Abstract
Arthropod-borne viruses (arboviruses) cause many diseases worldwide and their transmission is likely to change with land use and climate changes. La Crosse virus is historically transmitted by the native mosquito Aedes triseriatus (Say) in the upper Midwestern U.S., but the invasive congeners Aedes albopictus (Skuse) and Aedes japonicus (Theobald), which co-occur with A. triseriatus in water-holding containers, may be important accessory vectors in the Appalachian region where La Crosse encephalitis is an emerging disease. This review focuses on evidence for how climate, land use, and biological invasions may have direct abiotic and indirect community-level impacts on immature developmental stages (eggs and larvae) of Aedes mosquitoes. Because vector-borne diseases usually vary in space and time and are related to the ecology of the vector species, we propose that the ecology of its mosquito vectors, particularly at their immature stages, has played an important role in the emergence of La Crosse encephalitis in the Appalachian region and represents a model for investigating the effects of environmental changes on other vector-borne diseases. We summarize the health effects of La Crosse virus and associated socioeconomic costs that make it the most important native mosquito-borne disease in the U.S. We review of the transmission of La Crosse virus, and present evidence for the impacts of climate, land use, and biological invasions on Aedes mosquito communities. Last, we discuss important questions about the ecology of La Crosse virus mosquito vectors that may improve our understanding of the impacts of environmental changes on La Crosse virus and other arboviruses.
Introduction
In the past 60 years, many infectious diseases have emerged so that their epidemiology or symptoms are distinct from any disease seen previously (e.g., Ebola virus, severe acute respiratory syndrome, and Nipah virus) (Daszak et al., 2000). Many other infectious diseases were thought to be under control but are now re-emerging and causing morbidity or mortality at greater rates or in areas or populations not previously affected (Barrett et al., 1998; Weiss and McMichael, 2004; Wilcox and Colwell, 2005). Vector-borne diseases are a significant proportion of emerging and re-emerging infectious diseases (Jones et al., 2008). The most medically important vectors of disease are mosquitoes and among the most important pathogens they transmit are arboviruses (Weaver and Reisen, 2010).
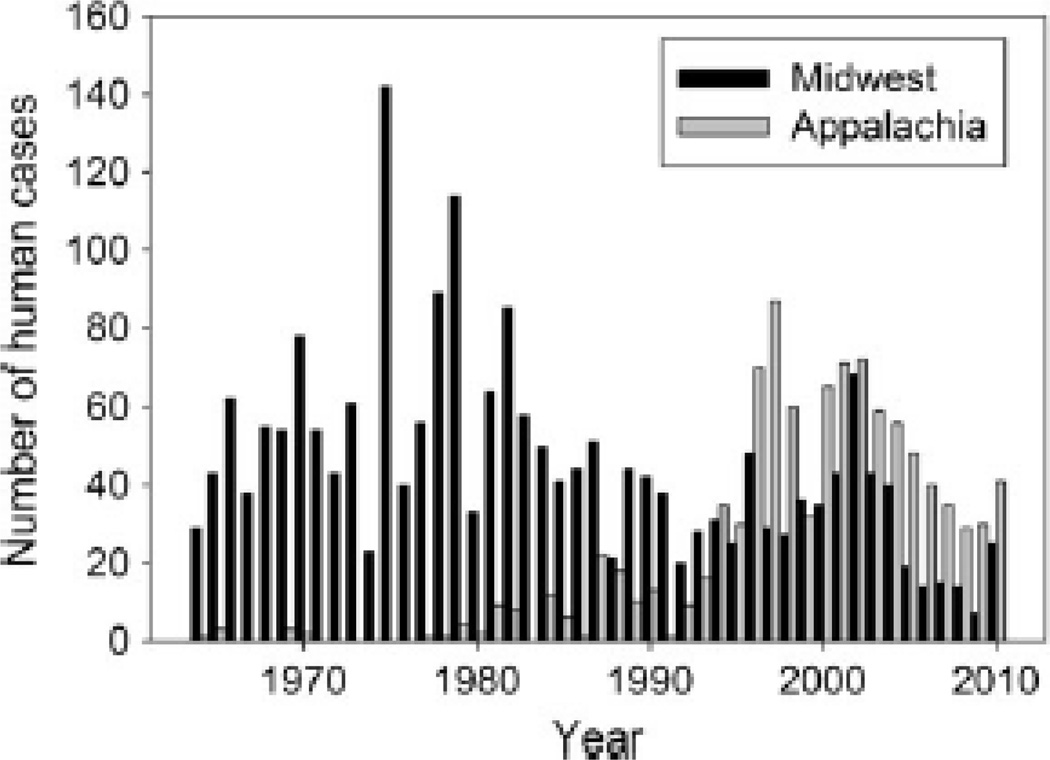
An important and emerging mosquito-borne disease is La Crosse virus (LACV) encephalitis. LACV encephalitis is the most common mosquito-borne disease native to North America (Calisher, 1994) and second most reported mosquito-borne disease behind introduced WNV encephalitis. LACV encephalitis is caused by LACV, which is a bunyavirus belonging to the Californian (CAL) serogroup of viruses (Family: Bunyaviridae, genus: Orthobunyavirus) (Tsai, 1991), and is historically transmitted by the native North American tree hole mosquito Aedes triseriatus (Say). LACV encephalitis has experienced a dramatic geographic shift in the last 20 years (Fig. 1). It was formerly almost entirely confined to sparsely populated areas in the upper Midwest with few cases in the Appalachian region (Kappus et al., 1982) but has emerged in the Appalachian region (Jones et al., 1999), which now yields the highest incidence risk in the nation among children 15 years and younger (Haddow and Odai, 2009). The emergence of LACV encephalitis in the Appalachian region has broadly coincided with the invasion and spread of the Asian Tiger mosquito, Aedes albopictus (Skuse), and the Asian bush mosquito, Aedes japonicus (Theobald) (=Ochlerotatus japonicus; Reinert, 2000), which co-occur with A. triseriatus as larvae in water-holding containers (e.g., Livdahl and Willey, 1991; Swanson et al., 2000; Szumlas et al., 1996; Barker et al., 2003a,b; Joy and Sullivan, 2005; Bevins, 2007a). Aedes albopictus and A. japonicus invaded the U.S. via used tires in the mid 1980s and 1990s, respectively. Both species have since spread throughout the Appalachian region, and A. japonicus is also present in the upper Midwest in apparently lower numbers (Darsie and Ward, 2005; Morris et al., 2007; Hughes et al., 2008). Aedes albopictus and A. japonicus are competent laboratory vectors of LACV (Grimstad et al., 1989; Cully et al., 1992; Sardelis et al., 2002). LACV has been isolated from field populations of both A. albopictus (Gerhardt et al., 2001; Haddow et al., 2009; Lambert et al., 2010; Westby et al., 2011) and A. japonicus (Westby et al., 2011). Thus, both invaders may affect the incidence of LACV in the Appalachian region by acting as important accessory vectors and by affecting the distribution and abundance of A. triseriatus by competing with the native species for resources in water-holding containers.
Fig. 1.
Number of cases with CAL serogroup viral disease in Midwestern (Ohio, Wisconsin, Minnesota, Illinois) and Appalachian (West Virginia, North Carolina, Tennessee) states of the United States (CDC 2011).
Despite this dramatic emergence in the Appalachian region and the invasions of A. albopictus and A. japonicus, LACV has received relatively little attention, even in reviews of emerging arboviruses (Jones et al., 2008; Weaver and Reisen, 2010). LACV cases are typically under-diagnosed (Calisher, 1994; Utz et al., 2003), which may contribute to the limited attention to this disease. However, we propose that the dramatic geographic shift of LACV towards the Appalachian region is unlikely to be due to differential rates of diagnosis between the Appalachian region and the upper Midwest and is more likely dependent on the environment. Aedes albopictus is the best-studied invasive mosquito (Juliano and Lounibos, 2005; Juliano, 2009). Despite this background, relatively little is known about how invasion of A. albopictus affects A. triseriatus, and how interactions among these species may alter LACV encephalitis risk. Even less is known about the recent invasion of A. japonicus and its interactions with A. triseriatus or A. albopictus (Juliano and Lounibos, 2005). We propose that management of LACV encephalitis requires an understanding of how climate change, land use change, and biotic invasions, affect the ecologies of A. triseriatus, A. albopictus, and A. japonicus. In this review, we define land use change as conversion of forest to peridomestic habitats including single rural homes, small towns, or larger suburbs, all of which increase numbers of artificial water-holding containers.
Incidence of arboviral diseases varies in space and time within a region (Reisen, 2010). For arboviral pathogens to persist and to spread, mosquito vectors must encounter vertebrate hosts and favorable environments so that spatiotemporal disease risk is directly related to the ecology of the vector (Andreadis et al., 2004; Yasuoka and Levins, 2007; Reisen, 2010). Thus, the ecologies of LACV vectors, particularly eggs and larvae, and their responses to environmental changes, may be the key processes driving the regional emergence of LACV encephalitis. In this review we postulate that human settlement facilitates invasions by A. albopictus and A. japonicus, increasing the risk of transmission of LACV encephalitis in the Appalachian region. Further, such land use changes combined with impending climate change will affect future transmission in the Appalachian region and other parts in the U.S. We also suggest that climate and land use changes may be altering ecological interactions among A. triseriatus, A. albopictus, and A. japonicus at the finer scale of individual water-holding containers that are development sites for larvae of these mosquitoes. Because of their tendency to bite humans, increased production of adult A. albopictus and A. japonicus with human settlement and climate change are likely to increase LACV risk. Regional coexistence of A. triseriatus, A. albopictus, and A. japonicus in a fragmented forest-peridomestic landscape and diverse local climates is especially likely to increase LACV transmission if they each contribute to simultaneous zoonotic and bridge transmission, or maintain LACV in a location throughout multiple seasons (Juliano and Lounibos, 2005; Juliano, 2009).
We begin with a review of the transmission cycle of LACV and describe recent changes in its geographic distribution. We summarize the health and socioeconomic effects of LACV encephalitis that make it an important mosquito-borne disease in the U.S. We then focus on the ecologies of A. triseriatus, A. albopictus, and A. japonicus, and how environmental changes may increase risk of LACV encephalitis by influencing the distributions and abundances of the egg and larval stages of these Aedes. Terrestrial adult mosquitoes are the direct cause of disease in humans, and ecological factors acting on adults (e.g., seasonal cold stress, desiccation, availability of hosts) have been an important traditional focus of investigations of ecology of mosquito-borne disease (e.g., Reisen, 2010). However, the ecology of mosquito host-vector interactions is only one component of mosquito population dynamics. Ecology of eggs and larvae is also critical for understanding and managing mosquito-borne disease and arguably may have a greater role than adult ecology in affecting the diseases transmitted by mosquitoes that develop in water-holding containers. Physical factors act directly on eggs and larvae to affect the production, distribution, and coexistence of adults (e.g., Hanson and Craig, 1995; Teng and Apperson, 2000), and we review evidence for differential responses of immature A. triseriatus, A. albopictus, and A. japonicus to these factors. Container-dwelling mosquito larvae are strongly affected by interspecific competition, predation, and parasitism, and by density dependent effects, which are largely absent at the adult stage. Such community-level effects can regulate the production and fitness of adults (Livdahl and Willey, 1991; Lounibos et al., 2003b). Thus, we focus also on how these biotic interactions regulate populations and coexistence of A. triseriatus, A. albopictus, and A. japonicus, and how climate and land use may modify these processes. We postulate that climate and land use play significant roles in LACV encephalitis risk by affecting the ecology of immature Aedes mosquitoes. Our goal is to outline a paradigm for investigating the ecology of these Aedes that will contribute to better management of LACV over different land uses and climates. Because all arthropod vectors are sensitive to environmental changes, we hope that ideas and approaches in our review will be relevant beyond understanding LACV, and will guide future efforts to understand how climate and land use changes affect mosquito ecology and public health.
Health impacts of LACV
LACV is an important cause of pediatric encephalitis in endemic regions of the U.S. LACV encephalitis is the second most common mosquito-borne encephalitis behind WNV encephalitis, with a total of 3,590 cases of confirmed and probable CAL serogroup viral illnesses from 1964 to 2009 (29–167 cases per year; CDC, 2011). Almost all of these CAL illnesses were LACV (Calisher, 1994; CDC, 2011). LACV cases usually exhibit symptoms of fever, headache, myalgia, malaise, and occasionally prostration (Calisher, 1994). It is likely that LACV infection is under-reported because LACV is often not specifically identified (Calisher, 1994). Mild LACV infections are often misdiagnosed as “flu” or “summer cold” (Utz et al., 2003) and severe LACV infections as herpes simplex encephalitis (Sokol et al., 2001). Cryptic infections may be as high as 300,000 per year in the U.S. (Rust et al., 1999).
Severe LACV cases can lead to encephalitis, with permanent neurologic sequelae or death in approximately 0.5% of cases (Rust et al., 1999). Although mortality is low compared to other arboviral diseases (Utz et al., 2003), a substantial socioeconomic burden is associated with LACV. For example, in North Carolina, direct and indirect costs of 25 cases totaled nearly $800,000 (mean ± SD: $32,974 ± $34,793 per case) over nearly 90 accumulated life years. Projected costs of lifelong neurologic sequelae are as high as $3,090,798 per patient. Lost workdays due to a LACV case are estimated to be an order of magnitude greater than those from dengue epidemics in Puerto Rico (Von Allmen et al., 1977; Torres, 1997).
Biology of LACV vectors
Aedes triseriatus is the natural vector and overwintering host of LACV. The virus has been repeatedly isolated from A. triseriatus in the field (e.g., Sudia et al, 1971; Watts et al., 1974; Szumlas et al., 1996; Nasci et al., 2000). Laboratory transmission experiments (Watts et al., 1972, 1973; Woodring et al., 1998), and the spatial and temporal association of A. triseriatus with zoonotic and human hosts with antibodies to LACV (e.g., Thompson and Evans, 1965; Wright and DeFoliart, 1970; Moulton and Thompson, 1971), all implicate A. triseriatus as the main vector. Aedes triseriatus circulates LACV naturally among eastern chipmunks (Tamias striatus), gray squirrels (Sciurus carolinensius), red foxes (Vulpes fulva), and possibly other small mammals (Calisher, 1994; Grimstad, 1988) in hardwood forests containing vector immature-stage (eggs and larvae) habitat of water-holding treeholes. Aedes triseriatus transovarially transmits LACV and the virus overwinters in diapausing A. triseriatus eggs (Watts et al., 1974).
Historically, most cases of LACV encephalitis have been reported from Wisconsin, Minnesota, Illinois, and Ohio (Fig. 1). However, in the past 20 years LACV has emerged in West Virginia, Tennessee, and North Carolina (Fig. 1). Encroachment of human dwellings into hardwood forests may have facilitated the emergence of LACV in the Appalachian region since A. triseriatus can also colonize artificial and natural containers in shaded peridomestic environments (Szumlas et al., 1996; Debboun et al., 2005; Kling et al., 2007; Yee, 2008). Complicating the epidemiology of LACV is the spread of the invasive congeners A. albopictus and A. japonicus into the Appalachian region. Aedes albopictus is a particularly aggressive day-time biter of small mammals and humans, making it an effective bridge vector of LACV (Estrada-Franco and Craig Jr., 1995). Aedes japonicus is also a daytime biter of a variety of hosts including humans (Andreadis et al., 2001; Goudarz et al., 2009). LACV has been isolated from A. albopictus eggs and adults (Gerhardt et al., 2001; Haddow et al., 2009; Lambert et al., 2010; Westby et al., 2011) and A. japonicus adults (Westby et al., 2011) in the field. For both species, LACV positive adults have been found at case sites of LACV encephalitis in eastern Tennessee (Haddow et al., 2009, Westby et al., 2011), implicating these species as vectors of human cases in the Appalachian region. Laboratory infection and oral transmission rates for A. albopictus may be equal to or greater than those for A. triseriatus (Grimstad et al., 1989; Cully et al., 1992), although disseminated infection (Hughes et al., 2006) and transoviral transmission (Tesh and Gubler, 1975) rates are lower.
Environmental effects on La Crosse vectors
Direct effects on immature stages
Aedes albopictus appears to require higher temperatures than A. triseriatus to complete larval development (Teng and Apperson, 2000). We may expect strong selection on adult A. albopictus to oviposit in areas that receive more radiant energy and thus be more likely to utilize containers in sunlit peridomestic areas warmed by built structures (McIntyre, 2000). Aedes albopictus and A. japonicus show a greater oviposition preference for sunlit peridomestic areas compared to A. triseriatus, which prefer to oviposit in forested areas (Barker et al., 2003 a,b; Joy and Sullivan, 2005). The role of microclimate in female oviposition choice and immature survival of these Aedes mosquitoes is still poorly understood.
Aedes triseriatus is widely regarded as extremely tolerant of a range of temperatures and is distributed from Florida to eastern Canada (Darsie and Ward, 2005). Since its arrival, A. japonicus has spread as far south as Georgia (Gray et al., 2005) and north into eastern Canada (Thielman and Hunter, 2006). Aedes albopictus has spread south into Florida and as far north as the latitude at which daily mean January temperatures reach −5°C in the eastern U.S., which was predicted by its northern distribution in its native range (Nawrocki and Hawley, 1987). The more limited distribution of A. albopictus to regions of warmer temperatures and higher humidity appears to be due to lower overwintering egg survivorship (Andreadis, 2009). Climate change is expected to increase mean temperatures and milder winters of across much of North America (IPCC, 2007), and thus may favor the northward spread of all Aedes mosquitoes and, more importantly, the movement of A. albopictus into areas already occupied by A. triseriatus and A. japonicus. Overwintering survival of Aedes eggs depends on temperature minimum and duration of exposure (Hawley et al., 1989; Hanson and Craig, 1994; Hanson and Craig, 1995). In laboratory experiments, the absolute minimum temperature that cold-acclimatized diapausing A. albopictus eggs can withstand can be as low as −12°C (Hanson and Craig, 1994), but that there has been survivorship of A. albopictus eggs in the field when briefly exposed to these temperatures (Hanson and Craig, 1995). It is likely that northern A. albopictus populations overwinter in areas where mean temperatures reach −5°C only if females oviposit in containers that are not subjected to prolonged extreme cold. Artificial containers in peridomestic areas (e.g., disused tires, trash receptacles) may be particularly well buffered against cold temperatures and increase overwintering success of A. albopictus. Thus, increasing conversion of forest into peridomestic areas may combine with climate change to promote the northward expansion of A. albopictus. Arboviral infections commonly affect overwintering Aedes mortality and the fitness of infected larvae. Mortality of overwintering LACV infected A. triseriatus eggs is greater than for uninfected eggs (McGaw et al. 1998). Species-specific overwintering mortality effects of LACV infection would affect LACV vectors but effects of LACV on overwintering A. albopictus or A. japonicus, vertical transmission of LACV in A. japonicus, and fitness of larvae infected with LACV have not been tested.
Aedes japonicus may be excluded from warm rock pool habitats, suggesting that a temperature barrier may inhibit A. japonicus populations from occupying southern areas of the U.S. with relatively high summer temperatures (Andreadis and Wolfe, 2010). Thus, climate change may also limit the spread southward of A. japonicus or even favor a retraction of this species’ southern range. However, as with A. albopictus, any regional climate changes are likely to interact strongly with climate variation among individual containers. Topographical diversity is also likely to contribute to local climate variability and affect both northern and southern distributions of Aedes mosquitoes in complex ways. Such regional and local scale variation in climate may be especially prominent in the Appalachian region, with its mountainous landscapes (Joy and Sullivan, 2005).
Community level effects on immature stages
Water-holding containers have low primary productivity (Fish and Carpenter, 1982; Carpenter, 1983). Almost all food resources are derived from allochthonous inputs of organic detritus and associated microorganisms (Carpenter, 1983; Merritt et al., 1992; Kitching, 2000; Kaufman et al., 2001). Thus, populations of Aedes in containers are often resource limited (Kitching, 2000, 2001) and competition for microbial food is likely the strongest ecological process structuring communities (Kitching 2001; Juliano and Lounibos, 2005). Some container habitats harbor predators and parasites that can regulate populations of Aedes mosquitoes. Prominent predators include the mosquito Toxorhynchites rutilus (Coquillet) and the midge Corethrella appendiculata (Graham) (Lounibos et al., 2001). Common parasites include the gregarine gut parasites Ascogregarina barretti (Vavra) and Ascogregarina taiwanensis (Lien and Levine) (Tseng, 2007), and obligate intracellular microsporidia (Andreadis, 2007). Pathogenic viruses have been isolated from A. triseriatus and A. albopictus (Becnel and White, 2007), but little is known about how they affect the ecology of either species.
Competition, predation, and parasitism can impact roles of A. albopictus and A. japonicus in disease transmission. When an invasive mosquito replaces a resident mosquito via competition, disease transmission changes if the invader is a more or less efficient vector (Juliano and Lounibos, 2005). Intensity of competition and resultant changes in adult body size or condition can affect the transmission of LACV (Grimstad and Haramis, 1984; Grimstad et al., 1989; Grimstad and Walker, 1991; Paulson and Hawley, 1991). When an invasive vector escapes enemies from its native range, distribution and abundance of that species may be enhanced. Likewise, native enemies may limit spread and impact of invasive mosquitoes (Juliano et al., 2010). These biotic interactions among larvae are well documented for A. triseriatus and A. albopictus, but not A. japonicus. Further, these effects likely will change with climate and land use.
Competition
Laboratory and field experiments consistently show A. albopictus to be superior in competition for food resources to A. triseriatus (Livdahl and Willey, 1991; Novak et al., 1993; Teng and Apperson, 2000; Aliabadi and Juliano, 2002; Yee et al., 2007; Juliano, 2009). Despite the competitive superiority of A. albopictus, there is little evidence for competitive exclusion of A. triseriatus (Juliano and Lounibos, 2005). Experiments on competition between A. albopictus and A. japonicus show clear competitive superiority for A. albopictus (Armistead and Lounibos, 2007). No studies have tested competition between A. japonicus and A. triseriatus but the spread of A. japonicus is associated with a decline of A. triseriatus and other native species in waste tire sites in Connecticut (allopatric to A. albopictus) suggesting competitive displacement (Andreadis and Wolfe, 2010). In the Appalachian region, Aedes japonicus are becoming the most abundant mosquito species in artificial containers where all three Aedes species coexist (Joy and Sullivan, 2005; Bevins, 2007a; Grim et al., 2007).
Superior overwintering survival and earlier hatching may enable A. triseriatus and A. japonicus to exploit vacant habitats early in the spring and to persist throughout the summer despite being competitively inferior to A. albopictus (Barker et al., 2003a,b; Swanson et al., 2000). The requirement for higher temperatures by A. albopictus to attain the same development rate as A. triseriatus, may also contribute to the dominance of A. triseriatus in northern, cooler regions of North America (Juliano and Lounibos, 2005). Alternative hypotheses for the regional persistence of A. triseriatus after the invasion of A. albopictus involve varying outcomes to interspecific competition across habitat gradients (Juliano, 2009). One form of competition among these Aedes, interference competition via direct physical or chemical negative effects, may relax the impacts of resource competition on A. triseriatus and promote its persistence. Aedes triseriatus and A. albopictus hatch when eggs are flooded and delay hatching, to varying degrees, in response to older larvae feeding in the water (Edgerly et al., 1993). Later-stage Aedes larvae are likely to be superior resource competitors and less susceptible to toxic effects of excretory products (e.g., ammonium) than younger Aedes larvae (Walker et el., 1991; Sunahara and Mogi, 2002). Aedes albopictus is both least sensitive to delayed hatching effects and more likely as a fourth-instar larva to produce this inhibitory effect than A. triseriatus (Edgerly et al., 1993). Delayed hatching of A. triseriatus may result in avoiding later stage A. albopictus and limited delayed-hatching response of A. albopictus may decrease A. triseriatus vulnerability to competition. However, as yet, no studies have considered how land use or climate changes may modify this asymmetrical interaction or interference competition involving A. japonicus.
Advantage in resource competition between A. triseriatus and A. albopictus appears to change with the nature of the container and resource, with A. albopictus having a strong advantage in discarded tires but coexistence expected in tree holes (Livdahl and Willey, 1991). Because the frequency of natural vs. artificial containers and the type and amount of detritus inputs likely change from forested to peridomestic areas, it is probable that land use change alters the competitive outcomes among A. triseriatus, A. albopictus, and perhaps A. japonicus, yielding another hypothesis for regional coexistence of these competitors. In the laboratory, the outcome of competition between A. triseriatus and A. albopictus appears to vary with the amount of total detritus and the ratio of leaf detritus to drowned insect detritus (Yee et al., 2007). The competitive advantage of A. albopictus is reduced with greater total detritus and greater proportions of insect detritus (Yee et al., 2007), suggesting that coexistence is more likely in habitats with greater total or animal detritus inputs. Different temporal patterns of detritus input also alter competitive interactions between A. triseriatus and A. albopictus, with multiple small, evenly-spaced inputs reducing competitive asymmetry compared to single large pulses (Bevins, 2007b). This result suggests that patterns of detritus input may also contribute to coexistence between competing Aedes species.
Forested areas are also less likely to be dominated by non-native vegetation than are urban areas (Thuiller et al., 2008). Different leaf species support different quantities (and possibly different species) of microorganisms (Walker et al., 1991; Yee and Juliano 2006), which in turn affects the quantity or quality of food for Aedes mosquitoes (Murrell and Juliano, 2008; Reiskind et al., 2009). Differential feeding of mosquito species on microorganism species or an overabundance of microorganisms available to both species alter or even reverse interspecific competitive advantage between A. triseriatus and A. albopictus, allowing their regional coexistence (Yee et al., 2007). To date no experiments have directly tested competitive outcomes among A. triseriatus, A. albopictus, and A. japonicus with detritus types chosen to represent different land use patterns. The nutritional quality of leaf detritus is highly dependent on the concentrations of nitrogen and carbon-based secondary compounds of the detritus (e.g., phenolics, tannins, and lignin) (Strand et al., 1999; Tuchman et al., 2003). Leaf species decomposition rate increases with nitrogen:carbon (N:C) ratio (Peterson and Cummins, 1974). High concentrations of carbon-based secondary metabolites are also likely to affect Aedes communities by being toxic to larvae, altering microbial food communities, and influencing the impacts of parasites (Sota, 1993; Mercer and Anderson, 1994).
Predation
Intraguild predation also may limit invasion of A. albopictus into containers with A. triseriatus. Fourth-instar larvae of A. triseriatus are more likely to prey upon newly hatched conspecifics and A. albopictus, and newly-hatched A. triseriatus are less vulnerable to such intraguild predation (Edgerly et al., 1999). Intraguild predation early in the season may be especially important for the persistence of A. triseriatus throughout the summer. However, no studies have considered how land use or climate change may modify intraguild predation or tested for intraguild predation involving A. japonicus. Top-down predation also may structure A. triseriatus and A. albopictus communities, and that predators are likely affected by land use and climate. Toxorhynchites rutilus and Corethrella appendiculata produce strong top-down effects in treeholes and other containers in forests, which are dominated by A. triseriatus and where A. albopictus is less common (Bradshaw and Holzapfel, 1983, 1985; Lounibos, 1983, 1985; Kesavaraju et al., 2008). Laboratory and field tests show that A. albopictus is more vulnerable to these predators (Kesavaraju and Juliano, 2004; Griswold and Lounibos, 2005; Juliano et al., 2010), in part because A. triseriatus shows reduced movement and foraging, which reduces predation risk, in response to water-borne cues from either T. rutilus or C. appendiculata predation, whereas A. albopictus show much more limited behavioral changes (Kesavaraju and Juliano, 2004, Kesavaraju et al., 2007). Few studies have examined the role of top-down predation in A. japonicus invasion, but as with A. triseriatus, A. japonicus shows greater reduction of movement and foraging in response to water-borne cues from T. rutilus than does A. albopictus (Kesavaraju et al., 2011). These predators are less common in open peridomestic areas and thus wooded areas appear to act as barriers to A. albopictus spread (Kesavaraju et al., 2008; Juliano et al., 2010). Thus, reduction in forested areas with increased urbanization would likely reduce predation and facilitate the spread of A. albopictus, while intact forest may act as a barrier to invasion of A. albopictus.
Parasitism
Parasitism by gregarines has been shown to affect the interaction of A. triseriatus and A. albopictus, and these parasites themselves are likely affected by climate. The gregarine parasites of A. triseriatus and A. albopictus [Ascogregarina barretti and Ascogregarina taiwanensis, respectively] cannot infect each other’s hosts, so species-specific effects of climate on the development and spread of each parasite would likely have an impact on mosquito assemblage composition. When A. albopictus invades a new area, gregarine parasitism is reduced for several years (Munstermann and Wesson, 1990; Blackmore et al., 1995; Aliabadi and Juliano, 2002). Aedes triseriatus in such newly-invaded areas are typically more heavily parasitized than A. albopictus, and this asymmetry in parasite load accentuates competitive advantage of A. albopictus (Aliabadi and Juliano, 2002). It is unclear how climate or land use change may impinge on gregarine parasites, but Van Rhein et al. (2000) showed that the drying of tree holes greatly reduced intensity and frequency of parasitism by A. barretti, suggesting that climate shifts toward aridity may reduce parasitism and increase Aedes populations. To our knowledge, no research has investigated community effects of parasites of A. japonicus.
Geographic variation
Predicting the impacts of climate and land use on LACV vectors may be complicated by geographic variation in ecological processes, which can result from varying abiotic or biotic environments favoring one species over the other, or from inherent geographic differentiation of local populations. Differences among populations of A. albopictus and A. triseriatus have been documented for numerous life-history traits. However, except for differences in diapause incidence (Shroyer and Craig, 1983; Lounibos et al., 2003a) and susceptibility to and transmission of LACV (Grimstad et al., 1977), no interregional differences can be attributed to differences in latitudinal climate, LACV distribution, predator abundances, or competitor abundances (Juliano, 1996; Frankino and Juliano, 1999; Armbruster and Conn, 2006; Leisnham et al., 2009). These findings suggest that highly local factors may select for life history differentiation, or that local variation results from strong founder effects, genetic drift, or other non-selective processes. Geographic variation in susceptibility to and transmission of LACV presents an interesting pattern of lower susceptibility and transmission among A. triseriatus from LACV-endemic areas of the upper Midwest (Grimstad et al., 1977), suggesting that LACV selects for resistance among its vectors (Grimstad et al., 1977). This study included only one A. triseriatus population from the Appalachian region (Cashiers, NC), which showed high susceptibility and transmission, as was typical of populations from non-endemic regions (Grimstad et al., 1977). This result suggests that: first, LACV was in fact rare or absent in the Appalachian region at the time of Grimstad et al.’s study, and that subsequent emergence of LACV in this region represents a real change in LACV distribution and abundance; second, susceptibility and transmission by newly invasive vectors A. albopictus and A. japonicus in a LACV-endemic region may be similarly high, making new invaders particularly important as LACV vectors.
Future Research and Conclusions
For a better understanding of LACV encephalitis we require investigations of the: i) impacts of biotic interactions (competition, predation, parasitism, vector-host interactions) involving A. triseriatus, A. albopictus, and A. japonicus in the field in both the Midwestern and Appalachian regions; ii) potential effects of climate change, land use change, and their interaction, on those biotic interactions; and iii) means to predict the temporal and spatial consequences of these biotic interactions on the distributions, abundances, animal and human host contacts of these vectors, and their vector competence. Knowing the composition of Aedes communities, including species co-occurrence patterns and abundances, across different land uses will be valuable. Such surveys must determine if containers along forest-to-peridomestic gradients have different leaf litter inputs, hydroperiods, and temperatures that may modify the impacts of climate change on mosquito communities. Experiments under controlled laboratory conditions can be useful for testing potential effects of particular factors (e.g., leaf detritus, hydroperiod, and temperature) on interspecific competition, predation and parasitism. Other experiments may test the effects of desiccation and temperature on survival of Aedes eggs and their effects on population growth rates. Field manipulations of climate or land use variables across latitude will be necessary to determine the actual importance of these factors. Models of the effects of microclimate on the population parameters of Aedes of under different climate scenarios will be necessary to integrate interactive effects of climate and land use on mosquito performance across the dynamic landscapes in both the Midwestern and Appalachian regions. Re-examination of susceptibility to and transmission of LACV by A. triseriatus populations from the Midwestern and Appalachian regions, and currently non-endemic areas (e.g., Florida), would be extremely interesting as a way of testing predictions of the hypothesis that LACV selects for resistance among A. triseriatus. Similar comparative studies of susceptibility to and transmission of LACV by invasive vectors A. albopictus and A. japonicus from these regions would also be informative for understanding the consequences of invasions of novel vectors into LACV endemic areas.
Contributor Information
Paul Leisnham, University of Maryland, Department of Environmental Science & Technology, 1443 Animal Sciences (Bldg 142), College Park, Maryland 20742-2315, 301.405.8569 TEL 301.314.9023 FAX, http://www.enst.umd.edu/People/Leisnham/index.cfm.
Steven A. Juliano, BEES section, School of Biological Sciences, Illinois State Univesity, Normal, IL 61790-4120, ph. (309) 438-2642 FAX (309) 438-3722, http://bio.illinoisstate.edu/sajulian/
References
- Aliabadi BW, Juliano SA. Escape from gregarine parasites affects the competitive interactions of an invasive mosquito. Biological Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Yanoviak SP, Lounibos LP, Drake BG. Effects of elevated atmospheric CO2 on water chemistry and mosquito (Diptera: Culicidae) growth under competitive conditions in container habitats. Florida Entomologist. 2005;88:372–382. doi: 10.1653/0015-4040(2005)88[372:EOEACO]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Diseases. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- Andreadis TG. Microsporidian parasites of mosquitoes. In: Floore TG, editor. Biorational Control of Mosquitoes. Vol. 23. American Mosquito Control Association, Supplement to the Journal of the Mosquito Control Association; 2007. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- Andreadis TG. Failure of Aedes albopictus to overwinter following introduction and seasonal establishment at a tire recycling plant in the northeastern USA. Journal of the American Mosquito Control Association. 2009;25:25–31. doi: 10.2987/08-5813.1. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Wolfe RJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the northeastern United States. Journal of Medical Entomology. 2010;47:43–52. doi: 10.1603/033.047.0106. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. Journal of Medical Entomology. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Armbruster P, Conn JE. Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae) Annals of the Entomological Society of America. 2006;99:1234–1243. [Google Scholar]
- Barker CM, Brewster CC, Paulson SL. Spatiotemporal oviposition and habitat preferences of Aedes triseriatus and Aedes albopictus in an emerging focus of La Crosse virus. Journal of the American Mosquito Control Association. 2003a;19:382–391. [PubMed] [Google Scholar]
- Barker CM, Paulson SL, Cantrell S, Davis BS. Habitat preferences and phenology of Aedes triseriatus and Aedes albopictus (Diptera: Culicidae) in southwestern Virginia. Journal of Medical Entomology. 2003b;40:403–410. doi: 10.1603/0022-2585-40.4.403. [DOI] [PubMed] [Google Scholar]
- Barrett R, Kuzawa CW, McDade T, Armelagos GJ. Emerging and re-emerging infectious diseases: The third epidemiologic transition. Annual Review of Anthropology. 1998;27:247–271. [Google Scholar]
- Becnel JJ, White SE. Mosquito pathogenic viruses: The last 20 years. In: Floore TG, editor. Biorational Control of Mosquitoes. Vol. 23. American Mosquito Control Association, Supplement to the Journal of the Mosquito Control Association; 2007. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Establishment and abundance of a recently introduced mosquito species Ochlerotatus japonicus (Diptera : Culicidae) in the Southern Appalachians, USA. Journal of Medical Entomology. 2007a;44:945–952. doi: 10.1603/0022-2585(2007)44[945:eaaoar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Timing of resource input and larval competition between invasive and native container-inhabiting mosquitoes (Diptera: Culicidae) Journal Vector Ecology. 2007b;32:252–262. doi: 10.3376/1081-1710(2007)32[252:torial]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Blackmore MS, Scoles GA, Craig GB. Parasitism of Aedes aegypti and Ae. albopictus (Diptera: Culicidae) by Ascogregarina spp. (Apicomplexa: Lecudinidae) in Florida. Journal of Medical Entomology. 1995;32:847–852. doi: 10.1093/jmedent/32.6.847. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CH. Predator-mediated, non-equilibrium coexistence of tree-hole mosquitoes in southeastern North America. Oecologia. 1983;57:239–256. doi: 10.1007/BF00379586. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CH. The distribution and abundance of treehole mosquitoes in North America: Perspectives from north Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop Florida Medical Entomology Laboratory; Vero Beach, FL. 1985. pp. 3–23. [Google Scholar]
- Calisher CH. Medically important arboviruses of the United States and Canada. Clinical Microbiology Reviews. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- CDC. Arboviral encephalitis cases reported in humans, by type, United States, 1964–2010. Centers for Disease Control and Prevention; 2011. [accessed 1 June, 2011]. Available: http://www.cdc.gov/ncidod/dvbid/arbor/arbocase.htm. [Google Scholar]
- Cully JF, Street TG, Heard PB. Transmission of La Crosse virus by four strains of Aedes albopictus to and from the eastern chipmunk (Tamias striatus) Journal of American Mosquito Control Association. 1992;8:237–240. [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Wildlife ecology - Emerging infectious diseases of wildlife - Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Debboun M, Green TJ, Rueda LM, Hall RD. Relative abundance of tree hole-breeding mosquitoes in Boone County, Missouri, USA, with emphasis on the vector potential of Aedes triseriatus for canine heartworm, Dirofilaria immitis (Spirurida: Filariidae) Journal of American Mosquito Control Association. 2005;21:274–278. doi: 10.2987/8756-971X(2005)21[274:RAOTHM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Edgerly JS, Willey MS, Livdahl TP. The community ecology of Aedes egg hatching: Implications for a mosquito invasion. Ecological Entomology. 1993;18:123–128. [Google Scholar]
- Edgerly JS, Willey MS, Livdahl TP. Intraguild predation among larval treehole mosquitoes, Aedes albopictus, Ae. aegypti, Ae. triseriatus (Diptera: Culicidae), in laboratory microcosms. Journal of Medical Entomology. 1999;36:394–399. doi: 10.1093/jmedent/36.3.394. [DOI] [PubMed] [Google Scholar]
- Estrada-Franco JG, Craig GB., Jr . Pan American Health Organization, Technical Publication 42. Washington, DC: PAHO; 1995. Biology, Disease Relationships, and Control of Aedes albopictus. [Google Scholar]
- Fish D, Carpenter SR. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology. 1982;63:283–288. [Google Scholar]
- Frankino WA, Juliano SA. Costs of reproduction and geographic variation in the reproductive tactics of the mosquito Aedes triseriatus. Oecologia. 1999;120:59–68. doi: 10.1007/s004420050833. [DOI] [PubMed] [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, et al. The first isolation of La Crosse virus from naturally occurring infected Aedes albopictus. Emerging Infectious Diseases. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EW, Harrison BA, Womack ML, Kerce J, Neely CJ, et al. Ochlerotatus japonicus japonicus (Theobald) in Georgia and North Carolina. Journal of the American Mosquito Control Association. 2005;21:144–146. doi: 10.2987/8756-971X(2005)21[144:OJJTIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Grim DC, Jackson BT, Paulson SL. Abundance and bionomics of Ochlerotatus J. japonicus in two counties in southwestern Virginia. Journal of the American Mosquito Control Association. 2007;23:259–263. doi: 10.2987/8756-971X(2007)23[259:AABOOJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Grimstad PR. California group virus disease. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology, Vol. II. Boca Raton, FL: CRC Press; 1988. pp. 99–136. [Google Scholar]
- Grimstad PR, Craig GB, Jr, Ross QE, Yuill TM. Aedes triseriatus and La Crosse virus: geographic variation in vector susceptibility and ability to transmit. American Journal of Tropical Medicine and Hygiene. 1977;26:990–996. doi: 10.4269/ajtmh.1977.26.990. [DOI] [PubMed] [Google Scholar]
- Grimstad PR, Haramis LD. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. III. Enhances oral transmission by nutrition-deprived mosquitoes. Journal of Medical Entomology. 1984;21:249–256. doi: 10.1093/jmedent/21.3.249. [DOI] [PubMed] [Google Scholar]
- Grimstad PR, Kobayashi JF, Zhang M, Craig GB., Jr Recently introduced Aedes albopictus in the United States: Potential vector of La Crosse virus (Bunyaviridae: California serogroup) Journal of American Mosquito Control Association. 1989;5:422–427. [PubMed] [Google Scholar]
- Grimstad PR, Walker ED. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. Journal of Medical Entomology. 1991;28:378–386. doi: 10.1093/jmedent/28.3.378. [DOI] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Competitive outcomes of aquatic container Diptera depends on predation and resource levels. Annals of the Entomological Society of America. 2005a;98:673–681. doi: 10.1603/0013-8746(2005)098[0673:COOACD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Moulton JK, Gerhardt RR, McCuiston LJ, Jones CJ. Description of the egg of Ochlerotatus japonicus japonicus (Diptera: Culicidae) using variable pressure scanning electron microscopy. Journal of Medical Entomology. 2009;46:9–14. doi: 10.1603/033.046.0102. [DOI] [PubMed] [Google Scholar]
- Haddow AD, Odai A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003–2007. PLoS ONE. 2009;4:e6145. doi: 10.1371/journal.pone.0006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Craig GB., Jr Relationship between cold hardiness and supercooling point in Aedes albopictus eggs. Journal of the American Mosquito Control Association. 1994;11:35–38. [PubMed] [Google Scholar]
- Hanson SM, Craig GB., Jr Aedes albopictus (Diptera: Culicidae) eggs: Field survivorship during northern Indiana winters. Journal of Medical Entomology. 1995;32:599–604. doi: 10.1093/jmedent/32.5.599. [DOI] [PubMed] [Google Scholar]
- Hawley WA, Pumpuni CB, Brady RH, Craig GB., Jr Overwintering survival of Aedes albopictus (Diptera, Culicidae) eggs in Indiana. Journal of Medical Entomology. 1989;26:122–129. doi: 10.1093/jmedent/26.2.122. [DOI] [PubMed] [Google Scholar]
- Hughes MT, Gonzalez JA, Reagan KL, Blair CD, Beaty BJ. Comparative potential of Aedes triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae) to transovarially transmit La Crosse virus. Journal of Medical Entomology. 2006;43:757–761. doi: 10.1603/0022-2585(2006)43[757:cpoata]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hughes TH, Irwin PM, Kaufman A, Sage H, Pagac BB, Jr, Paskewitz SM. First records of Aedes japonicus japonicus in Wisconsin. Journal of the American Mosquito Control Association. 2008;24:583–584. doi: 10.2987/5735.1. [DOI] [PubMed] [Google Scholar]
- IPCC. Climate Change 2007: Synthesis Report. IPCC’s Fourth Assessment Report. [accessed July 17, 2011];Intergovernmental Panel on Climate Change. 2007 Available at: http://www.ipcc.ch/publications_and_data/publications_and_data.htm.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TF, Craig AS, Nasci RS, Patterson LER, Erwin PC, Gerhardt RR, et al. Newly recognized focus of La Crosse encephalitis in Tennessee. Clinical Infectious Diseases. 1999;28:93–97. doi: 10.1086/515087. [DOI] [PubMed] [Google Scholar]
- Joy JE, Sullivan SN. Occurrence of tire inhabiting mosquito larvae in different geographic regions of West Virginia. Journal of the American Mosquito Control Association. 2005;21:380–386. doi: 10.2987/8756-971X(2006)21[380:OOTIML]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Geographic variation in Aedes triseriatus (Diptera: Culicidae): Temperature-dependent effects of a predator on survival of larvae. Environmental Entomology. 1996;25:624–631. [Google Scholar]
- Juliano SA. Species interactions among mosquitoes: Context dependence across habitat gradients. Annual Review of Entomology. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecology Letters. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, Nishimura N, Greene K. Your worst enemy could be your best friend: Predator contributions to invasion resistance and persistence of natives. Oecologia. 2010;162:709–718. doi: 10.1007/s00442-009-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappus KD, Calisher CH, Baron RC, Davenport J, Francy DB, et al. La Crosse virus infection and disease in western North Carolina. American Journal of Tropical Medicine and Hygiene. 1982;31:556–560. doi: 10.4269/ajtmh.1982.31.556. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Bland SN, Worthen ME, Walker ED, Klug MJ. Bacterial and fungal biomass responses to feeding by larval Aedes triseriatus (Diptera: Culicidae) Journal of Medical Entomology. 2001;38:711–719. doi: 10.1603/0022-2585-38.5.711. [DOI] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container dwelling mosquitoes. Annals of the Entomological Society of America. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Behavioral responses of Aedes albopictus to a predator are correlated with size-dependent risk of predation. Annals of the Entomological Society of America. 2008;101:1150–1153. doi: 10.1603/0013-8746-101.6.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Damal K, Juliano SA. Do natural container habitats impede invader dominance? Predator-mediated coexistence of invasive and native container-dwelling mosquitoes. Oecologia. 2008;155:631–639. doi: 10.1007/s00442-007-0935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Alto BW, Lounibos LP, Juliano SA. Behavioural responses of larval container mosquitoes to a size-selective predator. Ecological Entomology. 2007;32:262–272. doi: 10.1111/j.1365-2311.2006.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Khan DF, Gaugler R. Behavioral differences of invasive container-dwelling mosquitoes to a native predator. Journal of Medical Entomology. 2011;48:526–532. doi: 10.1603/me10200. [DOI] [PubMed] [Google Scholar]
- Kitching RL. Food Webs and Container Habitats: The Natural History and Ecology of Phytotelmata. New York: Cambridge University Press; 2000. [Google Scholar]
- Kitching RL. Food webs in phytotelmata: “Bottomup” and “top-down” explanations for community structure. Annual Review of Entomology. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Kling LJ, Juliano SA, Yee DA. Larval mosquito communities in discarded vehicle tires in a forested and unforested site: Detritus type, amount, and water nutrient differences. Journal of Vector Ecology. 2007;32:207–217. doi: 10.3376/1081-1710(2007)32[207:lmcidv]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Blair CD, D'Anton M, Ewing W, Harborth M, Seiferth R, et al. La Crosse Virus in Aedes albopictus Mosquitoes, Texas, USA, 2009. Emerging Infectious Diseases. 2010;16:856–858. doi: 10.3201/eid1605.100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P, Towler L, Juliano SA. Geographic variation of photoperiodic diapause but not adult survival or reproduction of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in North America. Annals of the Entomological Society of America. 2011;104:1309–1318. doi: 10.1603/AN11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Lounibos LP, O'Meara GF, Juliano SA. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology. 2009;90:2405–2413. doi: 10.1890/08-1569.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Sala LM, Juliano SA. Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. Journal of Medical Entomology. 2008;45:210–221. doi: 10.1603/0022-2585(2008)45[210:gviasa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenco-de-Oliveria R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Annals of the Entomological Society of America. 2003a;96:512–518. [Google Scholar]
- Lounibos LP, O’Meara GF, Nishimura N, Escher RL. Interactions with native mosquito larvae regulate the production of Aedes albopictus from bromeliads in Florida. Ecological Entomology. 2003b;28:551–558. [Google Scholar]
- Lounibos LP, O'Meara GF, Escher RL, Nishimura N, Cutwa M, et al. Testing predictions of displacement of native Aedes by the invasive Asian Tiger Mosquito Aedes albopictus in Florida, USA. Biological Invasions. 2001;3:151–166. [Google Scholar]
- McGaw MM, Chandler LJ, Wasieloski LP, Blair CD, Beaty BJ. Effect of La Crosse virus infection on overwintering of Aedes triseriatus. American Journal of Tropical Medicine and Hygiene. 1998;58:168–175. doi: 10.4269/ajtmh.1998.58.168. [DOI] [PubMed] [Google Scholar]
- McIntyre NE. Ecology of urban arthropods: A review and a call to action. Annals of the Entomological Society of America. 2000;93:825–835. [Google Scholar]
- Mercer DR, Anderson JR. Tannins in treehole habitats and their effects on Aedes sierrensis (Diptera: Culicidae) production and parasitism by Lambornella clarki (Ciliophera: Tetrahymenidae) Journal of Medical Entomology. 1994;31:159–167. doi: 10.1093/jmedent/31.1.159. [DOI] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding-behavior, natural food, and nutritional relationships of larval mosquitoes. Annual Review of Entomology. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Morris JA, Lampman RL, Ballmes G, Funes J, Halvorsen J, Novak RJ. First record of Aedes japonicus japonicus in Illinois: defining its spatial distribution and associated mosquito species. Journal of the American Mosquito Control Association. 2007;23:243–251. doi: 10.2987/8756-971X(2007)23[243:FROAJJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Munstermann LE, Wesson DM. First record of Ascogregarina taiwanensis (Apiomplexa: Lecudinidae) in North American Aedes albopictus. Journal of the American Mosquito Control Association. 1990;6:235–243. [PubMed] [Google Scholar]
- Nasci RS, Moore CG, Biggerstaff BJ, Panella NA, Liu HQ, et al. La Crosse encephalitis virus habitat associations in Nicholas County, West Virginia. Journal of Medical Entomology. 2000;37:559–570. doi: 10.1603/0022-2585-37.4.559. [DOI] [PubMed] [Google Scholar]
- Nawrocki SJ, Hawley WA. Estimation of the northern limits of distribution of Aedes albopictus in North America. Journal of the American Mosquito Control Association. 1987;3:314–317. [PubMed] [Google Scholar]
- Novak MG, Higley LG, Christianssen CA, Rowley W. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera: Culicidae) through replacement series experiments. Environmental Entomology. 1993;22:311–318. [Google Scholar]
- Peterson RG, Cummins KW. Leaf processing in a woodland stream. Freshwater Biology. 1974;4:343–368. [Google Scholar]
- Reinert JF. New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. Journal of the American Mosquito Control Association. 2000;16:175–188. [PubMed] [Google Scholar]
- Reisen WK. Landscape Epidemiology of Vector-Borne Diseases. Annual Review of Entomology. 2010;55:461–483. doi: 10.1146/annurev-ento-112408-085419. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Greene KL, Lounibos LP. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecological Entomology. 2009;34:447–456. doi: 10.1111/j.1365-2311.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust RS, Thompson WH, Matthews CG, Beaty BJ, Chun RWM. La Crosse and other forms of California encephalitis. Journal of Child Neurology. 1999;14:1–14. doi: 10.1177/088307389901400101. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre RG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae) Journal of Medical Entomology. 2002;39:635–639. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- Shroyer DA, Craig GB., Jr Egg diapause in Aedes triseriatus (Diptera, Culicidae): geographic variation in photoperiodic response and factors influencing diapause termination. Journal of Medical Entomology. 1983;20:601–607. doi: 10.1093/jmedent/20.6.601. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Kleiman MB, Garg BP. La Crosse viral encephalitis mimics herpes simplex viral encephalitis. Pediatric Neurology. 2001;25:413–415. doi: 10.1016/s0887-8994(01)00337-x. [DOI] [PubMed] [Google Scholar]
- Sota T. Performance of Aedes albopictus and A. riversi larvae (Diptera: Culicidae) in waters that contain tannic acid and decaying leaves: is the treehole species better adapted to treehole water? Annals of Entomological Society of America. 1993;86:450–457. [Google Scholar]
- Strand M, Herms DA, Ayres MP, Kubiske ME, Kaufman MG, Walker ED, et al. Effects of atmospheric CO2, light availability and tree species on the quality of leaf detritus as a resource for treehole mosquitoes. Oikos. 1999;84:277–283. [Google Scholar]
- Sudia WD, Newhouse VF, Calisher CH, Chamberlain RW. California group arboviruses: Isolation from mosquitoes in North America. Mosquito News. 1971;31:576–600. [Google Scholar]
- Sunahara T, Mogi M. Priority effects of bamboo-stump mosquito larvae: influences of water exchange and leaf litter input. Ecological Entomology. 2002;27:346–354. [Google Scholar]
- Swanson J, Lancaster M, Anderson J, Crandell M, Haramis L, Grimstad P. Overwintering and establishment of Aedes albopictus (Diptera: Culicidae) in an urban La Crosse virus enzootic site in Illinois. Journal of Medical Entomology. 2000;37:454–460. doi: 10.1093/jmedent/37.3.454. [DOI] [PubMed] [Google Scholar]
- Szumlas DE, Apperson CS, Powell EF, Hartig P, Francy DB, Karabotsos N. Relative abundance and species composition of mosquito populations (Diptera: Culicidae) in a La Crosse virus-endemic area in western North Carolina. Journal of Medical Entomology. 1996;33:598–607. doi: 10.1093/jmedent/33.4.598. [DOI] [PubMed] [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: Effects of density, food, and competition on response to temperature. Journal of Medical Entomology. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Gubler DJ. Laboratory studies of transovarial transmission of La Crosse and other arboviruses by Aedes albopictus and Culex fatigans. American Journal of Tropical Medicine and Hygiene. 1975;24:876–880. doi: 10.4269/ajtmh.1975.24.876. [DOI] [PubMed] [Google Scholar]
- Thuiller W, Albert C, Araujo MB, Berry PM, Cabeza M, Guisan A, Hickler T, Midgely GF, Paterson J, Schurr FM, Sykes MT, Zimmermann NE. Predicting global change impacts on plant species' distributions: Future challenges. Perspectives in Plant Ecology Evolution and Systematics. 2008;9:137–152. [Google Scholar]
- Tseng M. Ascogregarine parasites as possible biocontrol agents of mosquitoes. In: Floore TG, editor. Biorational Control of Mosquitoes. Vol. 23. American Mosquito Control Association, Supplement to the Journal of the Mosquito Control Association; 2007. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- Thompson WH, Evans AS. California encephalitis virus studies in Wisconsin. American Journal of Epidemiology. 1965;81:230–244. doi: 10.1093/oxfordjournals.aje.a120511. [DOI] [PubMed] [Google Scholar]
- Torres MI. Impact of an outbreak of dengue fever: a case study from rural Puerto Rico. Human Organization. 1997;56:19–27. [Google Scholar]
- Tsai TF. Arboviral infections in the United States. Infectious Disease Clinics of North America. 1991;5:73–102. [PubMed] [Google Scholar]
- Travis JT. The significance of geographical variation in species interactions. American Naturalist. 1996;148:51–58. [Google Scholar]
- Tuchman NC, Wahtera KA, Wetzel RG, Russo NM, Kilbane GM, Sasso LM, et al. Nutritional quality of leaf detritus altered by elevated atmospheric CO2: effects on development of mosquito larvae. Freshwater Biology. 2003;48:1432–1439. [Google Scholar]
- Utz JT, Apperson CS, MacCormack JN, Salyers M, Dietz EJ, McPherson JT. Economic and social impacts of La Crosse encephalitis in western North Carolina. American Journal of Tropical Medicine and Hygiene. 2003;69:509–518. [PubMed] [Google Scholar]
- Van Rhein SL, Flanary BE, Juliano SA. Effects of habitat type and drying on Ascogregarina barretti (Eugregarinida: Lecudinidae) infection in Aedes triseritatus (Diptera: Culicidae) Journal of Medical Entomology. 2000;37:950–956. doi: 10.1603/0022-2585-37.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Allmen SD, Lopez-Correa RH, Woodall JP, Morens DM, Chiriboga J, Caste-Velez A. Epidemic dengue fever in Puerto Rico, 1977: A cost analysis. American Journal of Tropical Medicine and Hygiene. 1977;59:265–271. doi: 10.4269/ajtmh.1979.28.1040. [DOI] [PubMed] [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]
- Watts DM, Morris CD, Wright RE, DeFoliart GR, Hanson RP. Transmission of La Crosse virus (California encephalitis group) by the mosquito, Aedes triseriatus. Journal of Medical Entomology. 1972;9:125–127. doi: 10.1093/jmedent/9.2.125. [DOI] [PubMed] [Google Scholar]
- Watts DM, Pantuwatana S, DeFoliart GR, Yuill TM, Thompson WH. Transovarial transmission of La Crosse virus (California encephalitis group) in the mosquito Aedes triseriatus. Science. 1973;182:1140–1143. doi: 10.1126/science.182.4117.1140. [DOI] [PubMed] [Google Scholar]
- Watts DM, Thompson WH, Yuill TM, DeFoliart GR, Hanson RP. Overwintering of La Crosse virus in Aedes triseriatus. American Journal of Tropical Medicine and Hygiene. 1974;23:694–700. doi: 10.4269/ajtmh.1974.23.694. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Research. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nature Medicine. 2004;10:S70–S76. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby K, Fritzen C, Huang J, Jaske E, Paulsen D, Jones C, Moncayo AC. La Crosse encephalitis in Eastern Tennessee: Evidence of invasive mosquito (Aedes albopictus and Ochlerotatus japonicus) involvement in the transmission of an indigenous disease. American Journal of Tropical Medicine and Hygiene. 2011;85(supplement):374. [Google Scholar]
- Wilcox BA, Colwell RR. Emerging and reemerging infectious diseases: Biocomplexity as an interdisciplinary paradigm. Ecohealth. 2005;2:244–257. [Google Scholar]
- Woodring J, Chandler LJ, Oray CT, McGaw MM, Blair CD, Beaty BJ. Short Report: Diapause, transovarial transmission, and filial infection rates in geographic strains of La Crosse virus-infected Aedes triseriatus. American Journal of Tropical Medicine and Hygiene. 1998;58:587–588. doi: 10.4269/ajtmh.1998.58.587. [DOI] [PubMed] [Google Scholar]
- Wright RE, DeFoliart GR. Associations of Wisconsin mosquitoes and woodland vertebrate hosts. Annals of the Entomological Society of America. 1970;63:777–786. doi: 10.1093/aesa/63.3.777. [DOI] [PubMed] [Google Scholar]
- Yee DA. Tires as habitats for mosquitoes: A review of studies within the eastern United States. Journal of Medical Entomology. 2008;45:581–593. doi: 10.1603/0022-2585(2008)45[581:tahfma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms and performance of the dominant consumer. Freshwater Biology. 2006;51:448–459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kaufman MG, Juliano SA. The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. Journal of Animal Ecology. 2007;76:1105–1115. doi: 10.1111/j.1365-2656.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka J, Levins R. Impacts of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Journal of Tropical Medicine and Hygiene. 2007;76:450–460. [PubMed] [Google Scholar]