Exhaled breath condensate (EBC) is a promising source of biomarkers of lung disease. It is important to note that EBC is not a biomarker, but rather a matrix in which biomarkers may be identified, in that way equivalent to blood, sweat, tears, urine and saliva. EBC may be thought of either as a body fluid or as a condensate of exhaled gas (and therefore not a body fluid). This issue is relevant because of potential regulatory issues involved with laboratory assessment of “body fluids”.
There are three principal contributors to EBC1. First are variable-sized particles or droplets that are aerosolized from the airway lining fluid (ALF)—such particles presumably reflect the fluid itself. Second is distilled water that condenses from gas phase out of the nearly water-saturated exhalate, substantially diluting the aerosolized ALF. Third are water soluble volatiles that are exhaled and absorbed into the condensing breath. Interest lies both in the non-volatile constituents mostly derived from the airway lining fluid particles and in the water-soluble volatile constituents which are found in substantially higher concentrations and are therefore more readily assayed than the non-volatile compounds.
The field of EBC research has advanced gradually, with the debates surrounding an emerging field helping to pose questions and gradually leading to answers. There are several key issues that are listed below.
1. Source of EBC biomarkers
Very little work has yet been done to help understand the nature and source of the exhaled particles/droplets that are part of the EBC matrix. That micron and sub-micron sized droplets emanate from the mouth or endotracheal tube in exhaled breath has been confirmed by laser particle counters2, 3, and indeed such particles serve as the only explanation for the presence of clearly non-volatile constituents in EBC such as cytokines4 and sodium ion5. However, how these particles form and change during exhalation before leaving the body is the subject only of speculation. Forwarded theories include that small amounts of ALF are torn from the airway surface when turbulence provides energy to the airway wall, similar to spray arising from whitecaps on the ocean on a windy day. Energy to overcome surface tension may also be applied to the wall when closed airways/alveoli pop open during inspiration, likewise potentially creating exhalable particles. Although surfactant and surfactant proteins found in EBC6 have been suggested to indicate an alveolar origin of the exhaled particles, this is not convincing, for alveolar fluids can move more proximal in the airway.
The size of particles that are measured exiting the mouth during expiration rapidly may be affected by condensation or evaporation. Size and numerical measurements by laser particle counters therefore reflect the particle size entering the counter, not necessarily the particle size initially generated from the airway lining surface.
2. Particle size
One 10 micron particle entering a sample of EBC can supply 1,000,000 times the quantity of non-volatiles to a sample of EBC as one 0.1 micron particle. However, there is skewing of the particle sizes exhaled towards the smaller particles3. Overall, the relative contribution to EBC non-volatile constituent of the different sized particles remains unknown.
3. Oropharyngeal contribution to EBC
In oral EBC collections, there is no reason to suspect that particles cannot be released from the oral and retropharyngeal mucosa into the airstream, with potential variably to contaminate what might otherwise be a pure lower airway sample. Furthermore, depending in part on the EBC collection equipment, gross or microscopic salivary contamination of EBC can and does occur7. Some subjects simply drool during collection, affirming the need for salivary trapping systems to be in place. Measures of salivary amylase are often used to test for the presence of salivary contamination. Most investigators find that no amylase is identified in the great majority of samples, although certainly those using higher sensitivity assays tend to report the presence of measurable amylase in a subset of samples7. It is important to mention that the amylase assays used are far from perfect and, similar to all other protein assays in EBC, suffer from some—potentially substantial—amount of false positivity and negativity. Overconfident reliance on any protein assay in EBC not uncommonly has led to mistaken conclusions, and this may be the case for amylase measures as well. Measurable phosphate has been suggested to be reliable indicators of salivary contamination as well8. One group concluded that saliva is the source of less than 10% of respiratory droplets9.
The ratios among various non-volatile compounds in EBC have been found to be substantially different than the ratio of compounds in saliva, suggesting a dominant (but not entire) lower airway source of EBC constituents9. In oral collections, there is currently no certainty that oral contribution can be completely excluded from the sample. In samples collected by endotracheal tube, there is confidence that the immediate source of volatiles and non-volatiles is the lower airway and lungs (although aspiration of saliva and gastric fluid can contaminate the lower airway fluid, of course).
4. Dilution
The ALF component of EBC is highly diluted by condensing vapor phase water. Estimates of the dilution of ALF particles in EBC range from 20-fold to 30,000- fold10. 2000 to 10000 fold seems to be a generally accepted number11. There may be relevant day-to-day and sample-to sample intrasubject variability in dilution, although debate occurs because the assays used for assessment of dilution are themselves a source of variability. As is common in much of medicine and biomedical science, in terms of a confident dilution marker for EBC there is as yet no gold standard. Within a given study, it seems worthwhile to attempt to standardize against a relevant additional EBC component, such as the conductivity of a lyophilized sample or ion measurements 10, 12, total protein, or urea measurement9. Indeed, in comparison to bronchoalveolar lavage, it may be easier to obtain a reliable dilution indicator for EBC, because unlike BAL, there is no reasonable mechanism by which collection of EBC significantly alters the ALF11.
There are two times when dilution markers are unnecessary. First are when multiple biomarkers are measured concurrently and their ratios considered. Ratios among inter-reactive or biologically related biomarkers can be of particular interest and eliminate the need for dilution markers. Examples of such ratios include IFN gamma (“Th1”) to IL4 (Th2) ratio13, 14, nitrite:nitrate (NO2−: NO3−) ratio15, reduced glutathione:oxidized glutathione (GSH:GSSG)16, and pH (which can be considered a ratio of acids and bases)17. The second time when dilution markers are unnecessary is when there is a confident assay for a substance which serves as an on-off indicator of an abnormality, such as present-not present. Examples might include the presence of M tuberculosis DNA (by PCR), gastric pepsin18, 19, rhinovirus RNA (by RT-PCR) and anthrax toxin. False positivity in such assays needs to be nil.
5. Lack of gold standards of lung disease assessment or diagnosis with which to compare EBC measurements
There is currently no gold-standard invasive or non-invasive method of determining absolute concentrations of ALF non-volatile constituents with which EBC can be readily compared. For example, bronchoalveolar lavage is subject to its own dilution concerns. Microsampling techniques that draw fluid from the airway wall by capillary action or suction alter the fluid itself, creating a lung biomarker equivalent to the Heisenberg Uncertainty Principle. Induced sputum suffers similarly to microsampling techniques in that the fluid expectorated appears at least somewhat affected by the sputum production process, at least on subsequent sampling. As a result, there is no consensus as to whether ALF is isotonic, hypo- or hypertonic in comparison to blood. The concentration of sodium ion in the human ALF remains subject to some uncertainty.
Our general disdain of invasively collecting samples from healthy lungs additionally limits our knowledge of normal airway fluid components, and normal variability. Invasiveness or discomfort of collection drastically limits our ability to study airway components. These issues underlie the attractiveness of EBC as a research and clinical tool. Importantly, the lack of ability to well access unadulterated ALF using other means should prompt caution if we are overly critical of EBC in light of the above delineated concerns. In fact, EBC has been used to assess the inflammatory effects of sputum induction which has been shown to increase two pro-inflammatory cytokines, IL6 and TNFα20. EBC may well be better than other alternatives in assessing the ALF milieu, as it may have fewer drawbacks than other methods.
6. Validation
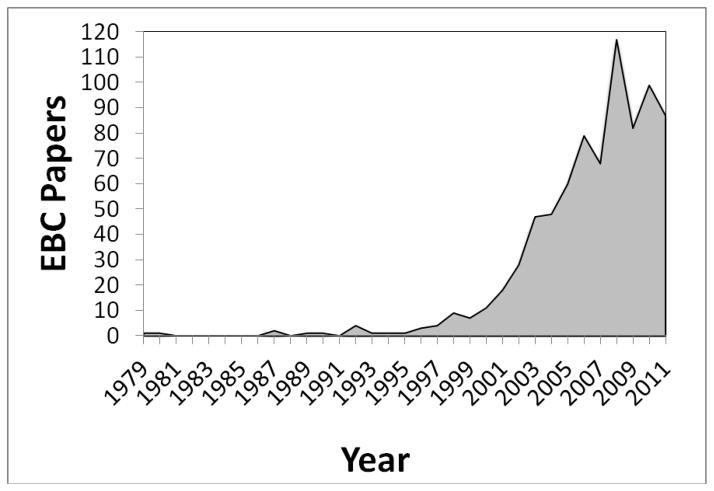
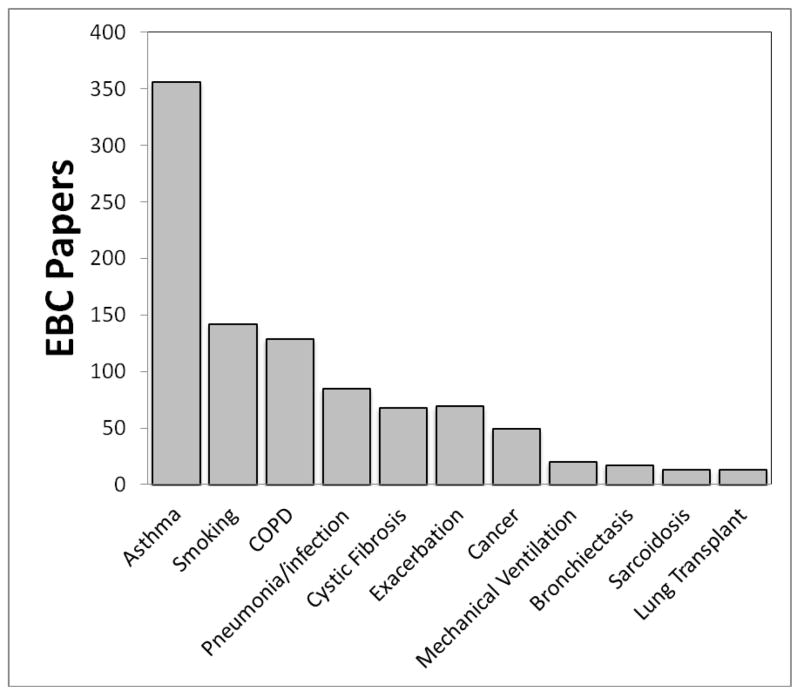
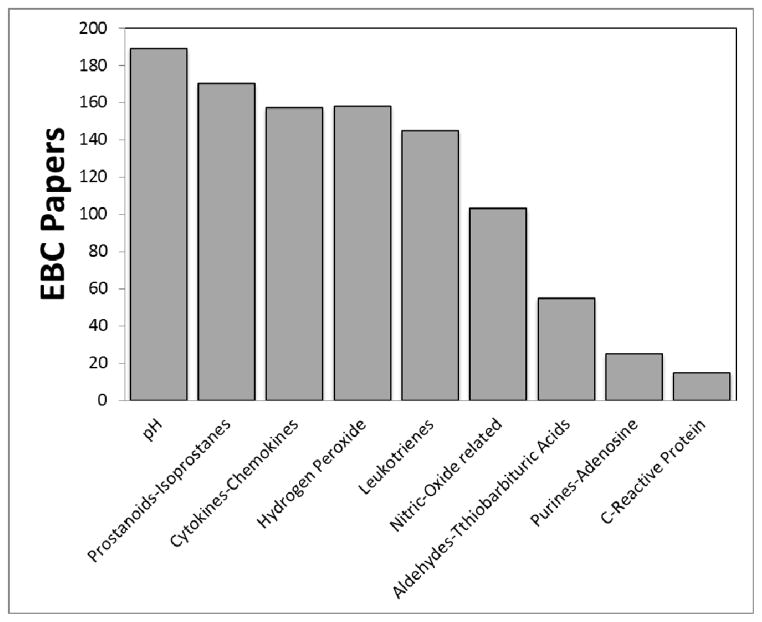
EBC is often lumped together with exhaled nitric oxide in review articles and insurance company briefings, but from a validation standpoint EBC is technically far behind exhaled nitric oxide (eNO)1. However this is not because exhaled NO is a “better” biomarker than EBC; we must remember that EBC is not a biomarker at all. Exhaled NO is one biomarker, whereas EBC is a matrix in which so many biomarkers have been identified that there is simply not the concentration of investigators studying any one EBC biomarker as there has been for eNO. A recent search of PubMed reveals 849 EBC papers, with the first paper in 197921. Publications involving EBC drastically increased at the turn of the century and are still coming out at a significant rate; 70 were published in 2011 and already 60 have been published as of June 2012 (see Figure 1). These papers cover over 11 diseases (not including different types of cancers, disease states, and infections) and over 100 biomarkers, with more being identified monthly. The most common diseases, conditions and biomarkers are depicted in Figures 2 and 3.
Figure 1.
EBC publications by year in the peer-reviewed literature.
Figure 2.
Common patient diagnoses and conditions reported in EBC publications, (including all manuscripts from single studies to reviews).
Figure 3.
Common biomarkers reported in EBC publications (including all manuscripts from single studies to reviews)
7. Collection of EBC
As noted, interest in EBC lies first and foremost with its ease of collection in nearly any setting. EBC can be safely collected orally from spontaneously breathing subjects or from patients undergoing mechanical ventilation22–24. The methods of collection for these settings, while both simple, vary somewhat.
Oral Collection
It takes as little as one breath to collect EBC, although in research practice, substantially longer collection times are often used to assure sufficient sample is available for repeated analysis of multiple biomarkers. Ten minutes of tidal breathing yields 1–2 milliliters of sample, and is well tolerated. Some centers focus on 1 or 2 biomarkers at a time allowing for smaller sample sizes. At our center, the most common oral EBC collection duration is 10 minutes.
Several options exist for collection of EBC samples from both spontaneously breathing and mechanically ventilated patients (Table 1). Multiple custom devices have been used throughout the years, using various cooling techniques, device shapes, materials and coatings. In terms of parts-which are often found about a respiratory laboratory-such home-made systems often can be made cheaply, although the expense in terms of personnel time may be surprisingly substantial. Commercially available equipment is also available. Certain biomarkers are seemingly best collected under set condensation conditions, but these conditions are markedly different for various biomarkers11. Although standardized methods of collection and storage for certain individual biomarkers are developing, there is no expectation that there will ever be a standardized EBC collection procedure that will be uniform for all biomarkers. Therefore any collection method that satisfies the needs of the user and biomarker is acceptable, but there is not, nor should there be, a one-size fit all standardized methodology.
Table 1.
Exhaled breath condensate collection systems currently used.
| EBC collection system | Manufacturer | Advantages | Disadvantages |
|---|---|---|---|
| ECoScreen I/II | Carefusion, Europe | Most commonly published EBC collection system. More common in European centers. Optional package for determination of total exhaled volume. Has been used to collect EBC during mechanical ventilation. | Not readily portable. Cleaning between patients may need to be extensive to abide by standard respiratory care practices. Limited ability to control condensation temperature. No longer distributed in the USA. |
| ECoScreen Turbo | Carefusion, Europe | Lightweight, portable condenser system. Controllable condenser temperature. Disposable collection circuit. Optional package for determination of total exhaled volume. | Few publications. No longer distributed in the USA. |
| RTube | Respiratory Research, USA | More total EBC collections performed using RTube than other systems. Multiple collections can be performed concurrently. More common in North American centers. Disposable (no cleaning between patients). Portable. Can be prepared for use in a standard freezer, enabling home collection. | Choice and maintenance of set condensing temperature requires optional cooling unit, otherwise condensation temperature is chosen by cooling sleeve preparation temperature and rises during collection. |
| RTube Vent | Respiratory Research, USA | Can be used inline with ventilator circuit or at expiratory port. Insignificant resistance regardless of placement in ventilator circuit. | Choice and maintenance of set condensing temperature requires optional cooling unit, otherwise condensation temperature is chosen by cooling sleeve preparation temperature and rises during collection. Few publications (safety data only). |
| Airway Lining Fluid Analyzer (ALFA) | Respiratory Research, USA | Has both non-disposable and disposable portions. Controllable collection temperature. Collects EBC continuously throughout the course of ventilation. Gas- standardizes and measures EBC pH continuously. Compatible with most ventilators. | Few publications. Complex system, requires skilled user. Only able to collect EBC at exhaust port of ventilator. |
Collection During Mechanical Ventilation
EBC can be collected in as little as 5 minutes during mechanical ventilation but there are several considerations specific to this patient population. The length of time needed to collect an adequate sample depends on the device used for collection (Table 1), the patient (i.e., higher volumes of EBC are obtained per given period of time in adults versus neonates) and the humidity devices/settings used (if any) during mechanical ventilation24. Samples collected from subjects receiving humidified mechanical ventilation will be diluted by the humidified bias flow that travels through the condenser (along with the exhaled breath). Reducing the humidity of the gas delivered to the patient may not be tolerated by the patient and will significantly reduce the amount of EBC sample collected over time, but will provide less dilute EBC. Dilution of the sample is less likely to affect the measurement of biomarker ratios than specific biomarkers. Another consideration is that EBC collected orally may have different biomarker ranges than EBC collected during mechanical ventilation because the upper airway is bypassed by the endotracheal or tracheostomy tube. Our group conducted a study of EBC pH pre/post intubation and it does not change in healthy subjects25, however the relatively low concentration of ammonia (a base) in endotracheally collected EBC allows for EBC pH to be a more sensitive indicator of airway acidification in intubated than non-intubated subjects.
EBC can be collected two ways during mechanical ventilation: in-line with the ventilator circuit or at the exhaust port of the ventilator (see Table 1). Both methods have pros and cons. In-line collection can be collected closer to the patient (on the expiratory end of the ventilator wye) and is therefore likely to trap larger particles in EBC that may rain out into the ventilator circuit prior to reaching the exhaust port. Downsides to in-line collection are the need to open the ventilator circuit in order to place and remove the collection device; this requires interrupting. Also, current in-line collection does not allow for analysis of EBC in real-time and, depending on the device, may limit the amount of EBC sample that can be collected (typically less than 5 ml). Collection at the exhaust port (post ventilator) does not require an interruption of ventilation and can allow for continuous collection EBC and even measurement of EBC pH in real-time22. Recent technological advances in mechanical ventilation EBC collection methods are stimulating this area of EBC research. Certain mechanical ventilators may alter their function in the presence of an EBC collection system, so it is important for a respiratory therapist or other experienced individual to formally assess how the devices interact. Be alert to the effects of any resistance added to the circuit or to the exhaust port.
8. Range of EBC biomarkers
Categorization of EBC biomarkers has been done in the past (Horvath Task Force11) although is open to change. There are several potential categorizations, and biomarkers may fall into one or more of the following groups:
-
Categorization group 1.
-
1
Volatile compounds
-
2
Non-volatile compounds
-
3
Non-volatile compounds derived from volatile compounds
-
1
-
Categorization group 2
-
4
Very low molecular weight compounds
-
5
Low molecular weight compounds
-
6
Polypeptides
-
7
Proteins
-
8
Nucleic acids
-
4
-
Miscellaneous differentiation
-
9
Lipid mediators
-
10
Inorganic molecules
-
11
Organic molecules
-
12
Redox relevant molecules
-
13
pH relevant molecules.
-
14
Cytokines, chemokines.
-
9
There is no reason to suspect that anything more than a tiny minority of potentially relevant compounds have been reported as of yet. Given a sufficiently sensitive assay, it is likely that any reasonably stable molecule in the ALF can be found in EBC and more useful EBC biomarker categories will result from new findings.
The most substantive difference among these categories is that between volatile and non-volatile constituents. As an introductory caveat, it is important to note that some clearly non-volatile compounds found in EBC may be derivatives of volatiles. For example, nitrate (NO3−) and nitrite (NO2−)—ionized and therefore not volatile—may arise in EBC in part from a reaction of volatile gaseous nitric oxide (NO) after reaction with oxygen26. Chloride ion (Cl−), another non-volatile, can be at least in part delivered as the volatile hydrochloric acid (HCl).
Volatiles
Volatiles such as acetic acid, formic acid and ammonia are found in much higher concentrations in EBC than non-volatile constituents, and tend, therefore, to be much easier to measure. Volatile biomarkers may be identified in the high micromolar or even low millimolar range. Their arrival and concentration in EBC is controlled by entirely different factors than the non-volatile biomarkers. Indeed, the amount and size of particles formed by turbulence (or other means) and dilution factors are irrelevant for volatile biomarkers. That dilution markers are not needed may enhance the value of the volatile biomarker assays.
However, other factors are important in regard to interpretation of volatile biomarker levels, including water solubility, gas-liquid partition coefficients, temperature of the source fluid (ALF), temperature of the condenser, pH of the source fluid and EBC, and the opportunity to react within (and therefore be captured by) the EBC matrix or collection device itself. So, what does this mean? It means we need to be careful about interpretation. An elevated level of formic acid in EBC may well not mean more formic acid production in the ALF, but rather may well indicate a lower pH of the ALF (and therefore enhanced volatility because non-volatile formate ion is protonated in acidic fluid to form the somewhat volatile species formic acid. The importance of EBC pH as an indicator of ALF pH is because of this feature of volatile acids and bases: Acids tend to be volatile from, while bases tend to be trapped by, acidic source fluid. When EBC pH is lower than normal, more acid and/or less base has been delivered to and captured in the EBC, primarily because more acid and less base has been volatilized from an acidic airway. Although EBC pH does not equal airway pH, an acidic EBC is indeed created from an acidic airway source fluid, so qualitative non-invasive assessments of airway pH deviation become achievable.
Some of the issues so far determined to be relevant to control for when planning to assay volatiles include:
Condensation temperature that is sufficiently cold to freeze the EBC may diminish the amount of volatiles (which are more readily absorbed into the liquid phase)25.
Frozen storage may protect reactive or unstable compounds, but may also allow sublimation of the volatiles into the airspace above the frozen EBC (unpublished observation). These volatiles will be lost when the storage container is opened, unless efforts are made to thaw and remix the sample before opening.
Volatile substances respond differently to sample manipulation. Each substance of interest should be studied well to control for potential effects of collection duration, temperature, storage conditions, and assay system.
Non-volatiles
Non-volatile constituents of EBC make up a broad category containing molecules as small as sodium ion (Na+) and as large as immunoglobulins. There are numerous publications in the literature presenting an individual compound that has been found in EBC (relying often on one assay) with levels depending upon disease state, with speculations added that the biomarker may be valuable in managing the disease of interest. There is optimism, but the more experienced researchers have also learned certain lessons:
Confirm the results of your assay with other assays using different methodology.
Assure that assay controls are performed appropriately and thoroughly. This is perhaps the single most important point for investigators studying EBC. EBC is a highly dilute, low-protein aqueous matrix. If one uses commercially available assay kits for EBC, it is important to assure that the standards used for comparison (standard curve generation) are done in a matrix as similar to that EBC sample as possible. The artifact-producing effect of using improper standards is often called a “matrix effect” and can be substantial in EBC assays27. Using proteinaceous standards (“cytokine X in BSA”), and attempting to compare to unaltered EBC will assuredly lead to misleading assay values. One choice is for the EBC sample to be altered so as to be substantially similar to the standards (such as by adding albumin, as the case may be). Because of inadequate recognition of matrix effect, it is likely that some of the published data are contaminated by artifacts sufficiently to invalidate their attached conclusions.
The pH of EBC does vary in disease states substantially, with pH values as low as 3.5 and has high as 9.0 reported17, 28. Because the reactivities and stabilities of many of the other biomarkers of interest are affected by the pH of the fluid in which they are found, and because accuracy of some assays can be affected as well, investigators need to be aware that EBC pH can cause assay artifact as well as loss (or gain) of biomarkers in EBC during storage.
Beware that, in the absence of dilution assessment different levels of a single EBC biomarker in a disease state may be interpreted either to represent different levels of the biomarker in the ALF, or a different amount or size of particles evolved from an otherwise identical ALF. To reiterate an earlier point, ratios among more than one related biomarker do not require dilution markers to be of more confident value.
Many if not most of the non-volatile constituents found within EBC are identified by assays pushing their lower limits of accuracy. On the one hand, great care should be taken to assure that the assay is reporting correctly. On the other hand, expectations for assay reproducibility at these low levels cannot be overly high. EBC biomarkers have often been critiqued as suffering from high intrasubject variability, and therefore of marginal value. In many cases, however, this variability may greatly result from assay variability as opposed to biological or EBC collection system variability. Such assay variability is found for dilution assessment efforts as well, which can mathematically compound the overall non-biologic variability and if not accounted for can lead to incorrect conclusions exaggerating the apparent biologic variability or EBC collection system variability.
Concentration of EBC samples by lyophilization/dehydration/freeze drying with resuspension of the lyophilate in small volumes of highly pure water has seemingly allowed for biomarker assessment by immunoassays in the many cases where the levels were previously simply too low to be measurable. A 1 ml EBC sample (collected in 7 minutes for example), can be lyophilized and resuspended into 50 uL—a 20-fold concentration. The advent of multiplex bead arrays has allowed for the tiny reconstituted volumes to be used for multiple immunoassays concurrently, opening up an exciting potential, with proof of concept in several studies, to gain a broad window on the balance among cytokines (or other mediators) in the ALF during any respiratory state. The issue of variable dilution remains (unless dilution factors are analysed), but the ratios among the cytokines are likely valid as long as sufficient assay controls have been performed. Cytokines present a particular challenge because in many cases they are at the lower limits of detection in serum/plasma and therefore often expected to be at even lower concentrations in EBC. One additional challenge in multiplex cytokine analysis lies in the variation between assay kits as their ability to detect cytokines varies from one platform to another. The reader is referred to Breen, et. al (2011) for further information29.
A key point is that conscientious assay technique will likely find in EBC any substance of substantially high concentration in the ALF. It is beyond the scope of this chapter to provide details regarding each of the biomarkers that has been reported in EBC, and the field is advancing with new discoveries weekly.
Interpretation of EBC Biomarkers
One of the major challenges in interpreting EBC biomarkers is the difficulty in comparing findings across studies. First, despite EBC methodological recommendations set forth by an ATS/ERS task force in 2005, many authors do not fully describe collection methods11. Second, there are multiple areas for variation in biomarker assays: 1) differences between laboratories or users, 2) differences between manufacturers or assay kits for the same biomarker and 3) differences between kit lot numbers from the same manufacturer. This makes it difficult to compare biomarker concentrations from one study to another. One recommendation is to analyze biomarker trends in terms of relative change rather than absolute concentration. Third, most biomarkers do not have established normal values or ranges. One method to overcome this deficiency is to describe biomarker variations in the context of the individual subject’s baseline where they serve as their own control. Clearly, one of the major needs in EBC research is the establishment of methodological standards and normative values specific to each EBC biomarker.
Future of EBC Research
With the formation of the International Association for Breath Research (IABR) in 2005, the development of the Journal of Breath Research in 2007 and the 2011 Breath Analysis Summit30, EBC research is advancing. As the field of EBC research and resulting literature expand, more methodological studies, reviews and meta-analyses will be conducted in order to fully develop EBC science and adequately assess the clinical relevance of EBC biomarkers. Exhaled biomarkers are ideal for many reasons but primarily because they are safe, non-invasive, can be repeatedly measured and represent the airway milieu. Exhaled breath may reveal more subtle changes in the airway, although it is yet unknown if exhaled biomarkers will show disease-induced variations earlier than traditional systemic markers (e.g., serum/plasma) and/or pulmonary diagnostics (e.g., pulmonary function testing, chest x-ray). Due to the array of biomarkers that can be assessed in exhaled breath, ease of collection and recent advances in technology, exhaled biomarkers have become an exciting field of research despite methodological issues. Development of sensitive tools for monitoring pulmonary diseases could potentially reduce patient burden, increase patient safety during diagnostic testing, aid in earlier diagnosis and give pulmonary-specific indicators of disease state, impending infection, and/or treatment effects. Exhaled breath research will expand our understanding of pathophysiologic mechanisms underlying pulmonary disease and provide a possible future for development of point-of-care testing. EBC research is an exciting, challenging and rapidly evolving line of inquiry.
Footnotes
Conflict of Interest: JH is a cofounder of Respiratory Research, Inc., which manufactures exhaled breath condensate collection equipment. MD and AM are supported by grant K99/R00 NR012016 from the National Institute of Nursing Research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael D. Davis, Email: mdavis35@vcu.edu, Project Director – Exhaled Breath Research, Virginia Commonwealth University, School of Nursing, Department of Adult Health and Nursing Systems, 804-828-3237.
Alison Montpetit, Email: ajmontpetit@vcu.edu, Assistant Professor, Virginia Commonwealth University, School of Nursing, Department of Adult Heath and Nursing Systems, 804-828-3422.
John Hunt, Email: Jfh2m@virginia.edu, Associate Professor of Pediatrics, Pulmonology, Allergy & Immunology, Box 800386, University of Virginia, Charlottesville, VA 22908, 434-243-9377.
References
- 1.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110(1):28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 2.Fairchild CI, Stampfer JF. Particle concentration in exhaled breath. Am Ind Hyg Assoc J. 1987;48(11):948–949. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- 3.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 4.Tufvesson E, Bjermer L. Methodological improvements for measuring eicosanoids and cytokines in exhaled breath condensate. Respir Med. 2005 doi: 10.1016/j.rmed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Zacharasiewicz A, Wilson N, Lex C, et al. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr Pulmonol. 2004;37(3):273–275. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]
- 6.Sidorenko GI, Zborovskii EI, Levina DI. [Surface-active properties of the exhaled air condensate (a new method of studying lung function)] Ter Arkh. 1980;52(3):65–68. [PubMed] [Google Scholar]
- 7.Gaber F, Acevedo F, Delin I, et al. Saliva is one likely source of leukotriene B4 in exhaled breath condensate. Eur Respir J. 2006;28(6):1229–1235. doi: 10.1183/09031936.00151905. [DOI] [PubMed] [Google Scholar]
- 8.Griese M, Noss J, Bredow CvC. Protein pattern of exhaled breath condensate and saliva. Proteomics. 2002;2(6):690–696. doi: 10.1002/1615-9861(200206)2:6<690::AID-PROT690>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Effros RM, Peterson B, Casaburi R, et al. Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J Appl Physiol. 2005;99(4):1286–1292. doi: 10.1152/japplphysiol.00362.2005. [DOI] [PubMed] [Google Scholar]
- 10.Effros RM, Hoagland KW, Bosbous M, et al. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165(5):663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 11.Horvath I, Hunt J, Barnes PJ, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 12.Effros RM, Biller J, Foss B, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 13.Shahid SK, Kharitonov SA, Wilson NM, et al. Increased interleukin-4 and decreased interferon-gamma in exhaled breath condensate of children with asthma. Am J Respir Crit Care Med. 2002;165(9):1290–1293. doi: 10.1164/rccm.2108082. [DOI] [PubMed] [Google Scholar]
- 14.Robroeks CM, Jobsis Q, Damoiseaux JG, et al. Cytokines in exhaled breath condensate of children with asthma and cystic fibrosis. Ann Allergy Asthma Immunol. 2006;96(2):349–355. doi: 10.1016/S1081-1206(10)61247-1. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TA, Woo-Park J, Hess M, et al. Assaying all of the nitrogen oxides in breath modifies the interpretation of exhaled nitric oxide. Vascul Pharmacol. 2005;43(6):379–384. doi: 10.1016/j.vph.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Yeh MY, Burnham EL, Moss M, et al. Non-invasive evaluation of pulmonary glutathione in the exhaled breath condensate of otherwise healthy alcoholics. Respir Med. 2008;102(2):248–255. doi: 10.1016/j.rmed.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paget-Brown AO, Ngamtrakulpanit L, Smith A, et al. Normative data for pH of exhaled breath condensate. Chest. 2006;129(2):426–430. doi: 10.1378/chest.129.2.426. [DOI] [PubMed] [Google Scholar]
- 18.Samuels TL, Johnston N. Pepsin as a marker of extraesophageal reflux. Ann Otol Rhinol Laryngol. 2010;119(3):203–208. doi: 10.1177/000348941011900310. [DOI] [PubMed] [Google Scholar]
- 19.Timms C, Thomas PS, Yates DH. Detection of gastro-oesophageal reflux disease (GORD) in patients with obstructive lung disease using exhaled breath profiling. J Breath Res. 2012;6(1):016003. doi: 10.1088/1752-7155/6/1/016003. [DOI] [PubMed] [Google Scholar]
- 20.Carpagnano GE, Foschino Barbaro MP, Cagnazzo M, et al. Use of exhaled breath condensate in the study of airway inflammation after hypertonic saline solution challenge. Chest. 2005;128(5):3159–3166. doi: 10.1378/chest.128.5.3159. [DOI] [PubMed] [Google Scholar]
- 21.Larson TV, Covert DS, Frank R. A method for continuous measurement of ammonia in respiratory airways. J Appl Physiol. 1979;46(3):603–607. doi: 10.1152/jappl.1979.46.3.603. [DOI] [PubMed] [Google Scholar]
- 22.Walsh BK, Mackey DJ, Pajewski T, et al. Exhaled-breath condensate pH can be safely and continuously monitored in mechanically ventilated patients. Respir Care. 2006;51(10):1125–1131. [PubMed] [Google Scholar]
- 23.Muller WG, Morini F, Eaton S, et al. Safety and feasibility of exhaled breath condensate collection in ventilated infants and children. Eur Respir J. 2006;28(3):479–485. doi: 10.1183/09031936.06.00063505. [DOI] [PubMed] [Google Scholar]
- 24.Carter SR, Davis CS, Kovacs EJ. Exhaled breath condensate collection in the mechanically ventilated patient. Respir Med. 2012;106(5):601–613. doi: 10.1016/j.rmed.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaughan J, Ngamtrakulpanit L, Pajewski TN, et al. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur Respir J. 2003;22(6):889–894. doi: 10.1183/09031936.03.00038803. [DOI] [PubMed] [Google Scholar]
- 26.Hunt J, Byrns RE, Ignarro LJ, et al. Condensed expirate nitrite as a home marker for acute asthma [letter] Lancet. 1995;346(8984):1235–1236. doi: 10.1016/s0140-6736(95)92947-9. [DOI] [PubMed] [Google Scholar]
- 27.Hom S, Walsh B, Hunt J. Matrix effect in exhaled breath condensate interferon-gamma immunoassay. J Breath Res. 2008;2(4):041001. doi: 10.1088/1752-7155/2/4/041001. [DOI] [PubMed] [Google Scholar]
- 28.Nicolaou NC, Lowe LA, Murray CS, et al. Exhaled breath condensate pH and childhood asthma: unselected birth cohort study. Am J Respir Crit Care Med. 2006;174(3):254–259. doi: 10.1164/rccm.200601-140OC. [DOI] [PubMed] [Google Scholar]
- 29.Breen EC, Reynolds SM, Cox C, et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol. 2011;18(8):1229–1242. doi: 10.1128/CVI.05032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corradi M, Mutti A. News from the Breath Analysis Summit 2011. J Breath Res. 2012;6(2):020201. doi: 10.1088/1752-7155/6/2/020201. [DOI] [PubMed] [Google Scholar]