Abstract
The authors report an adolescent who was found to have diabetes on routine blood testing. The initial diagnosis was type 2 diabetes because she was obese, did not have type 1 diabetes antibodies and both parents had diabetes. Highly sensitive C-reactive protein (hsCRP) was low in the proband and her father (≤0.1 mg/l) indicating that type 2 diabetes was unlikely, and that hepatocyte nuclear factor 1-α-maturity onset diabetes of the young (HNF1A-MODY) was the most likely diagnosis. Following a genetic diagnosis of HNF1A-MODY in the proband and her father, both patients were treated with gliclazide, with improvement in HbA1c. This case highlights the challenges of making a correct diagnosis of MODY in young onset diabetes. The authors report the first case where hsCRP, an easily available biomarker, has been used on an individual level to determine appropriate genetic testing of MODY in a family whose main differential diagnosis was familial type 2 diabetes.
Background
This case is important as it shows that highly sensitive C-reactive protein (hsCRP) measurement can be of considerable help in the diagnosis of maturity onset diabetes of the young (MODY).
MODY is a rare monogenic form of diabetes that affects 1%–2% of diabetes. It is usually misdiagnosed as either type 1 diabetes (T1D) in young slim patients or type 2 diabetes (T2D) in the older or obese patient who is non insulin dependent.1 Making a diagnosis of MODY is crucial for optimal management. Patients with both hepatocyte nuclear factor 1-α (HNF1A) and HNF4A-MODY are extremely sulphonylurea sensitive, up to four times more so than patients with T2D.2 Low dose sulphonylureas are the first line treatment in patients with HNF1A- and HNF4A-MODY, in contrast to metformin in T2D. No treatment is required routinely in glucokinase (GCK)-MODY.
A diagnosis of MODY can be made by molecular genetic sequencing of the common genes that cause MODY, HNF1A (52% cases with a genetic diagnosis), HNF4A (10%) and glucokinase (GCK, 32%).1 Genetic testing is expensive so combined clinical features, biochemical and immunological biomarkers are needed to help the clinician identify which patients are most appropriate for genetic testing.
Clinical features of MODY
Patients with MODY have unique clinical features but these can overlap with T1D and T2D.
MODY is an autosomal dominant, familial form of diabetes, in which diabetes is usually seen in at least one family member diagnosed under 25 years, with features of non-insulin dependency.3 However, parental family history may also occur in T1D (13%) and T2D (80%).4 5 Rising obesity also means that there are obese patients with T1D and MODY, and we now see paediatric patients with T2D. In the paediatric age range all of these forms of diabetes are diagnosed young and will not be insulin dependent if T2D or T1D in the honeymoon period.
Other clinical features can point towards a diagnosis of MODY, but are not specific enough to be used in isolation. Macrosomia (in 50%) (average increased birth weight 800g) and transient neonatal hypoglycaemia (in 15%) occur in HNF4A-MODY, but may also be seen in infants of diabetic mothers (T1D and T2D) due to maternal hyperglycaemia. In HNF1A-MODY, a low renal threshold for glucose results in glycosuria at minimally raised blood glucose levels (<8 mmol/l) due to decreased renal glucose reabsorption, but glycosuria is no longer routinely used to assess diabetes in young patients. An increased risk of cardiovascular mortality can be seen in patients with HNF1A-MODY,6 but accelerated atherosclerosis is also a feature of T1D and T2D.
Biochemical and immunological biomarkers to identify MODY
C-peptide and islet autoantibodies are routinely available biomarkers that can be used to discriminate MODY from T1D but are not useful in discriminating MODY from T2D.7 8 In T1D, C-peptide declines within 5 years of diagnosis and islet antibodies are usually positive.8–10 In MODY and T2D, C-peptide persists and islet antibodies are negative,2 3 7 8 11 12 which makes diagnosing MODY in the young obese patient with familial diabetes and negative islet antibodies difficult.
It has recently been shown that hsCRP is lower in patients with HNF1A-MODY compared with other diabetes subtypes, including T2D and HNF4A-MODY.13–15 This is because the CRP gene has HNF1A binding sites in its promoter region, and transcription of the protein is under control of HNF1A, so a heterozygous loss of function mutation in the HNF1A gene results in low levels of CRP.16
We report the first case in which hsCRP was used to confirm both the appropriateness of MODY testing and the identification of the correct gene to test in a family with a differential diagnosis of T2D.
Case presentation
Proband
A 14-year-old female patient presented to her general practitioner with hair thinning but without any symptoms of diabetes. On routine biochemical testing she was found to have an elevated fasting blood glucose of 9.3 mmol/l. She was obese with body mass index (BMI) SD score +3.2 (99.9th centile for age and gender), and HbA1c 78 mmol/mol (9.3%). She was born prematurely at 36 weeks gestation with birth weight of 3.24 kg (91st–98th centile for gestation and gender).
Family history
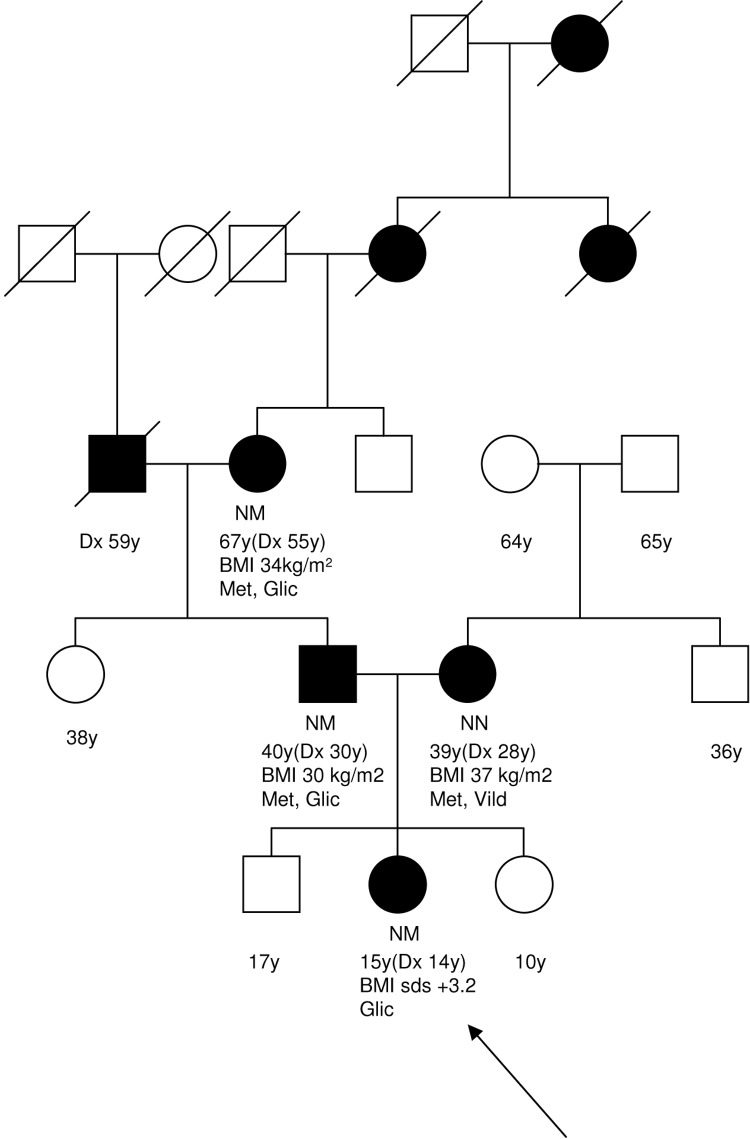
Both parents had a clinical diagnosis of T2D. The proband’s mother was diagnosed aged 28 years with BMI 37 kg/m2 and treated initially with diet, and later with slow release metformin 2 g daily and vildagliptin 50 mg twice daily. The proband’s father was diagnosed with diabetes following a myocardial infarction aged 30 years at which time his BMI was 30 kg/m2. He was initially treated with metformin 850 mg twice daily. Gliclazide 80 mg once daily was initiated 5 years after diagnosis due to progressive hyperglycaemia. Gliclazide was rapidly discontinued due to treatment associated hypoglycaemia. The father’s birth weight was 5.16 kg (>99.6th centile for age and gender). There was a five generation family history of diabetes on the father’s side, and two generation family history on mother’s side (figure 1).
Figure 1.
Partial family tree. Current age (age of diagnosis, Dx) years, body mass index and current treatment are indicated below the symbol, where known. Glic, gliclazide, Met, metformin, Vild, vildagliptin. The arrow indicates the proband.
Provisional diagnosis
The initial diagnosis was familial T2D and the patient was commenced on metformin 500 mg once daily, later increased to a twice daily regimen.
Investigations
Initial HbA1c in the proband was 78 mmol/mol (9.3%) and islet antibodies (GAD and IA2) were negative. In the proband’s father, 10 years after diagnosis HbA1c was 83 mmol/mol (9.7%). HsCRP was low both in the proband (0.1 mg/l, range 0–5 mg/l) and in the proband’s father (<0.05 mg/l), and normal in the proband’s mother (CRP 3 mg/l).
DNA sequencing revealed a known pathogenic heterozygous HNF1A gene mutation, p.R203H (c.608G>C) in the proband and the father, but no mutation in the mother. This confirmed a diagnosis of HNF1A-MODY in the proband and her father. This led to genetic testing in the paternal grandmother which also confirmed the presence of the same HNF1A mutation.
Differential diagnosis
Young-onset T2D and MODY were the main differential diagnoses in this family (table 1). Familial non-insulin dependent diabetes can be seen in both MODY and T2D but having two affected parents is more likely in young-onset T2D.17 The presence of marked obesity in the child also makes T2D more likely.18 HNF1A or HNF4A-MODY was suggested by the marked sulphonylurea sensitivity at a standard dose in the proband’s father. The increased birth weight in the patient and her father is a feature of HNF4A-MODY and not HNF1A-MODY although maternal hyperglycaemia is likely to contribute to this as well.19
Table 1.
Clinical characteristics of proband and typical features seen in HNF1A-MODY and type 2 diabetes (T2D) in young adults
| Diabetes Type | |||
|---|---|---|---|
| Proband | HNF1A-MODY | Type 2 diabetes | |
| Age of diagnosis (years) | 14 | Teens-young adulthood | Typically >35 but can be seen in children |
| Parent affected | Both | One | One or both |
| Insulin dependent | No | No | No |
| Obesity | Marked | Background population | Marked |
| Ethnicity | Low prevalence T2D population | Low prevalence T2D population | High prevalence T2D population |
| Sulphonylurea sensitivity | Yes | Yes | No |
| hsCRP (mg/l) | 0.1 | <0.75 | >0.75 |
hsCRP, highly sensitive C-reactive protein; T2D, type 2 diabetes.
The key feature pointing to this being HNF1A-MODY (and not T2D or HNF4A-MODY) was the hsCRP being low in the proband and the father especially as this is typically normal or elevated (>0.75 mg/L) in T2D, as seen in the mother. The molecular genetic testing of HNF1A confirmed the diagnosis.
Treatment
Following confirmation of HNF1A-MODY, the proband and father were started on sulphonylureas because patients with HNF1A-MODY are four times more sensitive to the glucose lowering effects than matched patients with T2D.2
Outcome and follow-up
The proband changed treatment from metformin to gliclazide 80 mg a.m./40 mg p.m. (with improvement in HbA1c from 77 mmol/mol (9.2%) to 61 mmol/mol (7.7%).
The proband’s father was treated with metformin 1850 mg/daily in divided doses. After the diagnosis of HNF1A-MODY was made gliclazide 40 mg once daily (half a tablet) was added, with an improvement in HbA1c within 3 months of changing treatment from 83 mmol/mol (9.7%) to 55 mmol/mol (7.2%).
Discussion
We report a case where hsCRP was able to determine whether genetic sequencing was appropriate in a patient whose main differential diagnosis was familial T2D and that HNF1A was the correct gene to test.
HsCRP to discriminate MODY from T2D
In our case, the low plasma hsCRP levels (≤0.1mg/l) in the proband and the proband’s father increased the likelihood of HNF1A-MODY, and the normal hsCRP (3 mg/l) in the proband’s mother made a diagnosis of HNF1A-MODY less likely.
Owen et al first reported that hsCRP was lower in HNF1A-MODY compared with other diabetes types.14 Two further studies have now replicated these findings.13 15 In these studies hsCRP levels <0.2–0.75 mg/l discriminated HNF1A-MODY from T2D.13–15 We used the same assay as McDonald et al (Roche Modular P800, Roche Diagnostics, Burgess Hill, UK), in which hsCRP<0.75 mg/l discriminated MODY from T2D with 79% sensitivity and 71% specificity.15 The very low levels of hsCRP in the proband and the proband’s father along with a history of sulphonylurea sensitivity increased the likelihood of HNF1A-MODY, which was then confirmed on genetic sequencing. In the proband’s mother, the hsCRP of 3 mg/l made a diagnosis of HNF1A-MODY less likely.
Discriminating HNF1A from HNF4A-MODY
Presence of greatly increased birth weight in the proband and proband’s father suggested HNF4A-MODY was likely but in this case the low hsCRP made HNF1A-MODY more likely. McDonald et al suggest that using a lower hsCRP cut-off of <0.55 mg/l helps discriminate HNF1A from HNF4A-MODY, with 71% sensitivity and 70% specificity.15 HNF1A-MODY was subsequently confirmed in the proband and her father. Large for gestational age in the proband and her father may be explained by maternal hyperglycaemia in pregnancy even though a formal diagnosis of gestational diabetes was not made.
Implications of making a diagnosis of MODY
Making a correct diagnosis of MODY rather than T2D had implications for the whole family. A genetic diagnosis of MODY resulted in treatment change from metformin to sulphonylureas in the proband and her affected father, with improvement in glycaemic control. Her diagnosis is important for genetic counselling of the risk of any offspring she might have in the future.
Cautions of using hsCRP
Caution is needed when interpreting CRP for use as a diagnostic test in diabetes. In adult patients the prevalence of HNF1A-MODY is much lower than T2D. This means that the majority of adult patients with a low hsCRP are still likely to have T2D. CRP should be used in conjunction with other clinical features and is not recommended for use in isolation. In children, the prevalence of T2D and HNF1A-MODY is similar.20 CRP is likely to be lower in children than adults but its use as a discriminatory tool in diabetes has not been tested in paediatric patients, and further studies are needed before its widespread use.
CRP is an inflammatory marker and can be increased during periods of infection or inflammation. For this reason, studies assessing hsCRP as a diagnostic tool to discriminate diabetes subtypes excluded patients with CRP >10 mg/l. If the CRP is elevated in a patient it would be advisable to repeat the result after a period of time, and should not be measured during any intercurrent illness.
In summary, hsCRP is a cheap, routinely available biomarker that can help discriminate HNF1A-MODY from familial T2D and result in appropriate requesting of molecular genetic testing.
Learning points.
MODY is frequently misdiagnosed as T1D or T2D.
Making a diagnosis of MODY is crucial for optimal management of the patient’s hyperglycaemia as HNF1A-MODY patients are very sensitive to sulphonylureas.
HsCRP can be used to discriminate HNF1A-MODY from T2D, as well as HNF4A and GCK-MODY.
Acknowledgments
The authors acknowledge Professor Sian Ellard and Mr Kevin Colclough in the Department of Molecular Genetics, Royal Devon & Exeter NHS Foundation Trust, for genetic testing of this family.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Shields BM, Hicks S, Shepherd MH, et al. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010;53:2504–8. [DOI] [PubMed] [Google Scholar]
- 2.Pearson ER, Starkey BJ, Powell RJ, et al. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003;362:1275–81. [DOI] [PubMed] [Google Scholar]
- 3.Tattersall RB. Mild familial diabetes with dominant inheritance. Q J Med 1974;43:339–57. [PubMed] [Google Scholar]
- 4.Dahlquist G, Blom L, Holmgren G, et al. The epidemiology of diabetes in Swedish children 0-14 years–a six-year prospective study. Diabetologia 1985;28:802–8. [DOI] [PubMed] [Google Scholar]
- 5.Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes 2009;10(Suppl 12):3–12. [DOI] [PubMed] [Google Scholar]
- 6.Steele AM, Shields BM, Shepherd M, et al. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet Med 2010;27:157–61. [DOI] [PubMed] [Google Scholar]
- 7.Besser RE, Shepherd MH, McDonald TJ, et al. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-{alpha}/hepatocyte nuclear factor 4-{alpha} maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes Care 2011;34:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald TJ, Colclough K, Brown R, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med 2009;55:2035–9. [DOI] [PubMed] [Google Scholar]
- 9.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes 2004;53:250–64. [DOI] [PubMed] [Google Scholar]
- 10.Sabbah E, Savola K, Ebeling T, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care 2000;23:1326–32. [DOI] [PubMed] [Google Scholar]
- 11.Byrne MM, Sturis J, Fajans SS, et al. Altered insulin secretory responses to glucose in subjects with a mutation in the MODY1 gene on chromosome 20. Diabetes 1995;44:699–704. [DOI] [PubMed] [Google Scholar]
- 12.Byrne MM, Sturis J, Menzel S, et al. Altered insulin secretory responses to glucose in diabetic and nondiabetic subjects with mutations in the diabetes susceptibility gene MODY3 on chromosome 12. Diabetes 1996;45:1503–10. [DOI] [PubMed] [Google Scholar]
- 13.Thanabalasingham G, Shah N, Vaxillaire M, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia 2011;54:2801–10. [DOI] [PubMed] [Google Scholar]
- 14.Owen KR, Thanabalasingham G, James TJ, et al. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care 2010;33:1919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald TJ, Shields BM, Lawry J, et al. High-sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care 2011;34:1860–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toniatti C, Demartis A, Monaci P, et al. Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J 1990;9:4467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Rahilly S, Spivey RS, Holman RR, et al. Type II diabetes of early onset: a distinct clinical and genetic syndrome? Br Med J (Clin Res Ed) 1987;294:923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbloom AL, Silverstein JH, Amemiya S, et al. Type 2 diabetes in children and adolescents. Pediatr Diabetes 2009;10(Suppl 12):17–32. [DOI] [PubMed] [Google Scholar]
- 19.Pearson ER, Boj SF, Steele AM, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med 2007;4:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schober E, Rami B, Grabert M, et al. Phenotypical aspects of maturity-onset diabetes of the young (MODY diabetes) in comparison with Type 2 diabetes mellitus (T2DM) in children and adolescents: experience from a large multicentre database. Diabet Med 2009;26:466–73. [DOI] [PubMed] [Google Scholar]