Abstract
Objective
Patients with severe alcohol withdrawal and delirium tremens are frequently resistant to standard doses of benzodiazepines. Case reports suggest that these patients have a high incidence of requiring intensive care and many require mechanical ventilation. However, few data exist on treatment strategies and outcomes for these subjects in the medical intensive care unit (ICU). Our goal was a) to describe the outcomes of patients admitted to the medical ICU solely for treatment of severe alcohol withdrawal and b) to determine whether a strategy of escalating doses of benzodiazepines in combination with phenobarbital would improve outcomes.
Design
Retrospective cohort study.
Setting
Inner-city municipal hospital.
Patients
Subjects admitted to the medical ICU solely for the treatment of severe alcohol withdrawal.
Interventions
Institution of guidelines emphasizing escalating doses of diazepam in combination with phenobarbital.
Measurements and Main Results
Preguideline (n = 54) all subjects were treated with intermittent boluses of diazepam with an average total and maximal individual dose of 248 mg and 32 mg, respectively; 17% were treated with phenobarbital. Forty-seven percent required intubation due to inability to achieve adequate sedation and need for constant infusion of sedative-hypnotics. Intubated subjects had longer length of stay (5.6 vs. 3.4 days; p = .09) and higher incidence of nosocomial pneumonia (42 vs. 21% p = .08). Postguideline (n = 41) there were increases in maximum individual dose of diazepam (32 vs. 86 mg; p = .001), total amount of diazepam (248 vs. 562 mg; p = .001), and phenobarbital use (17 vs. 58%; p = .01). This was associated with a reduction in the need for mechanical ventilation (47 vs. 22%; p = .008), with trends toward reductions in ICU length of stay and nosocomial pneumonia.
Conclusions
Patients admitted to a medical ICU solely for treatment of severe alcohol withdrawal have a high incidence of requiring mechanical ventilation. Guidelines emphasizing escalating bolus doses of diazepam, and barbiturates if necessary, significantly reduced the need for mechanical ventilation and showed trends toward reductions in ICU length of stay and nosocomial infections.
Keywords: alcohol withdrawal, benzodiazepines, phenobarbital, intensive care unit
Alcoholism and alcohol withdrawal syndromes (AWS) still represent a major problem both in the inpatient and outpatient Approximately 3% of the general population in the United States self-describe symptoms f alcohol withdrawal (1). In one study, 8% of all general hospital admissions, 16% of all postsurgical patients, and 31% of all trauma patients developed AWS (2). The higher incidence in trauma patients has been confirmed in other studies (3) and probably reflects the high association of alcohol use with numerous types of dangerous behaviors
Benzodiazepines are currently the mainstay of therapy for all of the alcohol withdrawal syndromes including delirium tremens, the most severe form of alcohol withdrawal. Multiple studies suggest that administration of benzodiazepines in a symptom-triggered fashion both reduces the total amount of benzodiazepine administered and shortens the duration of therapy (4, 5). In these studies, benzodiazepines are administered in repeated boluses (both intravenous and oral) of 10–20 mg of diazepam (or benzodiazepine equivalent) with current recommendations suggesting a maximal individual bolus of 20–30 mg of diazepam or benzodiazepine equivalent to be given until adequate sedation is achieved (4, 6, 7). Although widely accepted, these studies focus primarily on patients admitted to detoxification centers or the general hospital wards and exclude subjects admitted to the intensive care unit (ICU) (4, 5).
Alcoholism accounted for 21% of all admissions to a medical ICU in one study, with AWS being the most common alcoholism-related diagnosis (8). These patients are typically more difficult to manage than those in detoxification units or wards as they require massive doses of benzodiazepines or other drugs for treatment of alcohol withdrawal. This may relate to the presence of severe underlying illness in this population before their development of alcohol withdrawal and/or the overrepresentation in the ICU of patients with benzodiazepine-resistant AWS. Benzodiazepine-resistant AWS, defined as the need for >40 mg of diazepam in 1 hr in one study, is associated with the need for endotracheal intubation and mechanical ventilation for administration of continuous infusion of alternative sedative hypnotics such as barbiturates or propofol, alone or in combination (9 –12). In one study, five of six subjects with benzodiazepine resistance, as defined previously, required intubation (9). Once patients are admitted to the ICU, numerous studies document the course and treatment of surgical patients who develop AWS postoperatively and/or posttrauma (3, 13, 14). In these subjects, symptom-triggered therapy results in lower rates of complications and improved outcomes, although nearly 65% required reinstitution of mechanical ventilation (14). However, the preexisting surgical diagnosis makes it difficult to generalize these results to medical patients admitted to the ICU solely for treatment of AWS.
Therefore, the goal of this study was two-fold: first, to detail the clinical characteristics and outcomes of a large cohort of subjects admitted to a medical ICU solely for the treatment of AWS, and second, to ascertain whether treatment guidelines directed at more aggressive and combination symptom-triggered therapy of resistant AWS would alter outcome in these subjects.
METHODS
This was a retrospective cohort study of all subjects admitted to the Bellevue Hospital medical ICU from July 2000 to June 2002 (preguideline) and July 2003 to May 2005 (postguideline) with an admission diagnosis of AWS as identified by medical ICU admission logs. The definition of AWS was made according to Diagnostic and Statistical Manual of Mental Disorders classifications for alcohol withdrawal, delirium tremens, and alcoholic hallucinosis (15). Patients were included in the analysis if they were admitted to the ICU solely for the treatment of AWS and a complete medical record was available for review. Patients were excluded from the analysis if any of the following criteria were met: a) presence of a serious medical or surgical diagnosis that would have otherwise dictated admission to the ICU; b) evidence of use of other illicit substances as determined by urine toxicology screen. Subjects were not excluded for a positive urine toxicology screen for benzodiazepines if the urine was obtained after therapeutic administration of benzodiazepines for AWS. Collection of data was approved by the NYU Internal Review Board.
Once subjects were eligible for inclusion, all demographic and clinical data were recorded including Acute Physiology and Chronic Health Evaluation II score. This included presence or absence of withdrawal seizures on that admission, pharmacologic therapy, indication for intubation and mechanical ventilation if performed, ICU length of stay, admission liver function tests, chemistries, ethanol level, and hematologic variables. The criteria for admission to the ICU, which remained constant throughout the study, were the requirement for either 200 mg of diazepam in 4 hrs or an individual dose of >40 mg of intravenous diazepam for control of agitation. The decision to intubate subjects was left to the individual practitioner. Diazepam is the benzodiazepine of initial choice at our institution, and, for consistency, all benzodiazepine dosages are reported in diazepam equivalents (16). All subjects were treated in a symptom-triggered fashion although there were no established guidelines for dose escalation. The decision to administer benzodiazepines, and thus the definition of severe agitation, was the presence of a Riker Sedation Analgesia Scale of ≥5, with a goal of achieving a Sedation Analgesia Scale score of 3–4 (17). Maximum individual dose of diazepam is defined as the highest individual bolus received by a subject during treatment. Development of nosocomial pneumonia was defined as the development of a new infiltrate plus two of either leukocytosis (white blood cell count >10,000 cells/mm3), purulent secretions, or fever (≥38°C) (18).
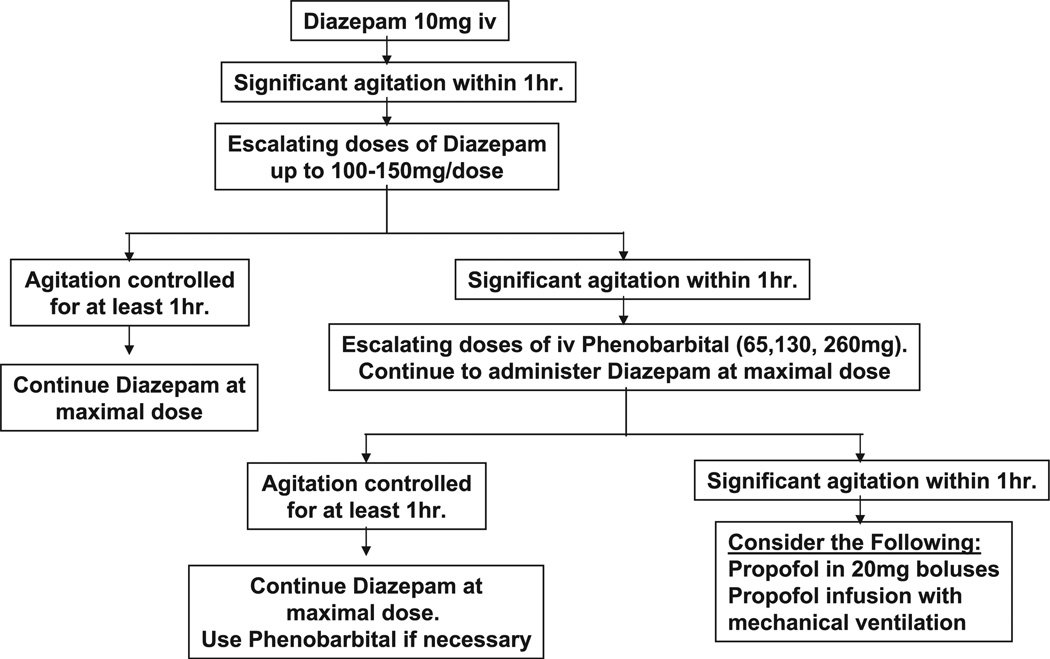
After initial analysis, data suggested that patients may be significantly undertreated and require more aggressive administration of sedative hypnotics. To achieve this, new guidelines were developed for the treatment of AWS and were fully implemented in July 2003. The basis of the guidelines was that for subjects for whom a given dose of benzodiazepine failed to control agitation for ≥1 hr, the dose of diazepam would be escalated until the patient remained calm for ≥1 hr. In cases where doses of >100–200 mg of diazepam were required, phenobarbital was added in similar fashion. These guidelines are summarized in Figure 1. The guidelines were posted on the hospital’s intranet and house staff intranet and were presented in educational lecture series to internal medicine and emergency medicine house staff. Postguideline, similar data collection was used from July 2003 to May 2005.
Figure 1.
Guidelines developed for the treatment of alcohol withdrawal in the intensive care unit (ICU). Guidelines were posted on the hospital intranet site and incorporated into regular lecture series for house staff. These were to be applied to subjects admitted to the medical ICU with a diagnosis of alcohol withdrawal and/or delirium tremens.
Statistics
Unless otherwise stated, a Mann-Whitney test was used to determine differences between groups for absolute variables. Chi-square test was used to determine differences in proportions between groups. Spearman’s test was used for univariate correlations. For all tests, p = .05 was considered significant. All analyses were performed with GraphPad Prism statistical software.
RESULTS
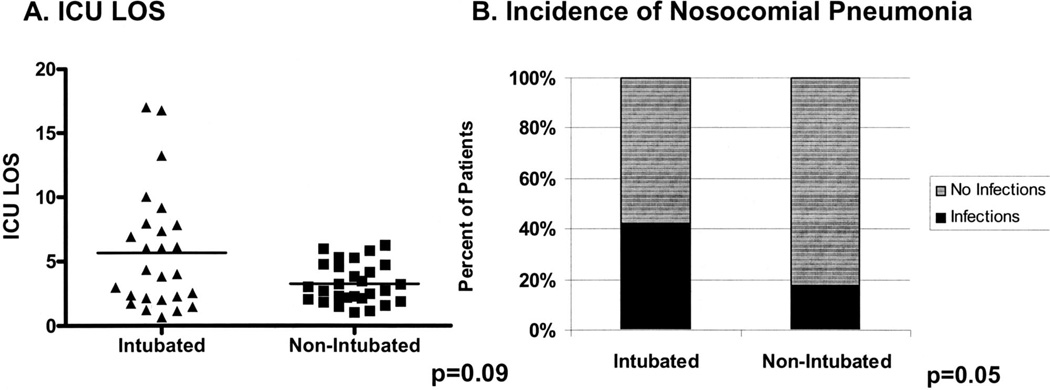
From July 2000 to June 2002 (preguideline), 54 subjects were admitted to the medical ICU with a primary diagnosis of AWS. Demographics can be found in Table 1. Of note, all patients were men, 98% met Diagnostic and Statistical Manual of Mental Disorders criteria for delirium tremens, and 27% had alcohol withdrawal seizures, all of which occurred before ICU admission. As a whole, the cohort had a median ICU length of stay (LOS) of 3.21 days (range 0.7–17.1). Forty-seven percent of subjects required mechanical ventilation, with the primary indication for intubation being inability to achieve adequate sedation with standard doses of diazepam and the subsequent “need” for constant infusion of benzodiazepines or propofol. Patients requiring mechanical ventilation had a trend toward a higher ICU LOS (Fig. 2A) and an increased incidence of nosocomial pneumonia (Fig. 2B). All subjects survived until hospital discharge. There was no difference in age, presence of withdrawal seizures, admission alcohol levels, or hematologic or biochemical variables between those requiring intubation and those not (data not shown).
Table 1.
Clinical characteristics
| Preguideline (n = 54) |
Postguideline (n = 41) |
|
|---|---|---|
| Epidemiological characteristics | ||
| Male, n (%) | 54 (100) | 41 (100) |
| Age, yrs | 45.7 ± 1.8 | 45 ± 1.2 |
| Delirium tremens, n (%) | 53 (98) | 40 (98) |
| Alcohol withdrawal seizures, n (%) | 15 (27) | 17 (38) |
| APACHE II | 13.0 ± 0.8 | 11.0 ± 0.8 |
| Biochemical characteristics | ||
| ETOH, g/dL | 71.7 ± 18.3 | 105.4 ± 24.8 |
| AST, units/L | 115.6 ± 14.2 | 140.1 ± 15 |
| ALT, units/L | 62 ± 6.2 | 82.1 ± 11 |
| HCT, % | 39.6 ± 0.8 | 40.9 ± 0.75 |
| MCV, fL | 95.9 ± 0.8 | 95.1 ± 0.86 |
APACHE, Acute Physiology and Chronic Health Evaluation; ETOH, ethanol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HCT, hematocrit; MCV, mean corpuscular volume.
Figure 2.
Subjects requiring mechanical ventilation have higher intensive care unit (ICU) length of stay (LOS) and incidence of nosocomial pneumonia. A, preguideline (n = 54), ICU LOS was compared between those requiring intubation (n = 26) and those not (n = 28). B, incidence of nosocomial pneumonia was compared between intubated and nonintubated subjects
All subjects received diazepam, and 52 (96%) subjects were treated with intravenous diazepam as the primary pharmacologic agent. Overall, diazepam accounted for 79% of all benzodiazepine doses in the first 24 hrs with the remaining doses given as either lorazepam or chlordiazepoxide. The mean amount of diazepam administered in the first 24 hrs was 248 mg. Consistent with previous reports (4, 6, 7), most subjects received diazepam in boluses between 10 and 40 mg with a mean maximum individual dose of 32.2 ± 3.6 mg and a median of 30 mg. Seventeen percent of subjects received phenobarbital, half of whom required mechanical ventilation, and 23% received propofol, all of whom were already receiving mechanical ventilation. Interestingly, patients who required mechanical ventilation received less diazepam in the first 24 hrs compared with those who did not (120 mg [25–75% quartile 50–310 mg] vs. 280 mg [125–365 mg]; p = .01). In addition, intubated subjects had a slightly lower maximal single dose of diazepam received before intubation compared with nonintubated subjects (29.7 ± 5.3 vs. 34 ± 4.9; p = .18). There was no correlation between total diazepam administered and ICU LOS. Finally, two subjects (4%) received at least one dose of haloperidol and no subject received β-adrenergic antagonists or clonidine.
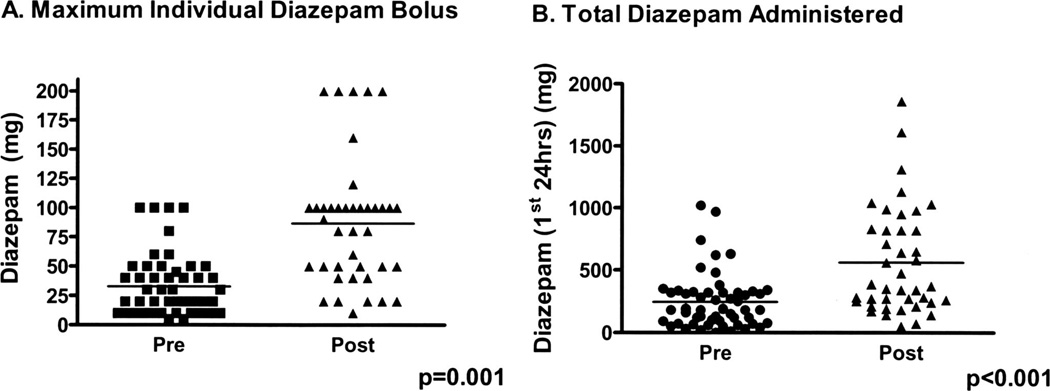
Following institution of the guidelines (postguideline) emphasizing escalation of the maximal individual dose of diazepam and use of barbiturates, there were significant changes in both the dose and type of pharmacologic therapy administered. Postguideline, 90% of all benzodiazepine doses were given as diazepam and the average dose of diazepam administered in the first 24 hrs increased twofold (Fig. 3A). This was associated with a significant three-fold increase in the mean maximal individual dose of diazepam used in symptom-triggered therapy (Fig. 3B). In addition, there was significantly greater use of phenobarbital postguideline (58% vs. 17%; p < .001) with a trend toward an increase in the median amount administered in the first 24 hrs (260 mg [25–75% quartile 87.5–650 mg] vs. 390 [130–1430 mg]; p = .1). Propofol remained the primary means of sedation in intubated subjects; however, postguideline, two subjects successfully received intermittent propofol dosing as a means of sedation without intubation. Consequently, there was only a nonsignificant trend toward less propofol use postguideline (17.8% vs. 23.1%; p not significant).
Figure 3.
Treatment guidelines were associated with increased total and individual dose diazepam administration. A, maximum individual bolus dose of diazepam administered pre- and postguideline. B, total amount of diazepam administered in the first 24 hrs.
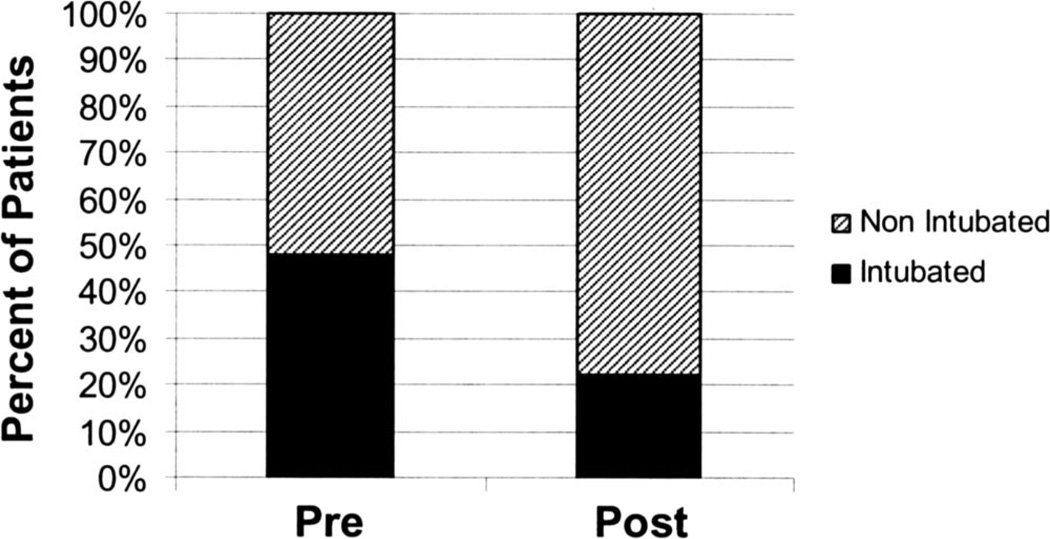
There was a significant reduction in the use of mechanical ventilation postguideline (21.9% vs. 47.3%; p = .008) (Fig. 4). Similar to preguideline, the primary indication for intubation was inability to control severe agitation with only one subject requiring intubation due to oversedation. Of note, two of nine subjects requiring intubation were not managed according to the guidelines. In one subject, the decision to intubate was made after only a 50-mg bolus of diazepam had proven ineffective. In the other, phenobarbital was not used when maximal doses of diazepam failed to control agitation. Similar to what was observed preguideline, those requiring mechanical ventilation postguideline had a higher ICU LOS (6.4 ± 1.6 vs. 3.1 ± 1.3 days; p = .001) and incidence of nosocomial pneumonia (55.5% vs. 12.5%; p = .02). Overall, there were nonsignificant reductions in total ICU LOS (3.8 ± 5.4 vs. 4.5 ± 4.7; p not significant) and the overall incidence of nosocomial complications (19.5% vs. 30.9%; p = .1) compared with patients admitted preguideline. Of note, postguideline there were no significant differences in demographics or biochemical characteristics of the patients compared with the initial survey (Table 1), and similar to the patients preguideline, there was no difference in demographics, Acute Physiology and Chronic Health Evaluation II score, presence of withdrawal seizures, or clinical biochemical characteristics in those requiring intubation and those not. However, there was now a correlation between ICU LOS and total amount of benzodiazepine administered postguideline (r = .48; p = .008).
Figure 4.
Institution of treatment guidelines was associated with a reduction in the need for mechanical ventilation.
DISCUSSION
Benzodiazepines have been the mainstay for the treatment of severe AWS since initially described by Kaim et al. (19). Subsequent to that report, numerous studies have investigated route, dose, and method of administration. Specifically, administration of benzodiazepines in a symptom-triggered fashion results in less benzodiazepine utilization and shorter duration of therapy (4, 5). However, these trials focused on subjects with relatively mild to moderate withdrawal who were stable enough to be treated in detoxification facilities or on the general medical ward. Based on the results of these trials, current recommendations are for administration of 10–30 mg of diazepam (or benzodiazepine equivalent) in repeated doses until adequate sedation (6).
Little is known about whether this method of therapy is adequate for the treatment of resistant AWS in the ICU. Spies et al. (14) demonstrated that in surgical/trauma patients who developed AWS in the ICU, symptom-triggered therapy with boluses of benzodiazepines was associated with less need for mechanical ventilation and a reduction in nosocomial infections. However, the main indication for ICU admission in those studies was their underlying surgical diagnosis, making it difficult to extrapolate these data to medical patients whose sole reason for ICU admission is severe AWS. In addition, the need for pain control, and thus opioid utilization, provides a significant confounder for treatment of these subjects when compared with those with isolated, severe AWS. In contrast to these studies, our cohort represents a population whose only indication for admission to the ICU was the inability to control severe psychomotor agitation due to AWS with standard doses of diazepam. In addition, the total size of our cohort (95 patients) is also one of the largest descriptors of AWS in the ICU to be reported, improving the generalizability of our results.
The most striking observation preguideline was the high use of mechanical ventilation in our patients. Although the incidence of mechanical ventilation was high (47%), it is in agreement with small case series of subjects with benzodiazepine-resistant AWS and in postoperative surgical patients treated with bolusdose therapy of benzodiazepines (9, 14). In concert with these studies, the use of mechanical ventilation was associated with a higher ICU LOS and development of nosocomial infections, suggesting that a strategy aimed at reducing the need for mechanical ventilation may improve overall clinical outcomes (14). The primary indication for mechanical ventilation was the failure of traditional bolus dosing of diazepam to control severe psychomotor agitation and the resultant need for continuous infusions of sedative hypnotics and subsequent intubation for airway protection. Although the total amount of diazepam administered was quite high, the maximal individual bolus was still only 30 mg, in accordance with most studies and recommendations (4, 6, 7). The lack of correlation between the total amount of diazepam administered and need for intubation or ICU LOS suggested that the method of sedative hypnotic administration may have an important role in achieving adequate mental status control in this population. Consequently, we instituted new treatment guidelines to incorporate escalating individual bolus doses of diazepam with the addition of barbiturates if necessary. Although these were only guidelines, the nearly three-fold increase in both the maximal bolus doses of diazepam administered and the frequency of barbiturate utilization strongly suggests that the guidelines altered clinical practice.
The significant reduction in the use of mechanical ventilation postguideline suggests a potential benefit to the incorporation of these guidelines. One important component of these guidelines is the use of escalating individual doses of benzodiazepines beyond currently recommended diazepam boluses of 10–30 mg (6). There are many reports of severe AWS and delirium tremens resistant to standard bolus doses of diazepam (9 –12, 20). The mechanism for benzodiazepine resistance is multifactorial. In animals, chronic ethanol abuse is associated with both altered structure of γ-aminobutyric acid type A (GABAA) receptor subunits as well as overall down-regulation of GABAA receptor expression (21–23). Consequently, rats suffering from chronic ethanol ingestion are highly resistant to GABA agonists such as benzodiazepines and may thus require higher doses to achieve the desired therapeutic effect (22). This has likely important clinical ramifications. Not only does it explain the potential benefit of and necessity for high doses of benzodiazepines in some subjects, but it also explains the safety of high-dose benzodiazepine administration in our cohort postguideline. Finally, the results of this study do not allow for any conclusions to be drawn as to which benzodiazepine is best suited for treatment of AWS in this population. Our institutional preference is to use diazepam for treatment of AWS based on its favorable pharmacologic profile (rapid peak clinical effect, long high life, active metabolites [desmethyldiazepam]) that may allow for better autotitration. However, it is likely that a similar effect would be observed with equivalently large bolus doses of other benzodiazepines such as lorazepam (20–30 mg), which also have proven efficacy for treatment of alcohol withdrawal seizures (24, 25).
The use of barbiturates as an adjunct is another important component to these guidelines. Our decision to use barbiturates as our primary adjunct was based on numerous factors. First, although antiadrenergic agents (β-blockers and clonidine) have been used in combination with benzodiazepines for treatment of AWS in surgical patients, randomized placebo-controlled trials document that the greatest benefit to these agents is modulation of autonomic signs of AWS (heart rate and blood pressure), with only modest effects for these agents on treatment of delirium with some reports documenting an increase in delirium with β-blocker treatment (3, 26–29). In similar fashion, a randomized controlled study of the α2-adrenergic agonist, lofexidine, documented an increase in delirium in subjects being treated for AWS with similar trends observed in postsurgical ICU subjects randomized to the combination of flunitrazepam/clonidine for treatment of AWS (3, 30, 31). We chose not to incorporate haloperidol based on both the lack of placebo-controlled data documenting benefit and the ability of neuroleptics to lower the seizure threshold and induce seizure in humans and animals with AWS (19, 32).
In contrast, many studies document the beneficial effects of barbiturates for the treatment of alcohol withdrawal and delirium tremens, with some studies even suggesting superiority of barbiturates to benzodiazepines (33, 34). Although no animal studies effectively document a synergistic effect to coadministration of benzodiazepines and barbiturates for AWS in vivo, neurophysiologic data suggest that this could be extremely beneficial. Similar to benzodiazepines, barbiturates also stimulate inhibitory GABAA receptors in the brain. However, barbiturates appear to augment benzodiazepines’ efficacy at the GABAA receptor (35). In addition, barbiturates can also inhibit stimulatory glutamate receptors, which are up-regulated in alcohol withdrawal (36 –38). The main drawback to the use of barbiturates is their narrow therapeutic window and potential to induce respiratory depression. However, in our cohort, this was not an issue postguideline as all but one patient was intubated for inability to achieve adequate sedation with bolus therapy. This is in part due to the fact that patients were given relatively low doses of phenobarbital (65–230 mg) compared with typical loading doses of 15 mg/kg. Another explanation is that benzodiazepines, with their greater safety profile, remained the mainstay of pharmacologic therapy, and barbiturates were only used when extremely high doses of benzodiazepines were unable to control extreme agitation as a sole agent.
Clinically, use of higher individual bolus doses of diazepam in combination with barbiturates was associated with a two-fold reduction in the utilization of mechanical ventilation. In addition, postguideline, there was a significant correlation between ICU LOS and benzodiazepine administration, suggesting that in contrast to what was observed preguideline, subjects were administered amounts of sedative-hypnotics in accordance with the severity of their withdrawal. The reduction in the need for mechanical ventilation was associated with a trend toward a reduction in ICU LOS and nosocomial infections compared with the preguideline group. One reason these secondary end points failed to reach significance is in part based on the number of patients studied. For example, there would need to be approximately 100 subjects in each arm to detect a 50% reduction in nosocomial pneumonia and a 1-day reduction in ICU LOS based on data in the preguideline cohort. Another potential explanation is that avoidance of intubation does not alter the underlying duration of severe AWS or susceptibility to nosocomial infections. This is much less likely, as postguideline ICU LOS and incidence of nosocomial pneumonia were significantly higher in intubated compared with nonintubated individuals. This further emphasizes that institution of mechanical ventilation is the largest contributor to excessive ICU LOS and nosocomial pneumonia, and strategies aimed at preventing this will overall improve outcomes in this population.
Finally, 19 subjects received propofol for the treatment of AWS, two of whom were not intubated at the time. Numerous case reports and small case series document successful use of propofol for benzodiazepine-resistant AWS (11, 12). Our study confirms these observations. The efficacy of propofol probably lies in its ability to activate GABAA chloride channels and inhibit N-methyl-d-aspartate-mediated neuroexcitation (39, 40). Although select reports suggest an increase in seizure-like activity with propofol administration (41), our data further validate the safety and efficacy of propofol in this population and reinforce its role in the treatment of AWS in subjects resistant to other sedative-hypnotics.
However, there are several limitations to our study. Most important is that this was not a randomized trial and treatment decisions, including the necessity for mechanical ventilation, were left up to the individual practitioner. Furthermore, the long period of data collection raises the possibility that other subtle systematic changes in ICU care could have significantly confounded the results. In addition, our admission criteria select for patients with isolated severe AWS and delirium tremens, and it is unclear if our results would be applicable in subjects with numerous other acute comorbid conditions. Another limitation is the lack of objective withdrawal categorization such as attained with use of the clinical institute withdrawal assessment for alcohol scale. CIWA scores have been used by numerous investigators for triggering bolus therapy for symptom-triggered therapy of alcohol withdrawal both in a detoxification setting and in the ICU in postsurgical/trauma patients (4, 5, 14). Consequently, we cannot fully exclude that differences were not due to subtle differences in the severity of withdrawal in the pre- and postguideline cohorts. However, there were no differences in either Acute Physiology and Chronic Health Evaluation II score or biochemical or epidemiologic characteristics between the two groups. This includes liver function tests and admission ethanol level, both of which have been shown to be predictors of outcome in subjects with mild withdrawal (42, 43). Furthermore, although use of CIWA for symptom-triggered therapy may have resulted in differences in total amounts of benzodiazepine required, the consistent use of other objective sedation scores to guide administration of sedative hypnotics makes it unlikely that differences in outcome were due to altered practitioner threshold for determination of the need, or effectiveness of, sedative hypnotics. Another potential limitation is the lack of a detailed set of guidelines for tapering the dose of benzodiazepines. Although it is possible that this could have been a significant confounder for ICU LOS, it is unlikely to have had a significant effect on the need for intubation, as the overwhelming majority (>90%) of intubations occurred in the first 24 hrs of therapy. Furthermore, the dramatic reduction in intubation rates, trends toward reduction in ICU LOS and nosocomial infections, and the relatively large number of patients and the lack of significant adverse events strongly suggest that this is a safe strategy to implement. It is extremely unlikely that these guidelines adversely affected patient care, and they are thus worthy of further study in a randomized controlled trial. Finally, it must be emphasized that the safety of these guidelines was in large part due to the monitoring available in the ICU, and consequently this strategy should not be used in nonmonitored settings.
CONCLUSIONS
This study documents the clinical outcomes of patients admitted to a medical ICU solely for the treatment of delirium tremens. Specifically, the need for mechanical ventilation is associated with both increased ICU LOS and nosocomial infection rate among patients with AWS. Implementation of guidelines that emphasize escalating individual bolus doses of diazepam with the addition of barbiturates if necessary resulted in a significant reduction in the need for mechanical ventilation and trends toward reductions in ICU LOS and development of nosocomial infections.
LEARNING OBJECTIVES.
On completion of this article, the reader should be able to:
Explain a rational treatment strategy for delirium tremens.
Describe the appropriate dosing of benzodiazepines and phenobarbital in this population.
Use this information in a clinical setting.
All authors have disclosed that they have no financial relationships with or interests in any commercial companies pertaining to this educational activity.
The authors have disclosed that propofol has not been approved by the U.S. Food and Drug Administration for use in the treatment of alcohol withdrawal. Please consult product labeling for the approved usage of this drug.
Lippincott CME Institute, Inc., has identified and resolved all faculty conflicts of interest regarding this educational activity.
Visit the Critical Care Medicine Web site (www.ccmjournal.org) for information on obtaining continuing medical education credit.
REFERENCES
- 1.Caetano R, Clark CL, Greenfield TK. Prevalence, trends, and incidence of alcohol withdrawal symptoms: Analysis of general population and clinical samples. Alcohol Health Res World. 1998;22:73–79. [PMC free article] [PubMed] [Google Scholar]
- 2.Foy A, Kay J, Taylor A. The course of alcohol withdrawal in a general hospital. QJM. 1997;90:253–261. doi: 10.1093/qjmed/90.4.253. [DOI] [PubMed] [Google Scholar]
- 3.Spies CD, Dubisz N, Neumann T, et al. Therapy of alcohol withdrawal syndrome in intensive care unit patients following trauma: Results of a prospective, randomized trial. Crit Care Med. 1996;24:414–422. doi: 10.1097/00003246-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: A randomized treatment trial. Arch Intern Med. 2002;162:1117–1121. doi: 10.1001/archinte.162.10.1117. [DOI] [PubMed] [Google Scholar]
- 5.Saitz R, Mayo-Smith MF, Roberts MS, et al. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272:519–523. [PubMed] [Google Scholar]
- 6.Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164:1405–1412. doi: 10.1001/archinte.164.13.1405. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WL, Johnson AD, Maddrey WL. Diazepam and paraldehyde for treatment of severe delirium tremens. A controlled trial. Ann Intern Med. 1975;82:175–180. doi: 10.7326/0003-4819-82-2-175. [DOI] [PubMed] [Google Scholar]
- 8.Marik P, Mohedin B. Alcohol-related admissions to an inner city hospital intensive care unit. Alcohol Alcohol. 1996;31:393–396. doi: 10.1093/oxfordjournals.alcalc.a008168. [DOI] [PubMed] [Google Scholar]
- 9.Dill C, Shin S. High-dose intravenous benzodiazepine. Acad Emerg Med. 2000;7:308–310. doi: 10.1111/j.1553-2712.2000.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 10.Nolop KB, Natow A. Unprecedented sedative requirements during delirium tremens. Crit Care Med. 1985;13:246–247. doi: 10.1097/00003246-198504000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Coomes TR, Smith SW. Successful use of propofol in refractory delirium tremens. Ann Emerg Med. 1997;30:825–828. doi: 10.1016/s0196-0644(97)70059-3. [DOI] [PubMed] [Google Scholar]
- 12.McCowan C, Marik P. Refractory delirium tremens treated with propofol: a case series. Crit Care Med. 2000;28:1781–1784. doi: 10.1097/00003246-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Spies CD, Nordmann A, Brummer G, et al. Intensive care unit stay is prolonged in chronic alcoholic men following tumor resection of the upper digestive tract. Acta Anaesthesiol Scand. 1996;40:649–656. doi: 10.1111/j.1399-6576.1996.tb04505.x. [DOI] [PubMed] [Google Scholar]
- 14.Spies CD, Otter HE, Huske B, et al. Alcohol withdrawal severity is decreased by symptom-orientated adjusted bolus therapy in the ICU. Intensive Care Med. 2003;29:2230–2238. doi: 10.1007/s00134-003-2033-3. [DOI] [PubMed] [Google Scholar]
- 15.First MB. Text Revision. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 16.Charney D, Mihi SC, Harris R. Hypnotics and sedatives. In: Hardman J, Limbird L, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. Tenth Edition. New York: McGraw-Hill; 2001. [Google Scholar]
- 17.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Rello J, Paiva JA, Baraibar J, et al. International Conference for the Development of Consensus on the Diagnosis and Treatment of Ventilator-Associated Pneumonia. Chest. 2001;120:955–970. doi: 10.1378/chest.120.3.955. [DOI] [PubMed] [Google Scholar]
- 19.Kaim SC, Klett CJ, Rothfeld B. Treatment of the acute alcohol withdrawal state: A comparison of four drugs. Am J Psychiatry. 1969;125:1640–1646. doi: 10.1176/ajp.125.12.1640. [DOI] [PubMed] [Google Scholar]
- 20.Hack J, Hoffman R, Nelson L. Resistant alcohol withdrawal: Does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2:55–60. doi: 10.1007/BF03161171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allan AM, Harris RA. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav. 1987;27:665–670. doi: 10.1016/0091-3057(87)90192-4. [DOI] [PubMed] [Google Scholar]
- 22.Cagetti E, Liang J, Spigelman I, et al. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 23.Follesa P, Biggio F, Talani G, et al. Neurosteroids, GABA(A) receptors, and ethanol dependence. Psychopharmacology (Berl) 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio G, Rathlev NK, Ulrich AS, et al. Lorazepam for the prevention of recurrent seizures related to alcohol. N Engl J Med. 1999;340:915–919. doi: 10.1056/NEJM199903253401203. [DOI] [PubMed] [Google Scholar]
- 25.Wretlind M, Pilbrant A, Sundwall A, et al. Disposition of three benzodiazepines after single oral administration in man. Acta Pharmacol Toxicol (Copenh) 1977;40(Suppl):28–39. [PubMed] [Google Scholar]
- 26.Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. N Engl J Med. 1985;313:905–909. doi: 10.1056/NEJM198510103131501. [DOI] [PubMed] [Google Scholar]
- 27.Worner TM. Propranolol versus diazepam in the management of the alcohol withdrawal syndrome: Double-blind controlled trial. Am J Drug Alcohol Abuse. 1994;20:115–124. doi: 10.3109/00952999409084061. [DOI] [PubMed] [Google Scholar]
- 28.Jacob MS, Zilm DH, MacLeod SM, et al. Propranolol-associated confused states during alcohol withdrawal. J Clin Psychopharmacol. 1983;3:185–187. [PubMed] [Google Scholar]
- 29.Stanley KM, Amabile CM, Simpson KN, et al. Impact of an alcohol withdrawal syndrome practice guideline on surgical patient outcomes. Pharmacotherapy. 2003;23:843–854. doi: 10.1592/phco.23.7.843.32719. [DOI] [PubMed] [Google Scholar]
- 30.Keaney F, Strang J, Gossop M, et al. A double-blind randomized placebo-controlled trial of lofexidine in alcohol withdrawal: Lofexidine is not a useful adjunct to chlordiazepoxide. Alcohol Alcohol. 2001;36:426–430. doi: 10.1093/alcalc/36.5.426. [DOI] [PubMed] [Google Scholar]
- 31.Adinoff B. Double-blind study of alprazolam, diazepam, clonidine, and placebo in the alcohol withdrawal syndrome: preliminary findings. Alcohol Clin Exp Res. 1994;18:873–878. doi: 10.1111/j.1530-0277.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 32.Blum K, Eubanks JD, Wallace JE, et al. Enhancement of alcohol withdrawal convulsions in mice by haloperidol. Clin Toxicol. 1976;9:427–434. doi: 10.3109/15563657608988141. [DOI] [PubMed] [Google Scholar]
- 33.Kramp P, Rafaelsen OJ. Delirium tremens: A double-blind comparison of diazepam and barbital treatment. Acta Psychiatr Scand. 1978;58:174–190. doi: 10.1111/j.1600-0447.1978.tb06930.x. [DOI] [PubMed] [Google Scholar]
- 34.Ives TJ, Mooney AJ, III, Gwyther RE. Pharmacokinetic dosing of phenobarbital in the treatment of alcohol withdrawal syndrome. South Med J. 1991;84:18–21. doi: 10.1097/00007611-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Amin J, Weiss DS. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald RL, McLean MJ. Cellular bases of barbiturate and phenytoin anticonvulsant drug action. Epilepsia. 1982;1(23 Suppl):S7–S18. doi: 10.1111/j.1528-1157.1982.tb06093.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman PL. Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metab Brain Dis. 1995;10:73–79. doi: 10.1007/BF01991784. [DOI] [PubMed] [Google Scholar]
- 38.Daniell LC. Effect of anesthetic and convulsant barbiturates on N-methyl-D-aspartate receptor-mediated calcium flux in brain membrane vesicles. Pharmacology. 1994;49:296–307. doi: 10.1159/000139246. [DOI] [PubMed] [Google Scholar]
- 39.Hans P, Bonhomme V, Collette J, et al. Propofol protects cultured rat hippocampal neurons against N-methyl-D-aspartate receptor-mediated glutamate toxicity. J Neurosurg Anesthesiol. 1994;6:249–253. doi: 10.1097/00008506-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Concas A, Santoro G, Mascia MP, et al. The general anesthetic propofol enhances the function of gamma-aminobutyric acid-coupled chloride channel in the rat cerebral cortex. J Neurochem. 1990;55:2135–2138. doi: 10.1111/j.1471-4159.1990.tb05807.x. [DOI] [PubMed] [Google Scholar]
- 41.Hickey KS, Martin DF, Chuidian FX. Propofol-induced seizure-like phenomena. J Emerg Med. 2005;29:447–449. doi: 10.1016/j.jemermed.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Wetterling T, Kanitz RD, Veltrup C, et al. Clinical predictors of alcohol withdrawal delirium. Alcohol Clin Exp Res. 1994;18:1100–1102. doi: 10.1111/j.1530-0277.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 43.Gorwood P, Limosin F, Batel P, et al. The A9 allele of the dopamine transporter gene is associated with delirium tremens and alcohol-withdrawal seizure. Biol Psychiatry. 2003;53:85–92. doi: 10.1016/s0006-3223(02)01440-3. [DOI] [PubMed] [Google Scholar]