SYNOPSIS
Glucocorticoid administration is the most common cause of secondary osteoporosis and the leading cause of nontraumatic osteonecrosis. In patients receiving long-term therapy, glucocorticoids induce fractures in 30 to 50% and osteonecrosis in 9 to 40%. This article reviews glucocorticoid-induced osteoporosis and osteonecrosis addressing the risk factors, pathogenesis, evaluation, treatment, and uncertainties in the clinical management of these disorders.
Keywords: glucocorticoid-induced apoptosis, bone strength, osteoblasts, osteocytes, osteoclasts, bone vascularity, bisphosphonates, teriparatide, denosumab, glucocorticoid-associated litigation
INTRODUCTION
“A marked osteoporosis of the skeleton was found, it being easily possible to cut the vertebral bodies with a knife, the spongy part of the bone having largely disappeared.” (1). Cushing described these adverse effects of long-term endogenous hypercortisolism on bone in his 1932 presentation to the Johns Hopkins Medical Society. Eighteen years later, only one year after the introduction of cortisone for the treatment of rheumatoid arthritis, clinicians became aware of the rapidly injurious skeletal effects of glucocorticoid administration (2,3). At first, it was uncertain whether the hip fractures that had occurred were the result of falls due to steroid myopathy or merely coincidental with cortisone therapy because vertebral fractures and radiographic osteoporosis had not yet been observed. However, within just a few more years, osteoporosis and fractures were clearly recognized as skeletal complications of treatment with cortisone, prednisolone, and prednisone (4). Collapse of the femoral and humeral heads after high-dose therapy was described shortly thereafter (5,6). Today, we know that glucocorticoid administration is the most common cause of secondary osteoporosis and the leading cause of nontraumatic osteonecrosis. In patients receiving long-term therapy, glucocorticoids induce fractures in 30 to 50% and osteonecrosis in 9 to 40% (7,8). Sadly, patients are seldom warned about these side effects and as a result, adverse skeletal events are the most common glucocorticoid-related complications associated with successful litigation (9).
GLUCOCORTICOID-INDUCED OSTEOPOROSIS (GIO)
RISK FACTORS
Bone loss in GIO is biphasic, with a relatively rapid reduction in bone mineral density (BMD) of 6–12% within the first year, followed by a slower annual loss of about 3% for as long as the glucocorticoids are administered (10). However, the relative risk of fracture escalates by as much as 75% within the first 3 months after initiation of glucocorticoid therapy and this often occurs before a significant decline in BMD (11). There is also a remarkable decrease in the risk of fractures within the first 3 months after the glucocorticoids are discontinued, well before any improvement in BMD. The rapid onset and offset of the fracture risk suggest a qualitative defect in bone material properties not captured by bone densitometry (11). Furthermore, more than one third of postmenopausal women receiving long-term glucocorticoid therapy may have one or more asymptomatic vertebral fractures without abnormal results on calcaneal ultrasound or lumbar and hip BMD determinations (12). Thus, the disparity between bone quantity and quality in glucocorticoid-induced osteoporosis makes ultrasound or BMD measurements inadequate for identifying who is at risk of fractures (13).
Several large case-controlled studies show clear and strong associations between glucocorticoid exposure and fracture (11,14). An increase in vertebral and hip fractures occurs with as little as 2.5 to 7.5 mg/day of prednisolone (equivalent to 3.1 to 9.3 mg of prednisone). In a cohort study of patients aged 18 to 64 years receiving glucocorticoids for a variety of disorders, the combination of higher dose, longer duration and continuous use had the greatest effect on the incidence of fractures (14). Continuous treatment with 10 mg/day of prednisone for more than 90 days was associated with a 7-fold increase in hip fractures and a 17-fold increase in vertebral fractures (14). At present, evidence suggests that the risk of fracture is small and intervention is not required with single dose-pack prescriptions, intermittent oral therapy with a cumulative exposure of less than 1 gram per year, or replacement therapy for patients with hypopituitarism, adrenal insufficiency, or congenital adrenal hyperplasia, provided that the replacement doses are not excessive and the recommendations for increased dosage during periods of stress are not supraphysiologic or inappropriate (e.g. as with mental stress as opposed to febrile or gastrointestinal illnesses) (7,15).
The risk of glucocorticoid-induced osteoporosis is probably the same in men and women of all ethnicities (20). Risk factors include advancing age, prolonged duration of treatment, increased daily dosage and cumulative dose (multiple courses of high-dose oral or intravenous therapy on a baseline of relatively low doses also increase risk), low body mass index, prevalent fractures, frequent falls, underlying disease (especially organ transplant recipients, inflammatory bowel disease and the accompanying malabsorption, rheumatoid arthritis, polymyalgia rheumatica, and chronic pulmonary disease), and polymorphisms in the glucocorticoid receptor (Table 1) (7,16–19). Another factor is the activity of the 11β-hydroxysteroid dehydrogenase (11β-HSD) system, a pre-receptor modulator of corticosteroid action (21). Two isoenzymes, 11β-HSD1 and 11β-HSD2, catalyze the interconversion of hormonally active glucocorticoids (such as cortisol or prednisolone) and inactive glucocorticoids (such as cortisone or prednisone). The 11β-HSD1 enzyme is an activator and the 11β-HSD2 enzyme is an inactivator. Increased fractures due to glucocorticoid administration in the elderly may be attributed to the increase in 11β-HSD1 that occurs with aging (21).
Table 1.
Risk Factors for Glucocorticoid-Induced Osteoporosis.
| Risk Factor | Explanation |
|---|---|
| Advancing age | Elderly patients receiving glucocorticoid therapy have a 26- fold higher risk of vertebral fractures than younger patients and a shorter interval between initiation of treatment and the occurrence of fracture (14) |
| Low body mass index | Significant risk factor for GIO and probably fractures as well (13). |
| Underlying disease | Rheumatoid arthritis, polymyalgia rheumatica, inflammatory bowel disease, chronic pulmonary disease, and transplantation are independent risk factors (7) |
| Family history of hip fracture, prevalent fractures, smoking, excessive alcohol consumption, frequent falls. |
All are independent risk factors for osteoporosis but have not been well studied in patients receiving glucocorticoids. |
| Glucocorticoid receptor genotype | Individual glucocorticoid sensitivity may be regulated by polymorphisms in the glucocorticoid receptor gene (16). |
| 11β-HSD isoenzymes | 11β-HSD1 expression increases with aging and glucocorticoid administration and thereby enhances glucocorticoid activation (17) |
| Glucocorticoid dose (peak, current, or cumulative, duration of therapy, interval) |
There may be no safe dose, although this is somewhat controversial. However, the risk of fracture unarguably escalates with increased doses and duration of therapy. Alternate day or inhalation therapy does not spare the skeleton (7,15). |
| LowBMD | Glucocorticoid-induced fractures occur independently of a decline in bone mass but patients with very low bone density may be at higher risk (7,13). |
11β-HSD, 11β-hydroxysteroid dehydrogenase; BMD, bone mineral density.
Natural and synthetic glucocorticoids differ in their vulnerability to 11β-HSD2, the inactivation enzyme. In dexamethasone, the 11-hydroxyl is already present as it is in prednisolone, but the fluorine atom at the 9α position of the B ring both extends the potency and occludes the 11β location. Dexamethasone causes more osteoporosis and osteonecrosis than prednisone possibly because it is resistant to inactivation by 11β-HSD2 (8).
PATHOGENESIS
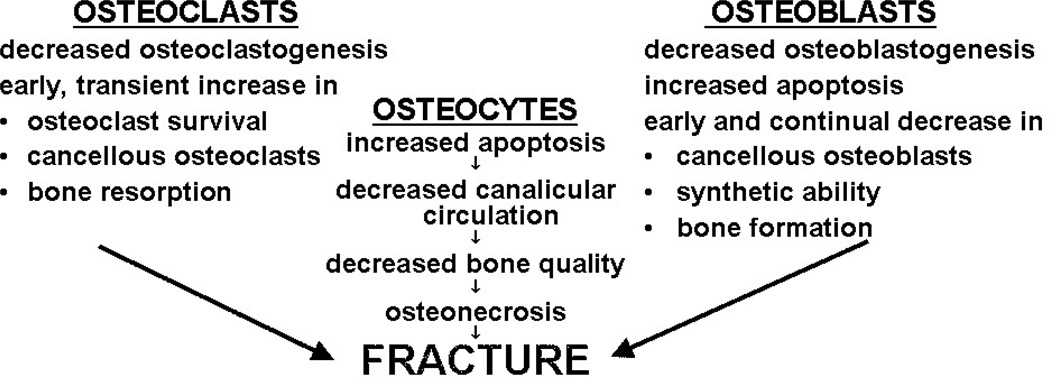
Histomorphometric studies in patients with GIO consistently show reduced numbers of osteoblasts on cancellous bone and diminished wall width, a measure of the work performed by these cells (7,22,23) (Fig. 1 and List 1). The decreased osteoblasts are due to the direct effects of glucocorticoids to decrease the production of new osteoblast precursors and cause premature apoptosis of the mature, matrix-secreting osteoblasts. Inadequate numbers of osteoblasts and incomplete erosion cavity repair during bone remodeling are the main cause of the reduction in cancellous bone area, wall width, trabecular width and bone formation rate typically found in GIO (22). Increased osteocyte apoptosis also occurs and is associated with decreases in vascular endothelial growth factor (VEGF), skeletal angiogenesis, bone interstitial fluid, and bone strength (24). Bone water represents at least 20-25% of the wet weight of bone and confers to bone much of its unique strength and resilience by reducing stress during dynamic loading. Tensile and compressive strength, modulus of elasticity, and hardness increase with decreasing bone water content but deformation and toughness, the energy absorbed before failure (i.e., fracture), are greater for wet bone as compared to dry bone. Both aging and glucocorticoid excess cause a reduction in bone blood flow and the volume of water present in the skeleton (24).
Figure 1.
Glucocorticoid Excess Causes Osteoporosis and Osteonecrosis by Direct Effects on Bone Cells.
List 1.
Fundamental Histological Features of Glucocorticoid-Induced Osteoporosis
| Cancellous Bone |
|---|
| Marked reduction in bone area with decreased trabecular width |
| Diminished wall width |
| Decreased osteoid area |
| Decreased numbers of osteoblasts |
| Increased prevalence of osteoblast and osteocyte apoptosis |
| Normal or slightly increased numbers of osteoclasts |
| Prolongation of the reversal phase |
| Decreased rate of bone formation |
| Decreased bone blood supply and interstitial fluid |
| Cortical Bone |
| Increased cortical porosity |
| Increased prevalence of osteocyte apoptosis |
| Decreased rate of bone formation |
Osteocytes and the lacunar-canalicular network are the strain sensing system of bone and signal the need for remodeling to accommodate prevailing loads or repair damage. Transmission of fluid shear stresses to the lacunar-canalicular network is critical for the mechano-sensing function of the osteocytes and the mechanical adaptation of bone to mechanical forces. Thus, glucocorticoid-induced osteocyte apoptosis could account for the loss of bone strength that occurs before loss of BMD (25) and the resultant mismatch between bone quantity and quality in patients with GIO (7,12,13,24).
Glucocorticoid excess also directly reduces osteoclast production but, in contrast to the increase in osteoblast apoptosis, the lifespan of osteoclasts is prolonged. Therefore, with longterm therapy, osteoclast numbers are usually maintained in the normal range, while osteoblasts and bone formation plummet (22,26). These histological features are quite distinct from those found in other forms of osteoporosis. Loss of gonadal function and secondary hyperparathyroidism are characterized by increased osteoclasts, osteoblasts, and bone formation rate. These different histological features and the evidence that the skeletal effects of glucocorticoid excess override those caused by sex steroid deficiency, fractures are similarly prevalent in amenorrheic and eumenorrheic women with Cushing’s syndrome, and parathyroid hormone levels are not increased by exogenous glucocorticoids indicates that hypogonadism and secondary hyperparathyroidism are not part of the pathogenesis of GIO (27–30).
The primary adverse effects of glucocorticoid excess on the skeleton are directly on the bone cells as is evident from experiments in transgenic mice overexpressing 11β-HSD2, the enzyme that inactivates glucocorticoids in a pre-receptor fashion (23–26). Mice harbouring the transgene in osteoblast and osteocytes are protected from prednisolone-induced apoptosis and decreased osteoblast number, osteoid production, and bone formation, but lost bone since the osteoclasts were still exposed to the prednisolone. However, bone strength was preserved in the transgenic animals in spite of the loss of bone, suggesting that osteocyte viability independently contributes to bone strength (23). Using the same approach, overexpression of 11β-HSD2 in osteoclasts preserved bone, but did not prevent the prednisolone-induced decrease in osteoblast number, osteoid production, and bone formation (26).
Glucocorticoids reduce osteoblast differentiation by attenuating Akt (protein kinase B) phosphorylation and increasing activation of the redox-sensitive forkhead box subgroup O transcription factor family (FoxOs), which in turn inhibit wingless (Wnt)/β-catenin signaling - a critical pathway for the generation of osteoblasts (31). Glucocorticoids also enhance the expression of Dickkopf-1, an antagonist of the Wnt pathway and suppress bone morphogenetic proteins, factors required to induce osteoblast differentiation (32). In addition, glucocorticoids increase production of peroxisome proliferator-activated receptor γ, a transcription factor that induces terminal adipocyte differentiation while suppressing osteoblast differentiation, potentially contributing to increased marrow fat and reduced osteoblasts (33).
In osteoblastic linage cells, glucocorticoids stimulate production of the receptor activator of NF-κB ligand (RANKL), which is essential for the generation and survival of osteoclasts, and reduce osteoblastic expression of osteoprotegerin (OPG), a decoy receptor for RANKL (34). However, exogenous glucocorticoids may not alter RANKL or OPG mRNA levels in vivo (26). Moreover, recent work indicates that RANKL produced by osteoblasts or their progenitors does not contribute to bone remodelling in mature animals and that osteocytes are the major RANKL producing cells that control osteoclast formation (35).
PATIENT EVALUATION
Physicians who prescribe glucocorticoids should educate their patients about side effects and complications including osteoporosis and osteonecrosis, cataract and glaucoma, hypokalemia, hyperglycemia, hypertension, hyperlipidemia, weight gain, fluid retention, easy bruisability, susceptibility to infection, impaired healing, myopathy, adrenal insufficiency and the steroid withdrawal syndrome. Patients receiving long-term glucocorticoid therapy should carry a steroid therapy card or wear medication identification jewelry. A document signed by the patient acknowledging disclosure of the possible side effects should be placed in the patient’s chart (7,36) (Lists 2 and 3). Malpractice suits precipitated by failure to document disclosure of the skeletal complications to patients are not rare (7,37). In a review of the WESTLAW database from 1996 to 2008, avascular necrosis was reported as a complication of glucocorticoid therapy in 39% and osteoporosis and fractures in 12% of the litigation that went to court (8). In spite of this, the bone complications are ignored by the majority of specialists who prescribe glucocorticoids, possibly because of the physicians’ greater concern for other coexisting disorders, their unfamiliarity with metabolic disorders of the skeleton, or lack of appreciation of the rapidity of the substantial increase in risk of fracture (20).
List 2.
Evaluation of the Patient About to Receive Glucocorticoids
| Explain the rationale behind the use of glucocorticoids, expected benefits, and alternatives |
| Use the lowest possible dose and shortest possible course of treatment |
| Inform patient of the side effects of glucocorticoid therapy |
| Document informed consent of the patient |
| Recommend a steroid identification card and medication identification jewelry |
Measure:
|
| Obtain baseline height, vertebral morphological assessment, and bone mineral density |
List 3.
Information for Patients About to Receive Glucocorticoids
| Under certain circumstances, serious medical problems must be treated with potent medications that are intended to improve the patient’s condition but have the potential for severe side effects. Your doctor has decided that the risk of allowing your present illness to progress without glucocorticoid treatment outweighs the risk of the possible side effects. There are several similar glucocorticoid medications produced by different manufactures under many different names. The most common names are corticosteroids, cortisone, cortisol or hydrocortisone (Cortef), prednisone (Deltasone), prednisolone, methyl prednisolone (Medrol), and dexamethasone (Decadron or Dexasone). While receiving glucocorticoids, medication identification jewelry and carrying a card listing all your medications is recommended. | |
| The following side effects are quite common and are seen in most individuals who receive these drugs for more than a few days:
| |
Other less common side effects may occur after longer periods of drug administration:
| |
| This list is not all-inclusive. Patients receiving glucocorticoids should never adjust or stop the medication without consulting a physician. Your physician will discuss these side effects with you. By signing this document, I acknowledge that I have been informed of the reason for the use of glucocorticoid medication, the alternative treatments, if any, as well as the potential benefits and risks that are involved. I have read the above statements and have been able to ask questions and express concerns, which have been satisfactorily responded to by my physician. I have been given a copy of this consent form. | |
| Patient’s signature_____________________ | Witness___________________________ |
| Physician’s signature___________________ | Date_____________________________ |
Laboratory testing should include measurement of serum 25-hydroxyvitamin D (25OHD), creatinine, and calcium (in addition to glucose, potassium, and lipids). It is particularly important to check the 25OHD level before the administration of anti-resorptive agents to avoid drug-induced hypocalcemia, especially as glucocorticoid use is independently associated with low serum 25OHD and the chances of severe vitamin D deficiency with levels less than 10 ng/mL in GIO are doubled (35). The association of glucocorticoid use and vitamin D deficiency may be due to glucocorticoid-induced enhancement of 24-hydroxylase transcription and increased inactivation of 25OHD or simply be a result of the underlying disease, nutritional status, and limited solar exposure of the patients (38). Adequate calcium and vitamin D supplementation should be recommended (7).
Bone turnover after long-term glucocorticoid therapy is low, so biochemical markers of bone metabolism are not usually helpful. However, if biomarkers have been already obtained and are elevated, another problem besides GIO may be present. Prevalent fractures, vertebral morphological assessment from digital images obtained by X-ray absortiometry, spinal radiographs, or loss of height may help identify patients at risk of additional fractures. BMD measurements are inadequate for identifying which patients are particularly at risk, but BMD is useful in follow up after intervention as the values should not decrease if the intervention was effective. The World Health Organization fracture prevention algorithm (FRAX) underestimates the risk of glucocorticoid-induced fractures as the current dose, cumulative dose, and duration of therapy are not entered into the calculation, the algorithm uses femoral neck density values but vertebral fractures are more common than hip fractures with glucocorticoid excess, and inclusion of the common risk factors for postmenopausal osteoporosis in the algorithm may not be applicable to GIO (7).
TREATMENT
Bisphosphonates are considered first-line options for GIO due at least in part to the long experience with these drugs in postmenopausal osteoporosis, their ease of use, and relatively lower cost (Table 2) (7). Randomized, placebo-controlled trials have shown that alendronate, risedronate, and zoledronic acid are effective for this indication and reduce the risk of vertebral but not hip fractures (39–41). Nitrogen-containing bisphosphonates induce apoptosis of osteoclasts and inhibit bone resorption (42), but glucocorticoids antagonize this effect (43) and this may account for the limited ability of these antiresorptive agents to protect BMD in GIO as compared to other forms of osteoporosis (38,44,45). In addition, alendronate decreases glucocorticoid-induced osteocyte apoptosis (46), which may play a role in the preservation of bone strength (25). The evidence for bisphosphonate treatment in GIO is not as strong as that for postmenopausal osteoporosis because the primary end point in the glucocorticoid treatment trials was BMD rather than fracture and glucocorticoids induce a susceptibility to fracture independent of BMD. In addition, most studies were only 12 to 18 months in duration and insufficient numbers of patients were recruited to study hip fractures. Side effects and disadvantages of bisphosphonate therapy are given in Table 2.
Table 2.
Treatment of Glucocorticoid-Induced Osteoporosis.
| Intervention | Advantages | Disadvantages |
|---|---|---|
| Alendronate (oral; 10 mg/d or 70 mg/wk), risedronate (5 mg/d or 35 mg/wk) |
Osteoclast inhibition reduces bone loss. Alendronate also prevents glucocorticoid- induced osteocyte apoptosis. If glucocorticoids are discontinued, these drugs can be stopped. |
Antiresorptive agents do not directly address the decreased bone formation characteristic of glucocorticoid-induced bone disease. Additional problems include gastrointestinal side effects, rare uveitis, poor compliance with oral therapy, and the time required to obtain skeletal protection. Avoid in patients with a creatinine clearance less than 30 mL/min. |
| Zoledronic acid (5 mg IV/yr) |
Osteoclast inhibition reduces bone loss. Increased compliance compared with oral treatment and rapid onset of skeletal effects. Gastrointestinal side effects are unlikely. |
Does not address the reduced bone formation caused by glucocorticoid excess. Avoid in patients with a creatinine clearance less than 30 mL/min. |
| Teriparatide (20 µg subcut/d) |
Directly addresses the pathogenesis of GIO. Reduces vertebral fractures. |
Cost, daily injections are required, reduced response with high dose glucocorticoids. Not studied in patients with elevated parathyroid hormone levels. Adverse effects: mild hypercalcemia, headache, nausea, leg cramps, dizziness. Caution with pre-existing nephrolithiasis. Check serum calcium at least once ≥16 hours after injection and adjust oral calcium intake as needed (54). |
| Denosumab (60 mg subcut every 6 mo) |
Potent inhibitor of osteoclasts with ease of administration. Can be stopped if glucocorticoids are discontinued. Useful in renal insufficiency. |
Does not address the reduced bone formation caused by glucocorticoid excess. Not yet approved for GIO. |
| Vertebroplasty, kyphoplasty |
Commonly used to treat recent painful vertebral fractures. |
Beneficial effects similar to sham procedures. Dangers of cement leakage. Increased incidence of additional fractures in patients receiving glucocorticoids (58). |
An advantage of oral bisphosphonates is that they can be stopped if glucocorticoids are discontinued but compliance with oral therapy is poor. Yearly infusions of zoledronic acid resolve this problem and provide rapid skeletal protection. If a patient presents for fracture protection and glucocorticoid administration has already been continuous with more than 10 mg/day of prednisone for longer than 90 days, there may not be time to wait for the delayed protective effects of oral bisphosphonates because of their average oral absorption of 0.7%, weekly or monthly administration, and lower molar potency as compared with intravenous zoledronic acid (47). For example, 63 mg of alendronate will be absorbed after 90 days of treatment with 70 mg/wk as compared to 5 mg of zoledronic acid in 15 minutes but the potency of alendronate is about 10-fold less than zoledronic acid. About 12-15% of patients with GIO who are receiving their first infusion of zoledronic acid experience an acute phase reaction within two to three days and lasting less than 3 days (41). The mild pyrexia, musculoskeletal pains, and flu-like symptoms are effectively managed with acetaminophen or ibuprofen and seldom occur with subsequent infusions. Substantial BMD loss occurs in patients who discontinue bisphosphonates while receiving glucocorticoids, so the recommended duration of therapy is typically at least as long as the steroids are prescribed (48). However, studies to determine the optimal duration of anti-fracture intervention have not been done. All the drugs used for the treatment of GIO require caution if the patient has childbearing potential.
Bisphosphonates are associated with osteonecrosis of the jaw, a disorder characterized by exposed maxillofacial bone for at least 8 weeks, typically diagnosed after a dental extraction or other invasive procedure, and associated with poor dental hygiene and infection with Actinomyces (49). The disorder occurs mainly in patients with osteolytic breast cancer or multiple myeloma who receive frequent high-dose intravenous bisphosphonates in addition to chemotherapy. In patients with osteoporosis treated with bisphosphonates, the risk of osteonecrosis of the jaw is between 1 in 10,000 to 100,000 patient-years (49). Before prescribing bisphosphonates, the clinician should perform an oral examination and encourage patients to be seen by a dentist. Bisphosphonates may also be associated with atypical subtrochanteric or diaphyseal femoral fractures, but if so, the risk is about 2 per 10,000 patient years (50). Glucocorticoid use has been reported in bisphosphonate-treated patients with osteonecrosis of the jaw and atypical femoral fractures but evidence for a causal effect of the steroids is absent.
An alternative treatment for GIO is teriparatide, recombinant human parathyroid hormone [1–34]. In an 18-month randomized, double-blind, controlled, head-to-head trial, teriparatide increased spinal BMD faster and to a greater extent than alendronate and also reduced vertebral fractures (0.6% vs. 6.1%, P=0.004) (51). Teriparatide represents a particularly rational approach to GIO by counteracting several fundamental aspects of its pathophysiology. The expected glucocorticoid-induced increase in osteoblast and osteocyte apoptosis and decrease in osteoblast number, bone formation, and bone strength are prevented by teriparatide. Decreased osteoblast apoptosis leads to an increase in bone formation and decreased osteocyte apoptosis is associated with preservation of bone strength (52). Furthermore, teriparatide abrogates the negative impact of glucocorticoids on Akt activation and Wnt signaling. However, the anabolic effect of teriparatide is somewhat compromised by high-dose glucocorticoid therapy (53). Dose and duration of glucocorticoid treatment as well as host factors such as severity of the underlying illness, weight loss, concurrent medications, renal function, and low insulin-like growth factor I levels may contribute to the diminished efficacy of teriparatide in GIO compared with other forms of osteoporosis (52). Disadvantages of teriparatide include the need for daily subcutaneous injections, refrigeration, cost, side effects (headache, nausea, dizziness, leg cramps), occasional mild hypercalcemia, and caution required in patients with nephrolithiasis or elevated baseline levels of parathyroid hormone (Table 2) (54).
Another potential treatment option is denosumab, a humanized monoclonal antibody to RANKL approved for the prevention of vertebral, nonvertebral, and hip fractures in women with postmenopausal osteoporosis, but not as yet for GIO (55). In a randomized, double-blind, placebo-controlled trial of denosumab in 61 patients with rheumatoid arthritis receiving less than 15 mg/day of prednisone and methotrexate, BMD of the spine and hip increased with denosumab to the same extent as in 88 patients receiving methotrexate and denosumab alone (56). Furthermore, there was no difference in adverse effects as compared with placebo and methotrexate. Denosumab may be considered for glucocorticoid-treated patients with renal insufficiency and stable serum calcium levels who are not candidates for bisphosphonates or teriparatide (Table 2). The ease of administration as a subcutaneous injection every 6 months may increase compliance. In a murine model of glucocorticoid-induced bone disease treated with osteoprotegerin, a decoy receptor for RANKL representing a similar strategy in GIO as treatment with denosumab, bone strength and osteocyte viability were protected and associated with preservation of the osteocyte-lacunar-canalicular network (57).
In patients with GIO, incapacitating adjacent vertebral fractures have been reported days after kyphoplasty, suggesting that great caution should be exercised before recommending the procedure in these patients (58).
GLUCOCORTICOID-INDUCED OSTEONECROSIS
Aseptic, avascular or ischemic necrosis, and bone infarctions are other terms for osteonecrosis. The most common joint involved is the hip and the most common cause of the disorder is trauma. Glucocorticoids are the second most common cause (8). There are many other etiologies in adults including alcohol, sickle cell disease, ionizing radiation, Gaucher’s disease, Caisson disease or decompression sickness, and idiopathic. However, before concluding that any case of osteonecrosis is idiopathic, a thorough history for pain shots is essential. A pain shot is a general term for a glucocorticoid, narcotic, non-steroidal anti-inflammatory drug, or local anesthetic injection. If it was intra-articular or epidural, glucocorticoid and/or local anesthetic injections were likely. The patient may have received many of these injections but still deny the use of corticosteroids, cortisone, cortisol or hydrocortisone (Cortef), prednisone (Deltasone), prednisolone, methyl prednisolone (Medrol), or dexamethasone (Decadron or Dexasone).
RISK FACTORS
The incidence of glucocorticoid-induced osteonecrosis increases with higher doses and prolonged treatment, although it may occur with short-term exposure to high doses, by intraarticular injection, and without osteoporosis (List 4). In the WESTLAW database, oral doses as low as 290 mg of prednisone and courses that lasted as short as 6 days were held responsible for osteonecrosis (9). Many of these trials could have been avoided if informed consent had been documented.
List 4.
Risk Factors for Glucocorticoid-Induced Osteonecrosis
| Risk Factors |
|---|
| Dose and duration of therapy |
| Intra-articular administration |
| Polymorphisms in VEGF, GR, 11β-HSD2, COL2A1, PAI1, P-glycoprotein |
| Underlying disorders: renal insufficiency, transplantation, graft vs. host disease, inflammatory bowel disease, HIV, acute lymphoblastic leukemia |
| Dexamethasone causes greater skeletal complications than prednisone |
VEGF, vascular endothelial growth factor; GR, glucocorticoid receptor; 11β-HSD2, 11β-hydroxysteroid dehydrogenase type2; COL2A1, collagen type II; PA1, plasminogen activator inhibitor 1; and HIV, human immunodeficiency virus.
Intra-articular glucocorticoids may be particularly dangerous because the injection may accelerate joint damage by alleviating pain, thus increasing weight bearing – a kind of Charcot’s arthropathy, in addition to the direct adverse effects of the steroids on bone. Osteonecrosis has been noted in just weeks to months after intra-articular injection of cumulative doses of 80 to 160 mg of methylprednisolone (8).
PATHOGENESIS
Osteonecrosis has been postulated to be due to fat embolism, vascular thrombosis, and fatigue fractures but recent attention is focused on the role of osteocyte apoptosis (7,8). Abundant apoptotic osteocytes and lining cells are found juxtaposed to the subchondral fracture crescent in femoral heads removed at surgery for glucocorticoid-induced osteonecrosis (Fig. 2) (59). Glucocorticoid-induced osteocyte apoptosis, a cumulative and irreparable defect, disrupts the mechanosensory function of the osteocyte-lacunar-canalicular system and thus starts the inexorable sequence of events leading to collapse of the joint.
Figure 2.
Osteocyte Apoptosis in Glucocorticoid-Induced Osteonecrosis of the Hip. Femoral head specimen taken from a patient with glucocorticoid-induced osteonecrosis during hip replacement. Apoptotic osteocytes (arrows) and lining cells (arrowheads) are stained brown by in situ end labeling (x200).
EVALUATION
Persistent hip or shoulder pain, especially with joint movement, tenderness or reduced range of motion, warrants magnetic resonance imaging because of the association of glucocorticoid therapy with osteonecrosis (8). The pathognomonic, subchondral crescent sign seen on radiographic examination may not be present in the early stages of osteonecrosis and MRI can reveal extensive osteonecrosis before any change in the shape of the femoral head or appearance of a fracture crescent on x-rays.
TREATMENT
For advanced disease with obliteration of the acetabular articular space and osteophyte formation, total hip replacement is usually required. Osteonecrosis is the most common cause of total hip replacement in young adults; but problems with infection, osteolysis, dislocation and revisions are worse with hip replacement for glucocorticoid-induced osteonecrosis than for osteoarthritis (8,37). Since these replacements have about a 10-year lifespan, any delay in the need for surgery would be welcome. For these reasons, recent evidence of the utility of bisphosphonates in the treatment of osteonecrosis is quite promising.
In a randomized, controlled, open-label trial, adults with osteonecrosis treated with alendronate had significant retardation of femoral head collapse and reduced need for total hip replacement compared to the control group over 24 months (60). Importantly, sustained improvement in pain and ambulation was reported within months. In another study, a ten-year follow-up of patients with early to moderately advanced osteonecrosis treated with alendronate for only the first 3 years showed decreased use of analgesics, increased ability to function as desired, and improvement in standing and ambulation time within months that lasted up to ten years (61).
A recent report assessed the efficacy of treatment of very early stage osteonecrosis of the femoral head with core decompression and autologous implantation of bone marrow-derived and culture-expanded mesenchymal stem cells (62). Five years after the procedure, 4% of the hips assigned to autologous implantation had deteriorated as compared to 23% of the hips that received core decompression alone. However, this procedure is both complicated and operatordependent and may not be widely reproducible. Furthermore, even without surgical treatment, as many as 40% of small, medially located, early osteonecrotic lesions may not progress (8).
AREAS OF UNCERTAINTY
Precise prediction of the risk of fracture is currently not possible in GIO and efforts to alert physicians prescribing glucocorticoids to their responsibilities to prevent bone complications have met with limited success. More data are needed to establish precise clinical thresholds for intervention and develop strategies to convey this information to physicians and patients. In addition, more research is required to ascertain the optimal duration of protective therapy, best treatment of premenopausal women, and efficacy of long-term use of teriparatide (63).
KEY POINTS.
Glucocorticoids are the most common cause of secondary osteoporosis and nontraumatic osteonecrosis.
Adverse skeletal events are the most common glucocorticoid-related complications associated with successful litigation.
Disparity between bone quantity and quality in GIO makes ultrasound or BMD measurements inadequate for identifying patients at risk of fracture.
Continuous treatment with 10 mg/day of prednisone for more than 90 days has a 7-fold increase in hip fractures and a 17-fold increase in vertebral fractures.
The adverse effects of glucocorticoids on the skeleton are due to direct actions on bone cells.
Laboratory testing should include measurement of serum 25-hydroxyvitamin D, creatinine, and calcium (in addition to glucose, potassium, and lipids).
The FRAX calculation underestimates fracture risk in GIO.
Bisphosphonates are first-line options for GIO and may also be useful in glucocorticoid-induced osteonecrosis.
Teriparatide counteracts several fundamental aspects of the pathophysiology of GIO.
Denosumab is useful in patients with renal insufficiency and stable serum calcium who are not candidates for bisphosphonates or teriparatide.
Do not wait. Start antifracture treatment at the onset of a course of glucocorticoid therapy of more than 10 mg/day of prednisone expected to last more than 90 days.
SUMMARY.
Glucocorticoid administration is the most common cause of secondary osteoporosis and the leading cause of nontraumatic osteonecrosis.
Patients are seldom warned about these side effects and as a result, adverse skeletal events are the most common glucocorticoid-related complications associated with successful litigation.
Disparity between bone quantity and quality in GIO makes ultrasound or BMD measurements inadequate for identifying patients at risk of glucocorticoid-induced fractures.
Continuous treatment with 10 mg/day of prednisone for more than 90 days is associated with a 7-fold increase in hip fractures and a 17-fold increase in vertebral fractures.
The adverse effects of glucocorticoids on the skeleton are primarily due to direct actions on osteoblasts and osteoclasts, decreasing the production of both osteoblasts and osteoclasts and increasing the apoptosis of osteoblasts while prolonging the lifespan of osteoclasts. Increased osteocyte apoptosis also occurs and is associated with decreases in VEGF, skeletal angiogenesis, bone interstitial fluid, and bone strength.
Laboratory testing should include measurement of serum 25OHD, creatinine, and calcium (in addition to glucose, potassium, and lipids).
The World Health Organization fracture prevention algorithm (FRAX) underestimates the risk of glucocorticoid-induced fractures.
Bisphosphonates are considered first-line options for GIO and may also decrease pain, delay lesion expansion, and reduce the need for surgery in glucocorticoid-induced osteonecrosis.
Teriparatide represents a particularly rational approach to GIO by counteracting several fundamental aspects of its pathophysiology.
Denosumab may be considered for glucocorticoid-treated patients with renal insufficiency and stable serum calcium levels who are not candidates for bisphosphonates or teriparatide.
Do not wait. Start antifracture treatment at the onset of a course of glucocorticoid therapy utilizing more than 10 mg/day of prednisone and expected to last more than 90 days.
ACKNOWLEDGEMENTS
The author would like to thank Drs. Stavros C. Manolagas, Robert L. Jilka, Maria Almeida, and Charles A. O’Brien for their advice and helpful discussions and to Drs. Stavros C. Manolagas, Robert L. Jilka, Maria Almeida, Fred H. Faas, and Irina Lendel for reviewing the manuscript.
SUPPORT
This material is based upon work supported by a VA Merit Review Grant from the Office of Research and Development, Department of Veterans Affairs and the National Institutes of Health (P01-AG13918).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no potential conflicts of interest relevant to this article.
References
- 1.Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism) Bull Johns Hopkins Hosp. 1932;50:137–195. [Google Scholar]
- 2.Boland EW, Headley NE. Management of rheumatoid arthritis with smaller (maintenance) doses of cortisone acetate. JAMA. 1950;144:365–372. doi: 10.1001/jama.1950.02920050005002. [DOI] [PubMed] [Google Scholar]
- 3.Freyberg RH, Traeger CH, Patterson M, et al. Problems of prolonged cortisone treatment for rheumatoid arthritis. JAMA. 1951;147:1538–1543. doi: 10.1001/jama.1951.03670330030008. [DOI] [PubMed] [Google Scholar]
- 4.Bollet AJ, Black R, Bumin JJ. Major undesirable side-effects resulting from prednisolone and prednisone. JAMA. 1955;157:459–463. doi: 10.1001/jama.1955.02960060017005. [DOI] [PubMed] [Google Scholar]
- 5.Heiman WG, Freiberger RH. Avascular necrosis of the femoral and humeral heads after high-dosage corticosteroid therapy. N Engl J Med. 1960;263:672–675. doi: 10.1056/NEJM196010062631404. [DOI] [PubMed] [Google Scholar]
- 6.Pietrogrande V, Mastromarino R. Osteopathia da prolungato trattmento cortisonico. Ortop e tramatol. 1957;25:791–810. [Google Scholar]
- 7.Weinstein RS. Clinical Practice: Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash JJ, Nash AG, Leach ME, et al. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144:10–15. doi: 10.1177/0194599810390470. [DOI] [PubMed] [Google Scholar]
- 10.LoCascio V, Bonucci E, Imbimbo B, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8:39–51. doi: 10.1016/0169-6009(91)90139-q. [DOI] [PubMed] [Google Scholar]
- 11.van Staa TP, Laan RF, Barton IP, et al. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arth Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 12.Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: A cross-sectional outpatient study. Bone. 2006;39:253–259. doi: 10.1016/j.bone.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein RS. Is long-term glucocorticoid therapy associated with a high prevalence of asymptomatic vertebral fractures in postmenopausal women? Nature Clin Practice Endocrinol Metab. 2007;3:86–87. doi: 10.1038/ncpendmet0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323–328. doi: 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 15.de Vries F, Bracke M, Leufkens HGM, et al. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arth Rheum. 2007;56:208–214. doi: 10.1002/art.22294. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JM, Modin GW, Arnaud CD, et al. Not all postmenopausal women on chronic steroids and estrogen treatment are osteoporotic: predictors of bone mineral density. Calcif Tissue Int. 1997;61:377–381. doi: 10.1007/s002239900351. [DOI] [PubMed] [Google Scholar]
- 17.Tatsuno I, Sugiyama T, Suzuki S, et al. Age dependence of early symptomatic vertebral fracture with high-dose glucocorticoid treatment for collagen vascular diseases. J Clin Endocrinol Metab. 2009;94:1671–1677. doi: 10.1210/jc.2008-1578. [DOI] [PubMed] [Google Scholar]
- 18.van Staa TP, Leufkins H, Cooper C. Use of inhaled glucocorticoids and risk of fractures. J Bone Miner Res. 2001;16:581–588. doi: 10.1359/jbmr.2001.16.3.581. [DOI] [PubMed] [Google Scholar]
- 19.Russcher H, Smit P, van den Akker ELT, et al. Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab. 2005;90:5804–5810. doi: 10.1210/jc.2005-0646. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JR, Westfall AO, Allison JJ, et al. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arth Rheum. 2005;52:2485–2494. doi: 10.1002/art.21194. [DOI] [PubMed] [Google Scholar]
- 21.Cooper MS, Rabbitt EH, Goddard PE, et al. Osteoblastic 11β-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J Bone Miner Res. 2002;17:979–986. doi: 10.1359/jbmr.2002.17.6.979. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein RS, Jilka RL, Parfitt AM, et al. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of the deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien CA, Jia D, Plotkin LI, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinol. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein RS, Wan C, Liu Q, et al. Endogenous glucocorticoids decrease vascularity and increase skeletal fragility in aged mice. Aging Cell. 2010;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeman E. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 26.Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their lifespan and reduce bone density. Endocrinol. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein RS, Jia D, Powers CC, et al. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinol. 2004;145:1980–1987. doi: 10.1210/en.2003-1133. [DOI] [PubMed] [Google Scholar]
- 28.Pearce G, Tabensky DA, Delmas PD, et al. Corticosteroid-induced bone loss in men. J Clin Endocrinol Metab. 1998;83:801–806. doi: 10.1210/jcem.83.3.4621. [DOI] [PubMed] [Google Scholar]
- 29.Tauchmanovà L, Pivonello R, Di Somma C, et al. Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab. 2006;91:1779–1784. doi: 10.1210/jc.2005-0582. [DOI] [PubMed] [Google Scholar]
- 30.Rubin MA, Bilezikian JP. The role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a reexamination of the evidence. J Clin Endocrinol Metab. 2002;87:4033–4041. doi: 10.1210/jc.2002-012101. [DOI] [PubMed] [Google Scholar]
- 31.Almeida M, Han L, Ambrogini E, et al. Glucocorticoids and tumor necrosis factor (TNF) α increase oxidative stress and suppress WNT signaling in osteoblasts via mechanisms involving pkcβ/P66SHC/JNK and AKT/FOXO. J Biol Chem. 2011;286:44326–44335. doi: 10.1074/jbc.M111.283481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohnaka K, Taniguchi H, Kawate H, et al. Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: Novel mechanism of glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun. 2004;318:259–264. doi: 10.1016/j.bbrc.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Lecka-Czernik B, Gubrij I, Moerman EJ, et al. Inhibition of Osf2/Cbfa1 expression and terminal differentiation by PPARγ2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 34.Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: Potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinol. 1999;140:4382–4389. doi: 10.1210/endo.140.10.7034. [DOI] [PubMed] [Google Scholar]
- 35.Xiong J, Onal M, Jilka RJ, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poetker DM, Smith TL. What rhinologists and allergists should know about the medico-legal implications of corticosteroid use: a review of the literature. Int Forum Allergy Rhinol. 2012 doi: 10.1002/alr.21016. [DOI] [PubMed] [Google Scholar]
- 37.Mankin HF. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 38.Skversky AL, Kumar J, Abramowitz MK, et al. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: results from the national health and nutrition examination survey (NHANES): 2001–2006. J Clin Endocrinol Metab. 2011;96:3838–3845. doi: 10.1210/jc.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arth Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 40.Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. J Bone Miner Res. 2000;15:1006–1013. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- 41.Reid DM, Devogelaer J-P, Saag K, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicenter, double-blind, double-dummy, randomized controlled trial. Lancet. 2009;373:1253–1263. doi: 10.1016/S0140-6736(09)60250-6. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein RS, Roberson PK, Manolagas SC. Giant Osteoclast Formation and Long-Term Oral Aminobisphosphonate Therapy. N Engl J Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinstein RS, Chen JR, Powers CC, et al. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 45.Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 46.Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleisch H. Bisphosphonates in bone disease: from the laboratory to the patient. 4th ed. San Diego, USA: Academic Press; 2000. p. 42. [Google Scholar]
- 48.Emkey R, Delmas PD, Goemaere S, et al. Changes in bone mineral density following discontinuation or continuation of alendronate therapy in glucocorticoid-treated patients: a retrospective, observational study. Arth Rheum. 2003;48:1102–1108. doi: 10.1002/art.10861. [DOI] [PubMed] [Google Scholar]
- 49.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American society for bone and mineral research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 50.Black DM, Kelly MP, Genant HK, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 51.Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–2039. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 52.Weinstein RS, Jilka RJ, Roberson PK, et al. Intermittent parathyroid hormone administration prevents glucocorticoid-induced osteoblast and osteocyte apoptosis, decreased bone formation, and reduced bone strength in mice. Endocrinol. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devogelaer J-P, Adler RA, Recknor C, et al. Baseline glucocorticoid dose and bone mineral density response with teriparatide or alendronate therapy in patients with glucocorticoid-induced osteoporosis. J Rheumatol. 2010;37:141–148. doi: 10.3899/jrheum.090411. [DOI] [PubMed] [Google Scholar]
- 54.Miller PD. Safety of parathyroid hormone for the treatment of osteoporosis. Curr Osteo Reports. 2008;6:12–16. doi: 10.1007/s11914-008-0003-y. [DOI] [PubMed] [Google Scholar]
- 55.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 56.Dore RK, Cohen SB, Lane NE, et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis. 2010;69:872–875. doi: 10.1136/ard.2009.112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinstein RS, O’Brien CA, Zhao H, et al. Osteoprotegerin prevents glucocorticoid-induced osteocyte apoptosis in mice. Endocrinol. 2011;152:3323–3331. doi: 10.1210/en.2011-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Syed MI, Patel NA, Jan S, et al. Symptomatic refractures after vertebroplasty in patients with steroid-induced osteoporosis. Am J Neuroradiol. 2006;27:1938–1943. [PMC free article] [PubMed] [Google Scholar]
- 59.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 60.Lai K-A, Shen W-J, Yang C-Y, et al. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. J Bone Joint Surg Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 61.Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: follow-up to eight years. J Bone Joint Surg Br. 2009;91:1013–1018. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- 62.Zhao D, Cui D, Wang B, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-deprived and cultures mesenchymal stem cells. Bone. 2012;50:325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Hansen KE, Wilson HA, Zapalowski C, et al. Uncertainties in the prevention and treatment of glucocorticoid-induced osteoporosis. J Bone Miner Res. 2011;26:1–8. doi: 10.1002/jbmr.362. [DOI] [PubMed] [Google Scholar]