Abstract
Purpose
Reported survival in patients with myeloproliferative neoplasms (MPNs) shows great variation. Patients with primary myelofibrosis (PMF) have substantially reduced life expectancy, whereas patients with polycythemia vera (PV) and essential thrombocythemia (ET) have moderately reduced survival in most, but not all, studies. We conducted a large population-based study to establish patterns of survival in more than 9,000 patients with MPNs.
Patients and Methods
We identified 9,384 patients with MPNs (from the Swedish Cancer Register) diagnosed from 1973 to 2008 (divided into four calendar periods) with follow-up to 2009. Relative survival ratios (RSRs) and excess mortality rate ratios were computed as measures of survival.
Results
Patient survival was considerably lower in all MPN subtypes compared with expected survival in the general population, reflected in 10-year RSRs of 0.64 (95% CI, 0.62 to 0.67) in patients with PV, 0.68 (95% CI, 0.64 to 0.71) in those with ET, and 0.21 (95% CI, 0.18 to 0.25) in those with PMF. Excess mortality was observed in patients with any MPN subtype during all four calendar periods (P < .001). Survival improved significantly over time (P < .001); however, the improvement was less pronounced after the year 2000 and was confined to patients with PV and ET.
Conclusion
We found patients with any MPN subtype to have significantly reduced life expectancy compared with the general population. The improvement over time is most likely explained by better overall clinical management of patients with MPN. The decreased life expectancy even in the most recent calendar period emphasizes the need for new treatment options for these patients.
INTRODUCTION
Myeloproliferative neoplasms (MPNs) are a group of clonal hematologic malignancies with great variation in reported patient life expectancy.1 MPNs, consisting of polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), and MPN unclassifiable (MPN-U), are characterized by a relatively indolent course, which can be complicated by thromboembolic events, and transformation to acute myeloid leukemia (AML). In 2005, new light was shed on the pathophysiology of MPNs with the discovery of an activating mutation in the Janus kinase 2 domain (JAK2 V617F) of the erythropoietin receptor, resulting in excess uni-, bi-, or trilinear myeloproliferation.2–5 Since then, several new mutations and a familial predisposition have been described in MPNs.6–8
Although progress has been made in the understanding of the pathogenesis and molecular biology of MPNs, there is still uncertainty regarding reported survival among patients with MPNs. To our knowledge, there have been only a small number of population-based studies published on survival in PV, ET, and PMF, which have either included a limited number of patients or had a limited follow-up time.9–12 In these studies as well as clinical trials, patients with PMF have consistently been reported to have reduced life expectancy.10–14 In the majority of studies, patients with PV have been observed to have moderately reduced survival.9,15–18 In contrast, according to most,10,15,19–22 but not all,23 reports, survival of patients with ET is not affected by the disease.
To establish patterns of survival in patients with MPNs, we conducted a large population-based study including more than 9,000 patients with MPNs diagnosed in Sweden from 1973 to 2008, with follow-up until 2009.
PATIENTS AND METHODS
Registries and Diagnostics
Sweden provides universal medical care for the entire population, currently approximately 9.5 million people. Information regarding patients diagnosed with a malignant disease in Sweden is by law reported to the population-based nationwide Swedish Cancer Registry, which was established in 1958.24 It is mandatory for every physician to report each patient with an MPN to the register; in 1984, the double reporting system (both clinicians and pathologists/cytologists) was introduced for MPNs, increasing the completeness of the registry.25 Each individual in Sweden receives a unique national registration number, and every death date is recorded in the Cause of Death Registry.
In Sweden, from the mid 1970s to the late 1990s, the diagnostics of PV, ET, and PMF were based on the Polycythemia Vera Study Group criteria.26,27 During the study period, certain modifications to the classification of MPNs were made. MPN-U was introduced into the Swedish Cancer Register in 1993. Diagnostic criteria were revised in 2001 by the WHO, introducing prefibrotic PMF as a new entity, separated from ET by bone marrow histology.28JAK2 status was incorporated into the 2008 WHO classification criteria.1 Information on the number of allogeneic stem-cell transplantations in patients with MPNs in Sweden during the study period was obtained from the European Group for Blood and Marrow Transplantation register, which was established in 1974.
Patient Cohort
We identified all patients diagnosed with an MPN reported to the Swedish Cancer Register between January 1, 1973, and December 31, 2008. Information was gathered on sex, date of birth, and date of diagnosis. By linking the national registration number to the Cause of Death Register, information on date of death was collected up to December 31, 2009 (end of follow-up). Patients underwent follow-up from the date of diagnosis until death, emigration, or end of follow-up, whichever occurred first.
The study was approved by the Stockholm Regional Ethics Review Board. Informed consent was waived, because we had no contact with study patients.
Survival Analyses
Relative survival ratios (RSRs) were computed as measures of patient survival.29,30 Relative survival is defined as the observed survival in the patient group (where all deaths are considered events) divided by the expected survival of a comparable group from the general population, which is assumed to be free from the cancer in question. RSR provides a measure of total excess mortality associated with a diagnosis of MPN, irrespective of whether the excess mortality was directly or indirectly associated with the MPN. Expected survival was estimated using the Ederer II method from the Swedish population life tables stratified by age, sex, and calendar period.31 One-, 5-, 10-, 15-, and 20-year RSRs with 95% CIs were calculated for patients with MPNs during four calendar periods: 1973 to 1982, 1983 to 1992, 1993 to 2000, and 2001 to 2008. The two latter calendar periods were made shorter to obtain a more even patient distribution and facilitate detection of effects on survival resulting from introduced changes in classification and treatment. In the most recent calendar period, 1-, 5-, and (because of the limited follow-up time) 8-year RSRs were calculated. RSRs were calculated separately in patients with different MPN subtypes (PV, ET, PMF, and MPN-U) for the whole cohort and for each of the four calendar periods. In addition, RSRs were analyzed in patients diagnosed before versus after 1993, which was when the category MPN-U was introduced. RSRs were calculated for patients in five age categories (< 50, 50 to 59, 60 to 69, 70 to 79, and ≥ 80 years) and separately for men and women.
Poisson regression was used to model excess mortality to estimate the effects of the factors described while controlling for potential confounding factors.30 The parameter estimates from this model are interpreted as excess mortality rate ratios (EMRRs). An EMRR of 1.5, for example, for men/women indicates that men experience 50% higher excess mortality than women. All EMRRs were adjusted for age, sex, and calendar period of diagnosis. All calculations were performed using STATA version 10.1 (STATA, College Station, TX).
RESULTS
A total of 9,384 patients with MPNs were identified (PV, n = 4,389; ET, n = 2,559; PMF, n = 1,048; and MPN-U, n = 1,388; Table 1). There was a higher proportion of women (53%). Median age at diagnosis was 71 years and was constant during the last three calendar periods. The reported number of patients during each calendar period increased over time, being more than twice as high in the most recent calendar period compared with the first (Table 1). Median year of diagnosis was 1995, and 6,180 patients (66%) died during follow-up. A total of 71 allogeneic stem-cell transplantations in patients with MPNs were reported to the European Group for Blood and Marrow Transplantation register during the study period. Of these, 72% were carried out during the most recent calendar period (2001 to 2008; Table 1). The majority of patients receiving transplants (63%) had PMF.
Table 1.
Patient Distribution
| Characteristic | Calendar Period |
Total | |||
|---|---|---|---|---|---|
| 1973 to 1982 | 1983 to 1992 | 1993 to 2000 | 2001 to 2008 | ||
| Total No. of patients | 1,644 | 2,413 | 2,292 | 3,035 | 9,384 |
| MPN subtype | |||||
| PV | 1,248 | 1,336 | 861 | 944 | 4,389 |
| ET | 157 | 728 | 697 | 977 | 2,559 |
| PMF | 239 | 349 | 171 | 289 | 1,048 |
| MPN-U | — | — | 563 | 825 | 1,388 |
| Age, years | |||||
| < 50 | 133 | 221 | 256 | 300 | 910 |
| 50-59 | 233 | 275 | 277 | 458 | 1,243 |
| 60-69 | 461 | 605 | 492 | 627 | 2,185 |
| 70-79 | 585 | 912 | 788 | 948 | 3,233 |
| ≥ 80 | 232 | 400 | 479 | 702 | 1,813 |
| Median age at diagnosis, years | 69 | 71 | 71 | 71 | 71 |
| Sex | |||||
| Male | 821 | 1,135 | 1,054 | 1,443 | 4,453 |
| Female | 823 | 1,278 | 1,238 | 1,592 | 4,931 |
| Male/female ratio | 50/50 | 47/53 | 46/54 | 48/52 | 47/53 |
| No. of SCTs | 1 | 4 | 15 | 51 | 71 |
Abbreviations: ET, essential thrombocythemia; MPN, myeloproliferative neoplasm; MPN-U, MPN unclassified; PMF, primary myelofibrosis; PV, polycythemia vera; SCT, stem-cell transplantation (allogeneic).
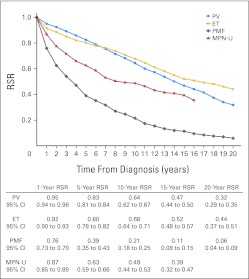
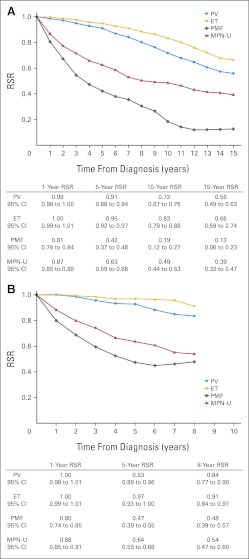
Patient survival was considerably lower in all MPN subtypes compared with expected survival in the general population; 10- and 20-year RSRs were 0.64 (95% CI, 0.62 to 0.67) and 0.32 (95% CI, 0.29 to 0.35) for PV, 0.68 (95% CI, 0.64 to 0.71) and 0.44 (95% CI, 0.37 to 0.51) for ET, and 0.21 (95% CI, 0.18 to 0.25) and 0.06 (95% CI, 0.04 to 0.09) for PMF, respectively (Fig 1). Ten- and 15-year RSRs were 0.49 (95% CI, 0.44 to 0.53) and 0.39 (95% CI, 0.32 to 0.47) for patients classified as MPN-U (Fig 1). Compared with those with PV, patients with PMF and MPN-U had higher overall excess mortality; EMRRs were 4.38 (95% CI, 3.90 to 4.91) and 4.57 (95% CI, 3.87 to 5.41) for PMF and MPN-U, respectively. Patients with ET had an EMRR of 1.83 (95% CI, 1.59 to 2.10) using PV as reference (P < .001). The inferior survival of patients with ET compared with those with PV was confined to those diagnosed before 1993 (data not shown). After 1993, 10-year RSRs were 0.72 (95% CI, 0.67 to 0.76) and 0.83 (95% CI, 0.79 to 0.88) for PV and ET, respectively (Fig 2A).
Fig 1.
Cumulative relative survival among patients with myeloproliferative neoplasms (MPNs) in Sweden, stratified by subtype, during the whole study period from 1973 to 2008. ET, essential thrombocythemia; MPN-U, MPN unclassifiable; PMF, primary myelofibrosis; PV, polycythemia vera; RSR, relative survival ratio.
Fig 2.
Cumulative relative survival among patients with myeloproliferative neoplasms (MPNs) in Sweden, stratified by subtype, diagnosed (A) during the years 1993 to 2008 and (B) during the most recent calendar period of 2001 to 2008. ET, essential thrombocythemia; MPN-U, MPN unclassifiable; PMF, primary myelofibrosis; PV, polycythemia vera; RSR, relative survival ratio.
There was an excess mortality in patients with any subtype during all calendar periods. During the most recent calendar period (2001 to 2008), 8-year RSRs were 0.84 (95% CI, 0.77 to 0.90), 0.91 (95% CI, 0.84 to 0.97), 0.48 (95% CI, 0.39 to 0.57), and 0.54 (95% CI, 0.47 to 0.60) in patients with PV, ET, PMF, and MPN-U, respectively (Fig 2B).
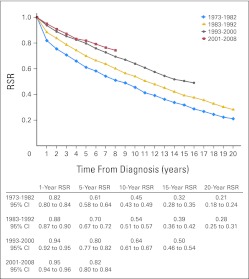
A significant improvement in RSRs was observed over time in an analysis of the whole MPN cohort (P < .001; Fig 3). Compared with patients diagnosed from 1973 to 1982, EMRRs were 0.60 (95% CI, 0.53 to 0.67) for patients diagnosed from 1983 to 1992, 0.29 (95% CI, 0.25 to 0.34) for patients diagnosed from 1993 to 2000, and 0.23 (95% CI, 0.19 to 0.27) for patients diagnosed from 2001 to 2008 (Table 2). The improvement between the two most recent calendar periods was of borderline significance (P = .046). In a stratified analysis of patients diagnosed before and after 1993, significant improvement in RSRs over time was seen in patients with PV and ET, whereas no improvement was observed in patients with PMF; 10-year RSRs were 0.21 (95% CI, 0.17 to 0.25) for those with PMF diagnosed before 1993 and 0.19 (95% CI, 0.12 to 0.27) after 1993.
Fig 3.
Cumulative relative survival among patients with myeloproliferative neoplasms in Sweden stratified by calendar period of diagnosis. RSR, relative survival ratio.
Table 2.
Excess Mortality Rate Ratios
| Characteristic | Excess Mortality Rate Ratio | 95% CI | P* |
|---|---|---|---|
| MPN subtype | |||
| PV | 1.00 (reference) | ||
| ET | 1.83 | 1.59 to 2.10 | < .001 |
| PMF | 4.38 | 3.90 to 4.91 | < .001 |
| MPN-U | 4.57 | 3.87 to 5.41 | < .001 |
| Year of diagnosis | |||
| 1973-1982 | 1.00 (reference) | ||
| 1983-1992 | 0.60 | 0.53 to 0.67 | < .001 |
| 1993-2000 | 0.29 | 0.25 to 0.34 | < .001 |
| 2001-2008 | 0.23 | 0.19 to 0.27 | < .001 |
| Age at diagnosis, years | |||
| < 50 | 0.33 | 0.26 to 0.40 | < .001 |
| 50-59 | 0.55 | 0.47 to 0.65 | < .001 |
| 60-69 | 1.00 (reference) | ||
| 70-79 | 1.50 | 1.34 to 1.67 | < .001 |
| ≥ 80 | 2.68 | 2.35 to 3.05 | < .001 |
| Sex | |||
| Male | 1.00 (reference) | ||
| Female | 0.72 | 0.66 to 0.78 | < .001 |
| Hospital type | |||
| Nonuniversity | 1.00 (reference) | ||
| University | 0.92 | 0.84 to 1.01 | .077 |
NOTE. Global tests for effect of subtype, calendar period, age, and sex were all significant (P < .001).
Abbreviations: ET, essential thrombocythemia; MPN, myeloproliferative neoplasm; MPN-U, MPN unclassified; PMF, primary myelofibrosis; PV, polycythemia vera.
P values are for test compared with reference; all variables adjusted for MPN subtype, calendar period of diagnosis, age at diagnosis, and sex.
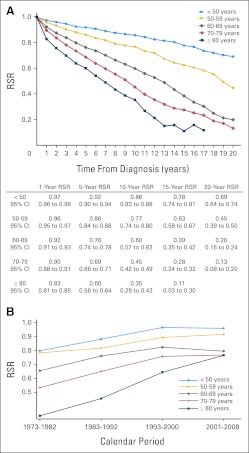
Older age at MPN diagnosis was associated with poorer survival (Fig 4A). Improved survival rates were seen in all age groups, in all but the most recent calendar period (Fig 4B).
Fig 4.
(A) Cumulative relative survival among patients with myeloproliferative neoplasms (MPNs) in Sweden stratified by age at diagnosis and (B) estimates of 5-year cumulative relative survival ratios (RSRs) among patients with MPNs stratified by age and calendar period of diagnosis.
Women had significantly superior survival, with an EMRR of 0.72 (95% CI, 0.66 to 0.78), compared with men (reference 1.00; Table 2). There was no significant difference in survival of patients diagnosed at university hospitals compared with nonuniversity hospitals (EMRR, 0.92; 95% CI, 0.84 to 1.01; Table 2).
DISCUSSION
In this large population-based study including more than 9,000 patients, we found patients with any MPN subtype to a have inferior survival compared with the general population during all calendar periods under study. There was continuous improvement in survival over time; however, this was less pronounced after the year 2000 as compared with the improvement seen earlier during the study period. The observed improvement in survival was restricted to patients with PV and ET, whereas no improvement was seen in patients with PMF. This underlines the assertion that all MPNs should be considered diseases that reduce life expectancy and highlights the need to improve treatment strategies for these patients.
In patients with PV, survival was 36% lower than expected in the general population. The finding that PV is associated with reduced life expectancy is supported by most published studies,9,15–18 but it has not, to our knowledge, been shown in a study this comprehensive before. Patients with PV in Sweden during the study period were treated according to international guidelines,32,33 and in 1998, the Swedish MPN Study Group published the first edition of national guidelines.34 In these guidelines, first-line cytoreductive treatment was hydroxyurea and radioactive phosphorus in patients with PV or ET. For those with PMF, recommended treatment was hydroxyurea or interferon.34 In a recent study of treatment-related risk factors for AML in patients with MPN diagnosed in Sweden from 1958 to 2005, the most common strategies in this patient cohort were treatment with hydroxyurea, treatment with radioactive phosphorus, or no cytoreductive treatment at all.32 In other studies involving patients with PV, major causes of death have been thromboembolic events and transformation to AML.16,17 Several factors may have contributed to the improved survival observed in those with PV. Aspirin prophylaxis and stringent adherence to the hematocrit goals for phlebotomy have been shown to be beneficial in preventing thromboembolic complications.35,36 In addition, prognosis after a vascular event has improved greatly for patients with PV as well as for the general population.37 The leukemogenic effect of alkylating agents and radioactive phosphorus has been recognized and has led to a decline in the use of these drugs.18,32,33 However, no cytoreductive therapy so far has been shown to significantly slow disease progression in PV. Even though a more widespread blood count screening, introduced during the second and third calendar periods,38 most likely led to an earlier establishment of MPN diagnoses (lead time bias29), our findings imply that better overall clinical management of these patients resulted in the observed improved outcome.
ET was associated with excess mortality throughout the study period. This novel finding is in contrast to results from previous smaller studies with short follow-up time, which reported normal life expectancy in patients with ET.10,15,19–21 A few studies with more than 10 years of follow-up have reported inferior survival, underlining the need for long follow-up to detect excess mortality.22,23 As in PV, the increased risk of thromboembolic events, both at diagnosis and during follow-up, in patients with ET is an important contributor to mortality in these patients.39,40 As discussed, prophylaxis and treatment of vascular events and the decline in use of leukemogenic drugs have most likely played an important role in the observed improvement in survival. The reported number of patients in each calendar period increased over time, most probably reflecting a better coverage of the registry rather than a true increase in incidence. There may also have been a certain selection in reporting of only the severe ET cases during first calendar period, resulting in a better survival rate with increasing registration of the patients with less aggressive disease during the later part of the study period. Possible misclassification with inclusion of patients with early PMF in the ET group may have contributed to the low RSR in ET before 1993, because patients with early PMF have inferior survival compared with those with ET.41,42 An accurate diagnosis differentiating prefibrotic PMF from ET is important not only for predicting overall survival but also for assessing the risk of bleeding complications and transformation to overt myelofibrosis and leukemia, both of which are higher in patients with prefibrotic PMF. Patients in Sweden, however, were classified according to the Polycythemia Vera Study Group criteria27 and later the WHO criteria,1,28 as in previously published studies from the corresponding calendar periods.10,15,19–23 Even after the introduction of the WHO classification in 2001, leading gradually to a group of more homogeneous patients with ET, this diagnosis was still associated with excess mortality during the most recent calendar period (2001 to 2008). Whether true ET is associated with normal survival needs to be investigated in future trials, but until then, ET should be recognized as a serious disease affecting life expectancy.
As previously reported,10–14 PMF was the subtype associated with the lowest relative survival; in addition, we found no improvement in survival over time. Patients with PMF have a higher rate of transformation to AML and myelodysplastic syndrome32,42 compared with patients with other subtypes; additionally, these patients experience a high risk of thromboembolic and hemorrhagic events, contributing largely to the excess mortality.43 Only a small number of patients underwent allogeneic stem-cell transplantation, which is the only known cure for PMF but is associated with substantial transplantation-related mortality.44 Several novel agents have been investigated in PMF, such as thalidomide, lenalidomide, and JAK2 inhibitors, but only a few patients during this study period were included in clinical trials of these drugs. Apart from smaller reports on interferon possibly reducing bone marrow fibrosis,45 no treatment has been shown to modify disease progression.8,46 There are, however, promising data on symptom relief with JAK2 inhibitors,47 and in a recent preliminary report, a survival benefit was seen in patients with PMF treated with JAK2 inhibitors when compared with placebo.48
MPN-U is a heterogeneous group including both patients in early as well as late disease stages who do not meet the criteria for PV, ET, or PMF.1 The excess mortality seen in patients with MPN-U may be explained by a predominance of patients in this group having late stage disease, including secondary myelofibrosis, which in turn is associated with high mortality.1
Age was a strong predictor of prognosis; higher age at diagnosis predicted poorer relative survival during all calendar periods. Similar results were reported in the ECLAP (European Collaboration on Low-dose Aspirin in Polycythemia Vera) trial.49 Possibly, older patients are affected by a more aggressive disease and/or higher rate of treatment-related complications. Some authors have reported less optimal stringency to treatment guidelines and less restrictive use of leukemogenic drugs in older patients because of the expected shorter survival.32,49,50 This needs to be addressed in the clinical setting to optimize treatment for elderly patients.
Female patients with MPNs had better outcome than male patients. Superior survival in female patients has been shown in many other studies of hematologic malignancies,15,51,52 but the underlying causes of the better outcome in women are currently unknown. It is possible that this could reflect variations in biology, treatment, and/or lifestyle factors.
The strengths of our study include a register-based cohort design including a large number of patients over a period of 36 years, ensuring a population-based setting and generalization of our findings. The Swedish Cancer Register consists of prospectively collected data and has a high diagnostic validity for hematologic malignancies.24
A limitation of this study is the lack of detailed medical information such as clinical and laboratory data in the Swedish Cancer Register; we were therefore not able to confirm individual diagnoses. In addition, as discussed, there were changes to the classification system during the study period, which may have influenced the comparison of survival and risk of complications in different MPN subtypes over time.
In summary, in this large population-based study, we found all MPN subtypes to have significantly reduced life expectancy compared with the general population, even in the most recent calendar period. Survival improved over time in patients with PV and ET and in all age groups. There was no improvement in survival in those with PMF, and only marginal improvement was observed between the two most recent calendar periods in the whole MPN cohort. Apart from better prophylaxis and management of disease complications, in contrast to chronic myelogenous leukemia, no disease-specific therapy has been shown to significantly improve survival in MPNs.50 Ongoing and future studies of the molecular background of MPNs and the underlying causes of the observed excess mortality are needed to enhance our understanding of these diseases. The reduced life expectancy seen in this study in all MPN subtypes underlines the need to optimize our current treatment strategies and highlights the need for new disease-modifying therapies to change the outlook for patients with MPNs.
Acknowledgment
We thank the European Group for Blood and Marrow Transplantation for providing information regarding stem-cell transplantations.
Footnotes
Supported by Grant No. CAN 2009/1203 from the Swedish Cancer Society; by Grant No. SLL 20090201 from the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet; by Grant No. 2009Fobi0072 from the Karolinska Institutet Foundations; by an unrestricted grant from Shire Pharmaceuticals; and by the Adolf H. Lundin Charitable Foundation.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Magnus Björkholm
Administrative support: Magnus Björkholm
Collection and assembly of data: Malin Hultcrantz, Sigurdur Yngvi Kristinsson, Therese M.-L. Andersson, Magnus Björkholm
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Swerdlow SH, Campo E, Harris NL, et al. Lyon, France: IARC Press; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 2.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 5.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 6.Landgren O, Goldin LR, Kristinsson SY, et al. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112:2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AV, Chase A, Silver RT, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tefferi A, Vainchenker W. Myeloproliferative neoplasms: Molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011;29:573–582. doi: 10.1200/JCO.2010.29.8711. [DOI] [PubMed] [Google Scholar]
- 9.Ania BJ, Suman VJ, Sobell JL, et al. Trends in the incidence of polycythemia vera among Olmsted County, Minnesota residents, 1935-1989. Am J Hematol. 1994;47:89–93. doi: 10.1002/ajh.2830470205. [DOI] [PubMed] [Google Scholar]
- 10.Mesa RA, Silverstein MN, Jacobsen SJ, et al. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: An Olmsted County study, 1976-1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Maynadié M, Girodon F, Manivet-Janoray I, et al. Twenty-five years of epidemiological recording on myeloid malignancies: Data from the specialized registry of hematologic malignancies of Cote d'Or (Burgundy, France) Haematologica. 2011;96:55–61. doi: 10.3324/haematol.2010.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 13.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 14.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 15.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–2232. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 17.Gruppo Italiano Studio Policitemia: Polycythemia vera: The natural history of 1213 patients followed for 20 years. Ann Intern Med. 1995;123:656–664. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: Final results of a randomized trial initiated in 1980. J Clin Oncol. 2011;29:3907–3913. doi: 10.1200/JCO.2011.36.0792. [DOI] [PubMed] [Google Scholar]
- 19.Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: Safety of cytoreductive therapy. Am J Hematol. 2009;84:215–220. doi: 10.1002/ajh.21360. [DOI] [PubMed] [Google Scholar]
- 20.Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer. 1991;67:2658–2663. doi: 10.1002/1097-0142(19910515)67:10<2658::aid-cncr2820671042>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Passamonti F, Rumi E, Arcaini L, et al. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: A study of 605 patients. Haematologica. 2008;93:1645–1651. doi: 10.3324/haematol.13346. [DOI] [PubMed] [Google Scholar]
- 22.Bazzan M, Tamponi G, Schinco P, et al. Thrombosis-free survival and life expectancy in 187 consecutive patients with essential thrombocythemia. Ann Hematol. 1999;78:539–543. doi: 10.1007/s002770050555. [DOI] [PubMed] [Google Scholar]
- 23.Wolanskyj AP, Schwager SM, McClure RF, et al. Essential thrombocythemia beyond the first decade: Life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81:159–166. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]
- 24.Turesson I, Linet MS, Björkholm M, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964-2003. Int J Cancer. 2007;121:2260–2266. doi: 10.1002/ijc.22912. [DOI] [PubMed] [Google Scholar]
- 25.Official Statistics of Sweden Stockholm: National Board of Health and Welfare, Centre for Epidemiology: Cancer Incidence in Sweden 2010. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18530/2011-12-15.pdf. [Google Scholar]
- 26.Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol. 1975;12:339–351. [PubMed] [Google Scholar]
- 27.Murphy S, Peterson P, Iland H, et al. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: A final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29–39. [PubMed] [Google Scholar]
- 28.Jaffe ES, Harris NL, Stein H, et al., editors. Lyon, France: IARC Press; 2001. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 29.Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–117. doi: 10.1111/j.1365-2796.2006.01677.x. [DOI] [PubMed] [Google Scholar]
- 30.Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 31.Ederer F. Bethesda, MD: End Results Evaluation Section; 1959. Instructions to IBM 650 programmers in processing survival computations [methodological note No. 10] [Google Scholar]
- 32.Björkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.34.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: Critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:2410–2415. doi: 10.1200/JCO.2010.31.8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birgegård G, Björkholm M, Kutti J, et al. Stockholm, Sweden: Swedish Orphan AB; 1998. Polycythemia vera, essential trombocythemia and myelofibrosis: Diagnosis, treatment and follow-up—Recommendations from a national working group 1998 [in Swedish] [Google Scholar]
- 35.Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–124. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 36.Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2:1219–1222. doi: 10.1016/s0140-6736(78)92098-6. [DOI] [PubMed] [Google Scholar]
- 37.Stenestrand U, Lindbäck J, Wallentin L. Long-term outcome of primary percutaneous coronary intervention vs prehospital and in-hospital thrombolysis for patients with ST-elevation myocardial infarction. JAMA. 2006;296:1749–1756. doi: 10.1001/jama.296.14.1749. [DOI] [PubMed] [Google Scholar]
- 38.Merk K, Mattsson B, Mattsson A, et al. The incidence of cancer among blood donors. Int J Epidemiol. 1990;19:505–509. doi: 10.1093/ije/19.3.505. [DOI] [PubMed] [Google Scholar]
- 39.Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood. 2011;117:5857–5859. doi: 10.1182/blood-2011-02-339002. [DOI] [PubMed] [Google Scholar]
- 40.Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 41.Thiele J, Kvasnicka HM. Chronic myeloproliferative disorders with thrombocythemia: A comparative study of two classification systems (PVSG, WHO) on 839 patients. Ann Hematol. 2003;82:148–152. doi: 10.1007/s00277-002-0604-y. [DOI] [PubMed] [Google Scholar]
- 42.Barbui T, Thiele J, Passamonti F, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: An international study. J Clin Oncol. 2011;29:3179–3184. doi: 10.1200/JCO.2010.34.5298. [DOI] [PubMed] [Google Scholar]
- 43.Barbui T, Carobbio A, Cervantes F, et al. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood. 2010;115:778–782. doi: 10.1182/blood-2009-08-238956. [DOI] [PubMed] [Google Scholar]
- 44.Abelsson J, Merup M, Birgegård G, et al. The outcome of allo-HSCT for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transplant. 2012;47:380–386. doi: 10.1038/bmt.2011.91. [DOI] [PubMed] [Google Scholar]
- 45.Silver RT, Vandris K, Goldman JJ. Recombinant interferon-alpha may retard progression of early primary myelofibrosis: A preliminary report. Blood. 2011;117:6669–6672. doi: 10.1182/blood-2010-11-320069. [DOI] [PubMed] [Google Scholar]
- 46.Merup M, Kutti J, Birgergård G, et al. Negligible clinical effects of thalidomide in patients with myelofibrosis with myeloid metaplasia. Med Oncol. 2002;19:79–86. doi: 10.1385/mo:19:2:79. [DOI] [PubMed] [Google Scholar]
- 47.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 48.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: An analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105:2664–2670. doi: 10.1182/blood-2004-09-3426. [DOI] [PubMed] [Google Scholar]
- 50.Björkholm M, Ohm L, Eloranta S, et al. Success story of targeted therapy in chronic myeloid leukemia: A population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29:2514–2520. doi: 10.1200/JCO.2011.34.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derolf AR, Kristinsson SY, Andersson TM, et al. Improved patient survival for acute myeloid leukemia: A population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113:3666–3672. doi: 10.1182/blood-2008-09-179341. [DOI] [PubMed] [Google Scholar]
- 52.Kristinsson SY, Dickman PW, Wilson WH, et al. Improved survival in chronic lymphocytic leukemia in the past decade: A population-based study including 11,179 patients diagnosed between 1973-2003 in Sweden. Haematologica. 2009;94:1259–1265. doi: 10.3324/haematol.2009.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]