Abstract
White matter development is important for efficient communication between brain regions, higher order cognitive functioning, and complex behaviors. Adolescents have a higher propensity for engaging in risky behaviors, yet few studies have explored associations between white matter integrity and risk taking directly. Altered white matter integrity in mid-adolescence was hypothesized to predict subsequent risk taking behaviors 1.5 years later. Adolescent substance users (predominantly alcohol and marijuana, n=47) and demographically similar non-users (n=49) received diffusion tensor imaging at baseline (ages 16–19), and risk taking measures at both baseline and an 18-month follow-up (i.e., at ages 17–20). Brain regions of interest were: fornix, superior corona radiata, superior longitudinal fasciculus, and superior fronto-occipital fasciculus. In substance using youth (n=47), lower white matter integrity at baseline in the fornix and superior corona radiata predicted follow-up substance use (ΔR2 =10–12%, ps < .01), and baseline fornix integrity predicted follow-up delinquent behaviors (ΔR2 = 10%, p < .01) 1.5 years later. Poorer fronto-limbic white matter integrity was linked to a greater propensity for future risk taking behaviors among youth who initiated heavy substance use by mid-adolescence. Most notable were relationships between projection and limbic system fibers and future substance use frequency. Subcortical white matter coherence along with an imbalance between the maturation levels in cognitive control and reward systems may disadvantage the resistance to engage in risk taking behaviors during adolescence.
Keywords: DTI, risk taking, white matter, marijuana, alcohol, adolescence
Introduction
Diffusion tensor imaging (DTI) measures reflect molecular water diffusion in brain tissue, specifically alterations in density, coherence, compactness, and fiber diameter (Le Bihan et al., 2001; Suzuki, Matsuzawa, Kwee, & Nakada, 2003; Taylor, Hsu, Krishnan, & MacFall, 2004). Early studies of white matter development through adolescence have shown increased myelination and organization of fiber tracts into coherent bundles (Benes, Turtle, Khan, & Farol, 1994; Brody, Kinney, Kloman, & Gilles, 1987; Yakovlev & Lecuors 1967). More recent studies have focused on DTI parameters, particularly fractional anisotropy (FA), a measure of the directionality of water movement within axons (Barnea-Goraly et al., 2005; Fryer et al., 2008; Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999; Muetzel et al., 2008; Schmithorst, Wilke, Dardzinski, & Holland, 2002; Snook, Paulson, Roy, Phillips, & Beaulieu, 2005). White matter maturation appears to improve conductivity between brain regions, so subtle alterations in fibers may affect neurocognitive capabilities and complex behavior (Cascio, Gerig & Piven, 2007). A recent study from our lab (Bava et al., 2010) yielded relatively large effect sizes for increased FA over 1.5 years during late adolescent development in long-association fiber bundles and projection fibers, including the corona radiata, superior longitudinal fasciculus, and fronto-occipital fasciculus, tracts that connect frontal and frontal-posterior cortices, as well as subcortical projections such as cortico-thalamic and cortical-limbic fiber tracts.
Adolescents tend to show gradual improvements in executive functioning and subtle increases in cognitive control capacity at the same time as macrostructural and microstructural tissue changes occur in prefrontal cortical and subcortical areas (Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001; Conklin, Luciana, Hooper, & Yarger, 2007; Levin et al., 1991; Luna, Padmanabhan, & O’Hearn, 2010; Rubia et al., 2006; Tamm, Menon, & Reiss, 2002; Van Leifenhorst et al., 2010). Research has emphasized the importance of efficient projections from subcortical regions to frontal cortices as contributors to executive functioning capabilities (Anderson et al., 2001; Cascio et al., 2007; Conklin et al., 2007; Levin et al., 1991; Liston et al., 2006; Luna et al., 2010; Qiu, Tan, Zhou, & Khong, 2008; Royall et al., 2002; Rubia et al., 2006; Tamm et al., 2002; Van Leifenhorst et al., 2010). Continuous development of projections among prefrontal, striatal, and limbic regions during adolescence likely reflects efficiency of neuronal communication and brain circuitry, coinciding with top-down control of problem solving strategies, response inhibition, mental flexibility, set-shifting, and tests of sustained attention (Barnea-Goraly et al., 2005; Ben Bashat et al., 2005; Eluvathingal, Hasan, Kramer, Fletcher & Ewing-Cobbs, 2007; Giorgio et al., 2010; Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008; Liston et al., 2006; Paus et al., 2001; Schmithorst et al., 2002).
Risk taking (both substance use and non-substance use behaviors) is a complex and dynamic construct, defined as engaging in behaviors with a significant probability of negative consequences or unintentional injury (Boyer, 2006; Centers for Disease Control and Prevention, 2010). It is influenced by individual state and trait factors such as mood, cognitive status, personality, sex, and culture (Boyer, 2006; Casey, Getz, & Galvan, 2008; Galvan, Hare, Voss, Glover, & Casey, 2007; Steinberg, 2004; Spear, 2010) From a neurobiological perspective of adolescent risk taking, the progressive development of two neural systems during adolescence, prefrontal (control) and limbic (reward), are postulated to leave all adolescents distinctly vulnerable to engaging in risk taking behaviors (Casey et al., 2008; Ernst et al., 2005; Galvan et al., 2006, 2007; Steinberg, 2004, 2008). Increases in subcortical activation as well as more diffuse prefrontal recruitment have been shown when adolescents make risky choices (Brown et al., 2005; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Casey et al., 1997, 2002; Crone, Donohue, Honomichl, Wendelken,, & Bunge, 2006; Galvan et al., 2006;; Moses et al., 2002; Tamm et al., 2002). The prefrontal cortex may not yet provide sufficient top down control, and delayed (or altered) white matter maturation in association fiber tracts (e.g., superior longitudinal fasciculus) and cortico-limbic tracts (e.g., anterior/superior corona radiata), and therefore cross-talk, between prefrontal and limbic areas may lead to adult-like, yet impulsive behavioral selections (Casey, Getz, & Galvan, 2008; Steinberg, 2008; Van Leifenhorst et al., 2010). To some extent, adolescent drug taking may be a consequence of poor functional connectivity, while at the same time substance use during adolescence may alter such neurodevelopmental processes. Therefore, neuroimaging biomarkers may help us identify individuals likely to engage in not only substance use behaviors, but risky behaviors in general during late adolescence and early adulthood (Squeglia, Spadoni, Infante, Myers, & Tapert, 2009; Tapert et al., 2004, 2007).
Risk taking, including adolescent substance use, follows a nonlinear trajectory, as adolescents engage in more risky behaviors than younger children or adults. Externalizing behaviors are often assessed throughout childhood and adolescence as a way to identify teens engaging in excessive rule breaking. While internalizing behaviors have been described as internal emotional states that are “overcontrolled,” externalizing is suggested as “undercontrol,” (Achenbach & Rescorla, 2001; Achenbach & Edelbrock, 1978), and assessing this construct has shown to have utility in predicting future engagement in risk taking behaviors (Thompson et al., 2011). In a comprehensive review by Boyer (2006), risk taking is discussed as taking on many labels throughout the literature (e.g., externalizing, norm-breaking, problem behaviors), however Boyer describes that generally these labels are attempting to look at similar behavior that may cause inherent harm or danger to the adolescent (2006). It is likely that personality (e.g., sensation seeking), biology, and culture all contribute to acting in these riskier ways (e.g., substance misuse), regardless of construct label. In fact, in the United States nearly 50% of adolescents (high school age students) have had a drink in the past month and 20% have used marijuana in the past month (Centers for Disease Control and Prevention, 2010). Among 8th, 10th, and 12th grade adolescents, alcohol continues to be the most widely used intoxicant, and marijuana use during this time period is on the rise after several years of decline, including an increase in daily marijuana use (Johnston, O’Malley, Bachman, & Schulenberg, 2011; Substance Abuse and Mental Health Services Administration, 2010). While the tendency to engage in some degree of risk taking and novelty seeking can increase opportunities for success and social status (Spear, 2010), risky behaviors can have acute and chronic consequences on health and quality of life. Few studies have explored the relationship between brain microstructure and risk taking; one recent cross-sectional study suggests a link between more coherent white matter in superior/anterior corona radiata fiber tracts and increased engagement in substance use-related risk taking behaviors (Berns, Moore, & Capra, 2009); however this finding is in the opposite directions of neurodevelopmental models of adolescent risk taking and therefore warrants further investigation.
Early identification of adolescents at risk for problematic behaviors may help target prevention efforts, and the degree to which neuroimaging indices can help predict future risky behaviors has not been adequately explored. The present study examined the influence of white matter microstructural integrity in mid-adolescence on real-world risk taking behaviors measured 1.5 years later as youth transitioned into the time of lifetime peak incidence of substance use and dependence (Substance Abuse and Mental Health Services Administration, 2010). It was expected that poorer white matter fiber integrity in association fiber tracts with prefrontal connections and cortico-limbic fiber tracts, measured using DTI at a baseline time point, would predict greater risk taking at 18-month follow-up in both heavy substance using teens at baseline and adolescents with limited substance use histories at baseline. We hypothesized that the relationship between white matter integrity in anterior association and projection fiber tracts and future risk taking behaviors (particularly substance use related risk taking) would be more pronounced in the sub-sample of heavy substance using teens due to increased vulnerability for even poorer fronto-subcortical connectivity associated with not only typical adolescent developmental trajectories, but also substance related white matter alterations and neurotoxicity.
Methods and Materials
Participants
Ninety-six adolescents from an ongoing longitudinal research project examining the effects of marijuana use on adolescent neurodevelopment (Bava et al., 2010; Mahmood, Jacobus, Bava, Scarlett, & Tapert, 2010; Tapert et al., 2007; Schweinsburg, Schweinsburg, Nagel, Eyler, & Tapert, 2011) were included in the present study. This prospective investigation includes participant overlap from our previous publications, including non-substance using controls from Bava et al., 2010 (N = 22) and substance users and demographically matched controls (N = 72; Bava et al., 2009) from a cross-sectional study comparing teens that engaged in marijuana and alcohol use with those who had limited substance use histories. The current sample of 96 adolescents includes all subjects (both controls and substance users) in our baseline dataset meeting eligibility criteria with valid DTI data, and complete risk taking data at the 18-month follow-up. Informed consent and assent were obtained from all youths and parents at project baseline and each follow-up using procedures approved by the University of California, San Diego Human Research Protections Program.
Participants were recruited from local schools when they were 16–19 years of age, and classified as substance users (SU) if they had >200 lifetime experiences with cannabinoids and as normal control youth (CON) if they had <10 lifetime experiences with cannabinoids at project baseline. Subjects were selected from an ongoing longitudinal study examining the effects of cannabis use on adolescent development, however as teens reported both significant marijuana and alcohol use, they are referred to as substance users throughout this manuscript; the present study includes 47 SU and 49 CON teens. A detailed screening procedure at baseline eliminated participants with the following potential confounds: history of a lifetime DSM-IV Axis I disorder (other than cannabis or alcohol abuse or dependence), history of learning disability, history of neurological disorder or head trauma with loss of consciousness >2 minutes, history of a serious physical health problem, complicated or premature birth; uncorrectable sensory impairments; left handedness; MRI contraindications, and use of psychoactive medications at project intake. Approximately 85% of subjects who were screened and completed the baseline assessment as part of the longitudinal project returned to complete the follow-up assessment 1.5 years later; while it is possible that individuals who returned for follow-up differ from those who did not, no between-group demographic differences between these individuals were found at baseline (e.g., age, gender, ethnicity, emotional functioning; ps > .05) SU and CON groups were similar demographically except substance users were about 5 months older than controls on average, more likely to have one or more first-degree biological relatives with a history of substance use disorder, had poorer academic grades at baseline and follow-up, and had less self-reported state anxiety on the day of the scan (ps < 05). Substance use was substantially higher for substance users than controls at baseline and 18-month follow-up, as expected (see Table 1).
Table 1.
Demographic and substance use characteristics of participants
| Baseline | 18-month Follow-up | |||
|---|---|---|---|---|
| Controls (n=49) | Substance Users (n=47) | Controls (n=49) | Substance Users (n=47) | |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Age (baseline: 16 to 19 years)*† | 17.6 (0.8) | 18.0 (0.9) | 19.0 (0.9) | 19.5(0.9) |
| % Male | 73% | 59% | - | - |
| % Caucasian | 65% | 65% | - | - |
| % Family history SUD* | 62% | 87% | - | - |
| % Postpubertal | 33% | 47% | - | - |
| Grade point average*† | 3.4 (0.6) | 3.1 (0.8) | 3.3 (0.5) | 3.0 (0.6)a |
| Household Income | $128K (74K) | $137K (117K) | - | - |
| Vocabulary T-score | 59.7 (9.2) | 56.2 (9.5) | - | - |
| Other risk taking T–score† | 48.7 (9.2) | 51.4 (10.4) | 47.6 (7.5) | 51.8 (9.2) |
| Other risk taking (raw)† | 4.2 (5.3) | 5.8 (6.0) | 5.8 (5.3) | 8.9 (6.7) |
| Spielberger State Anxiety total* | 25.7 (6.5) | 29.7 (8.4) | 25.8 (5.5) | 26.5(6.1) |
| Beck Depression Inventory total | 2.1 (2.6) | 3.4 (4.0) | 2.4 (4.3) | 3.7 (4.7) |
| Lifetime drinking occasions* | 25.5 (37.6) | 233.1 (234.7) | - | - |
| Lifetime marijuana use episodes* | 1.4 (2.4) | 471.0 (357.1) | - | - |
| Lifetime other drug use episodesb* | 0.4 (1.9) | 46.5 (139.8) | - | - |
| Days since last alcohol usec* | 323.4 (408.8) | 48.5 (82.0) | - | - |
| Alcohol use episodes over scan interval † | - | - | 64.6 (90.1) | 212.6 (228.7) |
| Marijuana use episodes over scan interval† | - | - | 31.6 (97.3) | 266.0 (284.9) |
| Days since last marijuana usec* | 385.2 (317.3)d | 77.9 (177.7) | - | - |
| Substance use days past 4 weeksef *† | 0.7 (1.3) | 12.6 (9.4) | 3.5 (5.2) | 13.6 (10.2) |
| Cigarettes smoked, past monthe*† | 0.1 (0.6) | 31.8 (101.0) | 0.2 (0.9) | 53.2 (130.7) |
| Fornix, FAg | 0.50 (0.1) | 0.49 (0.1) | - | - |
| S. Corona Radiata, FAg | 0.42 (0.1) | 0.41 (0.1) | - | - |
| S. Longitudinal Fasciculus, FAg | 0.44 (0.1) | 0.44 (0.1) | - | - |
| Fronto-occipital Fasciculus, FAg | 0.45 (0.1) | 0.45 (0.1) | - | - |
Note.
n = 46,
Other than marijuana, alcohol, and nicotine,
Days prior to DTI scan session,
n = 16 controls who had used any marijuana,
Reported prior to 28-day abstinence period,
Excludes nicotine and caffeine,
All fractional anisotropy (FA) values 10 −3
Group difference at baseline, p < .05,
Group difference at follow-up, p < .05
Measures
Substance Use
Drug use histories were assessed with the Customary Drinking and Drug Use Record (CDDR) (Brown et al., 1998). The CDDR, administered at baseline, obtained lifetime and past 3-month information on use of tobacco, alcohol, and other drugs, as well as withdrawal symptomatology and DSM-IV abuse/dependence criteria (DSM-IV), and was administered at baseline. In the sample of 47 substance users, 84% met criteria for a DSM-IV lifetime marijuana dependence and/or abuse diagnosis in their lifetime, 81% met criteria for a lifetime alcohol dependence and/or abuse diagnosis in their lifetime, and 10% met criteria for a lifetime tobacco use disorder at baseline. The follow-up version of the CDDR replaced lifetime items with a past 18-month follow-up interval. In the 47 substance users at follow-up, 70% met criteria for a marijuana use disorder (i.e., abuse or dependence), 45% met criteria for an alcohol use disorder, and 10% met criteria for nicotine dependence. The Timeline Followback (Sobell & Sobell, 1992) assessed self-reported substance use (excluding nicotine or caffeine) in the 28 days prior to initiating abstinence (described below), and in the 28 days prior to the 18-month follow-up. A substance use T-score was calculated for each individual at baseline and 18-month follow-up based on the means and standard deviations of the Timeline Followback for the sample (N=96).
Other Risk Taking Behaviors
The Child Behavior Checklist (CBCL) was given to all participants’ parents at baseline for reports on youth behavior (Achenbach & Rescorla, 2001). All participants completed the Youth Self Report (YSR; for ages ≤18) or Adult Self Report (ASR; for ages ≥19) at follow-up (Achenbach & Rescorla, 2001); this resulted in 21 individuals completing the YSR and 75 individuals completing the ASR. This well validated assessment system has shown excellent reliability and validity between assessment instruments. Cross-informant agreement (e.g., child/parent) on reports of externalizing behaviors show large effect sizes (Pearson rs= .48–.56) (Achenbach et al., 1987; Achenbach & Rescorla, 2001) and studies have shown consistency between youth and adult self report instruments over time (Hofstra et al., 2001).
Individual items (largely from the Aggression and Delinquency/Rule Breaking scales), were selected a priori to best reflect real-world risk taking (Thompson et al., 2011), defined as engaging in behaviors with a significant probability of negative consequence or unintentional injury (Boyer, 2006; Centers for Disease Control and Prevention, 2010). Internal consistency, measured with standardized Cronbach’s α coefficients, was assessed for all items that comprised other risk taking, including items from the CBCL (parent report) used at baseline (α = .91), items from the YSR used at follow-up if the participant was 18 or younger (adolescent self-report), (α = .90), and items from the ASR used at follow-up if the participant was over 18 (adolescent self-report) (α = .80); all three coefficient levels were ≥.80 suggesting excellent internal consistency. Correlations between raw composite scores of aggression/delinquency at both timepoints (e.g., CBCL, ASR/YSR) demonstrated consistency over time (r = .35, p < .01). Scores on these items were used to compute T-scores based on sample means and standard deviations to index aggressive and delinquent related risk taking behavior at baseline and 18-month follow-up.
Additional Baseline Measures
The Wechsler Abbreviated Scale of Intelligence Vocabulary subtest was administered to briefly estimate intellectual functioning (Wechsler, 1999). The Beck Depression Inventory-II and Spielberger State-Trait Anxiety Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961; Spielberger, Gorsuch, & Lushene, 1970)assessed depression and anxiety state on the day of imaging. The Family History Assessment Module (FHAM) assessed family history of substance use disorders (SUD) and major mental health problems (Rice et al., 1995). The Pubertal Development Scale (PDS) assessed physical development stage (Peterson, Crockett, Richards, & Boxer, 1988). Data on psychosocial functioning (e.g., hours worked at a job) was collected during a general clinical interview for all participants.
Procedures
At baseline, eligible adolescents agreed to remain abstinent from substances for 28 days, monitored by semi-weekly urine drug toxicology screens and breathalyzers (9 times over 28 days). Participants whose drug screen results indicated compliance with the protocol (Smith, Barnes, & Huestis, 2009) received a brain scan including DTI on Day 28. Participants were imaged in a 3.0-Tesla General Electric CXK4 short bore Excite-2 magnetic resonance system with an 8-channel phase-array head coil. A high-resolution 3d T1 anatomical MRI was acquired sagittally using a weighted spoiled gradient recalled acquisition sequence (repetition time = 7.784 ms, echo time = 2.988 ms, flip angle = 12°, field of view = 24 cm, resolution = 1 mm3, 176 continuous slices, acquisition time = 7:19 minutes). The DTI sequence was optimized for minimum echo time and included a single-shot dual spin echo excitation (Reese, Heid, Weisskoff, & Wedeen, 2003) to reduce eddy current artifacts (repetition time = 12400 ms, echo time = 93.4 ms, 4 averages, 15 directions, b ≈ 2000s/mm2, field of view = 24 cm, matrix 128 × 128, voxel resolution = 1.875 mm × 1.875 × 3 mm3, 36 slices, acquisition time = 13:39 minutes) (Frank, 2001). Two field map sequences were collected for unwarping (repetition time = 1000 ms, echo time = minimum full for the first and 5.5 ms for the second, same spatial dimensions as the DTI acquisition, acquisition time = 2:12 minutes for each).
Datasets were processed with Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Package (FSL) (Parker, 2004) and Analysis of Functional NeuroImages (AFNI) library (Cox, 1996). DTI acquisitions were unwarped with two field maps using FMRIB’s Phase Region Expanding Labeler for Unwrapping Discrete Estimates (PRELUDE) (Jenkinson, 2003) and FMRIB’s Utility for Geometrically Unwarping EPIs (FUGUE) (Jenkinson & Smith, 2001). A six-degree of freedom affine motion correction for head motion and a linear alignment to reduce the effects of gradient coil eddy currents were conducted using FMRIB’s Diffusion Toolbox (FDT) and Linear Image Registration Tool (FLIRT) (Smith et al., 2004; Jenkinson, Bannister, Brady, & Smith, 2002). Each image was visually inspected for quality, and non-brain voxels were removed from analyses by the AFNI program 3dAutomask. FA values were calculated using a log-linear estimation procedure that fits a diffusion tensor model at each voxel via FDT (Smith et al., 2004).
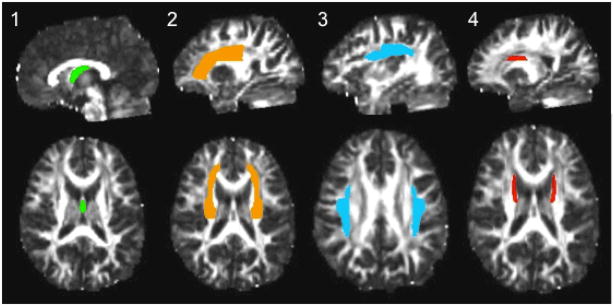
Given evidence for microstructural white matter tissue changes in frontal brain regions during adolescent development from our laboratory and others, data linking impulsivity and risk behavior to white matter integrity (Asato, Terwillinger, Woo, & Luna, 2010; Bava et al., 2010; Berns, et al., 2009; Li, Mathews, Wang, Dunn, & Kronenberger, 2005; Liston et al., 2006; Silveri et al., 2006;) and neurobiological models of adolescent risk taking (Casey et al., 2008; Ernst et al., 2005; Galvan et al., 2006; Steinberg, 2008) we chose to examine bilateral white matter regions containing fiber tracts associated with higher-order cognitive functioning and reciprocal frontal-subcortical and cortico-limbic projections in four regions of interest (ROIs) a priori. Regions of interest are: (1) body of the fornix, a large white matter bundle within the limbic system; (2) superior corona radiata, to capture cortico-limbic and cortico-thalamic fiber tracts; and two association fiber tracts identified in our previous prospective investigation as white matter tracts with ongoing development from ages 17.5 – 19 in healthy controls (Bava et al., 2010), namely (3) superior longitudinal fasciculus, and (4) the superior fronto-occipital fasciculus (see Figure 1). Regions were averaged across brain hemispheres in order to reduce the number of linear regression equations examined. The ICBM-DTI-81 stereotaxic white matter parcellation map (Mori et al., 2008) pre-defined the bilateral white matter ROIs in each subject’s FA dataset. Diffusion and anatomical images were linearly transformed into MNI-152 space using FLIRT (Jenkinson, Bannister, Brady & Smith, 2002; Evans, Collins, & Milner, 1992). FSL’s Automated Segmentation Tool (FAST) (Zhang, Brady, & Smith, 2001) was used to create and apply white matter masks to ROIs, to ensure that only white matter voxels were considered. All white matter ROIs were visually inspected with careful reference to landmarks and coordinates identified by the white matter parcellation map, and further verified by white matter anatomical atlases (Mori et al., 2008; Mori, Wakana, Nagae-Poetscher, & van Zijl, 2005; Schmahmann & Pandya, 2006). Datasets were multiplied by the binary parcellation map to obtain average FA coefficients for each ROI.
Figure 1.
Regions of interest on ICBM-DTI-81 white matter atlas (from left): (1) fornix (highlighted in green), (2) superior corona radiata (highlighted in orange), (3) superior longitudinal fasciculus (highlighted in blue), and (4) superior fronto-occipital fasciculus (highlighted in red).
Participants were interviewed about substance use, other behaviors, and psychopathological syndromes 18 months after their baseline visit with the protocol modified to accommodate an 18-month follow-up assessment interval and developmental changes suitable to 17–20 year-olds (e.g., YSR or ASR, Timeline Followback, CDDR). Valid baseline DTI following 28 days of monitored abstinence, and baseline and 18-month follow-up interviews data were available for all 96 participants.
Data Analysis
ANOVAs and chi-square tests examined between-group differences, and Pearson’s r correlation coefficients identified demographic variables related to 18-month risk taking (p< .05) for inclusion as covariates where appropriate. The following variables were assessed for contribution to variance in 18-month risk taking scores: age, pubertal development, gender, intellectual functioning, Hollingshead SES, family history of substance use disorders, and mood. BDI total (r = .30, p<.01) and family history of an alcohol use disorder (point-biserial r = .21, p<.05) were found to be related to 18-month risk taking. These variables, in addition to baseline risk taking, were included as covariates in follow-up regression analysis to determine the utility of white matter integrity as a significant predictor above and beyond baseline risk taking, family history, and emotional functioning in both groups of adolescents.
Hierarchical linear regressions examining the influence of white matter integrity on both follow-up substance use frequency and aggression/delinquency were conducted separately for each of the 4 regions of interest. Given expected between-group differences, the regression models were first examined in the whole sample to identify if relationships between white matter integrity and prospective risk taking differed based on substance use group status (i.e., moderation effect). If a significant interaction was found, models were re-examined within each group separately to determine the degree to which baseline DTI indices predicted future risk behaviors at follow-up, above and beyond identified covariates, in adolescent substance users compared to adolescents with more limited substance use histories.
Results
The sample consisted of 96 adolescents (66% male, 65% Caucasian) with a mean age of 17 (SD 1; range 16 to 19) at project intake (see Table 1). No between-group differences were found for diffusion indices in the four ROIs (ps > .05), and this did not change after controlling for age.
Hierarchical linear regressions were first conducted in the full sample (N = 96) to determine the influence of baseline white matter integrity on follow-up substance use and aggressive/delinquent behaviors 1.5 years later, and to identify if associations were dependent on group status. Preliminary regression models included the baseline substance use or aggressive/delinquent behaviors variable on step 1, FA averaged bilaterally across each ROI on step 2, and the corresponding interaction term (i.e., cross product of SU versus CON group by FA for the ROI) on step 3. Baseline FA significantly predicted 18-month substance use and, as anticipated, this relationship was found to be dependent on group status (ps < .03). Significant predictors were: FA in the fornix (β = −.22, p =.01) and SCR (β = −.21, p =.01), with lower FA values in these tracts associated with more days of substance use 1.5 years later. Similarly, lower FA values in the fornix (β = −.21, p =.04) predicted more delinquent and aggressive risk taking at follow-up.
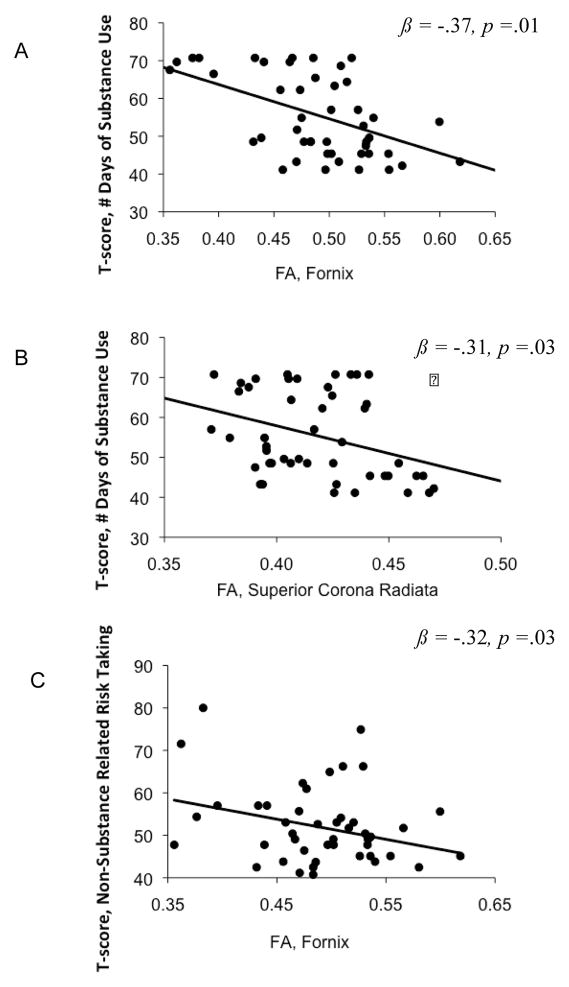
Follow-up regression models, to explore the significant interaction, were conducted separately in each group. Baseline substance use or aggressive/delinquent behavior, total depressive symptoms, and family history of an alcohol use disorder were entered on step 1, and baseline white matter integrity (FA) for each respective ROI (averaged bilaterally) on step 2. For the substance users group (n=47), baseline white matter integrity measures significantly predicted 18-month substance use frequency (in the past month), above and beyond covariates and baseline past-month substance use days. Significant predictors were: FA in the fornix (r = −.49, β = −.37, p =.01) and SCR ( r = −.34, β = −.31, p =.03), with lower FA values in these tracts associated with more days of substance use at 18-month follow-up. Similarly, lower FA values in the fornix r = −.30, β = −.32, p=.02) predicted more delinquent and aggressive risk taking at follow-up. To identify if the diffusion indices contributed to a significant model change above and beyond the covariates, an F-change statistic was calculated. FA in the fornix (ΔR2 = .12, F(1,42) = 4.4, p < .01) and SCR (ΔR2 = .10, F(1, 42) = 3.9, p <.01) contributed to a significant model change in substance use risk taking. FA in the fornix also accounted for variance in 18-month aggressive and deliquent risk taking behaviors beyond the covariates (ΔR2 = .10, F(1,42) = 4.8, p < .01) (see Figure 2).. For controls (n=49), no direct relationship between FA and follow-up substance use or other risk taking behavior was seen.
Figure 2.
Adolescent substance users (n=47) with lower baseline fractional anisotropy (FA) in the fornix (A, C) and superior corona radiata (B) averaged bilaterally showed significantly increased risk-taking behaviors at 18-month follow-up. T-scores (y-axes) reflect number of days of substance use in the past month at 18-month follow-up (A,B) and an index of other risk taking behaviors at 18-month follow-up.
Age and gender are known contributors to adolescent neurodevelopment; therefore, we reexamined the hierarchical linear regression models controlling for age and gender in step 1, along with family history of an alcohol use disorder and depressive symptoms. In the substance users, lower FA in the fornix (β = −.35, p =.03, ΔR2 = .09, F(1,40) = 2.9, p = .02) and SCR (β = −.30, p =.04, ΔR2 = .08, F(1,40) = 2.7, p = .03) were still found to predict more 18-month substance use frequency. Likewise, lower FA in the fornix accounted for variance in 18-month aggressive and delinquent risk taking (β = −.39, p < .01, ΔR2 = .14, F(1,40) = 4.3, p < .01) with age and gender included as covariates. Thus, results were unchanged after controlling for age and gender.
To evaluate if risk taking was related to psychosocial functioning at follow-up, both risk-taking constructs were examined in relationship to number of hours worked at a job, involvement in recreational activities, grade point average, and long-term career plans. In the substance user group, greater aggressive and deliquent related risk taking behaviors was linked to lower school grade point average (r = −.33, p=.02). In the control group, greater substance use risk taking was associated with fewer hours worked at a job (r = −.36, p=.01).
Discussion
White matter integrity was found to be a potential predictor of risk taking behaviors in substance using youth. Evidence for a direct relationship between white matter integrity and risk taking was found in adolescents who were already heavy substance users by mid-adolescence, but not in those with very limited substance use histories by mid-adolescence. This predictive relationship was above and beyond variability accounted for by baseline risk taking, emotional functioning (i.e., depressive symptoms), and family history of an alcohol use disorder.
In the user group, greater microstructural coherence significantly predicted less risky behaviors at follow-up. Specifically, higher FA values in the fornix and superior corona radiata were associated with less risky substance use and delinquent behaviors. The fornix has been implicated in memory (D’Esposito, Verfaellie, Alexander, & Katz, 1995; Gaffan, Gaffan, & Hodges, 1991; Papanicolaou, Hasan, Boake, Eluvathingal, & Kramer, 2007; Poreh et al., 2006; Rudebeck et al., 2009; Tsivilis et al., 2008) and plays a large role in the limbic system, as it connects the hippocampus to regions such as the mammillary bodies and hypothalamus, and projects to prefrontal cortical areas (Haines, 2008). The limbic system and connections to later-developing striatal and prefrontal circuits have important influences on the propensity to engage in risk taking behaviors (Ernst & Fudge, 2009). Likewise, the corona radiata contains reciprocal prefrontal-striatal connections and projection fibers central to detection of salient rewarding cues, positive affect related to cues, and higher order processing necessary to effectively monitor cues and resist impulsive action. These findings provide evidence that decreased white matter microstructural integrity in subcortical limbic and projection fiber circuits may be related to more risky behaviors, above and beyond indicators of emotional functioning.
Overall, this study supported the predictive relationships between risk taking behaviors and limbic and projection fiber pathways in the fornix and superior corona radiata, above and beyond commonly used measures (e.g., family history) shown to be related to white matter integrity in teens (Herting, Schwartz, Mitchell, & Nagel, 2011). Findings underscore the role of white matter connectivity in biological models of risk taking and support previous studies (Berns et al. 2009; Casey et al., 2008; Sturman & Moghaddam, 2011) suggesting distinct pathways for neurocognitive control, risk taking, and reward-related behavior. The dual-systems biological model suggests that adolescent risk taking results from an imbalance in two systems with unique biological underpinnings, a more structurally mature subcortical limbic system operating in conjunction with a less mature cognitive control system. Given that findings were less remarkable between white matter integrity in association fiber tracts compared to limbic and subcortical projection fibers and risk taking behaviors, it is possible that immature cortical association fiber connections, such as the fronto-occipital fasciculus, and more rapidly maturing subcortical limbic connections found to be associated with risk taking behaviors in our sample, represent an imbalance in two distinct neural systems (i.e., higher-order executive functioning/cognitive control and reward processing), further supporting this dual-systems neurobiological model (Asato et al., 2010; Casey, 2008; Steinberg, 2004, 2008, 2010; Van Leifenhorst et al., 2010). Nevertheless, in an important cross sectional study by Berns and colleagues (2009) the authors found that better white matter integrity (i.e., increased FA along with decreased transverse diffusivity) predicted increased rebelliousness (i.e., drinking, smoking, taking drugs) as measured by the Adolescent Risk Questionnaire in a sample of 60 adolescents ages 14 to 18 years old; these findings are in the opposite direction as we found that poorer white mater integrity was related to more substance use behaviors and aggressive/delinquent behaviors. Our prospective sample was imaged 2–3 years later on average, and the former study may reflect more adaptive risk taking as teens explore their environment throughout earlier adolescence. The discrepant findings may reflect different methodological approaches (longitudinal region of interest approach as compared to cross sectional voxelwise and clusterwise statistical correlations). It is important to point out that findings within our substance using group may also reflect pre-existing alterations in white matter tract integrity (e.g., remodeling of the myelin or axons) related to heavier alcohol and marijuana use leaving our group vulnerable to engage in high risk behavior as opposed to the sample examined by Berns and colleagues. Notably, both cross sectional and prospective studies suggest that subcortical white matter may contribute to likelihood to engage in risk taking. However, the differential finding is clearly interesting and emphasizes the need for further research in this area.
The link between limbic and subcortical white matter tracts and risk taking behavior may be more pronounced in the user group due to the purported neurotoxic effects of alcohol and marijuana on white matter tissue development (Ashtari, Cervellione, Cottone, Ardekani, & Kumra, 2009; Bava et al., 2009; Jacobus et al., 2009). It is possible that heavy alcohol and marijuana use may have altered subcortical fibers (specifically, tracts thought to develop earlier in neurodevelopment) necessary to efficiently monitor and regulate risk taking behavior (Asato et al., 2010; Liston et al., 2006; Luna, 2009). For instance, the literature suggests that heavy alcohol use (including binge drinking behaviors) during adolescence may alter fiber integrity via neurotoxic effects on ongoing myelination, axonal development, and neural circuitry (De Bellis et al., 2008; McQueeny et al., 2009; Tapert et al., 2003) While the effects of marijuana use remain more inconclusive (Delisi et al., 2006) there is some evidence to suggest changes in tract coherence associated with adolescent marijuana users (Arnone et al., 2008; Ashtari et al., 2009); the underlying mechanisms of these two commonly used substances may also interact in the microstructural development of the adolescent brain (e.g., excitotoxic cell death, oxidative stress) and be attributable to additive and/or synergistic effects that alter adolescent neurodevelopment (Bava et al., 2009; Jacobus et al., 2009). Taken together, adolescent alcohol and drug use likely affects fiber tract coherence within cortico-limbic regions important for quick signal communication between anatomical regions (still undergoing maturation at various stages) that require efficient transmission to regulate urges to engage in risky behaviors. Poor neural integrity may not only put substance-using teens at increased risk for poor resistance to impulses and future risk taking (particularly in the presence of distracters and emotionally salient cues), but also lead to problems with emotional regulation, psychopathology (e.g., anxiety disorders), and addiction later in life (Casey & Jones, 2010; Casey et al., 2010).
An inherent limitation to this study is that all contributing factors to risk taking cannot be controlled; however, the purpose of this investigation was to examine one potential biological component of risk taking. While this was a prospective study, any conclusion about causal effects must be interpreted with caution. The age range was limited to late adolescence and focused on risks related to substance use, delinquency, and aggressive behaviors. Risky sexual activity, gambling, and tobacco use also have serious consequences and should be the focus of future research. Further, while the vast majority of controls reported engaging in some risky behaviors (84%) and over half (approximately 60%) reported increasing their substance use at follow-up, it is possible that fewer risky behaviors in this group contributed to a lack of significant findings; it is still possible that similar associations exist in a sample of non-substance using teens despite the limited findings of our investigation. We utilized a theory-driven region of interest approach as opposed to a whole brain voxelwise approach used in previous investigations in our laboratory; while we did not find users and non-users to differ in integrity of these large bilateral white matter areas that were selected on the basis of linkage to risk and ongoing development, voxelwise analysis may be more sensitive to such effects and will be the focus of future longitudinal investigations. Finally, because the overall effect sizes were relatively modest and averaged across brain hemispheres replication would be helpful.
The project excluded individuals unable to remain abstinent prior to testing, as well as teens with histories of traumatic brain injury, psychiatric disorders, and learning disabilities. Risk taking should be examined closely in these higher-risk individuals. Identification of teens at increased risk for dangerous behaviors could help mental health providers design prevention programs and select intervention strategies that limit risk taking opportunity and encourage healthier outlets requiring less effective utilization of cognitive control (e.g., community activism, recreational activities). In the future, multi-domain risk taking assessment (e.g., imaging, personality) may be used to identify and guide high-risk teenagers. Future projects will examine interrelationships between structural predictors of brain maturation, such as white matter integrity and gray matter cortical thickness, to better understand if changes in multiple aspects of tissue architecture can be used together to predict prospective substance use behaviors in teens.
Table 2.
Hierarchical regressions representing baseline white matter integrity (independent variable) predicting substance use behaviors and delinquent/aggressive behaviors 1.5 years later (dependent variables) in the substance use group (n = 47).
| Independent, and Dependent Variable | R2 | ΔR2 | Sig. F. Change | F-value | p-value |
|---|---|---|---|---|---|
| Fornix FA, and substance use behaviors 1.5 years later | |||||
| Step 1 | 0.18 | 0.18 | 0.03 | 3.21 | 0.03 |
| Step 2 | 0.30 | 0.12 | 0.01 | 4.40 | < 0.01 |
| SCR FA, and substance use behaviors 1.5 years later | |||||
| Step 1 | 0.18 | 0.18 | 0.03 | 3.21 | 0.03 |
| Step 2 | 0.28 | 0.10 | 0.03 | 3.90 | < 0.01 |
| Fornix FA, and aggressive/delinquent behaviors 1.5 years later | |||||
| Step 1 | 0.25 | 0.25 | 0.01 | 4.01 | 0.02 |
| Step 2 | 0.35 | 0.10 | 0.02 | 4.80 | < 0.01 |
Note. Step 1 included baseline substance use or aggression/delinquency, total depression score, and family history of alcohol use disorder; Step 2 included baseline white matter integrity for the region of interest.
Table 3.
Comparable risk taking items (wording briefly summarized/revised for presentation purposes only) from the Child Behavior Checklist (CBCL), Youth Self-Report (YSR), and Adult Self-Report (ASR) based on definition from Youth Risk Behavior Surveillance System (Centers for Disease Control and Prevention, 2008)
| CBCL Cronbach’s α = .91 |
YSR Cronbach’s α = .90 |
ASR Cronbach’s α = .80 |
|---|---|---|
| 3. Argues b | 3. Argues b | 28. Gets along poorly with family b |
| 4. Fails to finish things d | 4. Fails to finish things d | 53. Trouble planning the future d |
| 16. Meanness b | 16. Meanness b | 16 Meanness b |
| 21. Destroys things b | 21. Destroys things b | 21. Destroys things a |
| 22. Disobedient, home b | 22. Disobedient, home b | 76. Irresponsible a |
| 23. Disobedient, school b | 23. Disobedient, school b | 23. Breaks rules a |
| 28. Breaks rules a | 28. Breaks rules a | 122. Trouble holding a job a |
| 36. Accident prone c | 36. Accident prone c | 36. Accident prone e |
| 37. Fights b | 37. Fights b | 37. Fights b |
| 39. Friends get in trouble a | 39. Friends get in trouble a | 39. Friends get in trouble a |
| 41. Impulsive d | 41. Impulsive d | 41. Impulsive a |
| 43. Lying/cheating behavior a | 43. Lying/cheating behavior a | 43. Lying/cheating behavior a |
| 57. Attacks people b | 57. Attacks people b | 57. Attacks people b |
| 61. School work poor d | 61. School work poor d | 61. Work performance poor d |
| 67. Runs away a | 67. Runs away a | 114. Financially irresponsible a |
| 72. Intentionally sets fires a | 72. Intentionally sets fires a | 89. Rushes into things d |
| 81. Steals, home a | 81. Steals, home a | 82. Steals a |
| 82. Steals, outside home a | 82. Steals, outside home a | 92. Illegal activities a |
| 90. Obscene language a | 90. Obscene language a | 95. Hot temper b |
| 95. Hot temper b | 95. Hot temper b | 97. Threatens people b |
| 97. Threatens others b | 97. Threatens others b | 124. Nicotine use a |
| 99. Tobacco use a | 99. Tobacco use a | 101. Skips work d |
| 101. Truancy a | 101. Truancy a | 121. Late for appointments d |
| 94. Teases b | 94. Teases b | 120. Drives fast a |
| 104. Louder than others b | 104. Louder than others b | 17. Trouble managing money a |
| 68. Screams b | 68. Screams b | - |
| 106. Destroys property a | - | - |
Numbers represent individual item numbers from assessment forms and letters represent item subscale;
Rule Breaking Scale;
Aggression Scale;
Social Problems Scale;
Attention Problems Scale;
Thought Problems Scale
Acknowledgments
This research was funded by the National Institute on Drug Abuse (R01 DA021182, PI: Tapert; F31 DA026263, PI: Jacobus;) and the National Institute of Alcohol Abuse and Alcoholism (T32 AA013525, PI: Edward Riley).
We would like to thank Drs. Edward Riley, Igor Grant, and Sarah Mattson whose support was vital to the completion of this research. We also thank Tim McQueeny, Jennifer Winward, Amanda Gorlick, Anthony Scarlett, Diane Goldenberg, and Megan Ward for their assistance with data collection.
Contributor Information
Joanna Jacobus, University of California, San Diego, VA San Diego Healthcare System.
Rachel E. Thayer, VA San Diego Healthcare System
Ryan S. Trim, VA San Diego Healthcare System, University of California, San Diego
Sunita Bava, University of California, San Diego, VA San Diego Healthcare System.
Lawrence R. Frank, University of California, San Diego, VA San Diego Healthcare System
Susan F. Tapert, VA San Diego Healthcare System, University of California, San Diego
References
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. [PubMed] [Google Scholar]
- Achenbach TM, Edelbrock CS. The classification of child psychopathology: A review and analysis of empirical efforts. Psychological Bulletin. 1978;85:1275–1301. [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for ASEBA school-age forms & profiles. Burlington, VT: University of VT, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41:1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwillinger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research: Neuroimaging. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, Assaf Y. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. 2005 doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS One. 2009;4(8):e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Horska A. Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer TW. The development of risk-taking: A multi-perspective review. Developmental Review. 2006;26(3):291–345. [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. Journal of Neuropathology and Experimental Neurology. 1987;46(3):283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: Application to the study of the developing brain. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52(3):225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. The Journal of Neuroscience. 2002;22:8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Youth risk behavior surveillance- United States, 2009 Surveillance Summaries, [2010] MMWR. 2010;59(SS-5) [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31:103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain regions mediating flexible rule use during development. The Journal of Neuroscience. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clinical Experimental Research. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal. 2006;9:3–17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Verfaellie M, Alexander MP, Katz DI. Amnesia following traumatic bilateral fornix transection. Neurology. 1995;45:1546–1550. doi: 10.1212/wnl.45.8.1546. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge J. A developmental neurobiological model of motivated behavior: Anatomy, connectivity, and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Milner B. An MRI-based stereotactic brain atlas from 300 young normal subjects. Proceedings of the 22nd Symposium of the Society for Neuroscience; 1992. p. 408. [Google Scholar]
- Frank LR. Anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance in Medicine. 2001;45(6):935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert S. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cognition. 2008;67(2):225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan EA, Gaffan D, Hodges JR. Amnesia following damage to the left fornix and to other sites. A comparative study. Brain. 1991;114:1297–1313. doi: 10.1093/brain/114.3.1297. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, James AC. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Haines DE. Neuroanatomy, An Atlas of Structures, Sections, and Systems. 7. Baltimore, MD: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure in youth with family history of alcoholism. Alcoholism, Clinical and Experimental Research. 2011;34(9):1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra MB, Van Der Ende J, Verhuslt FC. Adoelscents’ self-reported problems as predictors of psychopathology in adulthood: 10-year follo-up study. 2001 doi: 10.1192/bjp.179.3.203. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31(6):349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magnetic Resonance in Medicine. 2003;49(1):193–1977. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady J, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SA. Global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: overview of key findings, 2010. Ann Arbor: Institute for Social Research, The University of Michigan; 2011. [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. NeuroReport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: Concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ, Harward H, Fletcher JM. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. [Google Scholar]
- Li TQ, Mathews VP, Wang Y, Dunn D, Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Annals of the New York Academy of Science. 2005;1064:184–192. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. Advances in child development and behavior. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behavioral Research. 1995;30(1):41–62. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood OM, Jacobus J, Bava S, Scarlett A, Tapert SF. Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. Journal of Studies on Alcohol and Drugs. 2010;71(6):885–894. doi: 10.15288/jsad.2010.71.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitrious GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN. The occipitofrontal fascicule in humans: A quantitative, in vivo, DT-MRI study. NeuroImage. 2007;37:1100–1111. doi: 10.1016/j.neuroimage.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcoholism, Clinical and Experimental Research. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI atlas of human white matter. Amsterdam: Elsevier B.V; 2005. [DOI] [PubMed] [Google Scholar]
- Moses P, Roe K, Buxton RB, Wong EC, Frank LR, Stiles J. Functional MRI of global and local processing in children. Neuroimage. 2002;16:415–424. doi: 10.1006/nimg.2002.1064. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel MA, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou AC, Hasan KM, Boake C, Eluvathingal TJ, Kramer L. Disruption of limbic pathways in a case of profound amnesia. NeuroCase. 2007;13(4):226–228. doi: 10.1080/13554790701594854. [DOI] [PubMed] [Google Scholar]
- Parker GJ. Analysis of MR diffusion weighted images. The British Journal of Radiology. 2004;77:176–185. doi: 10.1259/bjr/81090732. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Peterson A, Crockett L, Richards M, Boxer AM. A self report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Poreh A, Winocur G, Moscovitch M, Backon M, Goshen E, Ram Z, Feldman Z. Anterograde and retrograde amnesia in a person with bilateral fornix lesions following removal of a colloid cyst. Neuropsychologia. 2006;44:2241–2248. doi: 10.1016/j.neuropsychologia.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41:223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Ready RE, Stierman L, Paulsen JS. Ecological validity of neuropsychological and personality measures of executive functions. The Clinical Neuropsychologist. 2001;15(3):314–323. doi: 10.1076/clin.15.3.314.10269. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magnetic Resonance in Medicine. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history of diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, Coffey CE. Executive control function: A review of its promise and challenges for clinical research. A report from the committee on research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck SR, Scholz J, Millington R, Rohenkohl G, Johansen-Berg H, Lee ACH. Fornix microstructure correlates with recollection but not familiarity memory. The Journal of Neuroscience. 2009;29:14987–14992. doi: 10.1523/JNEUROSCI.4707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford: University Press; 2006. [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106(3):564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magnetic Resonance Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. Journal of Analytic Toxicology. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow back. A technique for assessing self-reported alcohol consumption. Humana Press; 1992. [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. New York, NY: W.W. Norton; 2010. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory (self-evaluation questionnaire) Palo Alto, CA: Consulting Psychologists; 1970. [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23(4):715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk-taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. The neurobiology of adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neuroscience and Biobehavioral Reviews. 2011;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Summary of National Findings. I. Office of Applied Studies; Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health. NSDUH Series H-38A, HHS Publication No. SMA 10–4586 Findings. [Google Scholar]
- Suzuki Y, Matsuzawa H, Kwee IL, Nakada T. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR in Biomedicine. 2003;16(5):257–260. doi: 10.1002/nbm.848. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism, Clinical, and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Hsu E, Krishnan KR, MacFall JR. Diffusion tensor imaging: Background, potential, and utility in psychiatric research. Biological Psychiatry. 2004;55(3):201–207. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Thompson R, Tabone KJ, Litrownik AJ, Briggs E, Hussey JM, English DJ, Dubowitz H. Early adolescent risk behavior outcomes of childhood externalizing behavioral trajectories. Journal of Adolescence. 2011;31(2):234–257. [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nature Neuroscience. 2008;11:834–884. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Van Leifenhorst L, Moor BG, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence-administration and scoring manual. London: Psychological Corporation; 1999. [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]