Abstract
Purpose
To compare the mutational and copy number profiles of primary and metastatic colorectal carcinomas (CRCs) using both unpaired and paired samples derived from primary and metastatic disease sites.
Patients and Methods
We performed a multiplatform genomic analysis of 736 fresh frozen CRC tumors from 613 patients. The cohort included 84 patients in whom tumor tissue from both primary and metastatic sites was available and 31 patients with pairs of metastases. Tumors were analyzed for mutations in the KRAS, NRAS, BRAF, PIK3CA, and TP53 genes, with discordant results between paired samples further investigated by analyzing formalin-fixed, paraffin-embedded tissue and/or by 454 sequencing. Copy number aberrations in primary tumors and matched metastases were analyzed by comparative genomic hybridization (CGH).
Results
TP53 mutations were more frequent in metastatic versus primary tumors (53.1% v 30.3%, respectively; P < .001), whereas BRAF mutations were significantly less frequent (1.9% v 7.7%, respectively; P = .01). The mutational status of the matched pairs was highly concordant (> 90% concordance for all five genes). Clonality analysis of array CGH data suggested that multiple CRC primary tumors or treatment-associated effects were likely etiologies for mutational and/or copy number profile differences between primary tumors and metastases.
Conclusion
For determining RAS, BRAF, and PIK3CA mutational status, genotyping of the primary CRC is sufficient for most patients. Biopsy of a metastatic site should be considered in patients with a history of multiple primary carcinomas and in the case of TP53 for patients who have undergone interval treatment with radiation or cytotoxic chemotherapies.
INTRODUCTION
Genetic testing of patients with advanced colorectal carcinoma (CRC) for somatic mutations in KRAS has become routine clinical practice,1–5 and epidermal growth factor receptor inhibitors are now recommended only for use in patients with CRC whose tumors are KRAS wild type.6 There is also emerging evidence that mutations in BRAF and PIK3CA are associated with resistance to epidermal growth factor receptor–targeted agents.7–13 Finally, it has been suggested that inactivation of the TP53 gene, which is observed in 40% to 50% of CRCs, may influence response to therapy,14,15 although this requires validation in prospective clinical studies. Despite the routine use of KRAS mutational status to guide treatment selection, questions remain as to the optimal tissue source for genomic testing.
In this study, we performed a multiplatform genomic analysis of clinically relevant biologic events in a large cohort of primary and metastatic CRC tumors. We found the mutational concordance for the KRAS, NRAS, BRAF, PIK3CA, and TP53 genes between primary and metastatic disease to be high. Discordant results, when identified, were associated with multiple CRC primary tumors and, in the case of TP53, with tissue sampling and interval treatment.
PATIENTS AND METHODS
Tumor Specimens
In all cases, tissue and clinical data were collected on patients under an institutional review board–approved protocol or waiver of authorization. For each tumor, a hematoxylin and eosin–stained section was reviewed by a GI pathologist (E.V. or J.S.). When indicated, tumors were macrodissected to maximize tumor content. For all tumor-tumor pairs, DNA was checked for mislabeling, contamination, and misidentification using a multiplexed polymerase chain reaction (PCR)/mass spectrometry–based genetic fingerprinting assay, as previously reported.16 Normal DNA was used in the case of TP53 to establish the somatic nature of the mutations.
Genomic DNA Isolation
For frozen tissues, genomic DNA was extracted from two 30-μm frozen slices using the Genfind kit (Beckman Coulter Genomics, Beverly, MA), in a 96-well format, following the manufacturer's instructions. Tumor DNA was then whole genome amplified using the Repli-G Midi kit (Qiagen, Valencia, CA). The quality of whole genome–amplified DNA was verified by PCR reactions using two control amplicons.
Sequence Analysis
Mutations in KRAS (codons 12, 13, 22, 61, 117, and 146), NRAS (codons 12, 13, and 61), BRAF (codon 600), and PIK3CA (codons 345, 420, 542, 545, 546, 1043, and 1047) were detected using the iPLEX assay (Sequenom, San Diego, CA), as previously described.16 All mutations were confirmed either by a separate iPLEX assay or by Sanger sequencing. Mutations in TP53 were detected by Sanger sequencing of all coding exons, as previously reported.17 For 454 deep amplicon sequencing, PCR products for the desired targets were generated using primers designed with 5′ overhangs to facilitate emulsion PCR and sequencing. Primers for each sample were bar coded up to 10 per lane, followed by emulsion PCR and picotiter plate sequencing by-synthesis.
Array Comparative Genomic Hybridization
For comparative genomic hybridization (CGH) studies, labeled tumor DNA was cohybridized to Agilent 1M aCGH microarrays (Agilent, Santa Clara, CA) with a pool of reference normal. Raw copy number estimates were normalized18 and segmented with circular binary segmentation.19 Regions overlapping with copy number variations reported in the Database of Genomic Variants were excluded.20,21 Unsupervised hierarchical clustering was performed with one minus the Pearson correlation coefficient of the copy number profiles (segment means) as the distance measure and average linkage.22 Gains and losses were defined using a sample-specific threshold based on 2.2 median absolute deviations (approximately corresponding to 1.5 standard deviations) above and below the residual between the probe-level data and the segment means. The percent aberration was calculated per sample as the total number of gains and losses divided by the total number of probes. Patterns of gains and losses for primary-metastasis pairs were compared using a statistical methodology developed for testing clonality based on array CGH data23 using the Clonality R package.24
Statistical Analysis
The Fisher's exact test or χ2 test was performed to evaluate differences between data sets consisting of categorical variables. For analysis of the distribution of mutations between primary tumors and metastases, an unmatched single patient cohort was used, where patients with matched pairs were excluded from the analysis.
RESULTS
Clinical and Histologic Data
To compare the genomic profiles of primary and metastatic CRC, we collected 736 frozen CRC tumor samples from 613 patients; clinical characteristics are listed in Appendix Table A1 (online only). Primary invasive carcinomas comprised 57% of the specimens (n = 406), whereas 291 specimens were collected from metastatic foci and 39 were adenomas with or without high-grade dysplasia/intramucosal carcinoma. The metastatic sites included liver (n = 227, 78%), lung (n = 34), soft tissue (n = 14), brain (n = 7), ovary (n = 5), and distant lymph nodes (n = 4).
The cohort included two matched data sets. Matched data set A consisted of 84 pairs of primary tumor and metastasis, whereas matched data set B was a set of 31 pairs of metastatic foci, with each pair derived from the same patient. The majority of metastases in the matched data sets were from the liver (n = 208, 91%); however, lung (n = 11), soft tissue, (4) lymph node (n = 2), and brain (n = 1) metastases were also represented. For three of the primary tumors in matched data set A, the frozen tissue was from adenomas with high-grade dysplasia/intramucosal carcinoma.
Mutational Profiling
For all 613 patients, tumors were profiled for KRAS, NRAS, BRAF, PIK3CA, and TP53 mutations. To identify hotspot alterations in the KRAS, NRAS, BRAF, and PIK3CA genes, we used a mass spectrometry–based (Sequenom) assay.16 Because TP53 mutations are found scattered throughout the coding sequence, we performed Sanger sequencing of all TP53 coding exons. Mutations in KRAS or NRAS were identified in 277 patients (45.1%). Two hundred nineteen (35.7%) of the KRAS mutations were located at codon 12 or 13, whereas 58 patients (9.4%) had either NRAS mutation (2.9%) or mutations within exon 3 or 4 of KRAS (exon 3, 2%; exon 4, 4.6%). BRAF mutations were detected in 40 patients (6.5%), PIK3CA mutations were detected in 72 patient (11.7%), and TP53 mutations were detected in 247 patients (40.3%). The majority of PIK3CA mutations were in exon 9 (62.5%), whereas 29.2% of PIK3CA mutations were in exon 20. Mutations in KRAS, NRAS, and BRAF were all nonoverlapping in distribution. PIK3CA and TP53 mutations were found to co-occur with both RAS and BRAF mutations, although PIK3CA mutations were significantly more common in the KRAS mutant tumors than wild-type tumors (16.2% v 8.1%, respectively; P = .001)—and TP53 mutations were significantly more common in BRAF wild-type tumors than mutant tumors (9% v 2.6%, respectively; P = .002). PIK3CA mutations were present both in TP53 mutant and wild-type tumors (34.7% v 41%, respectively; P = .4).
The distribution of mutations between adenomas, primary invasive carcinomas, and metastases in an unmatched single patient cohort is shown in Figure 1. KRAS mutations were more prevalent in adenomas compared with primary carcinomas (68.6% v 42.7%, respectively; P = .004), whereas TP53 mutations were less common (8.6% v 30.3%, respectively; P = .005). Significantly fewer BRAF mutations were found in the metastases compared with the primary tumors (1.9% v 7.7%, respectively; P = .01), whereas TP53 mutations were significantly more prevalent (53.1% v 30.3%, respectively; P < .001).
Fig 1.
Frequency of RAS (KRAS, NRAS), BRAF, PIK3CA, and TP53 mutations in an unmatched, single-patient cohort of colon adenomas (n = 36), primary invasive colorectal adenocarcinomas (n = 323), and metastases (n = 160).
Concordance of Mutations in Matched Pairs of Primary Carcinomas and Metastases
To determine whether differences in the distribution of mutations between primary tumors and metastases were a reflection of their variable prognostic impact and not a result of differences in the genetic profiles of the predominant cell populations within the tumor-metastasis pairs, we examined the mutational concordance of a cohort of 84 pairs of primary and metastatic tumors. To minimize DNA quality as a confounding variable, we chose only patients for whom frozen tumor tissue from both the primary tumor and at least one metastasis was available. The mutational concordance rates for RAS/BRAF, PIK3CA, and TP53 after genotyping the frozen tissue were 92.8%, 96.4%, and 90.5%, respectively. Discrepant results were observed in 14 pairs affecting all genes studied (Appendix Table A2, online only). To exclude the possibility that the discordant results were attributable to tissue necrosis or low tumor content within the frozen samples, we analyzed formalin-fixed, paraffin-embedded (FFPE) material from all discordant pairs. After analysis of FFPE tissue from initially discordant pairs, the concordance rates increased to 97.6%, 98.8%, and 92.8% for RAS/BRAF, PIK3CA, and TP53, respectively, with discrepant results observed in only eight pairs (Fig 2).
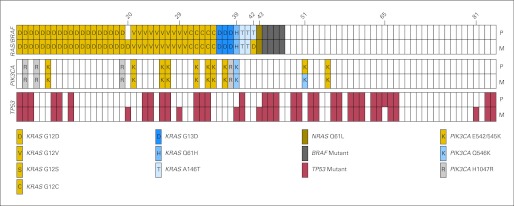
Fig 2.
Concordance of KRAS, NRAS, BRAF, PIK3CA, and TP53 mutations in 84 pairs of primary and metastatic colorectal carcinomas. The top rows represent the primary tumor (P), whereas the bottom rows represent the metastatic lesions (M). Mutations in KRAS, NRAS, and BRAF were mutually exclusive and thus grouped together. After analysis of both frozen and formalin-fixed, paraffin-embedded tissues, discordant results were observed in eight patients, with patient numbers shown above.
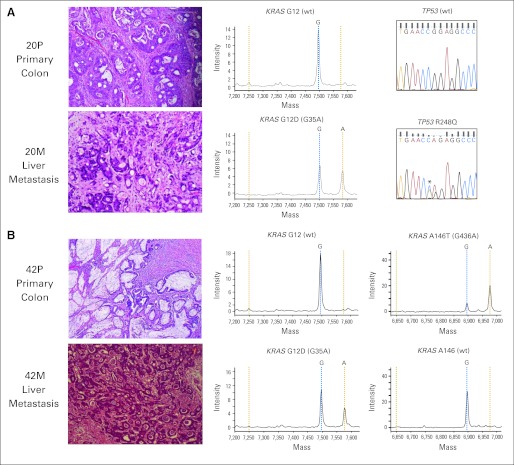
After analysis of both frozen and FFPE tissue, two of the 84 pairs had discordant KRAS mutational results (patients 20 and 42). In both patients, there was suspicion of a second primary adenocarcinoma. The first KRAS discordant pair was from a patient who underwent synchronous resection of a KRAS/TP53 wild-type, T3N0 cecal adenocarcinoma and a liver metastasis, the latter showing KRAS G12D and TP53 R248Q mutations (Fig 3A). Retrospective review of the patient's clinical record revealed that 6 months earlier, the patient had a T3N0 adenocarcinoma of the sigmoid colon resected, raising the possibility that the liver metastasis was derived from the preceding sigmoid colon carcinoma rather than the cecal adenocarcinoma resected at the time of her liver resection. Because the preceding procedure was performed at an outside institution, tissue from the sigmoid colon adenocarcinoma was not available for analysis.
Fig 3.
KRAS discordant pairs. (A) The primary tumor (P) was KRAS and TP53 wild type (wt), whereas the synchronously resected liver metastasis (M) harbored KRAS G12D and TP53 R248Q mutations. This patient had a sigmoid colon carcinoma removed 6 months prior at an outside institution. (B) The primary tumor harbored a KRAS A146T mutation, whereas the resected liver metastasis was KRAS G12D mutant. In this example, the tumor morphologies were also discordant between the primary and metastatic samples.
The second KRAS discordant pair was from a patient who had a synchronous resection of a T3N0 mucinous adenocarcinoma harboring a KRAS A146T mutation and a nonmucinous liver metastasis expressing a KRAS G12D mutation (Fig 3B). Although, no clinical history was available, the different tumor morphologies suggested that the liver metastasis may have been derived from a second primary lesion. Of note, our data set included another patient who had two primary invasive adenocarcinomas synchronously resected, a T3N2 cecal adenocarcinoma and a T3N1 rectal adenocarcinoma with mucinous features showing distinct mutational profiles (KRAS G12V, TP53 wild type v KRAS A146T, TP53 R306*, respectively). A liver metastasis removed during the same procedure was an adenocarcinoma with mucinous features whose mutational profile was identical to that seen in the rectal adenocarcinoma (KRAS A146T, TP53 R306*).
After analysis of frozen and FFPE tissues, six patients showed discrepancies in TP53 mutational status. In five of the six patients, the primary tumor was TP53 wild type, whereas the liver metastasis was TP53 mutant. In one patient (patient 65), a TP53 mutation was identified in the primary tumor but not in the liver metastasis. This patient was clinically notable because the patient had received neoadjuvant chemoradiotherapy treatment for a rectal primary tumor after resection of the liver metastasis. This clinical history suggests that the TP53 mutation found in the primary tumor may have been induced by the preceding chemoradiotherapy.
To further investigate the TP53 discordant pairs, we performed 454 deep amplicon sequencing of six primary-metastasis pairs (patient 8, 29, 39, 43, 65, and 81). In two of these pairs, the primary lesion contained invasive adenocarcinoma (patients 29 and 81), and in both pairs, we detected by 454 sequencing the mutant allele identified by Sanger sequencing in the metastasis at low allele frequency in the CRC primary tumor. A third pair that was discordant after analysis of the frozen samples (patient 8) was attributed to the absence of invasive adenocarcinoma in the original frozen section. When the invasive carcinoma and noninvasive components of this sample were analyzed separately using FFPE tissue, the TP53 mutation (R306*) found in the metastatic sample was detected in the invasive component but was absent in the noninvasive component (Fig 4). In two additional patients (patients 39 and 43), the primary frozen lesion was composed predominantly of adenoma with high-grade dysplasia/intramucosal carcinoma. In both of these latter patients, TP53 was wild type in the primary lesion by 454 sequencing, as was the liver metastasis of patient 65. In summary, the data suggest that TP53 status is highly concordant between primary and metastatic CRC tumors but that false-negative results may occur as a result of clonal/tissue heterogeneity and/or interval treatment with radiation or chemotherapy.
Fig 4.
Example of 454 TP53 sequencing results in a matched (A) primary and (B) liver metastasis pair (patient 8). The 454 sequencing showed the presence of a KRAS mutation (G12D) in 42.87%, 30.04%, and 53.67% of alleles in the adenomatous component, invasive component of the primary lesion, and metastasis, respectively. A TP53 R306* mutation was not detected in the adenomatous component of the primary lesion, whereas it was present in 15.27% and 39.5% of alleles in the invasive component of the primary tumor and in the metastasis, respectively. Genotyping of frozen primary tumor (intramucosal carcinoma) and the liver metastasis revealed concordant mutations in KRAS (G12D) but discordant TP53 results (data not shown).
Concordance of Mutations in Matched Pairs of Metastases
To examine the genetic heterogeneity of different metastatic foci, we compared the mutational profiles of 31 pairs of frozen metastases. After analysis of the frozen material and then FFPE in any initially discordant pairs, the concordance rates were 100%, 100%, and 96.7% for RAS/BRAF, PIK3CA, and TP53, respectively (Appendix Table A3, online only). The single discordant pair was from a 57-year-old woman who developed a solitary lung metastasis 2 years after resection of a stage III colon adenocarcinoma. This lung metastasis was TP53 wild type, whereas a second lung metastasis resected 2 years later, after treatment with several cytotoxic chemotherapeutic regimens, was TP53 mutant (R196*); 454 deep sequencing of the initial metastatic site failed to identify this TP53 mutant allele.
Analysis of Copy Number Aberrations in Primary and Metastatic CRCs
Whole genome copy number profiling has previously been shown to be of utility in distinguishing between metastatic and second primary carcinomas in patients with breast and lung cancers.25,26 Given the suspicion that the mutational discordance between several of the primary and metastatic samples was attributable to either multiple primary tumors or intervening chemotherapy or radiotherapy, we performed whole genome copy number analysis on 25 of the tumor-metastasis pairs, including three of the mutationally discordant pairs (patients 20, 42, and 65). Seventeen of the paired samples were derived from synchronous resections, whereas the remaining eight pairs were obtained from metachronous resections. Twenty samples (40%) were from patients who had received prior treatment.
Overall, the liver metastases showed more chromosomal aberrations compared with the primary tumors (9.6% v 7.5%, respectively). An analysis of the copy number data revealed no recurrent focal areas of copy number change in the metastatic samples that were not present in the primary lesions. However, focal aberrations in well-characterized cancer-associated genes were present in individual metastases but not in the primary lesions. For example, the metastasis from patient 38 had a focal deletion in the PARK2 gene, whereas the primary tumor from this patient was copy neutral at the PARK2 locus.
To determine whether pairs with discordant mutational profiles represented distinct primary cancers, we performed unsupervised, hierarchical clustering and clonality analyses of the array CGH data. The majority of pairs (20 of 25 pairs) clustered together (Appendix Fig A1, online only), and all 22 pairs with concordant mutational profiles were classified as clonal. Both pairs with discordant KRAS mutational results (patients 20 and 42) were deemed to be distinct primary tumors by this analysis (Table 1; Appendix Fig A2, online only). Furthermore, patient 65, who had received chemoradiotherapy to the primary lesion after resection of the liver metastasis, was deemed discordant based on both the hierarchical clustering and clonality assessments.
Table 1.
Clonality Analysis Based on Array Comparative Genomic Hybridization
| Patient Pair | Result | P |
|---|---|---|
| 65M, 65P | Equivocal | .68 |
| 20M, 20P | Equivocal | .25 |
| 42M, 42P | Equivocal | .19 |
| 4P, 4M | Clonal metastasis | .006 |
| 44P, 44M | Clonal metastasis | .005 |
| 5P, 5M | Clonal metastasis | .001 |
| 22P, 22M | Clonal metastasis | < .001 |
| 1P, 1M | Clonal metastasis | < .001 |
| 31P, 31M | Clonal metastasis | < .001 |
| 50P, 50M | Clonal metastasis | < .001 |
| 23P, 23M | Clonal metastasis | < .001 |
| 2P, 2M | Clonal metastasis | < .001 |
| 54P, 54M | Clonal metastasis | < .001 |
| 56P, 56M | Clonal metastasis | < .001 |
| 57P, 57M | Clonal metastasis | < .001 |
| 45P, 45M | Clonal metastasis | < .001 |
| 58P, 58M | Clonal metastasis | < .001 |
| 59P, 59M | Clonal metastasis | < .001 |
| 32P, 32M | Clonal metastasis | < .001 |
| 60P, 60M | Clonal metastasis | < .001 |
| 38P, 38M | Clonal metastasis | < .001 |
| 33P, 33M | Clonal metastasis | < .001 |
| 61P, 61M | Clonal metastasis | < .001 |
| 7P, 7M | Clonal metastasis | < .001 |
| 62P, 62M | Clonal metastasis | < .001 |
Abbreviations: P, primary tumor; M, metastasis.
DISCUSSION
With the development of cancer therapies that specifically target molecular alterations that mediate cancer progression, genotyping of patients with advanced CRC has become a component of routine clinical practice. Specifically, KRAS mutational testing has now been incorporated into several clinical practice guidelines for the treatment of patients with metastatic CRC. There is, however, no consensus as to how such testing should be performed, and it remains unknown whether testing of the primary lesion is sufficient. The National Comprehensive Cancer Network recommends testing either the primary tumor or a site of metastasis. This recommendation is based on several studies that have found a high (> 95%) KRAS mutational concordance between primary CRCs and metastases.21,27–29 In contrast to these results, several studies have reported a significant rate of discordance between the mutational profile of primary tumors and their corresponding metastatic lesions.30–33 These latter studies have led some to suggest testing of metastatic tumors when available. However, material from metastases is not routinely collected, and furthermore, its utility is often limited by low tumor content secondary to necrosis or prior treatment effect.
In this study, we sought to determine the incidence of clinically relevant mutations in primary CRC and metastases and their concordance in paired primary and metastatic samples. We genotyped tumors for mutations in the KRAS, NRAS, BRAF, PIK3CA, and TP53 genes using a multiplatform approach, which included mass spectrometry–based genotyping and Sanger sequencing. We found that the frequency of KRAS and PIK3CA mutations did not differ significantly between primary tumors and metastases. In contrast, TP53 mutations were significantly more frequent in metastases, whereas BRAF mutations were less frequent. Despite these differences, analysis of matched primary tumors and metastases showed a high concordance rate (> 90%) for the five genes examined. These results suggest that differences in the distribution of mutations are a reflection of their variable prognostic impact.
Consistent with the model that alterations in RAS and BRAF occur early in CRC pathogenesis,34 only two (2.4%) of 84 tumor-metastasis pairs were discordant for KRAS. Clinicopathologic assessment and a clonality analysis based on array CGH data suggested that both KRAS discordant pairs were not clonal. Thus, discordance in KRAS status between primary and metastatic lesions when it occurs may be attributable to the presence of more than one independent primary cancer, although larger studies will be needed to confirm this hypothesis. Synchronous colorectal adenocarcinomas are estimated to occur in 3.4% to 6.2% of patients with CRC.35–37 Although they remain a poorly studied group of tumors, one study found that nine of 13 pairs of synchronous primary colorectal neoplasms had discordant KRAS mutational profiles.38 Given these results and our results, it is prudent to recommend that, in this clinical context, genotyping should be performed on tissue obtained from a site of metastasis.
In the case of TP53, we found several reasons for discordant results, including the absence of invasive carcinoma in the frozen primary tumor and treatment effect. The former highlights the limitations of frozen tissue. Although current genotyping methods do not require the use of frozen tissue, it is considered by some to be the gold standard for molecular analyses, because it yields higher quality nucleic acids.39 However, only a small portion of a tumor is typically frozen, and the sample collected may not include the invasive component. This latter concern is of particular relevance in CRC because there is often an exophytic preinvasive component. Our data are thus consistent with the model that TP53 mutations occur later than KRAS mutations in CRC pathogenesis34 and suggest that TP53 genotyping should be performed using DNA derived from the invasive component of the primary lesion or from a metastatic site.
In summary, our results suggest that in most clinical scenarios, analysis of the primary colorectal tumor is sufficient for determining RAS, BRAF, PIK3CA, and TP53 mutational status. However, in patients with a history of more than one CRC primary tumor, a biopsy of a metastatic site should be considered if a treatment decision is being based on the mutational profiling results obtained.
Acknowledgment
We thank Igor Dolgalev, Sabrena Thomas, Kety Huberman, and Olga Aminova from the Geoffrey Beene Translational Oncology Core; Cora Mariano, Priscilla McNeil, Katrina Allen, and Anas Idelbi from the Tissue Procurement Service; and Irina Ostrovnaya and Venkatraman Seshan for their helpful discussion on the clonality analysis and Nick Socci for help with the bioinformatics analysis.
Appendix
Table A1.
Demographics and Clinical Characteristics of Study Group
| Demographic or Clinical Characteristic | % of Patients (N = 613) |
|---|---|
| Age, years | |
| Median | 62 |
| Range | 17-88 |
| Sex | |
| Male | 54.7 |
| Female | 45.3 |
| Tumor location | |
| Right colon | 37.4 |
| Left colon | 43.7 |
| Rectum | 18.9 |
| Disease stage at presentation | |
| I | 9.7 |
| II | 18.1 |
| III | 23.3 |
| IV | 48.9 |
Table A2.
Rate of Concordance in Matched Primary and Metastatic Colorectal Adenocarcinomas
| Gene | Frozen Samples |
After Analysis of FFPE Samples |
||
|---|---|---|---|---|
| No. of Concordant Pairs (n = 84) | % | No. of Concordant Pairs (n = 84) | % | |
| RAS/BRAF | 78 | 92.8 | 82 | 97.6 |
| PIK3CA | 81 | 96.4 | 83 | 98.8 |
| TP53 | 76 | 90.5 | 78 | 92.8 |
Abbreviation: FFPE, formalin-fixed, paraffin-embedded.
Table A3.
Rate of Concordance in Matched Colorectal Metastases
| Gene | Frozen Samples |
After Analysis of FFPE Samples |
||
|---|---|---|---|---|
| No. of Concordant Pairs (n = 31) | % | No. of Concordant Pairs (n = 31) | % | |
| RAS/BRAF | 30 | 96.7 | 31 | 100 |
| PIK3CA | 31 | 100 | 31 | 100 |
| TP53 | 29 | 93.5 | 30 | 96.7 |
Abbreviation: FFPE, formalin-fixed, paraffin-embedded.
Fig A1.
Copy number aberrations in primary and metastatic colorectal cancers. Hierarchical clustering of copy number aberrations for a set of 25 pairs of primary-metastasis colorectal cancers. Data were generated using the Agilent 1M feature aCGH array (Agilent, Santa Clara, CA). Segmented means were estimated from the raw copy number data using the circular binary segmentation algorithm and used for plotting the heat map. Sample names are labeled on the y-axis, and chromosomes are labeled on the x-axis. The hierarchical clustering dendrogram of the samples is plotted to the left of the heat map. Metastasis (black) or primary (white) status and KRAS/BRAF/PIK3CA/TP53 mutation status (white, wild type; black, mutant) are indicated on the right side of the heat map.
Fig A2.
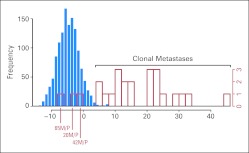
Likelihood ratio measures against a background reference distribution. The blue histogram consists of the log likelihood ratio measure calculated for all comparisons of tumors from different patients, and this serves as a reference distribution. The red histogram is a plot of the 25 tumor pairs from study patients. Three of these pairs (patients 65, 20, and 42) are consistent with the reference distribution, suggesting that they are not clonal metastases. M, metastasis; P, primary tumor.
Footnotes
See accompanying editorial on page 2937
Supported by grants from the National Institutes of Health (D.B.S), the Geoffrey Beene Foundation (D.B.S., L.B.S.), and the Society of Memorial Sloan-Kettering Cancer Center (M.W.).
Presented at the 47th Annual Meeting of the American Society of Clinical Oncology, June 3-7, 2011, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Leonard B. Saltz, Asuragen, Genomic Health, Genzyme Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Efsevia Vakiani, Leonard B. Saltz, David B. Solit
Provision of study materials or patients: Philip Paty, Timothy A. Chan
Collection and assembly of data: Efsevia Vakiani, Manickam Janakiraman, Zhaoshi Zeng, Jinru Shia, Andrea Cercek, Nancy Kemeny, Agnes Viale, Adriana Heguy, Philip Paty, Timothy A. Chan, Martin Weiser, David B. Solit
Data analysis and interpretation: Efsevia Vakiani, Manickam Janakiraman, Ronglai Shen, Rileen Sinha, Michael D'Angelica, Adriana Heguy, Timothy A. Chan, Leonard B. Saltz, Martin Weiser, David B. Solit
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 2.Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 3.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 4.Freeman DJ, Juan T, Reiner M, et al. Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer. 2008;7:184–190. doi: 10.3816/CCC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- 5.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 6.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 7.Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 9.Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 10.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 11.Tol J, Dijkstra JR, Vink-Börger ME, et al. High sensitivity of both sequencing and real-time PCR analysis of KRAS mutations in colorectal cancer tissue. J Cell Mol Med. 2010;14:2122–2131. doi: 10.1111/j.1582-4934.2009.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prenen H, De Schutter J, Jacobs B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–3188. doi: 10.1158/1078-0432.CCR-08-2961. [DOI] [PubMed] [Google Scholar]
- 13.Perrone F, Lampis A, Orsenigo M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 14.Oden-Gangloff A, Di Fiore F, Bibeau F, et al. TP53 mutations predict disease control in metastatic colorectal cancer treated with cetuximab-based chemotherapy. Br J Cancer. 2009;100:1330–1335. doi: 10.1038/sj.bjc.6605008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JS, Lee C, Bonifant CL, et al. Activation of p53-dependent growth suppression in human cells by mutations in PTEN or PIK3CA. Mol Cell Biol. 2007;27:662–677. doi: 10.1128/MCB.00537-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janakiraman M, Vakiani E, Zeng Z, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res. 2010;70:5901–5911. doi: 10.1158/0008-5472.CAN-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratilas CA, Hanrahan AJ, Halilovic E, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res. 2008;68:9375–9383. doi: 10.1158/0008-5472.CAN-08-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network: Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkatraman ES, Olshen AB. A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics. 2007;23:657–663. doi: 10.1093/bioinformatics/btl646. [DOI] [PubMed] [Google Scholar]
- 20.McCarroll SA, Kuruvilla FG, Korn JM, et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008;40:1166–1174. doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- 21.Knijn N, Mekenkamp LJ, Klomp M, et al. KRAS mutation analysis: A comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Development Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 23.Ostrovnaya I, Olshen AB, Seshan VE, et al. A metastasis or a second independent cancer? Evaluating the clonal origin of tumors using array copy number data. Stat Med. 2010;29:1608–1621. doi: 10.1002/sim.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrovnaya I, Seshan VE, Olshen AB, et al. Clonality: An R package for testing clonal relatedness of two tumors from the same patient based on their genomic profiles. Bioinformatics. 2011;27:1698–1699. doi: 10.1093/bioinformatics/btr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard N, Ostrovnaya I, Lau C, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res. 2009;15:5184–5190. doi: 10.1158/1078-0432.CCR-09-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bollet MA, Servant N, Neuvial P, et al. High-resolution mapping of DNA breakpoints to define true recurrences among ipsilateral breast cancers. J Natl Cancer Inst. 2008;100:48–58. doi: 10.1093/jnci/djm266. [DOI] [PubMed] [Google Scholar]
- 27.Etienne-Grimaldi MC, Formento JL, Francoual M, et al. K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropyrimidine therapy. Clin Cancer Res. 2008;14:4830–4835. doi: 10.1158/1078-0432.CCR-07-4906. [DOI] [PubMed] [Google Scholar]
- 28.Santini D, Loupakis F, Vincenzi B, et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: Implications for clinical practice. Oncologist. 2008;13:1270–1275. doi: 10.1634/theoncologist.2008-0181. [DOI] [PubMed] [Google Scholar]
- 29.Italiano A, Hostein I, Soubeyran I, et al. KRAS and BRAF mutational status in primary colorectal tumors and related metastatic sites: Biological and clinical implications. Ann Surg Oncol. 2010;17:1429–1434. doi: 10.1245/s10434-009-0864-z. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira C, Velho S, Moutinho C, et al. KRAS and BRAF oncogenic mutations in MSS colorectal carcinoma progression. Oncogene. 2007;26:158–163. doi: 10.1038/sj.onc.1209758. [DOI] [PubMed] [Google Scholar]
- 31.Albanese I, Scibetta AG, Migliavacca M, et al. Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem Biophys Res Commun. 2004;325:784–791. doi: 10.1016/j.bbrc.2004.10.111. [DOI] [PubMed] [Google Scholar]
- 32.Bossard C, Jamet P, Bezieau S, et al. Does testing of KRAS in patients with metastatic colorectal cancer offer valuable information in deciding treatment options? Mod Pathol. 2010;23:138A. abstr. [Google Scholar]
- 33.Houlle S, Lamy A, Blanchard F, et al. Metastatic CRCs KRAS and BRAF genotyping in routine diagnosis: Results and pitfalls. Mod Pathol. 2010;23:148A. abstr. [Google Scholar]
- 34.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 35.Chu DZ, Giacco G, Martin RG, et al. The significance of synchronous carcinoma and polyps in the colon and rectum. Cancer. 1986;57:445–450. doi: 10.1002/1097-0142(19860201)57:3<445::aid-cncr2820570307>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Finan PJ, Ritchie JK, Hawley PR. Synchronous and ‘early' metachronous carcinomas of the colon and rectum. Br J Surg. 1987;74:945–947. doi: 10.1002/bjs.1800741021. [DOI] [PubMed] [Google Scholar]
- 37.Lasser A. Synchronous primary adenocarcinomas of the colon and rectum. Dis Colon Rectum. 1978;21:20–22. doi: 10.1007/BF02586540. [DOI] [PubMed] [Google Scholar]
- 38.Ogino S, Brahmandam M, Kawasaki T, et al. Epigenetic profiling of synchronous colorectal neoplasias by quantitative DNA methylation analysis. Mod Pathol. 2006;19:1083–1090. doi: 10.1038/modpathol.3800618. [DOI] [PubMed] [Google Scholar]
- 39.Solassol J, Ramos J, Crapez E, et al. KRAS mutation detection in paired frozen and formalin-fixed paraffin-embedded (FFPE) colorectal cancer tissues. Int J Mol Sci. 2011;12:3191–3204. doi: 10.3390/ijms12053191. [DOI] [PMC free article] [PubMed] [Google Scholar]