Abstract
Background
Non-steroidal anti-inflammatory drugs (NSAIDs), including cyclo-oxygenase-2 (COX-2) inhibitors, are generally contraindicated in chronic kidney disease (CKD). This investigation sought to identify the frequency of NSAID/COX2 prescription and determine the influence of serum Cr versus estimated GFR on this practice pattern.
Methods
An established Veterans Health Administration (VHA) CKD safety cohort (n = 70,154) was examined to determine the frequency of NSAID/COX2 in fiscal year 2005 (FY05) for up to 30 days preceding the index hospitalization and as many as 365 days during that year. Binomial regression was used to determine adjusted prevalence ratios for prescription of NSAID/COX2 with respect to continuous eGFR measurement and serum creatinine (Cr) categories. CKD was defined as eGFR < 60 ml/min/1.73m2.
Results
15.4% of subjects had an NSAID/COX2 prescription during the observation period with the proportion prescribed these agents decreasing with declining renal function, but remained significant at any stage of CKD given the renal harm related to these medications. At specific GFR estimates, serum creatinine (Cr) remained a significant predictor of NSAID/COX prescription. At GFR set at 42 ml/min/1.73, the predicted proportion prescribed NSAID/COX2 was 0.29 (95% CI: 0.24,0.36); 0.23 (95% CI: 0.22,0.26); 0.20 (95%: 0.19,0,22); 0.12 (95% CI: 0.10,0.14) for Cr strata of ≤ 1.3 mg/dl, 1.4 – 1.6 mg/dl, 1.7 –2.1 mg/dl, ≥ 2.2 mg/dl, respectively (all p < 0.05).
Conclusion
A significant proportion of individuals with CKD continue to be prescribed NSAID/COX2 and serum Cr remains an influential guide to NSAID/COX2 prescription, even in GFR ranges where these agents are ill-advised.
Keywords: chronic kidney disease, safety, recognition, NSAIDs
Introduction
Consumption of non-steroidal anti-inflammatory drugs (NSAIDs) is widespread; they are frequently prescribed and can be easily obtained over the counter as analgesics. Use of NSAIDs has been shown to have adverse effects on renal function and prior studies have linked both NSAIDs and a subclass, cyclooxygenase-2 (COX-2) inhibitors, to an increased risk of kidney disease1–5. Therefore, guidelines for the care of patients advise against the use of NSAID/COX-2 in chronic kidney disease (CKD) 6. Despite these warnings, CKD is often under-recognized because of a common failure to check renal function in high risk populations, or an under-appreciation of elevations in serum creatinine obtained on routine blood tests7;8. This under-recognition of kidney disease may lead to patients not receiving appropriate therapies and failure to institute certain precautions to prevent further loss of renal function, or avert the potential exposure of patients to factors that hasten kidney damage. Improved recognition of CKD may prevent missed opportunities for the implementation of safety guidelines for patients with this disease and the prevention of adverse renal outcomes.
The increasing use of estimates of glomerular filtration rate (GFR) as part of routine laboratory reporting, has raised the expectation that providers would be more likely to recognize CKD and use this knowledge in prescribing patterns. Empirical evidence shows that the reporting of estimated GFR has, at least in studied health networks, increased RAAS blocker prescription and nephrology referrals.10,11 However it remains unknown to what extent practitioners acknowledge reporting of reduced GFR or still rely on serum creatinine in their practice decisions related to use of analgesics in patients with CKD. The objective of this study was to determine the prevalence of NSAID use in CKD patients and examine the role of eGFR versus serum Cr in influencing prescription patterns of NSAID/COX2 in CKD patients.
Methods
Study design
The study was a retrospective cross-sectional analysis of a national sample of patients with CKD from the Veterans Health Administration (VHA). The veterans were observed during the fiscal year 2005 (FY05: 10/01/2004 – 09/30/2005).
Setting and data sources
This study utilized a previously analyzed VHA CKD safety cohort that consists of a national sample of veterans who were followed during the 12 FY05,13. The VHA CKD safety cohort was compiled using VHA acute inpatient data files for FY05 (Medical SAS Inpatient Datasets), which were then merged with inpatient and outpatient laboratory values (Decision Support System Laboratory Result), outpatient event data sets, and vital statistics data for the study participants. For this analysis, the core data was appended with NSAID/COX-2 prescription records merged from the VA Pharmacy Benefits Management (PBM) file for that year. This study was classified as exempt by the Institutional Review Board of the University of Maryland, Baltimore and the Research & Development Committee of the Maryland VA Healthcare System.
Participants
Details on subject inclusion are described elsewhere 9;10 and summarized here. For inclusion in the cohort, study participants must have had one or more acute care hospitalizations at a VHA facility during the observation period, with a preceding outpatient serum creatinine (Cr) measured up to one year and greater than one week prior to the first (index) hospitalization, for the estimation of glomerular filtration rate (eGFR) and the determination of CKD status. For sensitivity analyses, and in the event that there was more than one serum Cr measurement available in the time period preceding the index hospitalization, the measurement that was closest to hospital admission and which preceded all, if any, NSAID/COX2 prescriptions was substituted for the index Cr. The index Cr was used to calculate the index eGFR with the abbreviated Modified Diet in Renal Disease (MDRD) equation. The choice of the abbreviated MDRD equation was made as this was the estimator of GFR being promulgated at that time and was viewed to more relevant to providers than more recent estimating equations such as the CKD-Epi equation.14 The index eGFR was used to determine CKD classification stage15. To be included in this study, participants had to have at least stage 3 or greater pre-dialysis CKD, defined as an index eGFR (calculated using the serum Cr most closely preceding the index hospitalization) < 60 ml/min/1.73m2. In sensitivity analyses described above, those individual who had a prior Cr substituted for the index value and the repeat GFR was ≥ 60 ml/min/1.73m2 were excluded from the sample for the repeat analyses.
Variables
The primary outcome variable was the exposure to NSAID/COX-2 at anytime during the observation period. Key predictors of NSAID/COX2 use included cohort-qualifying measure of renal function, along with demographics for each participant including gender, age, and self-reported race (categorized as Caucasian, African-American, or other). Additional covariates included the presence or absence of diabetes, number of hospitalizations during the observation period, and comorbidities including cancer (excluding non-melanomatous skin cancer), cardiovascular disease (a composite of cerebrovascular disease, myocardial infarction and/or congestive heart failure), and the Charlson Comorbidity Index (CCI).
Measurements
NSAID/COX-2 exposure was determined based on records from the VA Pharmacy Benefits Management file from the FY05. Any patient included in the data set with an NSAID/COX-2 prescription 30 days before or during the index hospitalization, or between the index hospitalization discharge date and the earlier of the end of FY05 or the next hospitalization was considered to be exposed to this class of drugs. Along with use of the index Cr to estimate eGFR it was used to categorize subjects into the following Cr groups: ≤1.4 mg/dl, 1.5 – 1.6 mg/dl, 1.7 – 2.1 mg/dl, and ≥2.2 mg/dl. These strata were selected in an attempt to identify relevant clinical thresholds and balance the study sample across strata. Due to the skew in the sample towards lower stages CKD, for some analyses we combined the groups as follows<60 ml/min/1.73m2 and ≥45 ml/min/1.73m2 (stage 3a CKD), and <45 ml/min/1.73m2 (stages 3b, 4, and 5 CKD).
The CCI was modified to exclude renal, cancer, diabetes and cardiovascular disease, which were considered separately in multivariate analyses. The International Classification of Diseases, 9th revision (ICD-9CM) was used to classify all comorbidities of interest from inpatient and outpatient VHA records from October 1, 1999, the earliest data available, to the date of index hospitalization. The number of hospitalizations during the study period was categorized as 0, 1, or greater than or equal to 2.
Statistical methods
Descriptive analyses were used for the prevalence estimates: we computed N (%) and used chi-square test to compare proportions among different groups. We used a binomial regression model to compute adjusted prevalence ratios and their confidence intervals of NSAID/COX-2 exposure for 10 units increase of eGFR within each index Cr categories. The prevalence ratio for NSAID/COX-2 prescriptions were calculated based on estimated regression coefficients of this log-linear model. The predictors included the indicator variables for index Cr categories (1.5 – 1.6 mg/dl, 1.7 – 2.1 mg/dl, and ≥2.2 mg/dl), the continuous index eGFR, the interaction terms between the index eGFR and the indicator variables for CR categories. Additional adjustment factors included patient characteristics (race, indicator variables for age categories, sex, and indicator variables for the modified Charlson comorbidity index categories). Then the adjusted prevalence ratio of NSAID/COX-2 prescription can be interpreted as the quotient of prevalence rates for every 10 units increase in eGFR (from c ml/min/1.73m2 to (c+10) ml/min/1.73m2) within each CR category. We also used this regression model to calculate predicted prevalence rates at fixed (and clinically relevant) values for all parameters in the regression. Sensitivity analysis with an identical regression strategy was performed in individuals who had an additional measure of renal function known to precede the NSAID/COX2 prescription to be sure that the qualifying measure of renal function was not the result of the NSAID/COX-2 prescription. Analyses were done using SPSS version 9 (SPSS Inc, Chicago, IL) and SAS Version 9 (SAS Institute Inc., Cary, NC).
Results
Participants
Of 71,156 CKD patients in the core VA patient safety cohort, 70,154 subjects had data obtainable from the VA PBM file to determine NSAID/COX2 exposure and were thus included in this analysis.
Descriptive Data
Demographic characteristics of study participants are enumerated in Table 1 according to whether or not they had any exposure to NSAID/COX-2 during the study period. Overall, 15.4% of patients in the cohort had been prescribed a NSAID/COX-2. The group prescribed a NSAID/COX2, had a slight preponderance of females, Caucasians, treatment with an ACEI and/or ARB, the subjects in this group were relatively younger and healthier than those not prescribed an NSAID/COX2 with a lower preponderance of diabetes, CVD, and malignancy, and a CCI more likely to be less than or equal to one.
Table 1.
Study population classified by NSAID/COX2 exposure
| Any NSAID or COX-2 exposure over the study period | |||
|---|---|---|---|
|
| |||
| N (% of total sample) | YES 10801 (15.4%) |
NO 59353 (84.6%) |
p-value |
|
| |||
| Age | |||
| ≤63 years | 3666 (33.9%) | 14199 (23.9%) | |
| 64–73 years | 3103 (28.7%) | 15380 (25.9%) | <0.001 |
| 74–80 years | 2307 (21.4%) | 15832 (26.7%) | |
| ≥81 years | 1725 (16.0%) | 13942 (23.5%) | |
|
| |||
| Sex | |||
| Male | 10202 (94.5%) | 57669 (97.2%) | <0.001 |
| Female | 599 (5.5%) | 1684 (2.8%) | |
|
| |||
| Race | |||
| Caucasian | 9165 (84.9%) | 48954 (82.5%) | <0.001 |
| African-American | 1516 (14.0%) | 9753 (16.4%) | |
| Other | 120 (1.1%) | 646 (1.1%) | |
|
| |||
| Diabetes | |||
| Yes | 4944 (45.8%) | 30463 (51.3%) | <0.001 |
| No | 5857 (54.2%) | 28890 (48.7%) | |
|
| |||
| CVD | |||
| Yes | 4765 (44.1%) | 32602 (54.9%) | <0.001 |
| No | 6036 (55.9%) | 26751 (45.1%) | |
|
| |||
| Malignancy | |||
| Yes | 2612 (24.2%) | 16711 (28.2%) | <0.001 |
| No | 8189 (75.8%) | 42642 (71.8%) | |
|
| |||
| Hospitalizations between index date and end of observation | |||
| Only index hospitalization | 6648 (61.5%) | 36325 (61.2%) | 0.494 |
| Additional hospitalizations | 4153 (38.5%) | 23028 (38.8%) | |
|
| |||
| ACE Inhibitor and/or Angiotensin II Receptor Blocker | |||
| Yes | 7282 (67.4%) | 38712 (65.2%) | <0.001 |
| No | 3519 (32.6%) | 20641 (34.8%) | |
|
| |||
| Charlson co-morbidity index | |||
| 0 | 4515 (41.8%) | 23145 (39.0%) | |
| 1 | 4106 (38.0%) | 22401 (37.7%) | <0.001 |
| 2 or more | 2180 (20.2%) | 13807 (23.3%) | |
Table 2 presents the study sample categorized into strata of index Cr, stages of CKD and stage of CKD within each of those strata. The table demonstrates the varying profile of individuals within groups of GFR across the range Cr values. As expected, stage of CKD increased with higher Cr values but a substantial number of patients with significantly elevated Cr values had Stage 3a CKD except among patients who entered the cohort patients with a serum creatinine of ≥ 2.2 mg/dl. Within all Cr strata, patients with more advanced stages of CKD tended to be older, more likely to be female (given the relatively small number of women), and Caucasian. In most Cr strata there was a tendency for patients with more advanced stages of CKD to have CVD, a malignancy, more than one (index) hospitalization during the study period, or greater co-morbidity based on the CCI.
Table 2.
Study participants classified by strata of index creatinine and Stage of CKD using abbreviated MDRD equation
| Serum creatinine at cohort entry
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Less than or equal to 1.3 mg/dl | 1.4 to 1.6 mg/dl | 1.7 to 2.1 mg/dl | Greater or equal to 2.2 mg/dl | |||||
|
| ||||||||
| CKD Stage* | CKD Stage* | CKD stage* | CKD stage* | |||||
|
| ||||||||
| 3a | 3b/4/5 | 3a | 3b/4/5 | 3a | 3b/4/5 | 3a | 3b/4/5 | |
|
| ||||||||
| Sample Size (Row %) | 12027 (98.7%) | 160 (1.3%) | 22624 (89.1%) | 2771 (10.9%) | 2527 (15.2%) | 14126 (84.8%) | 0 | 15908 (100%) |
|
| ||||||||
| Age | ||||||||
| ≤63 years | 1940 (16.1%) | 34 (21.3%) | 6125 (27.1%) | 99 (3.6%) | 1123 (44.4%) | 2855 (20.2%) | 02 | 5684 (35.7%) |
| 64–73 years | 3944 (32.8%) | 33 (20.6%) | 6619 (29.3%) | 81 (2.9%) | 556 (22.0%) | 3404 (24.1%) | 0 | 3841 (24.1%) |
| 74–80 years | 3393 (28.2%) | 21 (13.1%) | 5572 (24.6%) | 1151 (41.5%) | 448 (17.7%) | 4043 (28.6%) | 0 | 3510 (22.1%) |
| ≥81 years | 2750 (22.9%) | 72 (45.0%) | 4308 (19.0%) | 1440 (52.0%) | 400 (15.8%) | 3824 (27.1%) | 0 | 2873 (18.1%) |
|
| ||||||||
| Gender | ||||||||
| Male | 10725 (89.2%) | 0 | 22567 (99.7%) | 2432 (87.8%) | 2527 (100.0%) | 13921 (98.5%) | 02 | 15688 (98.6%) |
| Female | 1302 (10.8%) | 160 (100.0%) | 57 (0.3%) | 339 (12.2%) | 0 | 205 (1.5%) | 0 | 220 (1.4%) |
|
| ||||||||
| Ethnicity | ||||||||
| Caucasian | 11814 (98.2%)1 | 160 (100.0%) | 19901 (88.0%) | 2731 (98.6%) | 129 (5.1%) | 12695 (89.9%) | 02 | 10678 (67.1%) |
| African American | 84 (0.7%) | 0 | 2501 (11.1%) | 18 (0.6%) | 2390 (94.6%) | 1272 (9.0%) | 0 | 5004 (31.5%) |
| Other | 129 (1.1%) | 0 | 222 (1.0%) | 22 (0.8%) | 8 (0.3%) | 159 (1.1%) | 0 | 226 (1.4%) |
|
| ||||||||
| Diabetes | ||||||||
| Yes | 4775 (39.7%)1 | 57 (35.6%) | 10701 (47.3%) | 1135 (41.0%) | 1317 (52.1%)1 | 7648 (54.1%) | 02 | 9767 (61.4%) |
| No | 7252 (60.3%) | 103 (64.4%) | 11923 (52.7%) | 1636 (59.0%) | 1210 (47.9%) | 6478 (45.9%) | 0 | 6141 (38.6%) |
|
| ||||||||
| CVD | ||||||||
| Yes | 5510 (45.8%)1 | 160 (45.6%) | 11278 (49.8%) | 1560 (56.3%) | 1177 (46.6%) | 8402 (59.5%) | 02 | 9361 (58.8%) |
| No | 6517 (54.2%) | 87 (54.4%) | 11346 (50.2%) | 1211 (43.7%) | 1350 (53.4%) | 5724 (40.5%) | 0 | 6547 (41.2%) |
|
| ||||||||
| Malignancy | ||||||||
| Yes | 3323 (27.6%)1 | 39 (75.6%) | 6258 (27.7%) | 843 (30.4%) | 661 (26.2%) | 4137 (29.3%) | 02 | 4057 (25.5%) |
| No | 8704 (72.4%) | 121 (75.6%) | 16366 (72.3%) | 1928 (69.6%) | 1866 (73.8%) | 9989 (70.7%) | 0 | 11851 (74.5%) |
|
| ||||||||
| Hospitalization | ||||||||
| 1 (only index hosp.) | 8060 (67.0%)1 | 112 (70.0%) | 14648 (64.7%) | 1736 (62.6%) | 1583 (62.6%) | 8513 (60.3%) | 02 | 8313 (52.3%) |
| >1 (subsequent hosp.) | 3967 (33.0%) | 48 (30.0%) | 7976 (35.3%) | 1035 (37.4%) | 944 (37.4%) | 5613 (39.7%) | 0 | 7595 (47.7%) |
|
| ||||||||
| ACE or ARB | ||||||||
| Yes | 7509 (62.4%)1 | 103 (64.4%) | 15347 (67.8%) | 1798 (64.9%) | 1751 (69.3%)1 | 9832 (69.6%) | 02 | 9644 (60.6%) |
| No | 4518 (37.6%) | 57 (35.6%) | 7277 (32.2%) | 973 (35.1%) | 776 (30.7%) | 4294 (30.4%) | 0 | 6264 (39.4%) |
|
| ||||||||
| Charlson Comorbidity | ||||||||
| 0 | 4979 (41.4%)1 | 64 (40.0%) | 9226 (40.8%) | 991 (35.8%) | 1188 (47.0%) | 5099 (36.1%) | 02 | 6108 (38.4%) |
| 1 | 4606 (38.3%) | 63 (39.4%) | 8587 (38.0%) | 1119 (40.4%) | 841 (33.3%) | 5465 (38.7%) | 0 | 5822 (36.6%) |
| ≥2 | 2442 (20.3%) | 33 (20.6%) | 4811 (21.3%) | 661 (23.9%) | 498 (15.2%) | 3562 (25.2%) | 0 | 3978 (25.0%) |
CKD Stage based on estimated GFR using modified MDRD equation on Cr obtained at cohort entry.
CKD stage definitions in eGFR (mL/min/1.73m2): stage 3a, eGFR 45–59; stage 3b, eGFR 30–44; stage 4, eGFR 15–29; stage 5, eGFR <15
Denotes unable to determine p-value because of empty cells in contingency table. All other p-values significant (p < 0.05).
Table 3 shows the distribution of subjects based on strata of Cr and stage of CKD, and the proportion of individuals within each group prescribed an NSAID/COX-2. The proportion of individuals prescribed with an NSAID/COX-2 declines with increasing Cr, and also with more advanced CKD, However within CKD stages, the proportion of individuals being prescribed an NSAID/COX-2 is associated with varying Cr. The proportion of NSAID/COX2 prescriptions decreases in the more advanced stages (Stage 3B/4 and 5) of CKD within strata of Cr and also declines with increasing Cr within Stage of CKD. Yet in all groups the proportion of individuals with NSAID/COX-2 prescription were substantial. Of note there are no individuals with Stage 3A CKD and Cr ≥ 2.2 mg/dl.
Table 3.
Proportion of NSAID/Cox-2 prescriptions within sub-groups based on index Cr and stage of CKD
| Range in index serum Cr | ||||
|---|---|---|---|---|
| CKD stage | ≤1.3 mg/dl | 1.4 – 1.6 mg/dl | 1.7 – 2.1 mg/dl | ≥2.2 mg/dl |
| All CKD stages | 12187 (23.8%) | 25400 (44.1%) | 16658 (20.1%) | 15909 (12.0%) |
| CKD Stage 3A | 12027 (20.9%) | 22629 (19.4%) | 2530 (16.4%) | 0 (0%) |
| CKD Stage 3B, 4 and 5 | 160 (30.6%) | 2771 (13.3%) | 14128 (12.4%) | 15909 (8.2%) |
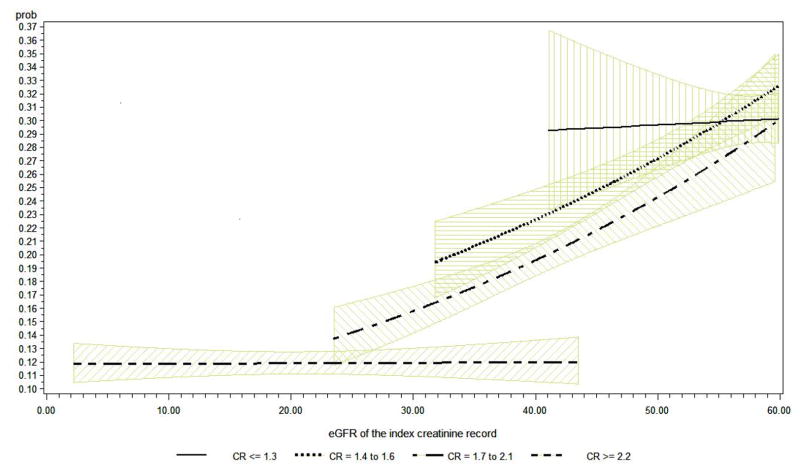
Table 4 shows the range in GFR within Cr strata, the adjusted prevalence ratios of NSAID/COX2 prescription across the range of GFR within each Cr strata, and estimated rates of NSAID/COX prescription for selected GFR values that span several Cr strata. In the lowest and highest Cr strata (Cr ≤ 1.3 mg/dl and Cr ≥ 2.2 mg/dl) the variation in the rate of NSAID/COX2 prescriptions, expressed as prevalence ratio of a prevalence rate in for a given GFR relative to a GFR lower by 10 ml/min/1.73 m2 shows no change across the range of GFR within that Cr strata. However within the 2nd and 3rd strata of Cr (1.4 – 1.6 mg/dl and 1.7 – 2.1 mg/dl) the likelihood of being prescribed a NSAID/COX2 increases significantly with rising GFR in that Cr stata. Of further note, when repeating the binomial regression in those individuals where the available GFR preceded any NSAID/COX-2 prescription or substituting an GFR using a serum Cr measured prior to the index GFR and preceding a NSAID/COX-2 prescription, the results were comparable (n = 69449). Table 4 also illustrates how serum Cr remains influential on the pattern of prescription by showing the predicted prevalence of NSAID/COX2 prescription from the binomial regression models for specific point estimates of GFR which span the maximum possible number of Cr strata. The table shows that the predicted probability of being prescribed a NSAID/COX2 increases with decreasing Cr – within fixed estimates of GFR (all p < 0.05). The influence of GFR on adjusted probability of being prescribed a NSAID/COX2 within strata of Cr and over the range of GFR values is further exhibited in Figure 1.
Table 4.
The influence of GFR versus serum Cr on prescription patterns for NSAID/COX2
| Cr group | Range in GFR (ml/min/1.73 m2) Within Cr strata | Proportion prescribed NSAID/COX-2 within strata | Rate ratio (RR) of NSAID/COX2 prescription per 10 ml/min/1.73m2 increase of GFR within Cr strata | p value | Predicted proportion prescribed NSAID/COX2 at specific estimates for GFR* | ||
|---|---|---|---|---|---|---|---|
| 35 ml/min/1.73m2 | 42 ml/min/1.73m2 | 50 ml/min/1.73m2 | |||||
| ≤ 1.3 mg/dl | 41.1 – 59.9 | 21.1 % | 1.07(0.89,1.16) | 0.82 | NA* | 0.29 (0.24, 0.36) | 0.29 (0.26, 0.33) |
| 1.4 – 1.6 mg/dl | 31.8 –59.9 | 18.8% | 1.20 (1.12,1.29) | < 0.001 | 0.21 (0.18,0.23) | 0.23 (0.22,0.26) | 0.27 (0.26,0.29) |
| 1.7 – 2.1 mg/dl | 23.5 –59.6 | 13.0% | 1.24(1.14,1.34) | < 0.001 | 0.18 (0.16,0.19) | 0.20 (0.19,0.22) | 0.24 (0.22,0.27) |
| ≥ 2.2 mg/dl | 2.2 –43.5 | 8.2% | 1.00 (0.95,1.06) | 0.9 | 0.12 (0.11,0.13) | 0.12 (0.10,0.14) | NA** |
Model set for patient with age less than or equal to 63, male, Caucasian, with charlson comorbidity index being 0, only index hospitalization, and no other co-morbidities.
Note: The difference in the predicted risk estimates is statistically significant for each pair of consecutive Cr categories at a fixed estimate of GFR; p values < 0.05.
NA refers to no actual data at that GFR within a Cr strata.
Figure 1.
Predicted probability of receiving NSAID/COX2 within Cr strata with varying eGFR, using a reference patient group set at age less than or equal to 63, male, Caucasian, with charlson comorbidity index being 0, only index hospitalization, and no other comorbidities.
Discussion
This study shows that NSAID/COX-2 prescriptions are not uncommon among CKD patients. While a majority of patients were not exposed to this class of drugs during the discrete study period, the proportion of the cohort prescribed an NSAID/COX2 was higher than expected. The frequency of NSAID/COX2 prescriptions was less common with more severe CKD; however eGFR was only one determinant of kidney disease severity that seemed to influence prescription patterns. Serum Cr was also a major determinant of prescription patterns as evidenced by the varying rate of predicted NSAID/COX2 prescriptions at fixed values of eGFR and across the range of Cr for that eGFR. The data suggests that health care providers continued to consider serum Cr measurements either as a compliment or without regard to eGFR when prescribing NSAID/COX-2 to CKD patients.
The prevailing recommendations in consensus-based clinical guidelines recommend that NSAID/COX-2 should be avoided in CKD6. However, a first step to adherence to such guidelines is recognition of patients who have this disease. Indeed, CKD is poorly recognized,7;8 and much of this lack of recognition relates to practitioners’ failure to identify the significance of apparently normal Cr values in patients who in fact have reduced eGFR16. The nephrology community has put great effort into promoting the automatic reporting of eGFR as a means to increase recognition of CKD. While the reporting has been increasing, it is not likely to yet have become universal and it is not known to what extent it was available throughout VA at the time of this study.17 Nevertheless, practice guidelines have promoted the message that serum Cr is an insensitive marker of CKD and should be interpreted as such in high risk populations.18 It is also worth noting that while automatic reporting of eGFR has been shown to increase recognition of CKD19, the provision of this clinical information may not translate into a reduction in NSAID/COX2 prescriptions or implementation of other disease-specific recommendations for management20. When providers self-identify patients with CKD, they are more likely to adhere to recommended practices including referral to a nephrologist, prescription of an ACE/ARB, ordering of urinalysis, and avoidance of contraindicated medications in diagnosed patients21. However, evidence from simulated CKD patient scenarios presented in a survey of primary providers suggest that when serum Cr instead of eGFR is used as the primary determinant of CKD, the providers are more likely to delay important recommendations for care such as timely referral to a nephrologist22. This practice pattern may be similar to the observations reported here.
Other systematic decision aids have been demonstrated to guide the safety of prescription patterns in CKD and may serve as an adjunct to renal function measurement in improving the safety of medication prescriptions. Automated order entry and alerts have been implemented in various healthcare settings to increase appropriate dosing and frequency of medications, and to decrease prescription of inappropriate medications and may be mechanism to reduce the use of NSAID/COX2 agents in the CKD population. The use of alerts, in one study, decreased the likelihood of contraindicated medications being prescribed to CKD patients by 42%.23 An automated order entry system that adjusted medication dosing parameters for renal insufficiency was shown to increase the frequency of CKD-appropriate dosing by 24% in the inpatient setting.24 Such interventions have also been found to be useful in the outpatient setting, which may be particularly pertinent for NSAID/COX2 use. An implemented system of alerts indicating interactions between laboratory values and medications being ordered in the outpatient setting found a 5.3% reduction in inappropriate medication ordering and a 12% increase in the ordering of indicated laboratory tests.25
Estimates of the prevalence of NSAID/COX2 use in the general population vary with some evidence suggesting a relationship between NSAID/COX2 use and the development of CKD or ESRD26–29. Reports of the prevalence of NSAID/COX2 use in CKD populations are fewer, but suggest rates comparable to this report. In a Taiwanese study of individuals with CKD defined by administrative ICD-9 codes, the prevalence of NSAID/COX2 use was almost 20% with a strong association with the development of ESRD30. In a smaller US clinic-based study of CKD patients who were being surveyed for the prevalence of pain, 15.4% of the 130 individuals in the sample reported some NSAID use31. The present study is unique in that it allowed us to determine to what extent severity of CKD plays in prescription patterns of NSAID/COX-2 agents and to examine the role of eGFR versus serum Cr in providers’ intended utilization of these agents.
Given that this study was a retrospective analysis, it has inherent limitations that need to be taken into account when considering the results. It is important to note that such a large sample of clinical data has the potential for recording errors and missing information since the data was not collected for the purpose of the intended study. However, the problem of errors and missing data is surpassed by the benefits of studying such a large clinical sample. It is impossible to determine whether the prescriber of an NSAID/COX2 was aware of the index level of renal function prior to prescription of such an agent, although use of electronic medical records in the VHA network ensures the availability of laboratory data to the vast majority of practitioners who use the same system for prescriptions. Moreover, renal function may have changed over the study year during which the patients were observed for NSAID/COX2 use and the VA CKD safety cohort was assembled, but in the sensitivity analysis we attempted to ensure that the index measure of Cr used preceded the observed prescription of an NSAID/COX2 and the results were no different. Moreover, the presence of a single indication of compromised renal function in a patient’s recent past, even if transient, should be a deterrent to usage of these agents, given that such an individual would be at risk for subsequent periods of acute kidney injury. Finally, it is not possible in this analysis to determine the frequency of over-the-counter use of NSAID/COX-2 agents among subjects in this study. However there is evidence that a majority of veterans prefer to use VHA services and in the case of prescriptions there is likely a financial incentive to do so, given the cost of non-prescription medications.32,33 Moreover, it is unclear how over-the-counter use of such medications would influence providers’ prescription patterns, which was the key outcome of this study.
Conclusion
Despite strong recommendations to avoid the use of NSAID/COX2 drugs in patients with reduced renal function, patients with CKD are still being prescribed these agents. While an impaired GFR is an important deterrent to the prescription of these agents, serum Cr values, independent of concomitant estimates of GFR often guide decisions about their prescription. Raising awareness in the health care community of the insensitivity of serum Cr as a measure of renal function and continued encouragement of the use of eGFR as an indicator of CKD may reduce the ill-advised usage of NSAID/COX2s and other medications which can contribute to the adverse outcomes common in this population.
Acknowledgments
KP: American Society of Nephrology Student Scholar Grant, 2010; JCF & MZ: NIH R21 DK07567
References
- 1.Sturmer T, Erb A, Keller F, Gunther K, Brenner H. Determinants of impaired renal function with use of nonsteroidal anti-inflammatory drugs: the importance of half-life and other medications. Am J Med. 2001;111:527. doi: 10.1016/s0002-9343(01)00942-1. [DOI] [PubMed] [Google Scholar]
- 2.Huerta C, Castellsaque J, Varas-Lorenzo C, Garcia Rodriquez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Disease. 2005;45:531–539. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Sandler DP, Smith JC, Weinberg CR, et al. Nonsteroidal anti-inflammatory drugs and the risk for chronic renal disease. Ann Intern Med. 1991;115:165–172. doi: 10.7326/0003-4819-115-3-165. [DOI] [PubMed] [Google Scholar]
- 4.Swan SK, Rudy RW, Lasseter KC, et al. Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low-salt diet. Ann Intern Med. 2000;133:1–9. doi: 10.7326/0003-4819-133-1-200007040-00002. [DOI] [PubMed] [Google Scholar]
- 5.Winkelmayer W, Waikar S, Mogun H, Solomon D. Nonselective and cyclooxygenase-2 selective NSAIDs and acute kidney injury. Am J Med. 2008;121:1098. doi: 10.1016/j.amjmed.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease Outcome Quality Initiative (K/DOQI) Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S242. [PubMed] [Google Scholar]
- 7.Kern EF, Maney M, Miller DR, et al. Failure of ICD-9 CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006;41:564–580. doi: 10.1111/j.1475-6773.2005.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16:2439–2448. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
- 9.Fink JC, Brown J, Hsu VD, Seliger SL, Walker L, Zhan M. CKD as an Underrecognized Threat to Patient Safety. Am J Kidney Dis. 2009;53:681–688. doi: 10.1053/j.ajkd.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain AK, McLeod I, Huo C, Cuerden MS, Akbari A, Tonelli M, van Walraven C, Quinn RR, Hemmelgarn B, Oliver MJ, Li P, Garg AX. When laboratories report estimated glomerular filtration rates in addition to serum creatinines, nephrology consults increase. Kidney Int. 2009;76:318–323. doi: 10.1038/ki.2009.158. [DOI] [PubMed] [Google Scholar]
- 11.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenback S, Quinn RR, Wiebe N, Tonelli M. Relation Between Kidney Function, Proteinuria, and Adverse Outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 12.Chapin E, Zhan M, Hsu VD, Seliger SL, Walker LD, Fink JC. Adverse safety events in chronic kidney disease: the frequency of “multiple hits”. Clin J Am Soc Nephrol. 2010;5:95–101. doi: 10.2215/CJN.06210909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seliger SL, Zhan M, Hsu VD, Walker LD, Fink JC. Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol. 2008;19:2414–2419. doi: 10.1681/ASN.2008010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;111:155A. [Google Scholar]
- 16.Minutolo R, De Nicola L, Mazzaglia G, Postorino M, Cricelli C, Mantovani LG, Conte G, Cianciaruso B. Detection and awareness of moderate to advanced CKD by primary care practitioners: a cross-sectional study from Italy. Am J Kidney Dis. 2008;52:444–453. doi: 10.1053/j.ajkd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Accetta NA, Gladstone EH, DiSogra C, Wright EC, Briggs M, Narva AS. Prevalence of Estimated GFR Reporting Among US Clinical Laboratories. Am J Kidney Dis. 2008;52:778–787. doi: 10.1053/j.ajkd.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 19.Akbari A, Swedko PJ, Clark HD, Hogg W, Lemelin J, Magner P, Moore L, Ooi D. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med. 2004;164:1788–1792. doi: 10.1001/archinte.164.16.1788. [DOI] [PubMed] [Google Scholar]
- 20.Quartarolo JM, Thoelke M, Schafers SJ. Reporting of estimated glomerular filtration rate: effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J Hosp Med. 2007;2:74–78. doi: 10.1002/jhm.172. [DOI] [PubMed] [Google Scholar]
- 21.Rothberg MB, Kehoe ED, Courtemanche AL, Kenosi T, Pekow PS, Brennan MJ, Mulhern JG, Braden GL. Recognition and management of chronic kidney disease in an elderly ambulatory population. J Gen Intern Med. 2008;23:1125–1130. doi: 10.1007/s11606-008-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greer RC, Powe NR, Jaar BG, Troll MU, Boulware LE. Effect of primary care physicians’ use of estimated glomerular filtration rate on the timing of their subspecialty referral decisions. BMC Nephrology. 2011;12 doi: 10.1186/1471-2369-12-1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanter WL, Didomenico RJ, Polikaitis A. A Trial of Automated Decision Support Alerts for Contraindicated Medications Using Computerized Physician Order Entry. J Am Med Inform Assoc. 2005;12:269–274. doi: 10.1197/jamia.M1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, Lee R, Mekala A, Song J, Komaroff AL, Bates DW. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839– 2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 25.Steele AW, Eisert S, Witter J, Lyons P, Jones MA, Gabow P, Ortiz E. The effect of automated alerts on provider ordering behavior in an outpatient setting. PLoS Med. 2005;2:e255. doi: 10.1371/journal.pmed.0020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agodoa LY, Francis ME, Eggers PW. Association of analgesic use with prevalence of albuminuria and reduced GFR in US adults. Am J Kidney Dis. 2008;51:573–583. doi: 10.1053/j.ajkd.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Curhan GC, Knight EL, Rosner B, Hankinson SE, Stampfer MJ. Lifetime nonnarcotic analgesic use and decline of renal function in women. Arch Intern Med. 2004;164:1519–1524. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]
- 28.Kurth T, Glynn RJ, Walker AM, Rexrode KM, Buring JE, Stampfer MJ, Hennekens CH, Gaziano JM. Analgesic use and change in kidney function in apparently healthy men. Am J Kidney Dis. 2003;42:234–244. doi: 10.1016/s0272-6386(03)00647-4. [DOI] [PubMed] [Google Scholar]
- 29.Gooch K, Culleton BF, Manns BJ, Zhang J, Alfonso H, Tonelli M, Frank C, Klarenbach S, Hemmelgarn BR. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120:e1–e7. doi: 10.1016/j.amjmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Kuo HW, Tsai SS, Tiao MM, Liu YC, Lee IM, Yang CY. Analgesic use and the risk of progression of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2010;19:745–751. doi: 10.1002/pds.1962. [DOI] [PubMed] [Google Scholar]
- 31.Pham PC, Dewar K, Hashmi S, Toscano E, Pham PM, Pham PA, Pham PT. Pain prevalence in patients with chronic kidney disease. Clin Nephrol. 2010;73:294–299. [PubMed] [Google Scholar]
- 32.Stroupe KT, Hynes DM, Giobbie-Hurder A, Oddone EZ, Weinberger M, Reda DJ, Henderson WG. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Med Care. 2005;43:453–460. doi: 10.1097/01.mlr.0000160377.82164.d3. [DOI] [PubMed] [Google Scholar]
- 33.Payne SM, Lee A, Clark JA, Rogers WH, Miller DR, Skinner KM, Ren XS, Kazis LE. Utilization of medical services by Veterans Health Study (VHS) respondents. J Ambul Care Manage. 2005;28:125–140. doi: 10.1097/00004479-200504000-00004. [DOI] [PubMed] [Google Scholar]