trajectory across a cycle of chemotherapy may provide insight as to when extra diligence is indicated in preventing, monitoring and intervening upon specific symptoms in adolescents.
BACKGROUND
Conceptual Framework
The Shifting Perspectives Model of Chronic Illness provides a framework for understanding the experience of living with chronic illness and proposes that individuals with chronic illness shift between perspectives of illness and wellness as a way of adapting.4 Perspectives are not static but fluctuate between wellness-in-the-foreground and illness-in-the-foreground. With the wellness perspective, the individual realizes the need to reframe and refocus on the positive aspects of life. With illness-in-the-foreground, the individual focuses on the illness—the toll the illness takes on the individual and family, and the suffering and loss associated with the illness. More importantly, when illness is the focus, the individual attends to dealing with his/her illness and coping with and managing symptoms.3 The shift from wellness-in-the-foreground to illness-in-the-foreground occurs when there is a perception of loss of control from a threat, transition, or suffering (e.g., physical suffering).4 Testing an intervention when the adolescent’s perspective is on wellness may force the adolescent to either take an illness perspective when they do not wish to or resist the shift; consequently they may be less receptive to information.3 We sought to identify periods of physical suffering during a cycle of chemotherapy, when the adolescent may assume the illness perspective. In addition, we extended the model to include certain biological and behavioral variables that have been linked to symptom severity, specifically anxiety, stress, and hematologic function.
Symptom Trajectory
Adolescents with cancer suffer a number of intolerable symptoms, particularly pain, sleep disturbances, fatigue, decreased appetite, and nausea,8,9 but the trajectories, or the changes over time of occurrence and severity, of these symptoms are not known. Few studies have been published on the trajectories of symptoms in adolescents with cancer. Comparison across studies is challenging because different methodologies and measures have been employed and a variety of symptoms have been examined, as have different trajectories. For example, using a case study approach, Docherty et al10 tracked a number of symptoms including pain, nausea, vomiting, and sleep disruptions, daily for the first 3 months following diagnosis in one adolescent with Hodgkin’s disease and identified a cyclic pattern with most of the symptoms. Van Cleve et al11 examined the trajectory of pain in children with leukemia across six time points during the first year of diagnosis (upon diagnosis and at the end of each phase of chemotherapy). Most of these children reported pain throughout the first year, with pain being most severe at diagnosis and least severe after completion of the first phase of maintenance. Lastly, in a series of studies to develop a fatigue scale, Hinds et al12 focused on fatigue during various periods of treatment (e.g., at the beginning and end of re-induction, and several times during a 10-day dexamethasone pulse therapy), and found that, in general, fatigue increased over time. Taken together, these findings suggest symptoms fluctuate across the course of cancer treatment but the exact nature of the trajectory across a cycle remains unclear.
Biological and Behavioral Factors Associated with the Symptom Experience
Across illnesses, anxiety, stress, and hematologic function have each been associated with various symptoms. Anxiety, a feeling of worry and apprehension,13 has been associated with greater symptom severity.14,15 In children, greater anticipatory anxiety has been correlated with higher postoperative pain ratings.15 Adolescents who have reported higher levels of worrying have also reported greater difficulty sleeping9,16,17 and more fatigue.16,17
Psychological stress has been associated with greater symptom severity in adolescents with diseases such as sickle cell anemia and chronic pain.18,19 In adolescents with cancer, relationships between psychological stress and symptom severity have not been fully examined but adolescents have reported that chemotherapy administration is very stressful.20,21 Physiologic measures of psychological stress have supported these findings. Specifically, salivary cortisol levels have been elevated around the time of chemotherapy administration10 and urine epinephrine levels have been elevated during clinic visits for children with cancer.20 These findings suggest adolescents with cancer are experiencing psychological stress, but whether stress has an effect on their symptoms is unknown.
Although cortisol has been used as a biological measure of psychological stress, it may not always be appropriate to measure in adolescents with cancer. Many childhood cancer protocols call for high dose corticosteroids. Steroids, particularly corticosteroids, can cause hypothalamic-pituitary-adrenal axis suppression,22 consequently affecting cortisol measures. An alternative surrogate biomarker of psychological stress that is not affected by corticosteroid use is salivary alpha amylase (sAA).
Salivary alpha amylase is a digestive enzyme produced by the acinar cells of the salivary glands. Salivary glands are innervated by the autonomic nervous system (ANS). During psychological stress there is increased activation of ANS resulting in increased production of saliva and increased levels of sAA.23-26 Salivary alpha amylase can be obtained by passive drool, which is non-invasive and does not cause pain, two characteristics that are important to consider when choosing biological markers for research in adolescents.27 Based on corticosteroid use with this population, and ease of collection, sAA was used as a biological marker of psychological stress in this study.
Hematologic function can be severely compromised by many chemotherapeutic agents. Myelosuppressive chemotherapy interferes with hematologic function by suppressing the bone marrow, generally causing below normal blood levels of hemoglobin (anemia), neutrophils (neutropenia), and platelets (thrombocytopenia). Typically, blood counts are near or within normal range at the beginning of the chemotherapy cycle, drop to their lowest at approximately 7 to 10 days after chemotherapy administration, and then recover. Limited research suggests low blood counts are associated with greater symptom severity. For example, anemia has been associated with greater fatigue and more sleep disruptions.16,17 Neutropenia can result in painful episodes of mucositis and infection. Individuals with thrombocytopenia have reported feeling fatigued.28 Whether abnormally low levels of hemoglobin, neutrophils, or platelets are associated with increased severity of symptoms in adolescents with cancer is unknown. Therefore, we sought to examine relationships between blood counts and symptom severity across a cycle of chemotherapy. In summary, certain biological and behavioral variables may influence the severity of symptoms but these relationships have not been adequately explored in adolescents with cancer. A more comprehensive understanding of the trajectory of symptoms and the effects of these factors on symptoms may guide development of symptom management interventions for adolescents with cancer.
METHODS
Design
This was a longitudinal, descriptive pilot study conducted over a cycle of chemotherapy. In consideration of factors that influence the symptom trajectory (chemotherapy administration and bone marrow suppression) and to decrease burden to participants, measures were obtained during scheduled clinic appointments at four time points: on day 1 of, but prior to, the start of a round/cycle of chemotherapy (T1); day 2 of chemotherapy (T2); 7-10 days after the start of the round/cycle of chemotherapy—when bone marrow suppression is typically most severe (T3); and day 1 of, but prior to, the start of the next cycle of chemotherapy (T4).
Participants
The sample consisted of adolescents who had been recently diagnosed with cancer and received treatment at a pediatric oncology clinic in the mid-Atlantic region. Inclusion criteria were ages 12 to 20 years, diagnosed with cancer for at least one month, and receiving anywhere from their 2nd through 6th cycle of chemotherapy. The period of the 2nd through 6th cycle was chosen so that all participants would have completed at least one cycle of chemotherapy prior to enrollment and so that their experiences earlier rather than later in their treatment could be examined, a preferable time to begin to aggressively manage symptoms. Of the few studies on symptoms in adolescents with cancer, including pain, fatigue, and sleep quality, there have been no significant differences in symptom severity reported during this early period of treatment.11, 29 Exclusion criteria were diagnosis of brain tumor because of the uniqueness of the treatment, participant’s or minor’s parents’ inability to read and write in English, or known cognitive disabilities.
Instruments
Symptoms
Pain was assessed with a 100 mm Visual Analog Scale (VAS) scale, with anchors of 0 (no pain) and 100 (pain as bad as you can imagine). Participants were asked to rate their worst pain in the last 24 hours. Visual analog scales have been used extensively to assess pain in children, are reliable and valid, and are simple to use and less sensitive to bias effects.30
Difficulty sleeping was assessed with a 100 mm VAS, with anchors of 0 (no difficulty sleeping) and 100 (a lot of difficulty sleeping). Participants were asked to rate how much difficulty they had sleeping (falling asleep or staying asleep) over the last 24 hours. Validity has been supported through findings of significant differences of scores between known groups (those with and without insomnia).31
Appetite was assessed with a 100 mm VAS, with anchors of 0 (normal appetite) and 100 (no appetite at all). Participants were asked to rate their worst level of appetite in the last 24 hours. Validity for the appetite VAS has been supported through findings of significant correlations between appetite (fullness) and food intake (r = −0.21) in young adults.32
Nausea was assessed with a 100 mm VAS scale, with anchors of 0 (no nausea) and 100 (nausea as bad as you can imagine). Participants were asked to rate their worst level of nausea in the last 24 hours. Validity for the nausea VAS has been supported through findings of significant correlations between the nausea VAS and vomiting (r = 0.51), bloating (r = 0.64), and appetite (r = −0.21) in the expected direction.33
Fatigue was measured using The Fatigue Scale–Adolescent (FS–A), a 14 item self-report instrument that measures cancer-related fatigue in the past 24 hours. Initial psychometric studies demonstrated that the FS-A is a reliable (coefficient alphas ranging from 0.67 to 0.95) and valid measure in adolescents with cancer (distinguishing between anemic and nonanemic patients).12 Response options ranged from 1 (not at all) to 5 (all the time). Scores are summative and can range from 14 to 70, with higher scores indicating greater fatigue. In this study, coefficient alphas for T1 through T4 ranged from 0.51 to 0.88.
Biological and behavioral variables
General anxiety was measured with the State-Trait Anxiety Inventory for adults and adolescents. The State anxiety scale consists of 20 items that assesses the current emotional state and will be measured at all data collection points. The Trait anxiety scale consists of 20 items that assesses anxiety proneness. Trait anxiety was measured only at the initial meeting because it is considered to be a stable characteristic over time. For both scales, response options range from 1 (not at all) to 4 (very much so). Scores are totaled for each scale and can range from 20 to 80; higher scores indicate greater anxiety. The STAI has demonstrated adequate reliability with a test-retest reliability in adolescents of 0.71 in females and 0.75 in males and internal consistency of 0.90 for males and females.34 Internal consistency in the present study for the Trait scale was 0.91 and for the State scale for T1 through T4 ranged from 0.91 to 0.95.
Anticipatory anxiety was assessed with a 100 mm VAS scale, with anchors of 0 (not worried at all) and 100 (very worried). Participants were asked to rate how worried they were about the chemotherapy treatment they were about to receive. Validity of this item has been demonstrated in children by its significant and inverse correlation with tolerance of upcoming painful procedures (r = −0.34) and its positive correlation with pain related to the procedure (r = 0.68).15
Salivary alpha amylase (sAA) was used as a biomarker of psychological stress. Salivary alpha amylase was collected by passive drool into a sterile cup at potential stressful times: prior to infusion of chemotherapy (T1 and T4), during chemotherapy administration (T2), and during a clinic visit to test for low blood counts (T3). Salivary alpha amylase collected by passive drool has not demonstrated a significant diurnal pattern thus time of day of is not critical.35 Participants were asked to avoid caffeine for several hours and to avoid drinking anything 15 minutes prior to each sAA collection. Participants were asked to briefly (30 seconds) refrain from swallowing and expectorate however much saliva was in the mouth from a single expectoration into the sterile cup. Each sample was assayed in duplicate, and the values of the two wells were averaged to obtain a value for that sample. The assay was done using a commercial kit (SALIVARY á-AMYLASE ASSAY KIT, Catalog No. 1-1902, Salimetrics, State College PA) which has <8% coefficient of variation for both intra- and inter-assay precision.
Data regarding hematologic function (blood counts of hemoglobin, platelets, and absolute neutrophil count [ANC]) were collected via chart review at T1, T3, and T4. Blood counts are not typically assessed on day 2 of chemotherapy because they are not expected to change from day 1 to day 2; therefore, we did not collect them at T2. Demographic and health information was obtained from self-report, including age, sex, ethnicity, race, and other health problems, and by chart review including diagnosis, time since diagnosis, blood counts, and medications.
Procedure
The institution’s review board granted approval for the study. Staff at the oncology clinic introduced the study to potential participants. Study personnel met with interested participants and their families, explained the study, and set up an initial meeting on a day of a scheduled admission for chemotherapy. At this initial meeting, as part of the informed consent process for minors, the researcher obtained written assent from minors and written permission from a parent. Written consent was obtained from adult participants. Participants had the choice of completing the self-report measures on a laptop computer or paper, which when provided the choice, all chose the laptop. The chart was reviewed for health information.
Data Analysis
Descriptive statistics were used to describe the occurrence and severity of symptoms. To assess the trajectory (change over time) of symptom severity and biological and behavioral factors, a simple random effects repeated measures analysis of variance, with subjects treated as random, was computed.36 In rare diseases, such as childhood cancer, large sample sizes are challenging to obtain. Efficient methods of analysis with small sample sizes are preferred to extract the maximum information from the data. The simple random effect model represented an ideal choice for testing for changes in the mean across time with the limited sample size for two reasons. First, the model appropriately accommodated the correlated within subject data. Second, the model allowed inclusion of subjects with missing data. Alternatives to the random effects model, such as multiple paired t-tests, would be less efficient, utilize an incorrect estimate of the overall variance and suffer from multiplicity issues within variables. To examine the relationships between symptom severity and the biological and behavioral variables at each timepoint, Pearson’s correlations for each of the variables were computed. Due to the pilot nature of the current study, alpha was set to 0.05 with no adjustment for multiplicity across variables.
RESULTS
Sample Description
Ten adolescents were invited to participate, one declined. Nine adolescents with cancer, age 13 to 18 years, participated. One participant did not complete measures at T4 due to death. See Table 1 for sample characteristics. Most participants (n=8) received chemotherapy as inpatients and all received myelosuppressive chemotherapy with moderate to high emetogenic potential. Mean (SD) time since diagnosis was 2.61 (1.89) months; all but one adolescent was within 4 months of being diagnosed. Mean weight did not differ significantly from T1 to T4 (P = 0.76). Only one adolescent reported other health problems not related to cancer (migraine) but did not attributed this problem to present symptoms. One adolescent reported pain unrelated to cancer but it is unclear from the data whether this participant was currently experiencing pain from this other cause.
Table 1.
Sample Characteristics (N = 9)
| Variable | M | SD |
|---|---|---|
| Age (Range 13 -18 years) | 15.3 | 1.7 |
| Time since diagnosis (Range 1 -7 months) | 2.6 | 1.9 |
| n | % | |
| Gender | ||
| Female | 4 | 44.4 |
| Male | 5 | 55.6 |
| Race | ||
| African-American | 5 | 55.6 |
| Asian | 1 | 11.1 |
| Caucasian | 3 | 33.3 |
| Cancer Diagnosis | ||
| Bone tumor | 3 | 33.3 |
| Leukemia | 2 | 22.3 |
| Lymphoma | 3 | 33.3 |
| Soft tissue tumor | 1 | 11.1 |
Trajectory of Symptoms and Biological and behavioral Variables
Our first aim was to describe the trajectory of symptoms and biological and behavioral variables across one cycle of chemotherapy. See Table 2 for mean scores on study variables. The mean (SD) number of symptoms out of the five assessed at each time point were 3.1 (1.27) at T1; 4.22 (0.44) at T2; 2.86 (1.35) at T3; and 2.86 (1.07) at T4. Most of the adolescents experienced two or more symptoms at each time point. Prior to the start of the chemotherapy cycle a number reported a decreased appetite (67%, 33%), nausea (57%, 43%), and difficulty sleeping (100%, 71%) at T1 and T4 respectively. At T2, while receiving chemotherapy, all the adolescents reported some level of fatigue, sleep disruption, and decreased appetite, and a majority (55.6%) experienced all five symptoms.
Table 2.
Means (SE) of Symptoms and Biological and Behavioral Variables
| Variable (Scale Range) | Time 1 | Time 2 | Time 3 | Time 4 |
|---|---|---|---|---|
| Symptoms | ||||
| Pain VAS (0–100) | 10.8 (21.1) | 20.2 (17.5) | 15.3 (29.1) | 23.9 (29.0) |
| Sleep VAS (0–100) | 44.9 (32.2) | 62.2 (19.7) | 19.6 (30.0) | 28.6 (33.7) |
| Appetite VAS (0–100) | 26.6 (36.1) | 53.8 (35.5) | 25.1 (41.4) | 12.6 (24.7) |
| Nausea VAS (0–100) | 14.7 (24.2) | 43.2 (41.3) | 15.9 (21.2) | 0.9 (1.5) |
| Fatigue FS-A (14–70) | 30.0 (8.2) | 37.9 (4.9) | 26.3 (6.1) | 27.4 (5.7) |
| Biological and Behavioral Variables | ||||
| Anxiety | ||||
| STAI: Traita (20–80) | 32.7 (9.3) | |||
| STAI: State (20–80) | 33.3 (12.7) | 36.2 (10.6) | 30.0 (11.5) | 29.3 (9.4) |
| Anticipatory (0–100)b | 28.3 (18.1) | 21.5 (22.3) | ||
| Salivary Alpha Amylase | 93.4 (17.9) | 135.7 (42.9) | 59.0 (13.3) | 75.2 (18.9) |
| Hematologic Functionc | ||||
| Hemoglobin | 10.5 (0.5) | 9.4 (0.9) | 10.2 (0.6) | |
| Platelets (× 109/L) | 347.0 (36.1) | 203.1 (40.6) | 339.1 (36.1) | |
| ANC | 5478.4 (982.4) | 4090.0 (1151.4) | 5120.5 (827.9) | |
Abbreviation: ANC, Absolute neutrophil count. FS-A, Fatigue Scale – Adolescent. STAI, State Trait Anxiety Inventory. VAS, visual analog scale.
Trait anxiety was measured at T1 only.
Anticipatory anxiety was measured at T1 and T4 only.
For hematologic indices, blood counts were obtained at T1, T3, and T4 only.
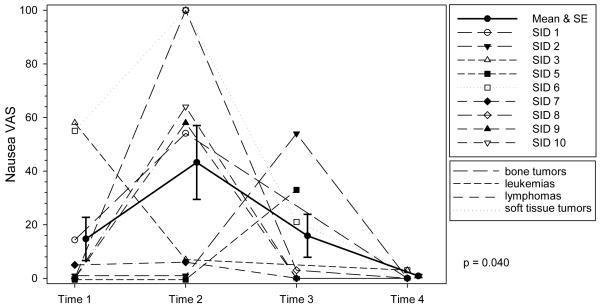
For the trajectory over the cycle, we were interested in knowing whether the severity of each symptom was constant or changed over time. If a symptom has a significant trajectory, this indicates that significant differences exist between timepoints in the mean severity of a symptom. The trajectories for both pain and appetite were not significant (P = 0.71 and P = 0.09 respectively), indicating there were no significant differences in mean severity between timepoints for either symptom. Across the cycle, mean pain severity scores were mild, ranging from 10.8 to 23.9, but the ranges indicated that some were experiencing moderate to severe pain at times. Although the trajectory for appetite was not significant, mean severity scores were in the mild to moderate range over the cycle, with scores ranging from 12.6 to 53.8. See Figures 1 - 5 for symptom trajectories. Profile plots were used in the figures because they simultaneously display the dispersion (variance) at each time point, the patient level changes over time, and the group means and standard errors. In addition, cancer type is identified for each patient.
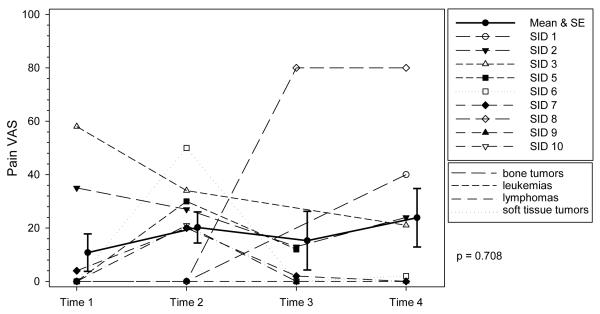
Figure 1. Profile Plot of Severity of Pain by Diagnosis over Time.
Individual ratings of severity of pain at each time point. VAS indicates Visual Analog Scale. The solid line represents the mean severity scores of pain and the error bars represent ± 1 standard error. The trajectory of pain was not significant indicating mean pain severity scores did not change over time.
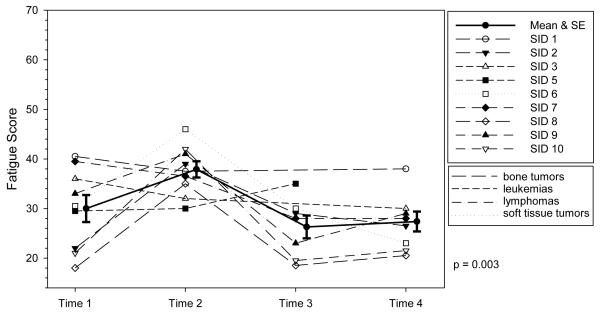
Figure 5. Profile Plot of Severity of Fatigue by Diagnosis over Time.
Individual ratings of severity of fatigue at each time point. VAS indicates Visual Analog Scale. The solid line represents the mean severity scores of fatigue and the error bars represent ± 1 standard error. The trajectory of nausea was significant indicating mean scores changed over time.
The trajectories of three symptoms were significant: fatigue, difficulty sleeping, and nausea. The fatigue trajectory was significant (P = 0.003), with mean scores numerically highest at T2 (37.9), during chemotherapy administration, and lowest at T3, when blood counts are dropping. All of the adolescents reported some level of fatigue at each timepoint, though scores generally ranged in the mild to moderate level.
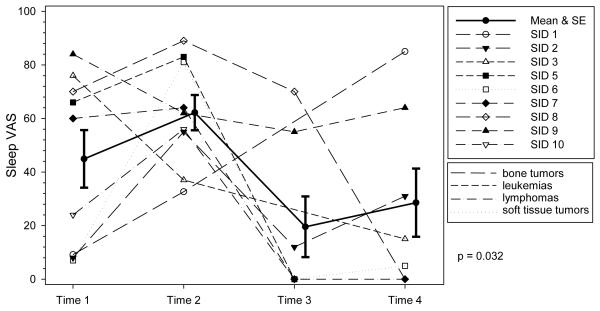
Difficulty sleeping displayed a significant trajectory (P = 0.032). Although mean scores were numerically highest at T2 (62.2) and lowest at T3 (19.6), mild to moderate levels of difficulty sleeping were evident on the nights prior to being admitted for chemotherapy (T1 and T4). See Table 2.
The trajectory of nausea was significant (P = 0.04), with mean scores numerically highest at T2 and lowest at T4. Mean nausea scores ranged from 0.9 to 43.2 across the cycle. At T1, prior to starting chemotherapy and T3, approximately 7 to 10 days after chemotherapy, at least one-third of the adolescents experienced mild to moderate levels of nausea, with individual scores ranging from 0 to 58 at T1and 0 to 54 at T3. Post hoc analyses of specific mean differences for each of the symptom trajectories were not conducted because of the small sample size.
Mean scores for the biological and behavioral variables of anxiety, stress and hematologic function are reported in Table 2. For anxiety, most of the adolescents reported at least some anticipatory anxiety about the impending chemotherapy (89% at T1, 75% at T4). Mean anticipatory anxiety scores were 21.5 (range 0 to 59) and 28.3 (range 0 to 60) at T1 and T4, respectively, and did not differ significantly (P = 0.26). The mean (SE) score for Trait anxiety, measured only at T1, was 32.7 (9.3). The mean (SE) State anxiety scores ranged from 36.2 (10.6) to 29.3 (9.4). The State anxiety trajectory trended toward but was not significant (P = 0.054). However, more than half of the adolescents reported some level of state anxiety at each timepoint.
Psychological stress was measured by sAA. The trajectory of sAA was not significant (P = 0.23), though mean sAA levels at each timepoint were somewhat elevated, ranging from 59.0 to 93.4. Normal adult levels of sAA, achieved by 5 to 6 years of age, range from 3.1 to 423.1 U/ml.36 The highest mean numeric score was at T2, during chemotherapy (135.7 U/ml).
Findings varied on the trajectories of blood counts. The only blood count that had a significant trajectory was platelets, P = 0.037. As expected due to the myelosuppressive effects of chemotherapy, numeric scores were lowest at T3. Neither hemoglobin nor ANC exhibited significant trajectories (P = 0.10 and P = 0.51, respectively). However, mean blood counts were numerically lowest at T3 for both hemoglobin and ANC.
Relationships between Symptoms and Biological and behavioral Variables
Our second aim was to examine the relationships among symptoms and biological and behavioral variables. At T1, day 1 of, but prior to the start of chemotherapy, correlations were significant between anticipatory anxiety and nausea (r = 0.86, P = 0.003) and sleep disruptions and platelets (r = 0.73, P = 0.024). At T2, the second day of chemotherapy, correlations were significant between trait anxiety and fatigue (r = −0.82, P < 0.001) and nausea and appetite (r = 0.75, P = 0.02). At T3, 7 to 10 days after chemotherapy, the only significant correlation was between psychological stress (sAA) and pain (r = 0.78, P = 0.039). Lastly, at T4, the correlation between fatigue and sleep was significant (r = 0.78, P = 0.039).
DISCUSSION
In this pilot study we examined the trajectory of five symptoms and investigated relationships between symptoms and biological and behavioral variables in order to begin to identify patterns of physical suffering over a cycle of chemotherapy. Most of the adolescents were in early phases of treatment, with all but one within 4 months of being diagnosed. Prior to starting chemotherapy (T1 and T4), at a time when they theoretically should be feeling their best, more than half of these adolescents reported 3 or more symptoms. However, the adolescents reported the greatest number of symptoms during chemotherapy (T2), at least 4 symptoms each, consistent with findings in some studies.8, 38 In contrast, others have found no difference between the number of symptoms reported at the start of chemotherapy compared to during chemotherapy.39 In examining the trajectory of the five symptoms, three exhibited significant changes in severity over the cycle: fatigue, difficulty sleeping, and nausea. Neither appetite nor pain displayed a significant trajectory.
Fatigue was the ever-present symptom that all adolescents reported. This is consistent with other findings where fatigue was a frequently reported symptom,29 was greater immediately following several days of chemotherapy40 and was less but still present between treatments.41 At T2, when hospitalized for chemotherapy, the mean FS-A fatigue score was 37.9, which is in the same range as FS-A fatigue scores reported by other adolescents with cancer receiving chemotherapy: mean ranges of 31.59 to 39.18.12 Hospitalization has been identified as a key contributor to fatigue as well as boredom, worry, and side effects of treatment.41,42 In this study the trajectory of fatigue was significant, even with the small sample, though across studies fatigue has not consistently displayed a predictable pattern. What appears to be predictable is that fatigue is an ongoing symptom that generally increases during treatment.39,40,43,44 There are many interventions for cancer-related fatigue, including exercise and cognitive-behavioral therapy45 but the extent to which adolescents know of and use these strategies is not known.
Sleeping difficulties changed significantly over time. Some level of difficulty sleeping in the past 24 hours was reported by all participants at T1 and by the majority at T4, indicating the adolescents were having difficulty sleeping on the night before being admitted. All experienced difficulty sleeping on the second day of chemotherapy (T2). Adolescents with cancer have reported that sleep is challenging,41 particularly when in the hospital.46 Using actigraphy, Gedaly-Duff et al47 demonstrated that adolescents hospitalized for chemotherapy experience numerous sleep disruptions. Linder et al48 have identified numerous environmental sources of nighttime noise for hospitalized adolescents with cancer. Our preliminary findings indicate that sleep and fatigue may be problematic prior to being admitted, however validation in larger samples is needed. Pediatric oncology nurses could assess whether their adolescent patients are having difficulty sleeping on the night prior to admission, seek to identify the underlying cause (e.g., anxiety, nervousness), and suggest interventions to improve sleep.
Although fatigue and sleep disturbances were reported by all participants at both T1 and T2, the only time these symptoms were significantly correlated with each other was at T4. Thus, findings differed regarding the relationships between fatigue and sleep disturbances on the first days of chemotherapy (T1 and T4). Similarly, findings have been inconsistent between these variables in other studies of adolescents with cancer on the first day of a cycle of chemotherapy.29,40 The conflicting findings may be due to our small sample size, or to differences in measures and timeframes of the symptom assessment (severity of symptom over past week versus past 24 hours). We used the same measure of fatigue as Hockenberry et al40 but a different measure of sleep disturbance—Hockenberry used actigraphy to measure sleep disturbance and we used a self-report measure. Erickson et al29 used self-report measures for both variables but they were not the same measures as was used in this study.
The trajectory for nausea was significant, with the highest numeric mean score at T2 (during chemotherapy). Medications for minimizing nausea from chemotherapy have improved dramatically over the last decade, but nausea has not been completely controlled. Nausea was reported by 55.6% of these participants during chemotherapy, consistent with some studies,49,50 though lower than the 81.3% reported in Hockenberry’s study.40 This difference may be attributed in part to differences in chemotherapy regimens. Hockenberry examined symptoms specific to cisplatin, doxorubicin, and ifosfamide, whereas we included adolescents early in treatment but receiving any chemotherapy; though all of the participants in this study received myelosuppressive chemotherapy with moderate to high emetogenic potential.
Of equal importance is that nausea was occurring throughout the cycle: approximately 56% at T1 and 29% at T4 reported nausea prior to chemotherapy, and 57% at T3 (around nadir). The nausea occurring at T1 and T4, just prior to the start of chemotherapy, might be anticipatory nausea. We assessed anticipatory anxiety but not anticipatory nausea. The nausea at T3 may have been delayed nausea, that is, nausea occurring after the completion of chemotherapy. Delayed nausea is a problem in a small percentage of adolescents receiving chemotherapy,49 suggesting a need for nurses to assess for nausea between treatments, such as when the adolescent comes to the clinic for blood counts or transfusions. In this study, nausea and appetite were significantly correlated during chemotherapy (T2) but not at the other time points. Indications are that these symptoms may not be as closely linked as in adults with cancer49 thus we cannot assume that because an adolescent is eating, they are not nauseated. Ongoing assessment and management of nausea is vital, not just during chemotherapy administration but across the cycle.
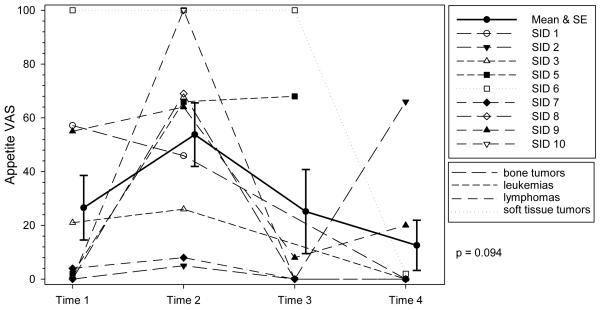
Appetite demonstrated no significant change over time, which may be attributed to the small sample size. However, appetite did appear to be decreased during chemotherapy administration. It was not surprising to find that all the adolescents reported some loss of appetite at T2, during chemotherapy, however more significantly, almost half reported some level of appetite loss at every other time point. This finding is consistent with other studies where, overall, 30 to 50% of adolescents with cancer reported a decreased appetite.8,39,50 Loss of appetite can potentially lead to inadequate nutrition which, in turn, can have detrimental effects on the individual such as lower tolerance of chemotherapy and increased risk of infection.51,52
The trajectory for pain was not significant. There are multiple causes of cancer related pain; therefore it is not surprising that there was no significant change over time. However, again, the small sample size may explain the lack of a significant trajectory over time. Similarly, Van Cleve et al11 did not find any significant differences in pain over time in 8 to 17 year olds with acute lymphocytic leukemia who were in early phases of treatment (induction and consolidation). Other studies on pain in adolescents with cancer have consistently revealed that pain has no specific pattern but is related to treatments, side effects, and procedures.10,53 Nurses are on the forefront in helping adolescents manage pain, and in the case of adolescents with cancer, can anticipate and plan to aggressively manage known painful events. However, nurses should be aware that adolescents may be hesitant to report their pain.54
The key biological and behavioral variables we investigated in relation to the symptoms were anxiety, stress and hematologic function. Self-reported anticipatory anxiety regarding impending chemotherapy ranged from mild to moderate in most of the adolescents, and the level did not differ significantly between T1 and T4. Over time, anxiety related to treatment has been shown to significantly decrease in adolescents with cancer,55 though it is generally higher just prior to a chemotherapy treatment compared to immediately following that treatment.3 We found the mean score of anticipatory anxiety was numerically but not significantly lower at T4 than T1 possibly because of the short period of time between measurements (approximately 4 weeks) or from diagnosis.
In examining relationships between anticipatory anxiety and symptom severity, the only significant correlation was between anticipatory anxiety and nausea at T1, such that higher anticipatory anxiety scores were significantly correlated with higher levels of nausea. These variables were measured before chemotherapy had been started. In adults with cancer, similar relationships have been found: higher levels of anxiety have been linked to higher levels of anticipatory nausea.56 Whether the nausea assessed at T1 was anticipatory nausea is unknown, but these findings warrant further investigation.
The Trait Anxiety numeric mean score of 32.7 for these participants was slightly lower than the mean norms for male (40.17) and female (40.97) high school students54 and lower than other adolescents with cancer (males 37.68, females 42.31).56 Trait anxiety was correlated with fatigue at T2 but not in the expected direction. Higher Trait anxiety scores were significantly associated with less fatigue. Although this contrary finding may be due to the small sample size, a systematic review of anxiety and fatigue in individuals with cancer revealed a significant association between the variables but of low magnitude.58
State Anxiety mean scores for the four timepoints (29.3 to 36.2) were not high overall. They were consistent with mean norms for this age group: 39.95 for males and 40.54 for females (Spielberg manual) and with other children and adolescents who were receiving chemotherapy (28 to 38.25).59 In general, anxiety levels are not higher than adolescents who do not have cancer, but a small percentage of adolescents with cancer may have higher than average anxiety that may require close scrutiny and attention.57,60 Most of the adolescents reported some level of either anticipatory or State anxiety, and although these findings are preliminary, pediatric oncology nurses are in key positions to screen for and are trained to provide interventions to reduce anxiety.
The trajectory for sAA was not significant, indicating there were no significant differences in sAA levels across timepoints. Mean sAA levels were somewhat elevated at T1 and T2, suggesting that some individuals were experiencing psychological stress at the time of starting the cycle and during administration. This is consistent with findings in other studies.10,20 In examining the relationships between psychological stress (sAA) and symptoms at each timepoint, the only significant correlation was between sAA and pain at T3, that is, higher levels of pain were associated with higher levels of sAA. Pain and stress may have been correlated only at T3 because of the small sample size, though pain is known to be one of the more distressing symptoms for adolescents.8 In light of the somewhat elevated levels of sAA across the cycle, further examination of the relationships between stress and symptom severity is warranted.
For hematologic function, at all time points, and for all symptoms except one, none of the variables were correlated with symptom severity. The only significant correlation was between sleep and platelets at T1, though not in the expected direction. Higher levels of disrupted sleep were significantly associated with higher platelet counts. This unexpected finding may have been due to the small sample size. We were particularly curious to see whether the hematologic function variables at T3, when blood counts are expected to be low, were associated with greater symptom severity. None of the correlations were significant perhaps because not all of the participants exhibited severe bone marrow suppression at the time of data collection. At T3, some appeared to have reached nadir while others had not. Myelosuppression can occur at slightly different rates and to different degrees among individuals, making it challenging in research to capture participants at their nadir.
This study had some limitations. Pediatric cancer is fortunately a rare disease, but this poses problems for obtaining a homogenous sample regarding cancer type and for recruiting large numbers of participants. The sample size is small and meaningful interpretation of changes over time is limited. Our sample was heterogeneous in type of cancer. Instead of having a homogenous sample based on cancer type, we chose to include adolescents who were in their early phase of chemotherapy treatment and who received myelosuppressive chemotherapy with moderate to high emetogenic potential. The inclusion criteria of being within first the first 6 cycles of chemotherapy limited the pool of potential participants; however we think that understanding the symptom experience in the early phase of diagnosis is important for determining whether more aggressive approaches to symptom management are necessary at such a critical time.
CONCLUSION
Findings from this pilot study revealed interesting patterns in the trajectories of symptoms over a cycle of chemotherapy in adolescents with cancer worth further exploration. Several of the symptoms displayed significant trajectories (fatigue, nausea, and sleeping difficulties) while others did not (pain, appetite), indicating we may be able to identify patterns, thus potential periods of suffering. The majority suffered at least two of the five symptoms at any one time across the cycle. Most of these adolescents experienced several symptoms prior to starting a cycle of chemotherapy and when blood counts were low. Sleep disruptions and loss of appetite, in particular, were experienced by many prior to the first day of the cycle. Stress and anticipatory anxiety were also somewhat elevated at this time. There is a dearth of research on improving self-management of symptoms in adolescents with cancer. For a couple of reasons these pilot findings suggest that a potential time to target interventions may be just prior to starting a cycle of chemotherapy. First, anticipatory anxiety related to treatment and stress were both elevated before beginning a cycle, possibly indicating a shift from a focus on wellness to illness. Second, symptoms were evident throughout the cycle but tended to worsen during chemotherapy administration. However, larger studies are needed to further examine the trajectories of symptoms and the nature of their relationships with relevant biological and behavioral factors in order to confirm these pilot findings. Intervening when the shift to illness begins but prior to symptoms escalating as a result of chemotherapy may be an ideal time to test interventions.
As chemotherapy is given in repetitive cycles, extending knowledge of physical suffering across a cycle is useful for guiding nursing care of adolescents with cancer. In the clinical setting, nurses know that vigilant monitoring and management of symptoms when chemotherapy is being administered is critical. However, these pilot findings suggest that nurses may need to monitor more closely and intervene upon symptoms that adolescents experience on the days prior to being admitted for chemotherapy and when their counts are expected to be low. Stress and anxiety may influence the symptom experience. Nurses can help adolescents reduce stress and anxiety by preparing them both emotionally and physically for the impending chemotherapy and associated symptoms.
Figure 2. Profile Plot of Severity of Difficulty Sleeping by Diagnosis over Time.
Individual ratings of severity of difficulty sleeping at each time point. VAS indicates Visual Analog Scale. The solid line represents the mean severity scores of difficulty sleeping and the error bars represent ± 1 standard error. The trajectory of difficulty sleeping was significant indicating mean scores changed over time.
Figure 3. Profile Plot of Severity of Appetite Loss by Diagnosis over Time.
Individual ratings of severity of appetite loss at each time point. VAS indicates Visual Analog Scale. The solid line represents the mean severity scores of appetite loss and the error bars represent ± 1 standard error. The trajectory of appetite loss was not significant indicating mean scores did not change over time.
Figure 4. Profile Plot of Severity of Nausea by Diagnosis over Time.
Individual ratings of severity of nausea at each time point. VAS indicates Visual Analog Scale. The solid line represents the mean severity scores of nausea and the error bars represent ± 1 standard error. The trajectory of nausea was significant indicating mean scores changed over time.
Acknowledgements
This work was supported in part by a grant from the National Institute of Nursing Research (P20 NR008988, “Biobehavioral Research in Critical Health Experiences;” N. McCain, PI)
The authors are incredibly grateful to the adolescents and families from the pediatric oncology clinic at the VCU Health System for generously being involved in the study, and to the staff for their unwavering support.
Footnotes
The authors have no conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hinds PS, Hockenberry-Eaton M, Rai SN, et al. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. J Pain Symptom Manage. 2007;33(6):686–697. doi: 10.1016/j.jpainsymman.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Schneider SM, Workman ML. Effects of virtual reality on symptom distress in children receiving chemotherapy. Cyberpsychol & Behavior. 1999;2(2):125–134. doi: 10.1089/cpb.1999.2.125. [DOI] [PubMed] [Google Scholar]
- 3.Hinds PS. Adolescent-focused oncology nursing research. Oncol Nurs Forum. 2004;31(2):281–287. doi: 10.1188/04.ONF.281-287. [DOI] [PubMed] [Google Scholar]
- 4.Paterson BL. The shifting perspectives model of chronic illness. J Nurs Scholarsh. 2001;33:21–26. doi: 10.1111/j.1547-5069.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- 5.Ljungman G, Gordh T, Sorensen S, Kreuger A. Pain variations during cancer treatment in children: a descriptive survey. Pediatr Hematol Oncol. 2000;17(3):211–221. doi: 10.1080/088800100276389. [DOI] [PubMed] [Google Scholar]
- 6.Woodgate RL, Degner LF. Expectations and beliefs about children’s cancer symptoms: perspectives of children with cancer and their families. Oncol Nurs Forum. 2003;30(3):479–491. doi: 10.1188/03.ONF.479-491. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Nursing Research [Accessed November 4, 2011];Strategies for building the science. http://www.ninr.nih.gov/NR/rdonlyres/54FB62BD-21F5-4F84-BFEF-19A71D2AA46F/0/StrategicStrategies.pdf.
- 8.Collins JJ, Byrnes ME, Dunkel IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage. 2000;19(5):363–377. doi: 10.1016/s0885-3924(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 9.Williams DP, Schmideskamp J, Ridder EL, Williams AR. Symptom monitoring and dependent care during cancer treatment in children. Cancer Nurs. 2006;29(3):188–197. doi: 10.1097/00002820-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Docherty SL, Sandelowski M, Preisser JS. Three months in the symptom life of a teenage girl undergoing treatment for cancer. Res Nurs Health. 2006;29:294–310. doi: 10.1002/nur.20143. [DOI] [PubMed] [Google Scholar]
- 11.Van Cleve L, Bossert E, Beecroft P, Adlard K, Alvarez O, Savedra MC. The pain experience of children with leukemia during the first year after diagnosis. Nurs Res. 2004;53(1):1–10. doi: 10.1097/00006199-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Hinds PS, Hockenberry M, Tong X, et al. Validity and reliability of a new instrument to measure cancer-related fatigue in adolescents. J Pain Symptom Manage. 2007;34(6):607–618. doi: 10.1016/j.jpainsymman.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Hockenberry-Eaton M, DiIorio C, Kemp V. The relationship of illness longevity and relapse with self-perception, cancer stressors, anxiety, and coping with cancer. J Pediatr Oncol Nurs. 1995;12(2):71–79. doi: 10.1177/104345429501200206. [DOI] [PubMed] [Google Scholar]
- 15.Tsao JCI, Myers CD, Craske MG, Bursch B, Kim SC, Zeltzer LK. Role of anticipatory anxiety and anxiety sensitivity in children’s and adolescents’ laboratory pain responses. J Pediatr Psychol. 2004;29(5):379–388. doi: 10.1093/jpepsy/jsh041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson F, Mulhall AB, Richardson A, Edwards JL, Ream E, Sepion BJ. A phenomenologic study of fatigue in adolescents receiving treatment for cancer. Oncol Nurs Forum. 2005;32:651–660. doi: 10.1188/05.ONF.651-660. [DOI] [PubMed] [Google Scholar]
- 17.Hockenberry-Eaton M, Hinds PS, Alcoser P, et al. Fatigue in children and adolescents with cancer. J Pediatr Oncol Nurs. 1998;15:172–182. doi: 10.1177/104345429801500306. [DOI] [PubMed] [Google Scholar]
- 18.Gil KM, Carson JW, Porter LS, Scipio C, Bediako SM, Orringer E. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol. 2004;23(3):267–274. doi: 10.1037/0278-6133.23.3.267. [DOI] [PubMed] [Google Scholar]
- 19.Hechler TPMP, Dobe MMP, Kosfelder JPMP, et al. Effectiveness of a 3-week multimodal inpatient pain treatment for adolescents suffering from chronic pain: statistical and clinical significance. Clin J Pain. 2009;25(2):156–166. doi: 10.1097/AJP.0b013e318185c1c9. [DOI] [PubMed] [Google Scholar]
- 20.Hockenberry-Eaton M, Kemp V, DiIorio C. Cancer stressors and protective factors: Predictors of stress experienced during treatment for childhood cancer. Res Nurs Health. 1994;17:351–361. doi: 10.1002/nur.4770170506. [DOI] [PubMed] [Google Scholar]
- 21.McCaffery CN. Major stressors and their effects on the well-being of children with cancer. J Pediatr Nurs. 2006;21(1):59–66. doi: 10.1016/j.pedn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook. 15th ed Lexi-Comp; Hudson: 2008. [Google Scholar]
- 23.Ehlert U, Erni K, Hebisch G, Nater U. Salivary alpha-amylase levels after yohimbine challenge in healthy men. J Clin Endocrinol Metab. 2006;91(12):5130–5133. doi: 10.1210/jc.2006-0461. [DOI] [PubMed] [Google Scholar]
- 24.Nater U, Lamarca R, Florin L, et al. Stress-induced changes in human salivary alpha-amylase activity—associations with adrenergic activity. Psychoneuroendocrinology. 2006;31(1):49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Speirs RL, Herring J, Cooper WD, Hardy CC, Hind CR. The influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Arch Oral Biol. 1974;19(9):747–752. doi: 10.1016/0003-9969(74)90161-7. [DOI] [PubMed] [Google Scholar]
- 26.van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31(1):137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Moore IM. Advancing biobehavioral research in childhood cancer. J Pediatr Oncol Nurs. 2004;21(3):128–131. doi: 10.1177/1043454204264400. [DOI] [PubMed] [Google Scholar]
- 28.Kalpatthi R, Bussel JB. Diagnosis, pathophysiology and management of children with refractory immune thrombocytopenic purpura. Curr Opin Pediatr. 2008 Feb;20(1):8–16. doi: 10.1097/MOP.0b013e3282f45bb9. [DOI] [PubMed] [Google Scholar]
- 29.Erickson JM, Beck SL, Christian BR, et al. Fatigue, sleep-wake disturbances, and quality of life in adolescents receiving chemotherapy. J Pediatr Hematol Oncol. 2011;33(1):e17–25. doi: 10.1097/MPH.0b013e3181f46a46. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 31.Rumble ME, Keefe FJ, Edinger JD, Porter LS, Garst JL. A pilot study investigating the utility of the cognitive-behavioral model of insomnia in early-stage lung cancer patients. J Pain Symptom Manage. 2005;30(2):160–169. doi: 10.1016/j.jpainsymman.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Parker BA, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58(2):212–218. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- 33.Bruera E, Belzile M, Neumann C, Harsanyi Z, Babul N, Darke A. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427–435. doi: 10.1016/s0885-3924(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 34.Spielberger CD. State-trait anxiety inventory STAI. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- 35.Harmon AG, Towe-Goodman NR, Fortunato CK, Granger DA. Differences in saliva collection location and disparities in baseline and diurnal rhythms of alpha-amylase: A preliminary note of caution. Horm Behav. 2008;54(5):592–596. doi: 10.1016/j.yhbeh.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J. 2006;48(2):286–301. doi: 10.1002/bimj.200510192. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell MD, Miller NJ. Plasma pancreatic and salivary-type amylase and immunoreactive trypsin concentrations: variations with age and reference ranges for children. Clin Chim Acta. 1980;104(3):265–273. doi: 10.1016/0009-8981(80)90384-8. [DOI] [PubMed] [Google Scholar]
- 38.Yeh CH, Chiang YC, Lin L, et al. Clinical factors associated with fatigue over time in paediatric oncology patients receiving chemotherapy. Br J Cancer. 2008;99(1):23–29. doi: 10.1038/sj.bjc.6604434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker AJ, Gedaly-Duff V, Miaskowski C, Nail L. Differences in symptom occurrence, frequency, intensity, and distress in adolescents prior to and one week after the administration of chemotherapy. J Pediatr Oncol Nurs. 2010;27(5):259–265. doi: 10.1177/1043454210365150. [DOI] [PubMed] [Google Scholar]
- 40.Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K, Sambuco G, Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010;37(1):E16–27. doi: 10.1188/10.ONF.E16-E27. [DOI] [PubMed] [Google Scholar]
- 41.Gibson F, Mulhall AB, Richardson A, Edwards JL, Ream E, Sepion BJ. A phenomenologic study of fatigue in adolescents receiving treatment for cancer. Oncol Nurs Forum. 2005 May;32(3):651–660. doi: 10.1188/05.ONF.651-660. [DOI] [PubMed] [Google Scholar]
- 42.Hockenberry-Eaton M, Hinds PS. Fatigue in children and adolescents with cancer: evolution of a program of study. Semin Oncol Nurs. 2000;16(4):261–272. doi: 10.1053/sonu.2000.16577. [DOI] [PubMed] [Google Scholar]
- 43.Erickson JM, Beck SL, Christian B, et al. Patterns of fatigue in adolescents receiving chemotherapy. Oncol Nurs Forum. 2010;37(4):444–455. doi: 10.1188/10.ONF.444-455. [DOI] [PubMed] [Google Scholar]
- 44.Yeh CH, Chiang YC, Chien LC, Lin L, Yang CP, Chuang HL. Symptom clustering in older Taiwanese children with cancer. Oncol Nurs Forum. 2008;35(2):273–281. doi: 10.1188/08.ONF.273-281. [DOI] [PubMed] [Google Scholar]
- 45.Health NCIatNIo Fatigue (PDQ®). (n.d.) http://www.cancer.gov/cancertopics/pdq/supportivecare/fatigue/Patient/AllPages.
- 46.Davies B. A typology of fatigue in children with cancer. J Pediatr Oncol Nurs. 2002;19(1):12–21. doi: 10.1053/jpon.2002.30012. [DOI] [PubMed] [Google Scholar]
- 47.Gedaly-Duff V, Lee KA, Nail L, Nicholson HS, Johnson KP. Pain, sleep disturbance, and fatigue in children with leukemia and their parents: a pilot study. Oncol Nurs Forum. 2006;33(3):641–646. doi: 10.1188/06.ONF.641-646. [DOI] [PubMed] [Google Scholar]
- 48.Linder L, Christian B. Characteristics of the nighttime hospital bedside care environment (sound, light, and temperature) for children with cancer. Cancer Nurs. 2011;34(3):176–184. doi: 10.1097/NCC.0b013e3181fc52d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupuis LL, Lau R, Greenberg ML. Delayed nausea and vomiting in children receiving antineoplastics. Med Pediatr Oncol. 2001;37(2):115–121. doi: 10.1002/mpo.1179. [DOI] [PubMed] [Google Scholar]
- 50.Yeh C-H, Wang C-H, Chiang Y-C, Lin L, Chien L-C. Assessment of symptoms reported by 10- to 18-year-old cancer patients in Taiwan. J Pain Symptom Manage. 2009;38(5):738–746. doi: 10.1016/j.jpainsymman.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 51.Donaldson SS, Wesley MN, DeWys WD, Suskind RM, Jaffe N, vanEys J. A study of the nutritional status of pediatric cancer patients. Am J Dis Child. 1981;135(12):1107–1112. doi: 10.1001/archpedi.1981.02130360015007. [DOI] [PubMed] [Google Scholar]
- 52.Hays DM, Merritt RJ, White L, Ashley J, Siegel SE. Effect of total parenteral nutrition on marrow recovery during induction therapy for acute nonlymphocytic leukemia in childhood. Med Pediatr Oncol. 1983;11(2):134–140. doi: 10.1002/mpo.2950110213. [DOI] [PubMed] [Google Scholar]
- 53.Bossert EA, Van Cleve L, Adlard K, Savedra M. Pain and leukemia: the stories of three children. J Pediatr Oncol Nurs. 2002;19(1):2–11. doi: 10.1053/jpon.2002.30011. [DOI] [PubMed] [Google Scholar]
- 54.Ameringer S. Barriers to pain management among adolescents with cancer. Pain Manag Nurs. 2010;11(4):224–233. doi: 10.1016/j.pmn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinds PS, Gattuso JS, Billups CA, et al. Aggressive treatment of non-metastatic osteosarcoma improves health-related quality of life in children and adolescents. Eur J Cancer. 2009;45(11):2007–2014. doi: 10.1016/j.ejca.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y, Morrow GR. The effects of family support, anxiety, and post-treatment nausea on the development of anticipatory nausea: A latent growth model. J Pain Symptom Manage. 2007;34(3):265–276. doi: 10.1016/j.jpainsymman.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Allen R, Newman SP, Souhami RL. Anxiety and depression in adolescent cancer: Findings in patients and parents at the time of diagnosis. Eur J Cancer. 1997;33(8):1250–1255. doi: 10.1016/s0959-8049(97)00176-7. [DOI] [PubMed] [Google Scholar]
- 58.Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: A systematic review. Psychosomatics. 2009;50(5):440–447. doi: 10.1176/appi.psy.50.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hockenberry-Eaton M, Kemp V, DiIorio C. Cancer stressors and protective factors: predictors of stress experienced during treatment for childhood cancer. Res Nurs Health. 1994;17(5):351–361. doi: 10.1002/nur.4770170506. [DOI] [PubMed] [Google Scholar]
- 60.Jörngården A, Mattsson E, von Essen L. Health-related quality of life, anxiety and depression among adolescents and young adults with cancer: A prospective longitudinal study. Eur J Cancer. 2007;43(13):1952–1958. doi: 10.1016/j.ejca.2007.05.031. [DOI] [PubMed] [Google Scholar]