Abstract
There is a critical need for efficacious therapeutic strategies to improve the outcome of patients afflicted by malignant peripheral nerve sheath tumors (MPNST). Multiple lines of evidence suggest a role for deregulated PI3K/mTOR signaling in MPNST, making this axis an attractive target for therapeutic manipulation. Based on previous observations obtained from in vitro experimentation, here we aimed to assess the effects of PI3K/mTOR blockade on MPNST growth in vivo. The anti-MPNST impact of XL765, a dual PI3K/mTOR inhibitor currently being evaluated in human cancer clinical trials, was tested in two human MPNST xenograft models (STS26T and MPNST724) and an experimental model of pulmonary metastasis (STS26T). XL765 abrogated human MPNST local and metastatic growth in SCID mice. Notably, this therapeutic approach failed to induce apoptosis in MPNST cells but rather resulted in marked productive autophagy. Importantly, genetic and pharmacologic autophagy blockade reversed apoptotic resistance and resulted in significant PI3K/mTOR inhibition-induced MPNST cell death. The addition of the autophagy inhibitor, chloroquine, to the therapeutic regimen of MPNST xenografts after pre-treatment with XL765 resulted in superior anti-tumor effects as compared to either agent alone. Together, pre-clinical studies described here expand our previous findings and suggest that PI3K/mTOR inhibition alone and (most importantly) in combination with autophagy blockade may comprise a novel and efficacious therapy for patients harboring MPNST.
Keywords: MPNST, Targeted-Therapy, PI3K/mTOR inhibitors, Autophagy, chloroquine
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a highly aggressive and frequently fatal soft tissue sarcoma histological subtype exhibiting a predilection for development in young adults (1,2). Neurofibromatosis type I (NF1) is a well established MPNST risk factor (3,4). Exhibiting marked chemo- and radio-therapy resistance, the prospects for MPNST cure are currently heavily dependent on the ability to achieve complete tumor extirpation which frequently necessitates extensive and highly debilitating surgical procedures (1,5,6). However, even in cases where complete surgical resection is initially achievable, local and systemic relapses are a common subsequent and devastating consequence (1-6). The five year MPNST patient survival rate of less than 40% points to the critical need to identify and implement improved therapeutic strategies (1-6).
The PI3K/AKT/mTOR signaling axis has received much attention in recent years given its potential role in cancer (7,8). This critical pathway acts as a convergence point for a multitude of upstream signals and in turn stimulates the activity of numerous downstream effectors, thereby mediating enhanced cellular survival, growth, protein synthesis, motility and other functions of pro-tumorigenic impact (7). Consequently, it is not surprising that deregulation and aberrant activation of the PI3K/AKT/mTOR signaling axis componentry is a common molecular event in a wide range of malignancies (8). These insights have led to the development of novel therapies targeting single (e.g., mTORC1 inhibitors) or multiple (e.g., dual PI3K/mTOR inhibitors) constituents of this pathway; several are currently in clinical evaluation (9,10). Multiple lines of evidence strongly support a role for deregulated PI3K/AKT/mTOR signaling in MPNST (11). Studies from our laboratory have recently shown enhanced expression of the activated AKT and mTOR downstream effectors S6RP and 4EBP1 in a relatively large cohort of human MPNST specimens as well as in human tumor derived cell lines (12). Aberrant PI3K/AKT/mTOR signaling in MPNST is (at least in part) mediated by the loss of neurofibromin (Nf1) function, the critical molecular event responsible for NF1, as recently demonstrated in genetically engineered mouse models (GEMMs; 13). Loss of function Nf1 mutations have also been identified in a portion of sporadic MPNSTs (14). The Nf1 protein is a known RAS-GAP; consequently, Nf1 loss results in constitutive RAS activation leading to enhanced downstream PI3K/AKT/mTOR signaling. Other MPNST-associated deregulations possibly contributing to the noted constitutive activation of this axis include the common overexpression and aberrant signaling of multiple upstream tyrosine kinase receptors (e.g., EGFR, MET, PDGFR, and IGF-R1 and others noted in MPNST (1,11,12,15,16) as well as loss of the PI3K inhibitor, PTEN, which has recently been shown as contributory to MPNST malignant transformation (17). These insights highlight the relevance of the PI3K/AKT/mTOR axis as a potential novel target for anti-MPNST therapy.
Pre-clinical studies using rapamycin (an mTORC1 complex inhibitor) or its derivatives have yielded promising results. MPNST cells isolated from NF1 patients were found to be highly sensitive to rapamycin which was also found to effectively abrogate tumor growth in MPNST GEMMs (13). Furthermore, the rapamycin analogue RAD001 inhibited the growth of human NF1-associated and sporadic MPNST cells; RAD001 treatment of human MPNST xenografts significantly delayed tumor growth (18). These findings form the rationale for several currently ongoing clinical trials to assess the effect of such inhibitors (as monotherapy or in combination with conventional chemotherapy) in patients with non-operable NF1-associated neurofibromas and/or those with advanced MPNST (19). However, accumulating data from other solid malignancies suggest that the clinical effects of mTORC1 inhibitors are at best cytostatic, resulting in transient tumor stabilization with evidence of re-growth during and/or after treatment discontinuation (13,18). Identifying additional molecular targets for inhibition in combination with mTORC1 blockade is critical if enhanced anti-tumor effects are to result. Taking into account that PI3K/AKT pro-tumorigenic signals are mediated through multiple downstream effectors (i.e., not exclusively via mTOR; 7,8,11) and the recently identified feedback loops by which mTORC1 inhibition further activates PI3K/AKT (20,21) provides a sound rationale for the development of dual PI3K/mTOR inhibitors (12,22). A recent study from our laboratory has identified enhanced anti-MPNST effects for one such inhibitor, PI103, when tested in vitro (12). However, to the best of our knowledge, pre-clinical testing of such inhibitors in vivo, a critical step prior to the conduct of human clinical trials, has yet to be reported.
Interestingly, our initial in vitro based studies using transmission electron microscopy (TEM) image analyses and LC3 western blotting (WB) identified PI103 to induce the accumulation of autophagosomes in MPNST cells (12). Notably, this morphological change might represent either enhanced autophagic flux or halted, blocked macroautophagy (designated herein autophagy; 23,24); multiple experiments are needed in order differentiate between these two potential consequences (23). Recent published data suggest that PI3K/mTOR blockade potentially induce the former, i.e. enhanced productive autophagy, in preclinical models of lung and pancreatic cancer (25,26); whether this is the case in MPNST remains to be elucidated. Autophagy is a multi-step catabolic process characterized by the appearance of cytoplasmic vacuoles, leading to eventual self-digestion of cellular organelles and other constituents within autolysosomes (24). While initially described as a mechanism of cell death (type II; 27), a large body of evidence supports a role for drug-induced autophagy in tumor cell survival, thereby a potential mechanism of therapeutic resistance (28). These effects might be tumor type-, compound-, or even context-dependent (29). Unraveling the role of autophagy in a particular therapeutic context is of significant clinical relevance.
The goal of the current study was to bridge several knowledge gaps noted above and to: 1) assess the anti-tumor effect of dual PI3K/mTOR blockade on the local and metastatic growth of MPNST xenografts; 2) determine whether PI3K/mTOR inhibition results in enhanced productive autophagy or autophagy blockade in MPNST cells; and, 3) if the former is the case, to assess the role of drug-induced autophagy in therapeutic response. XL765 (Sanofi-Aventis, Vitry/Seine, France), a highly potent PI3K/mTOR inhibitor, was specifically selected for testing; this compound is now undergoing clinical evaluation in a broad range of other cancer types (30; 19).
Materials and Methods
Cell-lines and reagents
MPNST cell lines included the NF1-associated: S462 (provided by Dr Kluwe, University Hospital Eppendorf, Hamburg, Germany), ST88-14 (provided by Dr Fletcher, Brigham and Women’s Hospital, Boston, MA), MPNST642 isolated in our laboratory (31), and the sporadic MPNST cell lines STS26T (provided by Dr Porcelli, Albert Einstein College of Medicine, Bronx, NY) and MPNST724 (provided by Dr Fletcher); these were propagated and maintained as previously described (32). We acquired these cell lines between 2008-2011; all were authenticated using DNA fingerprinting (short tandem repeat [STR]) as previously described (31), confirming that no cross contamination has occurred. Cell lines utilized were re-fingerprinted, as per above, during their use for the current study. Compounds utilized in our studies included the small molecule dual PI3K/mTOR inhibitor, XL765 (provided by Exelixis, South San Francisco, CA and Sanofi-Aventis, Vitry/Seine, France), the dual PI3K/mTOR inhibitor, PI103 (Tocris, Ellisville, MO), and the mTORC1 inhibitor rapamycin (Calbiochem, Darmstadt, Germany). XL765 chemical structure will be described in a manuscript currently in preparation by Sanofi (personal communication), structure of other compounds is provided in Fig 1A. Further information can be found in Supplementary data.
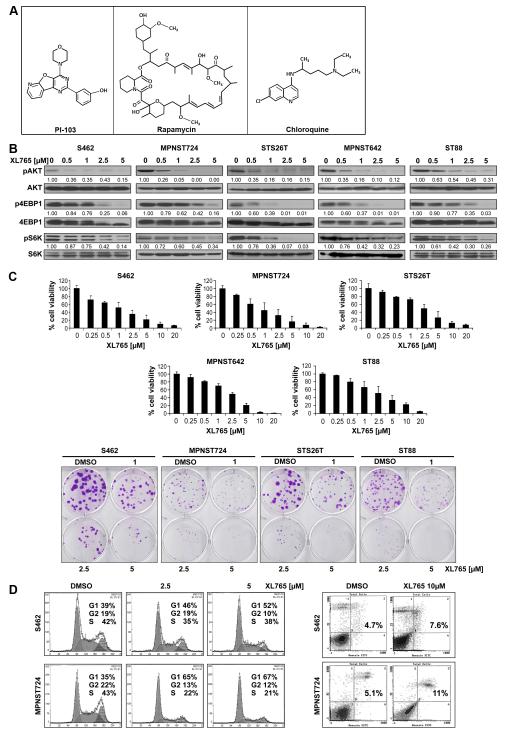
Figure 1. XL765 blocks PI3K/mTOR signaling in MPNST cells resulting in marked growth inhibition and G1 cell cycle arrest.
(A) Chemical structures of compounds used in the study; (B) XL765 inhibits AKT/mTOR activation (protein expression levels were determined via densitometry; WB analyses); (C) XL765 (96h) induces a dose-dependent decrease in MPNST cell growth (MTS assays). A marked decrease in colony-forming capacity can also be observed; (D) XL765 (48h) induces G1 cell cycle arrest but not pronounced apoptosis in MPNST cells.
Cellular growth and autophagy related assays
Experiments were conducted in multiple cell lines and with either or/both inhibitors as previously described (33-35). Assessing autophagy mandates the conduct of multiple complementary assays evaluating both autophagosome accumulation as well as autophagic flux. Studies conducted here followed recently published guidelines (23) and were performed as previously described (12,31). Transfection procedures were performed as previously described (30). Additional information can be found in supplementary data.
In vivo xenograft therapeutic experiments
All animal procedures and care were approved by the MD Anderson Cancer Center Institutional Animal Care and Usage Committee. Animals received humane care as per the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals”. Animal experiments were conducted as previously described (31). Detailed information regarding animal models, therapeutic regimens, schedule and doses can be found in Supplemental Data.
Results
The dual PI3K/mTOR inhibitor XL765 inhibits MPNST cell growth
We have previously demonstrated that PI3K/mTOR blockade exerts marked anti-MPNST effects in vitro utilizing the experimental inhibitor PI103 (12). Seeking to expand these initial studies and evaluate the impact of this therapeutic strategy on MPNST local and metastatic growth in vivo, we opted to test the effect of a novel PI3K/mTOR inhibitor, XL765, currently in human clinical trials (19). First, we confirmed the anti-MPNST effects of this compound on cultured human MPNST cells. Dose range selected was in accordance with previously published pre-clinical studies (26,36) and per company’s recommendation. XL765 was found to induce a marked dose dependent decrease in the phosphorylation of AKT and the mTOR downstream targets 4EBP1 and S6K (Fig 1B). MPNST cell treatment with increasing XL765 doses (0.25-20μM/96hr) induced significant growth inhibition (Fig 1B); extrapolated XL765 IC50 concentrations were found to be S462 = 0.81μM, MPNST724 = 0.86μM, STS26T= 1.75μM, MPNST642 = 1.93μM, and ST88 = 2.49μM. Similarly, a XL765 dose dependent decrease in MPNST cell colony forming capacity was noted (Fig 1C). Concurring with our previous PI103 studies, XL765 treatment (48h) resulted in G1 cell cycle arrest in MPNST cells (Fig 1D). Of note, no evidence for increased sub-G1 cell populations or pronounced XL765-induced apoptotic cell death was observed (Fig 1D). Together, these findings confirm that XL765 abrogates MPNST cell growth and justify further testing the effects of this compound in experimental models in vivo.
XL765 abrogates local and metastatic MPNST xenograft growth
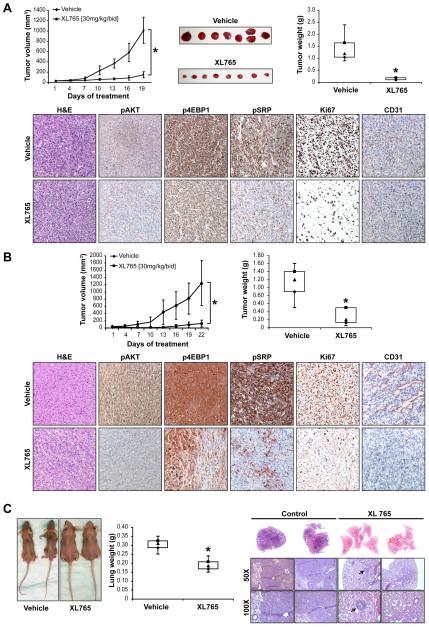
To determine whether the in vitro effects of PI3K/mTOR blockade could be recapitulated in vivo, we conducted a series of therapeutic experiments using xenograft mouse models. A XL765 dose of 30mg/kg/bid given orally was selected (per manufacturer recommendation) based on previous toxicity and pharmacodynamic studies (exelixis.com). First, we investigated the effect of XL765 on MPNST724 xenograft growth (Fig 2A); therapy was initiated after tumor establishment (~4-5mm in larger dimension; control and treatment groups included 7 and 8 mice, respectively). This treatment regimen was well tolerated; no significant weight loss was observed. XL765 markedly inhibited tumor growth; average tumor size at study termination was 151mm3 (±57) for treated group as compared to 1015mm3 (±151) for control group (p<0.0000001). Moreover, treatment with XL765 significantly reduced tumor weight compared to control (p<0.00001); average tumor weights at study termination were 1.41g (±0.59) and 0.15g (±0.05) in control and XL765 groups, respectively (Fig 2A). To confirm that XL765 blocked PI3K and mTOR activity in vivo, immunostainings for pAKT, p4EBP1, and pSRP were performed. Fig 2A demonstrates the marked inhibition of the pathway components in the XL765-treated group. Ki67 immunostaining confirmed a pronounced decrease in tumor cell proliferation. Furthermore, a marked decrease in the number of large blood vessels was noted, confirming the previously reported (37) effect of PI3K/mTOR inhibitors on tumor angiogenesis.
Figure 2. XL765 abrogates MPNST local and metastatic growth in vivo.
(A) XL765 markedly inhibits MPNST724 xenograft growth [graph X axis represents days of treatment] and weight as compared to control vehicle-treated tumors. IHC studies demonstrate marked inhibition of the PI3K/mTOR pathway components in the XL765 treated group. Furthermore, a decrease in tumor cell proliferation (Ki67) and in the number of tumor associated large blood vessels (CD31) can be seen; (B) a similar effect on tumor growth and biomarker expression was seen after treatment of STS26T xenografts; (C) XL765 decreases the growth of STS26T experimental lung metastasis. Untreated mice exhibited marked cachexia and poor body condition while XL765 treated mice retained their weight and well being. These effects were further reflected in marked difference in average lung weight between control and treated mice. Macroscopic findings were confirmed on H&E staining, demonstrating large lung tumor deposits in control and only small (arrow) microscopic lesions in XL765 treated mice. Statistically significant effects; P < 0.05
To demonstrate that XL765 anti-MPNST effects were not MPNST724 xenograft-specific, we also utilized the STS26T model to assess therapeutic effects (control and treatment groups included 7 and 8 mice, respectively; Fig 2B). This treatment regimen was well tolerated; no significant weight loss was observed. At the time point mandating control mouse euthanasia, average volumes of vehicle treated tumors were 1243mm3±619 as compared to 119mm3±93 for the XL765 treated tumors (p<0.001). Average tumor weights at study termination were 1.13g (±0.43) and 0.35g (±0.3) in control and XL765 groups, respectively (p<0.05; Fig 2B). Immunohistochemical analyses concurred with the findings for MPNST724-treated xenografts as described above (28).
Finally, to evaluate whether XL765 resulted in pulmonary metastatic outgrowth inhibition, we utilized the STS26T experimental MPNST lung metastasis model (control and treatment groups included 7 and 8 mice, respectively). Treatment was initiated 10 days after tail vein injection (microscopic lung metastases can usually be found at this time), and continued for ~three weeks, a time point when a portion of control mice exhibited poor body condition and/or decreased body weight mandating euthanasia (Fig 2C). Of note, no significant change in mouse well being or body weight was noted in XL765-treated mice. Lungs of all control mice (n=8) exhibited large and diffuse metastases while macroscopic lesions were observed in only two of the eight XL765-treated mice. These effects were further reflected in marked differences in average lung weight noted comparing control (0.3g±0.03) and treated mice (0.19g±0.03; p<0.0001). Macroscopic findings were also confirmed on hematoxylin and eosin (H&E) staining, demonstrating large pulmonary tumor deposits in control and only small microscopic lesions in XL765 treated mice. In summary, these data align with our previous cell culture-based findings, demonstrating that XL765 markedly inhibits the local and metastatic growth of MPNST in vivo.
PI3K/mTOR inhibitors induce productive autophagy in MPNST cells
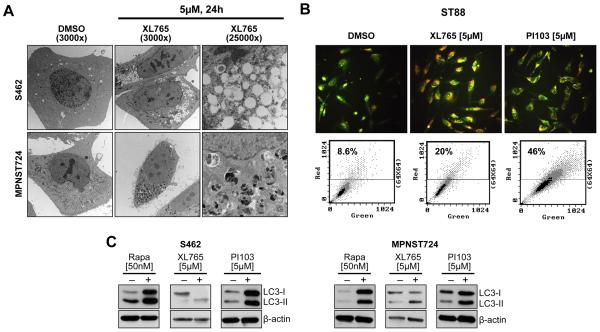
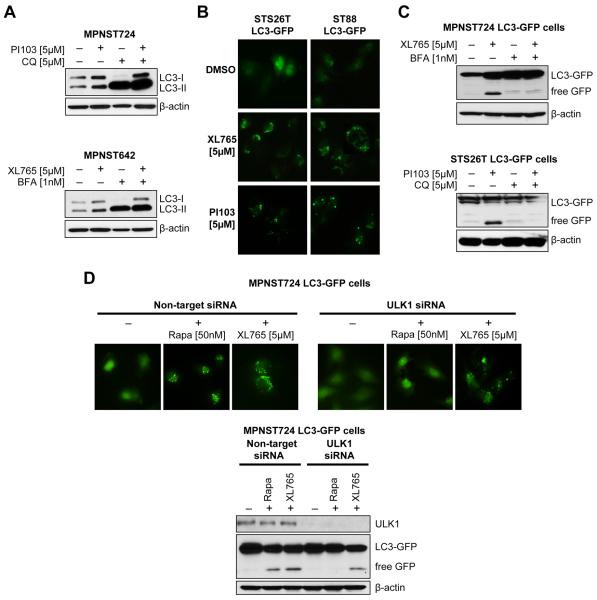
We have previously demonstrated that PI3K/mTOR blockade via PI103 induces autophagosome accumulation in MPNST cells (12), so we wanted to determine whether a similar response was observed with XL765 treatment and if this effect represented enhanced (productive) or blocked autophagy. Transmission electron microscopy (TEM) revealed a large number of autophagosomes at different maturational stages in MPNST cells treated with XL765 but no apparent signs of apoptosis (Fig 3A). Acridine-orange staining demonstrated increased acidic vesicular organelles in XL765-treated cells as was further confirmed via FACS analysis (Fig 3B). Increased LC3 conversion and LC3-II expression (normalized to actin) were also noted in response to treatment (Fig 3C). In that these experimental results could represent either productive autophagy or blocked, reduced autophagosome turnover (23), several additional experiments were conducted to discriminate between these possibilities. Cells were pretreated (1h) with the autophagy inhibitors Bafilomycin A1 (BFA; 1 nmol/L) or chloroquine (CQ; 5 μmol/L) prior to PI3K/mTOR blockade (XL765 or PI103; 24 hours). CQ and BFA block the final steps of the autophagy process, i.e. prevent cargo degradation through neutralizing lysosomal pH and/or autophagosome:lysosome fusion (23); consequentially, increase in LC3-II can be observed in response to these inhibitors representing autophagosome accumulation. Treatment with XL765 or PI103 produced increased LC3B-II expression even in the presence of these lysosomal inhibitors, providing evidence of efficient autophagic flux (Fig 4A). Furthermore, cells stably transduced to express LC3-GFP exhibited increased GFP puncta in response to PI3K/mTOR blockade (Fig 4B). WB analyses demonstrated increased GFP cleavage following XL765/PI103 that was inhibited by pre-treatment with chloroquine or bafilomycin, further supporting PI3K/mTOR blockade-induced productive autophagy (Fig 4C).
Figure 3. PI3K/mTOR inhibition induces autophagosome accumulation in MPNST cells.
(A) TEM pictures showing ultrastructural changes in MPNST cells in response to XL765 (5μM/L per 24 h). A large number of autophagic vesicles were identified without evidence of chromatin condensation, nuclear membranes remained intact further supporting lack of apoptotic effect; (B) Acridine-orange staining demonstrating increased acidic vesicular organelles in XL765 (5μM/L/24h) or PI103 (5μM/L/24) treated MPNST cells as compared to control DMSO-treated cells, as was further confirmed via FACS analysis; (C) Increased LC3-II expression is noticed after treatment with either of the PI3K/mTOR inhibitors;
Figure 4. PI3K/mTOR inhibition induces productive autophagy in MPNST cells.
(A) Cells were pretreated (1h) with the autophagy inhibitors Bafilomycin A1 (BFA; 1nM/L) or chloroquine (CQ; 5μM/L) prior to PI3K/mTOR-blockade (24h). Additional increased LC3-II expression in response to PI3K/mTOR-blockade was noticed when combined with these lysosomal inhibitors; (B) Cells stably transduced to express LC3-GFP exhibited increased GFP puncta in response to PI3K/mTOR-blockade treatment; (C) Free-GFP expression was found in response to PI3K/mTOR-inhibition and was blocked by pretreatment with autophagy inhibitors; (D) MPNST724LC3-GFP cells were transiently transfected with ULK1 siRNA (20nM/L pool); non-targeting constructs were used as controls. ULK1 knockdown blocked rapamycin-induced GFP puncta formation but not XL765-induced puncta. These results are further reflected in the WB analyses where ULK1 knockdown abrogated rapamycin induced LC3-GFP cleavage but not XL765-induced free-GFP expression.
mTORC1 is known to be a master autophagy regulator, mediating blockade of this process through phosphorylation of ULK1 (38). To determine whether PI3K/mTOR inhibition-induced autophagy noted in MPNST cells is solely dependent on mTORC1 and/or ULK1, the later was knocked down in LC3-GFP transduced MPNST cells using target-specific siRNA constructs; non-targeting siRNA was used as control. Cells were treated with rapamycin or XL765. As depicted in Fig 4D, ULK1 knockdown abrogated rapamycin-induced, but not XL765- induced puncta formation. Similarly, ULK1 knockdown blocked LC3-GFP cleavage and free GFP expression as induced by rapamycin but not by XL765. Together, these data suggest that PI3K/mTOR blockade induces productive autophagy in MPNST cells. This effect is probably regulated by multiple molecular mechanisms and is not exclusively dependent on mTORC1/ULK1 inhibition.
Autophagy blockade enhances PI3K/mTOR inhibition-induced apoptosis
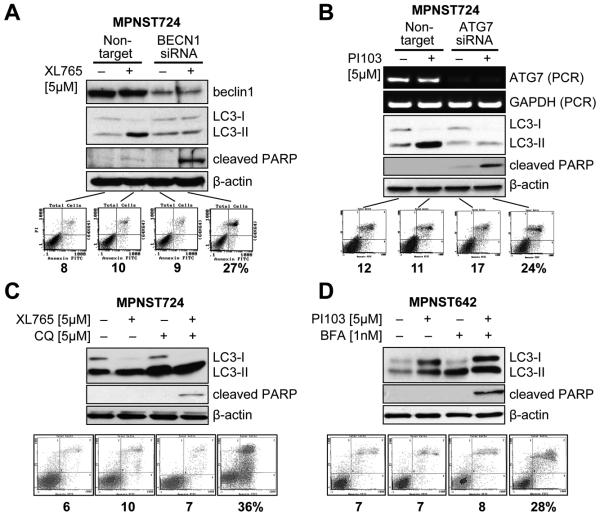
Next, we wanted to determine the impact of PI3K/mTOR blockade-induced autophagy on therapeutic response. Autophagy inhibition was accomplished using complementary genetic and pharmacologic manipulations. Knockdown of the autophagy constituent, beclin and ATG7 was conducted using target-specific siRNAs and cells were treated with PI3K/mTOR inhibitors (Fig 5A&B). WB analyses confirmed that the knockdown of these genes blocked XL765-induced autophagy. Most importantly, both beclin and ATG7 knockdown resulted in pronounced MPNST cell apoptosis in response to PI3K/mTOR inhibition. Similar effects were noted after pharmacologic autophagy blockade (using bafilomycin or chloroquine; Fig 5C&D). Taken together, these data suggest that PI3K/mTOR inhibition-induced autophagy serves as a survival mechanism in MPNST cells, enabling them to escape from the proapoptotic effects of these compounds.
Figure 5. Autophagy blockade sensitizes MPNST cells to the proapoptotic effects of PI3K/mTOR inhibitors in vitro.
(A) Beclin1 siRNA (20 nM/L pool) knockdown was confirmed via WB and autophagy blockade was validated using LC3-II WB. Enhanced apoptosis in response to XL765 (5μM/L/24h) was determined via PARP cleavage and Annexin-V/PI FACS analyses; (B) Similar effects were noted after ATG7 knockdown (confirmed by RT-PCR); (C&D) Pharmacologic autophagy inhibition was achieved using CQ and BFA. None of the compounds alone induced apoptotic cell death, however, apoptosis was noticed after CQ (5μM/L) or BFA (1nM/L) pretreatment of cells (1h) followed by PI3K/mTOR-blockade for 24h.
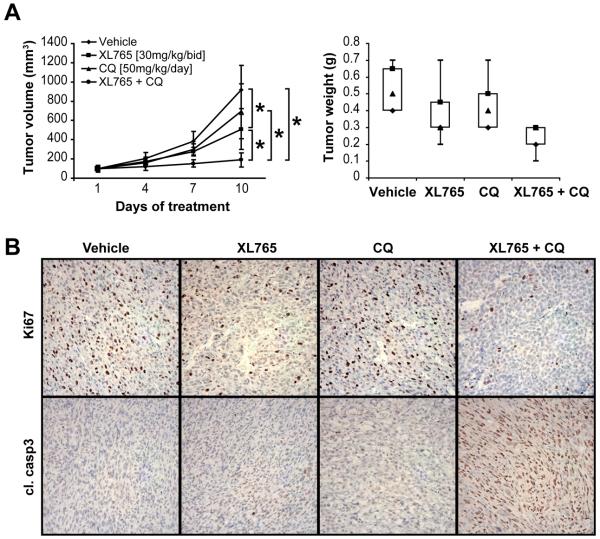
To further determine whether autophagy blockade can perhaps enhance the anti-MPNST effects of PI3K/mTOR inhibitors in vivo, we tested the impact of the XL765/chloroquine combination on the growth of STS26T xenografts (Fig 6A). The study was designed to possibly recapitulate a clinically relevant scenario as following: once palpable tumors (~4-5mm) were identified, all mice were first treated with XL765 alone for 10 days, a time point where a small increase in average tumor size (to ~6mm) was noted; at this juncture mice were then randomly divided into four treatment arms: 1) control (vehicle only; 7 mice); 2) XL765 alone (7 mice); 3) chloroquine alone (7 mice); and, 4) XL765 + chloroquine (8 mice). No major side effects were noted throughout the study and it was terminated when mice in control group mandated euthanasia. Average tumor volumes at the end of the study were control: 918mm3 (±255), XL765: 510mm3 (±211), chloroquine: 696mm3 (±286), and combination: 191mm3 (±74). While no statistically significant difference was found between the chloroquine and control arms (p=0.15), the differences in tumor volume between XL765 and control, combination and control, and combination and XL765 arms were significant (p=0.007, p<0.00001, and p=0.0014, respectively; Fig 6A). Furthermore, combination-treated tumors exhibited a significantly (p<0.001) lower average tumor weight at study termination as compared to control (Fig 6A). Finally, a pronounced decrease in tumor cell proliferation (Ki67) and increase in apoptosis (cleaved caspase-3) were noted in combination-treated xenografts based on immunostaining (Fig 6B). Taken together, these data recapitulate the observations made in vitro and demonstrate that autophagy blockade enhances the anti-MPNST treatment effects of XL765. These findings have potential significant clinical implications.
Figure 6. Autophagy blockade sensitizes MPNST cells to the proapoptotic effects of PI3K/mTOR inhibitors in vitro and in vivo.
(A) XL765+CQ combination results in superior local MPNST growth inhibition in vivo. Tumor growth curves [X axis represents days of treatment from start of the four armed therapeutic regimen] and weight bars showing a significant impact of XL765 alone and to a higher extent XL765+CQ combination on STS26T growth; (B) IHC analysis depicting decreased tumor proliferation (Ki67) and increased apoptosis (cleaved caspase 3) in combination treated tumors.
Discussion
Novel therapeutic strategies that can efficaciously target MPNST are desperately needed to improve the currently unfavorable outcome of afflicted patients. Multiple studies have provided compelling evidence of a critical role for aberrant PI3K/mTOR pathway signaling in these aggressive malignancies (11), supporting the evaluation of compounds targeting this axis (12). Studies here complement our previous cell culture-based observations (12), demonstrating that dual PI3K/mTOR blockade via the clinically relevant XL765 markedly inhibits the local and metastatic growth of human MPNST xenografts. This compound is an orally bioavailable, potent, and selective class-I PI3K/mTORC1/mTORC2 inhibitor previously shown to exhibit broad anticancer efficacy (39). An initial human phase-I XL765 clinical study has demonstrated favorable toxicity and tolerability profiles with no established maximally tolerated dose (39). Several clinical trials (19) are currently ongoing, including evaluation of XL765 as a single agent (for patients with solid tumors or recurrent glioblastoma) as well as in combination with other compounds. Our results here support the development of XL765-based therapeutic strategies for testing in human MPNST clinical trials.
However, it is critical to note that the anti-MPNST effects secondary to PI3K/mTOR blockade reflected growth arrest rather than apoptotic cell death. These effects were found using either of the tested inhibitors, PI103 and XL765, and are in alignment with the effects of PI3K/mTOR dual inhibitors in several different tumor systems (e.g., lung cancer and glioblastoma; 25,40). Taking into account the established role of PI3K/AKT signaling in cellular survival, negating apoptosis directly through phosphorylation of apoptosis-associated downstream effectors or indirectly by modulating the transcription of critical pro- and anti-survival molecules (41) would suggest that a marked pro-apoptotic response secondary to the inhibition of this axis might be expected. However, as previously found (12) and further exemplified in our study, apoptosis is not necessarily the primary response to PI3K/AKT inhibition, especially in cancer cells where marked apoptosis suppression can be the consequence of multiple genetic alterations. While targeting the highly proliferative state of locally advanced and/or metastatic MPNST is an attractive therapeutic stratagem, it might not be sufficient for disease eradication. Given that the anti-MPNST effects of PI3K/mTOR inhibitors are cytostatic rather than cytotoxic diminishes enthusiasm for their use as single agents. Consequentially, identifying PI3K/mTOR blockade-based therapeutic combinations having superior anti-MPNST efficacy appears highly warranted. Examples of recent preclinical investigations of such an approach include combining dual PI3K/mTOR inhibitors with conventional chemotherapy, TRAIL, and EGFR blockade demonstrate enhanced effects as compared to results when agents were used alone (42, 43). While not yet evaluated in MPNST, such a strategy may have particular relevance in this context in that EGFR deregulation commonly occurs in these malignancies and is demonstrably contributory to their tumorigenic phenotype (1,11,16). EGFR blockade alone appears to exert only modest anti-MPNST effects as observed in the preclinical MPNST setting (11), and a phase-II clinical trial failed to demonstrate any objective responses to the EGFR inhibitor Tarceva in patients with relapsed MPNST (11). However, a recent study of combined EGFR blockade with mTOR inhibition resulted in additive anti-proliferative, proapoptotic effects in MPNST cells in vitro and in vivo (18). Based on these findings, evaluating the impact of dual PI3K/mTOR inhibitors in combination with EGFR blockade might therefore be useful for identifying potential therapies for MPNST.
Searching for strategies that can enhance the anti-MPNST effects of dual PI3K/mTOR inhibitors, we sought to expand on our previous observation that these compounds induce the accumulation of autophagosomes in MPNST cells. The current study suggests that the PI3K/mTOR inhibitors induce productive autophagy in MPNST cells, in accord with effects previously observed in other tumor models in response to such compounds (25,26). mTOR is a master regulator of autophagy, and mTORC1 activation blocks this process through the phosphorylation of its downstream target ULK (38). Thus, it is not surprising that mTOR blockade (e.g., using rapamycin) leads to autophagy induction. While PI3K/AKT inhibition can result in autophagy through downregulation of mTORC1 activity, additional mTOR-independent mechanisms have been suggested, including PI3K/AKT inhibition-induced activation of FoxO proteins (44,45) as well as increased mitochondrial superoxide and cellular ROS signals (46). Our data further supports these findings, demonstrating that while ULK knockdown is sufficient to abrogate rapamycin-induced autophagy in MPNST cells, this genetic manipulation does not completely block XL765-induced autophagy in these cells.
Autophagy induced by the blockade of the PI3K/AKT axis has been identified in several studies as a mechanism of cell death, while others have provided data demonstrating the role of this process in therapeutic resistance. Recent examples of the former include the finding that PI3K/AKT inhibition increases radiosensitivity by augmenting autophagic response (47), and that combining the PI3K/mTOR inhibitor BEZ235 with the mTORC1 inhibitor temsirolimus results in cell death secondary to massive autophagic response (48). Conversely, autophagy blockade has been identified as enhancing the proapoptotic effects of dual PI3K/mTOR inhibitors in preclinical models of lung and pancreatic cancer (25,26). Our data suggest that in MPNST, as in the latter examples discussed above, PI3K/mTOR blockade-induced autophagy acts as a mechanism of apoptotic resistance and that combining PI3K/mTOR inhibitors with autophagy blockers can result in marked cytotoxicity in vitro and in vivo. These results are similar to our recent findings using a different anti-MPNST therapeutic strategy, HDAC inhibition, where autophagy blockade was found to augment anti-tumor effects (31). Taken together, these findings possibly suggest that MPNST commonly utilizes autophagy to avoid the cytotoxic effects of therapeutic agents. The observation that the lysosomotropic agent chloroquine enhances the pro-apoptotic effects of XL765 is of potential major translational relevance. Chloroquine is currently being evaluated in human clinical trials as a single agent or in combination with other therapies (19); initial studies already have confirmed its safety (49). The development of an MPNST clinical trial to test the effect PI3K/mTOR inhibitors in combination with chloroquine is supported by our study. However, it is noteworthy that chloroquine and other compounds within its class are not specific autophagy blockers and do exhibit known off-target effects (50). Much effort is currently devoted to the development of autophagy-specific inhibitors; when available, future studies evaluating the anti-MPNST effects of these novel compounds in combination with PI3K/mTOR inhibitors might be warranted.
Supplementary Material
Acknowledgments
We thank Dr Douglas Laird (Exelixis, South San Francisco, CA) and Drs Coumaran Egile and Sukhvinder Singh Sidhu (Sanofi-Aventis, Vitry/Seine, France) for kindly providing XL765. We also thank Dr Juan Fueyo-Margareto for providing the GFP-LC3 construct, Mr. Kenneth Dunner Jr. for electron microscopy assistance, and Ms. Kim Vu for her aid in figure preparation.
Grant Support: This manuscript was supported in part by a NIH/NCI RO1CA138345 (to D. Lev), an Amschwand Foundation Seed Grant (to D. Lev), a Deutsche Forschungsgemeinschaft Fellowship grant (supporting M.P. Ghadimi), and a NIH/NCI 5T32CA009599-21 training grant (supporting K. Lusby). MDACC cell-line characterization and electron microscopy core facilities were further supported by an NCI Cancer Center Support Grant (CA#16672)
Footnotes
Conflict Of Interests: None To Declare. It Is Noted That Exelixis (South San Francisco, Ca) Has Kindly Provided The Pi3K/Mtor Inhibitor Xl765. During The Study Period The Compound Was Licensed To Sanofi-Aventis (Vitry/Seine, France).
References
- 1.Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–22. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 2.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 3.Evans DGR, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis. J Med Genet. 2002;39:311–4. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology. 2005;65:205–11. doi: 10.1212/01.wnl.0000168830.79997.13. [DOI] [PubMed] [Google Scholar]
- 5.Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A, Mussi C, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107:1065–74. doi: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 6.Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42:351–60. doi: 10.1016/s0360-3016(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 7.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 8.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 9.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 10.Kong D, Yamori T. Advances in development of phosphatidylinositol 3-kinase inhibitors. Curr Med Chem. 2009;16:2839–54. doi: 10.2174/092986709788803222. [DOI] [PubMed] [Google Scholar]
- 11.Katz D, Lazar A, Lev D. Malignant peripheral nerve sheath tumour (MPNST): the clinical implications of cellular signalling pathways. Expert Rev Mol Med. 2009;11:e30. doi: 10.1017/S1462399409001227. [DOI] [PubMed] [Google Scholar]
- 12.Zou CY, Smith KD, Zhu QS, Liu J, McCutcheon IE, Slopis JM, et al. Dual targeting of AKT and mammalian target of rapamycin: a potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–68. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]
- 13.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottillo I, Ahlquist T, Brekke H, Danielsen SA, van den Berg E, Mertens F, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol. 2009;217:693–701. doi: 10.1002/path.2494. [DOI] [PubMed] [Google Scholar]
- 15.Torres KE, Zhu QS, Bill K, Lopez G, Ghadimi MP, Xie X, et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res. 2011;17:3943–55. doi: 10.1158/1078-0432.CCR-11-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres KE, Liu J, Young E, Huang KL, Ghadimi M, Lusby K, et al. Expression of ‘drugable’ tyrosine kinase receptors in malignant peripheral nerve sheath tumour: potential molecular therapeutic targets for a chemoresistant cancer. Histopathology. 2011;59:156–9. doi: 10.1111/j.1365-2559.2011.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregorian C, Nakashima J, Dry SM, Nghiemphu PL, Smith KB, Ao Y, et al. PTEN dosage is essential for neurofibroma development and malignant transformation. Proc Natl Acad Sci U S A. 2009;106:19479–84. doi: 10.1073/pnas.0910398106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson G, Mahller YY, Collins MH, Kim MO, Nobukuni T, Perentesis J, et al. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2008;7:1237–45. doi: 10.1158/1535-7163.MCT-07-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials. National Institutes of Health (US); Bethesda (MD): c2009. gov [homepage on the Internet] [updated 2012 May 2; cited 2012 May 3]. Available from: http://clinicaltrials.gov. [Google Scholar]
- 20.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 21.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 23.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu CX, Zhao L, Yue P, Fang G, Tao H, Owonikoko TK, et al. Augmentation of NVP-BEZ235’s anticancer activity against human lung cancer cells by blockage of autophagy. Cancer Biol Ther. 2011;12:549–55. doi: 10.4161/cbt.12.6.16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirzoeva OK, Hann B, Hom YK, Debnath J, Aftab D, Shokat K, et al. Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTOR pathway in pancreatic adenocarcinoma. J Mol Med. 2011;89:877–89. doi: 10.1007/s00109-011-0774-y. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12:1528–34. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 28.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 29.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 30.Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J Hematol Oncol. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez G, Torres K, Liu J, Hernandez B, Young E, Belousov R, et al. Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res. 2011;71:185–96. doi: 10.1158/0008-5472.CAN-10-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller SJ, Rangwala F, Williams J, Akerman P, Kong S, Jegga AG, et al. Large-scale molecular comparison of human Schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 2006;66:2584–91. doi: 10.1158/0008-5472.CAN-05-3330. [DOI] [PubMed] [Google Scholar]
- 33.Lahat G, Zhu QS, Huang KL, Wang S, Bolshakov S, Liu J, et al. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS ONE. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Zhu QS, Ren W, Korchin B, Lahat G, Dicker A, Lu Y, et al. Soft tissue sarcoma cells are highly sensitive to AKT blockade: a role for p53-independent up-regulation of GADD45α. Cancer Res. 2008;68:2895–903. doi: 10.1158/0008-5472.CAN-07-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Z, Lahat G, Korchin B, Nguyen T, Zhu QS, Wang X, et al. Midkine enhances soft-tissue sarcoma growth: a possible novel therapeutic target. Clin Cancer Res. 2008;14:5033–42. doi: 10.1158/1078-0432.CCR-08-0092. [DOI] [PubMed] [Google Scholar]
- 36.Prasad G, Sottero T, Yang X, Mueller S, James CD, Weiss WA, Polley MY, Ozawa T, Berger MS, Aftab DT, Prados MD, Haas-Kogan DA. Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with temozolomide. Neuro Oncol. 2011 Apr;13(4):384–92. doi: 10.1093/neuonc/noq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellino RC, Durden DL. Mechanisms of disease: the PI3K-Akt-PTEN signaling node--an intercept point for the control of angiogenesis in brain tumors. Nat Clin Pract Neurol. 2007;3:682–93. doi: 10.1038/ncpneuro0661. [DOI] [PubMed] [Google Scholar]
- 38.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LoRusso P, Markman B, Tabernero J, Shazer R, Nguyen L, Heath E. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765, a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced solid tumors. J Clin Oncol. 2009;27:15s. [Google Scholar]
- 40.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–9. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, et al. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 42.Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–63. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 43.Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, et al. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–5. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, Brault JJ, Schild A, Goldberg AL. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy. 2008;4:378–80. doi: 10.4161/auto.5633. [DOI] [PubMed] [Google Scholar]
- 46.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–74. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara K, Iwado E, Mills GB, Sawaya R, Kondo S, Kondo Y. Akt inhibitor shows anticancer and radiosensitizing effects in malignant glioma cells by inducing autophagy. Int J Oncol. 2007;31:753–60. [PubMed] [Google Scholar]
- 48.Yang S, Xiao X, Meng X, Leslie KK. A mechanism for synergy with combined mTOR and PI3 kinase inhibitors. PLoS One. 2011;6:e26343. doi: 10.1371/journal.pone.0026343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625:220–33. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 50.Chen N, Debnath J. Autophagy and tumorigenesis. FEBS Lett. 2010;584:1427–35. doi: 10.1016/j.febslet.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.