Abstract
In Drosophila melanogaster, mutations in the gene drop-dead (drd) result in early adult lethality, with flies dying within 2 weeks of eclosion. Additional phenotypes include neurodegeneration, tracheal defects, starvation, reduced body mass, and female sterility. The cause of early lethality and the function of the drd protein remain unknown. In the current study, the temporal profiles of drd expression required for adult survival and body mass regulation were investigated. Knockdown of drd expression by UAS-RNAi transgenes and rescue of drd expression on a drd mutant background by a UAS-drd transgene were controlled with the Heat Shock Protein 70 (Hsp70)-Gal4 driver. Flies were heat-shocked at different stages of their lifecycle, and the survival and body mass of the resulting adult flies were assayed. Surprisingly, the adult lethal phenotype did not depend upon drd expression in the adult. Rather, expression of drd during the second half of metamorphosis was both necessary and sufficient to prevent rapid adult mortality. In contrast, the attainment of normal adult body mass required a different temporal pattern of drd expression. In this case, manipulation of drd expression solely during larval development or metamorphosis had no effect on body mass, while knockdown or rescue of drd expression during all of pre-adult (embryonic, larval, and pupal) development did significantly alter body mass. Together, these results indicate that the adult-lethal gene drd is required only during development. Furthermore, the mutant phenotypes of body mass and lifespan are separable phenotypes arising from an absence of drd expression at different developmental stages.
Keywords: Drosophila, lifespan, body mass, metamorphosis, drop-dead
1. Introduction
Over the past several decades, Drosophila has been a popular model organism for the study of neurodegeneration (Lessing and Bonini, 2009). While some such studies involve genes that were identified through their association with human disease (Lu and Vogel, 2009), many neurodegeneration-linked genes were originally discovered in the fly through forward genetic screens for adult lethality, retinal defects, or neurodegeneration itself (Cook et al., 2011; Knust, 2007; Kretzschmar, 2009; Min and Benzer, 1997). The very first adult lethal mutation connected to neurodegeneration, reported by Benzer and coworkers, was in the gene drd (Benzer, 1971). Flies that carry the strong alleles drd1 or drdlwf have a median survival of four days and display widespread degeneration of the brain at the time of death (Benzer, 1971; Blumenthal, 2008; Buchanan and Benzer, 1993). Despite the early discovery of drd, the function of this gene remains unknown.
Flies with mutations in drd manifest diverse and seemingly unrelated phenotypes, many of which may contribute to their short lifespan. Newly eclosed drd mutants display abnormal glial morphology that has been postulated to lead to the neurodegeneration that accompanies death (Buchanan and Benzer, 1993). Moreover, the tracheae in the brain appear fragile, and it is hypothesized that tracheal breakdown leads to hypoxia and subsequent neurodegeneration (Kim et al., 2012; Lehmann and Cierotzki, 2010; Tschäpe et al., 2003). Also, these flies exhibit a gut phenotype; the movement of ingested food from the crop into the midgut is impaired (Peller et al., 2009). The decreased ability to digest food results in depletion of triglyceride and glycogen stores, suggesting that the flies may also be starving to death. In addition to these phenotypes, drd mutants have a reduced body mass on the day of eclosion and are female sterile (Blumenthal, 2008; Buchanan and Benzer, 1993).
The drd gene encodes a member of the NRF (nose resistant to fluoxetine)-domain protein family, a group of putative integral membrane proteins with limited homology to prokaryotic acyltransferases (Blumenthal, 2008; Choy and Thomas, 1999). The drd protein is localized to an intracellular membrane-bound compartment (Kim et al., 2012). To date, no biochemical function has been reported for DRD, the other 16 NRF-domain proteins encoded by the Drosophila genome, or any other eukaryotic NRF-domain protein.
The two phenotypes under investigation in this work are adult lifespan and body mass. These phenotypes share a dependence on signaling by insulin-like peptides (ilps) and by ecdysone and related steroid hormones (ecdysteroids). In holometabolous insects such as Drosophila, adult body mass is determined by body mass at the time of pupariation; this in turn is regulated by the timing of pupariation and the growth rate of the larva up until that point (Edgar, 2006; Mirth and Riddiford, 2007; Riddiford, 2011; Shingleton, 2005). Larvae commit to pupariation upon reaching a “critical mass”; the growth rate up until this point determines the overall length of larval development, while the growth rate after this point affects the mass upon pupariation and the resulting adult mass. Pupariation itself is triggered by a pulse of ecdysteroid production by the prothoracic gland, and mutants that fail to produce this pulse do not pupariate (Garen et al., 1977). The overall growth rate of larval tissues is positively regulated by ilps in response to nutrient availability (Britton et al., 2002), and larvae with defects in ilp signaling become very small adults due to their lack of growth post-critical mass (Clancy et al., 2001; Tatar et al., 2001). In addition, both positive and negative interactions exist between the ilp and ecdysteroid signaling pathways, resulting in a complex regulatory network controlling larval growth and development (Caldwell et al., 2005; Colombani et al., 2005; Mirth et al., 2005). In the adult, ilp signaling is a major determinant of lifespan; mutants in the ilp signaling pathway are long-lived (Clancy et al., 2001; Giannakou and Partridge, 2007; Tatar et al., 2001). Modest reductions in ecdysteroid receptor expression also increase lifespan in male flies while conflicting results have been reported in females (Schwedes and Carney, 2012; Simon et al., 2003; Tricoire et al., 2009). As in larvae, ilp and ecdysteroid signaling in the adult are highly interconnected (Francis et al., 2010; Tu et al., 2002).
The dual effects of ilp signaling mutations on body mass and lifespan result from a lack of ilp signaling at different stages of the insect lifecycle. Nutritional deprivation of late third-instar larvae, which inhibits ilp signaling and results in low body mass, has no effect on adult lifespan (Tu and Tatar, 2003). Conversely, inhibition of ilp signaling specifically in the adult fat body results in an extension of lifespan with no change in body mass (Giannakou et al., 2004; Hwangbo et al., 2004).
In this study, we ascertained the temporal requirement for drd expression in determining rapid adult lethality and body mass. We find that drd expression during mid to late metamorphosis is both necessary and sufficient for survival beyond the first month of adult life. Adult body mass requires a different period of drd expression that spans the larval-pupal transition and potentially encompasses all of pre-adult development. Together, these results demonstrate that drd is an essential developmental gene that separately modulates adult lifespan and body mass.
2. Materials and Methods
Drosophila Stocks
All fly stocks were maintained on standard cornmeal-yeast-agar food (http://flystocks.bio.indiana.edu/Fly_Work/media-recipes/molassesfood.htm) at 25°C on a 12h:12h light:dark cycle unless otherwise noted. UAS-RNAi stocks (w1118; P{GD3367}v37404 (FBst0461992) and w1118; P{GD15915}v51184 (FBst0469325)) were obtained from the Vienna Drosophila RNAi Center. Other stocks (y1 w*; P{Act5C-GAL4}17bFO1/TM6B, Tb1 (FBst0003954), w*; P{GAL4-Hsp70. PB}2 (FBst0002077), and w1118; P{UAS-Dcr-2. D}2 (FBst0024650)) were obtained from the Bloomington Drosophila Stock Center. The genes and alleles referenced in this work include drd (FBgn0260006), drdlwf (FBal0193421), and rp49 (FBgn0002626). Stocks were not outcrossed prior to this study.
For RNAi experiments, the UAS-Dcr-2 transgene was recombined onto the two UAS-RNAi chromosomes by a standard crossing scheme. Recombinants were identified by eye color.
Generation of UAS-drd transgenic flies
The entire coding sequence of drd was amplified from the cDNA clone RE74651 (Drosophila Genomics Resource Center, Bloomington, IN) by PCR with PfuUltra II Fusion HS DNA polymerase (Agilent Technologies, Santa Clara, CA) and the primers drd CDS 5′ BglII 5′ GTC AGA TCT ATG TCG CGT ATG TCG CAT ATG 3′ and drd CDS 3′ Xbal 5′ GTC TCT AGA CCA GAC TAA TCC GAG TGC GG 3′, which included BglII and XbaI sites, respectively. The PCR product was purified (QIAquick, Qiagen, Valencia, CA), and digested with XbaI and BglII (New England Biolabs, Ipswich, MA). The digested insert was ligated into XbaI/BglII-digested pUAST vector (Quick Ligation kit, NEB) and transformed into competent DH5alpha bacteria (NEB). A single clone was isolated and miniprepped (Wizard Plus SV, Promega, Madison, WI) and its integrity confirmed by sequencing the entire drd coding sequence. Maxi-prep DNA was prepared (QIAfilter Plasmid Maxi kit, Qiagen) and injected commercially to create transgenic lines (Genetic Services Inc., Cambridge, MA).
The resulting transgenic lines were crossed onto a drdlwfba ckground to screen for “leaky” expression of the UAS-drd transgene. Lines that did not display a reduced lifespan were discarded (four out of 11 autosomal insertions). The remaining lines were crossed with y w; Act5C-Gal4/TM6B to drive ubiquitous overexpression of the UAS-drd transgene. Real-time PCR was performed on 3-day old flies and the line that displayed the greatest fold increase in drd expression relative to sibling controls was used for all experiments.
Synthesis of cDNA
RNA was isolated from whole flies or pupae using Trizol reagent (Life Technologies, Grand Island, NY). One μg total RNA was treated with either RQ1 RNase-free DNase (Promega) for 30 minutes at 37°C or DNase I, amplification grade (Life Technologies) for 20 minutes at room temperature and then cDNA synthesis was performed with qScript cDNA supermix (Quanta Biosciences, Gaithersburg, MD). To determine the genotype of individual pupae, PCR was performed (GoTaq Hot Start Polymerase, Promega) for the presence of Gal4. Primers used: Gal4 F: 5′ GGC TAG AAA GAC TGG AAC AGC T 3′ and Gal4 R: 5′ AGG GCA AGC CAT CCG ACA TG 3′.
Quantitative Real Time PCR
Real-time PCR was performed on cDNA using the MyiQ thermocycler (Bio-Rad, Hercules, CA) and iQ SYBR Green supermix (Quanta Biosciences). Each sample was run in triplicate. A melt curve was performed directly after amplification to verify the authenticity of the PCR products. drd transcript levels, relative to the housekeeping gene rp49, were calculated in MyiQ software (Bio-Rad) using a dilution series of whole fly cDNA that was included in each PCR run. For the pupal heat shock experiments, these relative expression levels were then further normalized to the average expression at either the beginning of the heat pulse or 24 hours after the end of the heat pulse, depending on the experiment. Primers used: rp49F: 5′ AAG ATC GTG AAG AAG CGC ACC AA 3′, rp49 R: 5′ CTG TTG TCG ATA CCC TTG GGC TT 3′, drdL: 5′ GCT CTG AAG ATG CAC GAC TC 3′, and drdR: 5′ CCT CGA ATT GTG TGG CAA AA 3′.
Lifespan Assays
Flies were collected on the day of eclosion, transferred to fresh vials every 2–7 days, and scored daily for survival for 40 days, except for the experiment shown in figure 1b, which was continued until all flies died. A minimum of 50 flies per genotype were used for each survival curve.
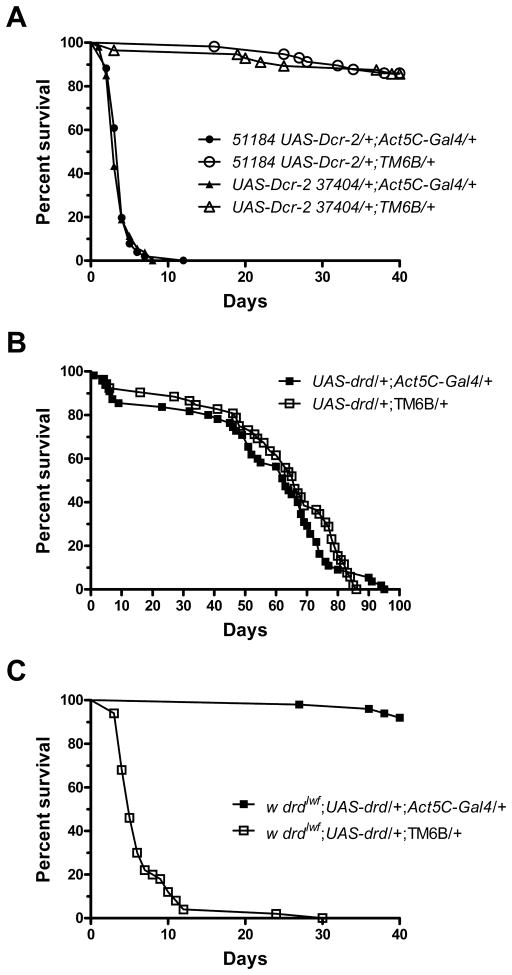
Figure 1.
Establishment of a system for knockdown and expression of drd. a) Ubiquitous knockdown of drd by crossing y w; Act5C-Gal4/TM6B males with either w; {GD15915}v51184 UAS-Dcr-2 or w; UAS-Dcr-2 {GD3367}v37404 females recapitulates the early lethality phenotype. b) Overexpression of drd on a wild-type background by crossing y w; Act5C-Gal4/TM6B males with w; UAS-drd females has no effect on lifespan. c) Ubiquitous expression of drd on a drdlwf background by crossing y w; Act5C-Gal4/TM6B males with drdlwf/FM7a; UAS-drd females rescues the early lethality phenotype. n=50–57 flies/genotype.
Body Mass Assays
The mass of the flies was performed as previously described (Blumenthal, 2008). Flies were collected in the early afternoon of the day of eclosion, killed by placing on dry ice for 3 minutes, and weighed in groups containing 2–13 flies. The average fly mass for each group was determined by dividing the total mass by the number of flies. The legend of figure 5 indicates the number of groups of flies weighed for each experimental condition and the total number of flies contained in those groups. Large, newly eclosed flies were excluded from analysis. We do not expect the measured differences in body mass to be biased by changes in mass that occur during the day of eclosion, as we have not observed any effect of drd on the timing of eclosion (E.M.B., unpublished observations).
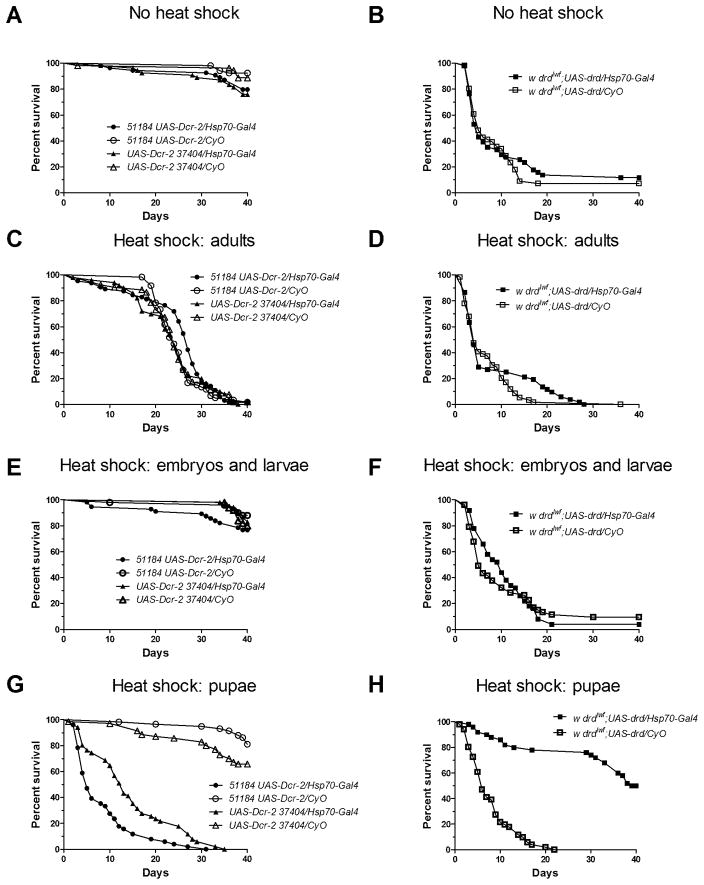
Figure 5.
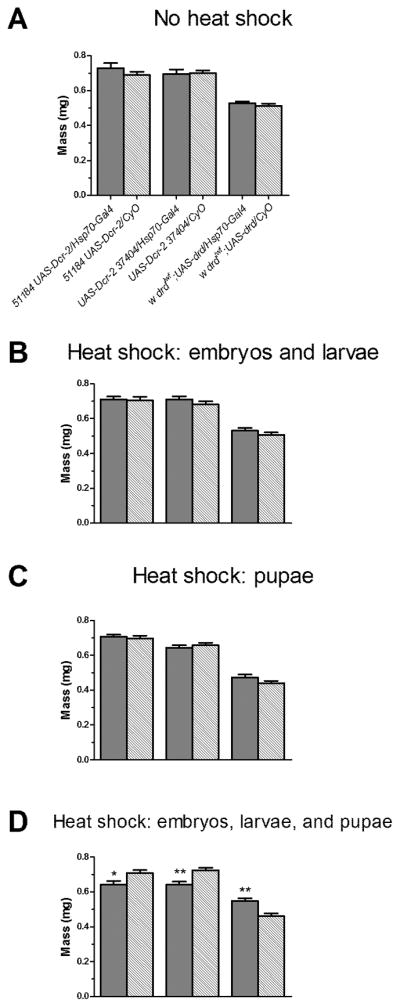
Temporal requirement of drd expression for normal body mass on the day of eclosion. Crosses and heat shock were performed as in fig. 2. The legend shown in (a) applies to all four graphs. a) No heat shock. b) Heat shock from embryogenesis until wandering third larval instar. c) Heat shock throughout metamorphosis. d) Heat shock for all of pre-adult development from embryogenesis until eclosion. Asterices indicate significant effect of rescue or knockdown by 1-way ANOVA and Bonferroni post-test. *:p<0.05; **:p<0.01. n=50–55 flies weighed in 10–23 groups per condition. Error bars represent S.E.M.
Statistics and Data Analysis
Data were graphed and analyzed using GraphPad Prism v5 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com). For survival curves (fig 1–3), pair-wise comparisons of each experimental group with its sibling control were carried out using a Mantel-Haenszel test. For body mass data (fig 5), each dataset was first tested for normality using a D’Agostino-Pearson normality test, and all sets were found to be Gaussian. The data for each experiment (based on the timing of the heat shock) was then analyzed by 1-way ANOVA followed by a Bonferroni Multiple Comparison Test to compare each experimental group with its sibling control. For real-time data (fig 4), a 1-way ANOVA followed by a Bonferroni Multiple Comparison Test was used to compare each time point either to the 0 hr value (start of heat shock) or the 24 hr value (recovery from heat shock).
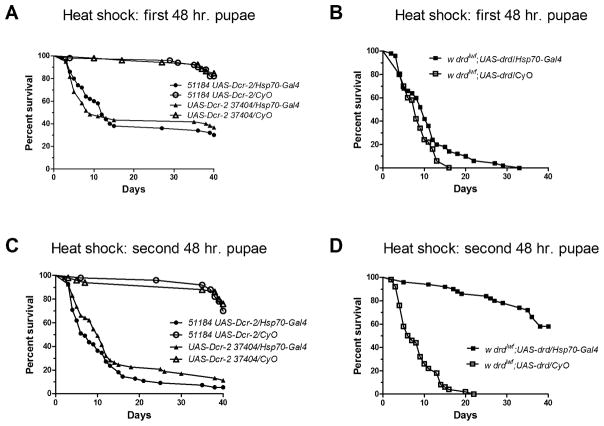
Figure 3.
Requirement for drd expression within metamorphosis. Survival curves of adult male progeny of crosses as detailed in fig. 2 following knockdown (a, c) or rescue (b, d) of drd during the first 48 hours of metamorphosis (a, b) or the final 48 hours of metamorphosis (c, d). n=50–60 flies/genotype.
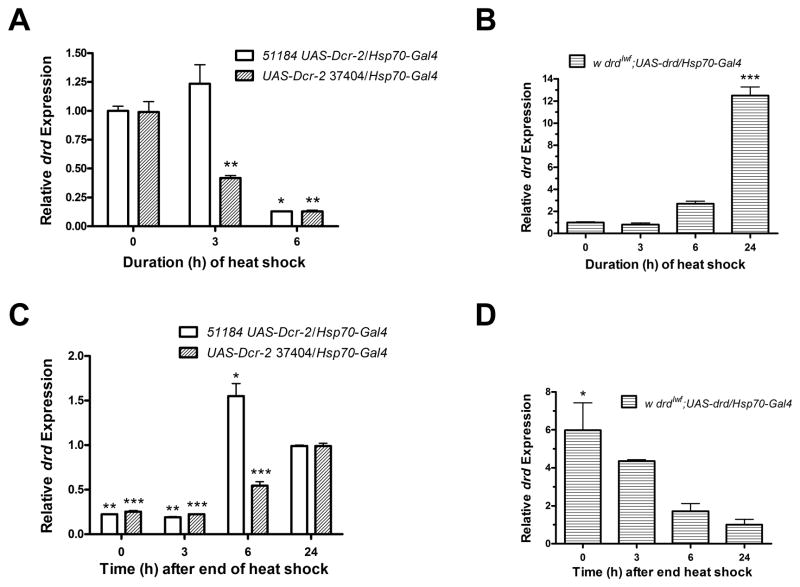
Figure 4.
Time course of drd knockdown and rescue during and following heat shock in 2-day pupae. Pupae were derived from crosses as detailed in fig. 2. a) knockdown of drd and b) rescue of drd after different durations at 30°C. Transcript levels were normalized to the level at 0 hours. c, d) Recovery of drd transcript from knockdown (c) and rescue (d) at different times after the end of a 48-hour heat shock. Transcript levels were normalized to the level at 24 hours of recovery. Asterices indicate significant difference from 0 hours (a, b) and 24 hours (c, d) by 1-way ANOVA and Bonferroni post-test. *:p<0.05; **:p<0.01; ***:p<0.001. n=2. Error bars represent S.E.M.
3. Results
We first established a system for targeted knockdown and expression of drd. The UAS-RNAi lines w1118; {GD15915}v51184 and w1118; {GD3367}v37404 (referred to as 51184 and 37404, respectively) were obtained from the Vienna Drosophila RNAi center (Dietzl et al., 2007). The hairpin from 51184 is targeted to the first exon of drd, while the hairpin from 37404 is targeted against the sixth exon; neither is predicted to have any off-target effects (http://stockcenter.vdrc.at). Both UAS-RNAi lines were recombined with a UAS-Dicer-2 line to ensure efficient cleavage of the dsRNA. All experiments using the UAS-RNAi lines also included the UAS-Dicer-2 transgene. To test our ability to knock down drd, the UAS-RNAi lines were crossed with the ubiquitous Actin5C-Gal4 driver. Male progeny from both crosses recapitulated the early lethality phenotype, with flies from the 51184 cross surviving 4 days and flies from the 37404 cross surviving 3 days (Fig 1a). Sibling controls that lacked the Gal4 driver survived the duration of the experiment (40 days, p<0.0001 for pairwise comparisons of each knockdown population with its sibling control). Quantitative real-time PCR of 3 day-old males displayed 8.8±0.8 fold and 7.9±2.0 fold knockdown of transcript for progeny of the 51184 and 37404 crosses, respectively, relative to sibling controls (n=3, mean±SD). For targeted expression of drd, we created a line carrying an inducible UAS-drd transgene (see methods). Flies carrying both the UAS-drd transgene and the Act5C-Gal4 driver displayed a 32.7±1.3 fold upregulation of drd transcript relative to sibling controls (n=3). This level of overexpression did not affect lifespan (Fig 1b, p=0.59). To test the ability of UAS-drd to rescue lifespan, the UAS-drd transgene was placed on a drdlwf background and crossed with the Act5C-Gal4 line. The progeny ubiquitously expressing drd survived for the entire experiment and sibling controls had a median survival of 5 days (Fig 1c, p<0.0001). This result indicated that we were able to rescue the early lethality phenotype and that the UAS-drd transgene is only expressed under Gal4 control.
To temporally control the expression and knockdown of drd, we crossed each UAS-RNAi line and the UAS-drd line with flies carrying the Heat Shock Promoter 70 (Hsp70)-Gal4; all experiments below were conducted on the progeny of these crosses. We first examined progeny at 24°C to ensure that the driver did not cause expression at a non-inducing temperature. Survival was wild-type for both of the UAS-RNAi lines (Fig 2a, p=0.06 and 0.08 for 51184 and 37404, respectively) and the early lethality phenotype was observed in drdlwfrescue flies (Fig 2b, p=0.60). For the remainder of the experiments, we heat-shocked flies at 30°C for different stages of life to determine the temporal requirement of drd expression. This temperature is optimal for inducing and maintaining expression of a gene, while minimizing the adverse effects of increased temperature on lifespan (Chavous et al., 2001).
Figure 2.
Temporal requirement of drd expression for survival. Survival curves of adult male progeny from crossing w; Hsp70-GAL4 males with either w; {GD15915}v51184 UAS-Dcr-2 or w; UAS-Dcr-2 {GD3367}v37404 females for drd knockdown (a, c, e, g) and w; Hsp70-GAL4 males with drdlwf/FM7a; UAS-drd females for drd rescue (b, d, f, h). a, b) flies were maintained at 24°C for the entire life cycle. c, d) flies were maintained at 30°C after eclosion. e, f) flies were maintained at 30°C from embryogenesis until wandering third larval instar. g, h) flies were maintained at 30°C during metamorphosis. n=50–70 flies/genotype.
To test the necessity of drd expression in adults, flies were maintained at 24°C until eclosion and then moved to 30°C for the duration of the experiment. As shown in Figure 2c, there was no significant difference between drd knockdown flies and sibling controls (p=0.06 and 0.70). Flies of all genotypes had a median survival between 24 and 27 days. We attribute the relatively short lifespan observed in this experiment to the increased temperature (Hollingsworth, 1968). Similarly, the complementary rescue experiment did not extend lifespan of the drd mutants, with both the experimental and control lines surviving a median of 5 days (Fig 2d, p=0.23). These results indicate that drd expression in the adult fly does not affect the early lethality phenotype.
We next tested the requirement for drd expression during the embryonic and larval stages by crossing flies at 30°C and maintaining the progeny at this temperature until the wandering third larval instar. Under these conditions, knockdown of drd did not have an effect on lifespan; flies, experimental and control, survived the duration of the experiment for both UAS-RNAi lines (Fig 2e, p=0.11 and 0.67). Similarly, induction of drd expression during these developmental stages did not significantly rescue early adult lethality in drdlwf mutants (Fig 2f, p=0.77).
In contrast to the previous experiments, expression of drd during metamorphosis did have an effect on lifespan. Knockdown of drd from the white prepupal stage until eclosion resulted in a median survival of 5 and 13 days for 51184 and 37404, respectively (Fig 2g). Sibling controls survived the duration of the experiment (p<0.0001 for each RNAi line). Similarly, induction of drd during this developmental stage resulted in complete rescue of the early lethality phenotype (Fig 2h, p<0.0001).
To narrow down the requirement for drd expression within the four days of metamorphosis, white prepupae were collected, immediately moved to 30°C for 48 hours, and then returned to 24°C. We observed partial effects when manipulating drd expression during the first half of metamorphosis. Knockdown of drd during this period resulted in a significant reduction in median lifespan to 12 and 9 days for 51184 and 37404, respectively (Fig 3a, p<0.0001 for each RNAi line). However, 30–40% of the experimental flies survived for the duration of the 40-day experiment. In addition, we observed a significant effect on survival (p=0.03) of rescuing drd expression during the first two days of metamorphosis, but an inspection of the survival curves indicates that this rescue was marginal (Fig 3b). To examine the second half of metamorphosis, white prepupae were collected and left at 24°C for 48 hours and moved to 30°C until eclosion, after which they were returned to 24°C. Knockdown of drd during this stage resulted in a median survival of 7 and 9 days for 51184 and 37404, respectively (Fig 3c, p<0.0001 for each RNAi line). Moreover, driving drd expression during the last two days of metamorphosis resulted in a complete rescue of early adult lethality (Fig 3d, p<0.0001).
Quantitative real-time PCR was performed to determine the time course of drd knockdown and induction and subsequent recovery following the beginning and end of a 30°C heat shock starting on day 2 of metamorphosis. For both UAS-RNAi lines, there was a significant reduction in transcript abundance after 6 hours of heat shock. Moreover, 37404 showed significant knockdown of transcript by 3 hours (Fig 4a). The UAS-drd line required 6 to 24 hours to induce an increase in the transcript level (Fig 4b). For the UAS-RNAi line 51184, drd transcript levels recovered within 6 hours after removal from a 48-hour heat shock and in fact showed a slight overshoot. Transcript levels in the 37404 UAS-RNAi line returned to normal between 6 and 24 hours after the end of heat shock (Fig 4c). Recovery in the UAS-drd rescue line also appeared to take 6–24 hours, although there was no statistically significant change in transcript abundance from 3 hours after the end of the heat shock (Fig 4d).
On the day of eclosion, drd mutants have a significantly lower body mass than sibling controls (Blumenthal, 2008). We set out to determine when drd must be expressed during development to cause the reduced body mass phenotype. The same experimental strategy utilized for lifespan determination was employed for body mass assays. Heat shock was performed at different stages of development and then flies were weighed on the day of eclosion.
As expected, flies that did not undergo heat shock and were maintained at 24°C did not differ in body mass from their control siblings (Fig 5a). Since body mass is primarily determined during the larval stages, we next manipulated drd expression from embryogenesis until third instar wandering larvae. Knocking down and rescuing drd expression during this developmental time did not have a significant effect on body mass (Fig 5b). Moreover, there was no significant difference in body mass when drd was knocked down or rescued during metamorphosis (Fig 5c). However, when flies were heat-shocked throughout all of pre-adult development, we observed a significant difference in body mass. Knocking down drd throughout all of development resulted in a 10% decrease in body mass relative to sibling controls. Additionally, expressing drd throughout all of pre-adult development in a drdlwf background resulted in flies that were 19% heavier than sibling controls (Fig 5d).
4. Discussion
The defining phenotype of drd mutants is an extremely short adult lifespan, and we have demonstrated that this phenotype results from an absence of drd expression during pupal metamorphosis. We can both rescue early adult lethality in drd mutants by temporarily expressing drd and recapitulate the adult-lethal phenotype in wild-type flies by temporarily knocking down drd expression. Therefore, we conclude that pupal expression of drd is both necessary and sufficient to ensure adult survival during the first weeks after eclosion. Consistent with this interpretation, manipulation of drd expression during embryogenesis, larval development, or adulthood had no effect on rapid adult lethality. In addition, ubiquitous overexpression of drd on a wild-type background had no apparent detrimental consequences.
Within the four days of metamorphosis, expression of drd is required during the later stages. Complete rescue/recapitulation of the adult lethal phenotype was achieved by heat-shocking pupae beginning 48 hours after prepupa formation and ending at eclosion. We can therefore rule out a requirement for drd expression during the first half of metamorphosis. It remains possible, however, that drd is required early in the first day of adult life because in this experiment drd transcript levels remained suppressed/elevated for several hours after eclosion and the end of the heat pulse. In the complementary experiment, heat-shocking pupae for the first 48 hours of metamorphosis had little effect on rescue of adult lethality but resulted in approximately half of the RNAi flies dying within two weeks of eclosion. There is some overlap in the developmental stages probed by these two experiments, as metamorphosis is accelerated at higher temperatures (Powsner, 1935). Taking our data as a whole, we conclude that for survival during early adulthood, drd expression is required beginning in the middle of the third day of metamorphosis, and this critical period may extend into the first several hours following eclosion. Defining this critical period further will require not only shorter heat pulses but also knowledge of drd protein dynamics following the induction of expression or knockdown.
Our model for the timing of drd expression is consistent with recent immunostaining data, in which high levels of drd protein are reported in several pupal tissues (Kim et al., 2012). Our results also agree to some extent with the modENCODE temporal expression data, which show a modest 3–4 fold increase in drd expression on the third day of metamorphosis relative to the previous two days (Graveley et al., 2011; McQuilton et al., 2012). However, the modENCODE data also indicate that the highest level of drd expression occurs during the final third of embryogenesis, when expression levels are up to 7-fold higher than during metamorphosis. While our results are not consistent with a role for embryonic expression of drd in the determination of adult lifespan, it is possible that this early expression peak is required for other aspects of Drosophila development or physiology. No larval phenotypes have been reported for drd mutants, but to our knowledge this topic has not been thoroughly investigated.
One drd phenotype that could arise during metamorphosis is a defect in glial morphology. On the day of eclosion, glial cells in drd mutants appear morphologically immature and fail to wrap around the neurons (Buchanan and Benzer, 1993). The process of glial cell maturation and neuronal wrapping occurs in late pupae; eighty hours after pupal formation, glia in drd mutants are morphologically wild-type. It has been hypothesized that drd expression is required for glial maturation during late pupal development and that the failure of glial maturation is related to the neurodegeneration that occurs several days after eclosion (Buchanan and Benzer, 1993).
Some of the phenotypes seen in adult drd mutant flies are hypothesized to be associated with defects in epithelial integrity or function. The tracheae and air sacs in mutant flies are reported to be fragile, and drd mutants express genes associated with hypoxia (Kim et al., 2012; Lehmann and Cierotzki, 2010; Tschäpe et al., 2003). Mutant flies also exhibit a specific defect in the movement of food from the crop through the cardia and into the midgut (Peller et al., 2009). It is noteworthy that both the foregut and the tracheae are lined with cuticle (Nation, 2002), and the cardia synthesizes another chitinous structure, the peritrophic matrix (PM) (Hegedus et al., 2009). The expression of multiple genes encoding cuticular or PM components, such as Peritrophin A, Crystallin, resilin, Acp65Aa, and 12 Cpr genes, peak on day 3 of metamorphosis (Graveley et al., 2011) —the same day on which drd expression is required for adult survival. The causal relationships among neurodegeneration, hypoxia associated with fragile tracheae, and starvation associated with defective food movement remain to be elucidated, as do the relative roles of these phenotypes in causing rapid adult death. However, all three of these phenotypes are potentially connected with developmental events that occur during the second half of metamorphosis.
The other phenotype examined in this study was adult body mass, which is significantly reduced in drd mutants (Blumenthal, 2008). In contrast to the effect on adult survival, knockdown/rescue of drd expression during metamorphosis had no effect on body mass. This result indicates that the reduced body mass observed in drd mutants is an independent phenotype, separate from adult lethality. In this respect, mutation of drd is similar to mutations in ilp signaling, which, as reviewed above, have separable effects on body mass and lifespan. However, the control of lifespan by ilp signaling is an adult-specific effect, in contrast to the actions of drd during metamorphosis. Furthermore, knockdown/rescue of drd expression from embryogenesis through the wandering third larval instar, which encompasses the entirety of larval feeding, failed to affect body mass. Thus, the drd body mass phenotype appears to be distinct from the nutritionally regulated ilp-dependent control of larval growth rate discussed above. We were able to rescue/recapitulate the body mass phenotype by heat-shocking flies continuously from embryogenesis until eclosion. This effect was significant but did not fully eliminate the difference in body mass between the different genotypes; for example, even after heat shock, the experimental rescue flies still had a lower mass than the control RNAi flies. We attribute this residual mass difference to differences in the genetic backgrounds of the stocks used in this experiment. We conclude from these results that the critical period for the body mass effects of drd either extends throughout pre-adult development or encompasses the transition between the end of larval development and the beginning of metamorphosis. It is interesting to note that both insulin receptor activity and the ilp dILP6 are reported to have effects on adult body mass after the cessation of feeding, during the wandering third instar (Okamoto et al., 2009; Shingleton et al., 2005; Slaidina et al., 2009). Thus, our data are consistent with an interaction between drd and ilp signaling late in larval development. We also cannot rule out a role for drd in ecdysteroid production or signaling that would affect the timing of pupariation.
The primary finding of this work is that the early adult lethality observed in drd mutants arises from an absence of drd expression at an earlier stage of the life cycle. To our knowledge, this is without precedent among neurodegeneration mutants. While many neurodegeneration-associated genes are expressed both during development and in adults, this expression has either been demonstrated or is believed to be required for the maintenance of larval, pupal, or adult neuronal integrity (Khodosh et al., 2006; Lee et al., 2011; Lim and Kraut, 2009; Mühlig-Versen et al., 2005; Sone et al., 2009). In contrast, drd expression is clearly not required for the maintenance of the adult nervous system. Rather, drd expression appears to be required for the development of one or more adult tissues during metamorphosis, and the improper development of such tissues in drd mutants leads, within 1–2 weeks, to the death of the adult. It has also been reported that appearance of several age-dependent markers or phenotypes is accelerated in drd mutant adults, suggesting that drd mutants age more quickly than wild-type flies (Reenan and Rogina, 2008; Rogina et al., 1997). In light of this finding, our data raise the intriguing possibility that the rate of aging in adults could be controlled to some degree by events that occur during metamorphosis.
Highlights.
Knockdown and rescue of drd expression was temporally controlled by heat shock.
Adult lifespan was affected only when drd expression was manipulated during mid to late metamorphosis.
Adult body mass was affected only when drd expression was manipulated through all of pre-adult development.
Acknowledgments
We wish to thank the Vienna Drosophila RNAi Center (VDRC) and the Bloomington Drosophila Stock Center at Indiana University for providing fly stocks, Dr. Gail Waring for the pUAST vector, and Dr. Dale Noel for the use of his 30° room. We also received materials through the Drosophila Genomics Resource Center, which we gratefully acknowledge. Support was provided by Marquette University, a U.S. Department of Education GAANN (Graduate Assistance in Areas of National Need) fellowship to C.L.S. and National Institutes of Health grant 1R15 GM080682-01 to E.M.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christine Lynn Sansone, Email: christine.sansone@marquette.edu.
Edward M. Blumenthal, Email: edward.blumenthal@marquette.edu.
References
- Benzer S. From the gene to behavior. J Am Med Assoc. 1971;218:1015–1022. [PubMed] [Google Scholar]
- Blumenthal EM. Cloning of the neurodegeneration gene drop-dead and characterization of additional phenotypes of its mutation. Fly. 2008;2:180–188. doi: 10.4161/fly.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Buchanan RL, Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron. 1993;10:839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Chavous DA, Jackson FR, O’Connor CM. Extension of the Drosophila lifespan by overexpression of a protein repair methyltransferase. Proc Natl Acad Sci U S A. 2001;98:14814–14818. doi: 10.1073/pnas.251446498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy RK, Thomas JH. Fluoxetine-resistant mutants in C. elegans define a novel family of transmembrane proteins. Mol Cell. 1999;4:143–152. doi: 10.1016/s1097-2765(00)80362-7. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- Cook T, Zelhof A, Mishra M, Nie J. 800 facets of retinal degeneration. Prog Mol Biol Transl Sci. 2011;100:331–368. doi: 10.1016/B978-0-12-384878-9.00008-X. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Francis VA, Zorzano A, Teleman AA. dDOR is an EcR coactivator that forms a feed-forward loop connecting insulin and ecdysone signaling. Curr Biol. 2010;20:1799–1808. doi: 10.1016/j.cub.2010.08.055. [DOI] [PubMed] [Google Scholar]
- Garen A, Kauvar L, Lepesant JA. Roles of ecdysone in Drosophila development. Proc Natl Acad Sci U S A. 1977;74:5099–5103. doi: 10.1073/pnas.74.11.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophilawi th overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, Brown JB, Cherbas L, Davis CA, Dobin A, Li R, Lin W, Malone JH, Mattiuzzo NR, Miller D, Sturgill D, Tuch BB, Zaleski C, Zhang D, Blanchette M, Dudoit S, Eads B, Green RE, Hammonds A, Jiang L, Kapranov P, Langton L, Perrimon N, Sandler JE, Wan KH, Willingham A, Zhang Y, Zou Y, Andrews J, Bickel PJ, Brenner SE, Brent MR, Cherbas P, Gingeras TR, Hoskins RA, Kaufman TC, Oliver B, Celniker SE. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MJ. Environmental temperature and lifespan in poikilotherms. Nature. 1968;218:869–870. [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Khodosh R, Augsburger A, Schwarz TL, Garrity PA. Bchs, a BEACH domain protein, antagonizes Rab11 in synapse morphogenesis and other developmental events. Development. 2006;133:4655–4665. doi: 10.1242/dev.02650. [DOI] [PubMed] [Google Scholar]
- Kim JY, Jang W, Lee HW, Park E, Kim C. Neurodegeneration of Drosophila drop-dead mutants is associated with hypoxia in the brain. Genes Brain Behav. 2012;11:177–184. doi: 10.1111/j.1601-183X.2011.00743.x. [DOI] [PubMed] [Google Scholar]
- Knust E. Photoreceptor morphogenesis and retinal degeneration: lessons from Drosophila. Curr Opin Neurobiol. 2007;17:541–547. doi: 10.1016/j.conb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kretzschmar D. Swiss cheese et allii, some of the first neurodegenerative mutants isolated in Drosophila. J Neurogenet. 2009;23:34–41. doi: 10.1080/01677060802471635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Bagley JA, Lee HY, Jan LY, Jan YN. Pathogenic polyglutamine proteins cause dendrite defects associated with specific actin cytoskeletal alterations in Drosophila. Proc Natl Acad Sci U S A. 2011;108:16795–16800. doi: 10.1073/pnas.1113573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann FO, Cierotzki V. Locomotor performance in the Drosophila brain mutant drop-dead. Comp Biochem Physiol A Mol Integr Physiol. 2010;156:337–343. doi: 10.1016/j.cbpa.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Lessing D, Bonini NM. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet. 2009;10:359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A, Kraut R. The Drosophila BEACH family protein, blue cheese, links lysosomal axon transport with motor neuron degeneration. J Neurosci. 2009;29:951–963. doi: 10.1523/JNEUROSCI.2582-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuilton P, St Pierre SE, Thurmond J. FlyBase 101--the basics of navigating FlyBase. Nucleic Acids Res. 2012;40:D706–D714. doi: 10.1093/nar/gkr1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KT, Benzer S. Spongecake and eggroll: two hereditary diseases in Drosophila resemble patterns of human brain degeneration. Curr Biol. 1997;7:885–888. doi: 10.1016/s0960-9822(06)00378-2. [DOI] [PubMed] [Google Scholar]
- Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insects. Bioessays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- Mühlig-Versen M, Bettencourt da Cruz A, Tschäpe JA, Moser M, Buttner R, Athenstaedt K, Glynn P, Kretzschmar D. Loss of Swiss cheese/neuropathy target esterase activity causes disruption of phosphatidylcholine homeostasis and neuronal and glial death in adult Drosophila. J Neurosci. 2005;25:2865–2873. doi: 10.1523/JNEUROSCI.5097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation JL. Insect Physiology and Biochemistry. CRC Press; Boca Raton: 2002. [Google Scholar]
- Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O’Connor MB, Mizoguchi A. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell. 2009;17:885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peller CR, Bacon EM, Bucheger JA, Blumenthal EM. Defective gut function in drop-dead mutant Drosophila. J Insect Physiol. 2009;55:834–839. doi: 10.1016/j.jinsphys.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powsner L. The effects of temperature on the durations of the developmental stages of Drosophila melanogaster. Physiol Zool. 1935;8:474–520. [Google Scholar]
- Reenan RA, Rogina B. Acquired temperature-sensitive paralysis as a biomarker of declining neuronal function in aging Drosophila. Aging Cell. 2008;7:179–186. doi: 10.1111/j.1474-9726.2008.00368.x. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. When is weight critical? J Exp Biol. 2011;214:1613–1615. doi: 10.1242/jeb.049098. [DOI] [PubMed] [Google Scholar]
- Rogina B, Benzer S, Helfand SL. Drosophila drop-dead mutations accelerate the time course of age-related markers. Proc Natl Acad Sci U S A. 1997;94:6303–6306. doi: 10.1073/pnas.94.12.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedes CC, Carney GE. Ecdysone signaling in adult Drosophila melanogaster. J Insect Physiol. 2012;58:293–302. doi: 10.1016/j.jinsphys.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Shingleton AW. Body-size regulation: combining genetics and physiology. Curr Biol. 2005;15:R825–R827. doi: 10.1016/j.cub.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Shingleton AW, Das J, Vinicius L, Stern DL. The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol. 2005;3:e289. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Shih C, Mack A, Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Uchida A, Komatsu A, Suzuki E, Ibuki I, Asada M, Shiwaku H, Tamura T, Hoshino M, Okazawa H, Nabeshima Y. Loss of yata, a novel gene regulating the subcellular localization of APPL, induces deterioration of neural tissues and lifespan shortening. PLoS One. 2009;4:e4466. doi: 10.1371/journal.pone.0004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech Ageing Dev. 2009;130:547–552. doi: 10.1016/j.mad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Tschäpe JA, Bettencourt da Cruz A, Kretzschmar D. Progressive neurodegeneration in Drosophila: a model system. J Neural Transm Suppl. 2003:51–62. doi: 10.1007/978-3-7091-0643-3_3. [DOI] [PubMed] [Google Scholar]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Tu MP, Yin CM, Tatar M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell. 2002;1:158–160. doi: 10.1046/j.1474-9728.2002.00016.x. [DOI] [PubMed] [Google Scholar]