Hydrogen sulfide (H2S) has been known as a toxic pollutant for years. However, this molecule has been recently recognized as the third gaseous transmitter (the other two are nitric oxide and carbon monoxide).[1–3] The production of H2S in mammalian systems has been attributed to at least three endogenous enzymes:[4–7] cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfur-transferase (MPST). These enzymes use cysteine or cysteine derivatives as substrates and convert them into H2S within different organs and tissues. In addition to these enzymatic pathways, there are also a range of comparably simple chemical events which may liberate H2S from the intracellular pool of `labile' sulfur, for instance from the `sulfane sulfur' pool (compounds containing sulfur atoms bound only to other sulfur atoms).[8] The production of endogenous H2S and exogenous administration of H2S have been demonstrated to exert protective effects in many pathologies. For example, H2S has been shown to relax vascular smooth muscle, induce vasodilation of isolated blood vessels, and reduce blood pressure. H2S can also inhibit leukocyte adherence in mesenteric microcirculation during vascular inflammation in rats, suggesting H2S is a potent anti-inflammatory molecule. Additionally, it has become evident that H2S is a potent antioxidant and, under chronic conditions, can up-regulate antioxidant defense. Despite the rising interest in H2S research, fundamental questions regarding regulation of its production, its mechanism of action, and its destruction remain. A critical debate in the field involves the biologically relevant levels of H2S as current reports varying over 105-fold concentration range.[9–12] Obviously, accurate and reliable measurement of H2S concentrations in biological samples is needed and can provide useful information to understand the function of H2S. Currently the major methods for H2S detection are colorimetric and electrochemical assays, gas chromatography, and sulfide precipitation.[12–16] These methods often require complicate sample processing. Given the high reactivity of H2S, these methods can yield variable results.[9–12] Fluorescence based assays could be useful in this field due to the high sensitivity and convenience. However, fluorescence method for H2S detection, especially for real-time detection in biological samples, is still very limited so far.[17–19] Here, we report a reaction-based fluorescent turn-on strategy for the detection of H2S.
We envisioned that H2S is a reactive nucleophile in biological systems which can participate in nucleophilic substitution. In order to selectively detect H2S, the key is to differentiate H2S from other biological nucleophiles, especially thiols such as cysteine and glutathione. Theoretically, H2S can be considered as a non-substituted thiol. It can undergo nucleophilic reaction two times, while other thiols like cysteine are mono-substituted thiols which can only undergo nucleophilic reaction one time. Based on this property, we expected that compounds containing bis-electrophilic enters could be useful reagents for H2S detection. As shown in Scheme 1, H2S should react with the most electrophilic component of a fluorescent probe like A to form a free SH containing intermediate A1. If another electrophile is presented at suitable position, like the ester group shown in A1, the SH group should undergo a spontaneous cyclization to release the fluorophore and form product B. This strategy not only can capture H2S as a stable and analyzable product B, but also will allow us to visualize H2S-related signal via convenient and sensitive fluorescence measurement. We envisioned that substrate A could also react with biological thiols like cysteine. However, the product A2 should not undergo the cyclization to release the fluorophore. Therefore, the fluorescent signal should be selective only for H2S.
Scheme 1.
Proposed fluorescent turn-on strategy
With this idea in mind, we designed a reactive disulfide-containing probe (compound 1). This compound was prepared from thiosalicylic acid 2 in two steps using the procedure shown in Scheme 2. The fluorescence property of this probe was tested in aqueous PBS buffer solution (pH 7.4). Compound 1 (fluorescence q uantum yield: Φ = 0.003) adopted a closed lactone conformation and exhibited no absorption features in the visible region (supporting information). We found that probe 1 reacted rapidly with H2S to generate fluorophore 6 (Φ = 0.392) and benzodithiolone 7 in good yields (Scheme 3). In these experiments, NaHS was used as the equivalent of H2S. It is known that in aqueous state under the physiological pH of 7.4, the major form of H2S exists as HS−; the ratio of HS−/H2S is ~3:1.[9]
Scheme 2.
Synthesis of fluorescent probe 1
Scheme 3.
Fluorescent probes and reaction with H2S
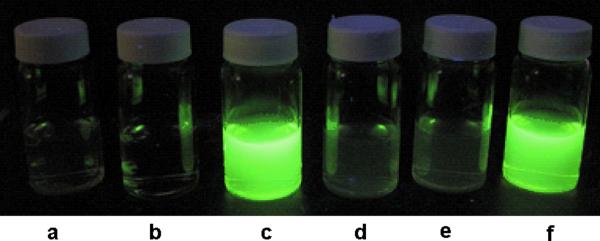
As shown in Figure 1, the reaction of 1 with H2S yielded significant fluorescence signal. Control experiments using cysteine or glutathione did not lead to any fluorescence increase. As expected, when H2S and thiols like GSH co-existed, we still observed strong fluorescence. These results demonstrated that 1 was a selective fluorescent probe for H2S.
Figure 1.
. Fluorescent images of probe 1: a) 1 only (100 μM), b) NaHS only (50 μM), c) 1 (100 μM) + NaHS (50 μM); d) 1 (100 μM) + cysteine (50 μM); e) 1 (100 μM) + glutathione (50 μM), f) 1 (100 μM) + glutathione (50 μM) + NaHS (50 μM), in a mixture of PBS buffer (pH 7.4 mM) and CH3CN (9:1).
The turn-on responses of 1 to H2S and other biological thiols were also measured by a spectrofluorometer. As indicated in Figure 2, the fluorescence intensity of 1 increased dramatically (50~60 fold) if H2S was presented in the solution (even when H2S and other thiols were presented together). In addition, the maximum intensity was reached in 1 hour, which suggested the reaction was fast.
Figure 2.
Fluorescence response of probe 1 toward H2S and other thiols. 1) 1 only (100 μM), 2) 1 (100 μM) + NaHS (50 μM); 3) 1 (100 μM) + cysteine (50 μM); 4) 1 (100 μM) + glutathione (50 μM), 5) 1 (100 μM) + glutathione (50 μM) + NaHS (50 μM); measured in a mixture of PBS buffer (pH 7.4) and CH3CN (9:1), λex 465 nm, 25 °C.
To demonstrate the efficiency of probe 1 in the measurement of H2S concentration, 1 was treated with H2S under a series of different concentrations in order to obtain a standard curve of emission intensity versus H2S concentration. The concentration of compound 1 was maintained at 100 μM, while the concentrations of NaHS varied from 0 to 10 μM. As shown in figure 3, the fluorescent signal was indeed linearly related to the concentration of NaHS in such concentration range. These results demonstrated that probe 1 could detect H2S both qualitatively and quantitatively.
Figure 3.
Linear correlation of fluorescent intensity toward H2S concentration. NaHS concentration: 0, 1, 2.5, 5, 7.5, 10 μM.
Next, we used plasma to investigate the potential of probe 1 for use in the detection of H2S in complex systems. Bovine plasma containing NaHS at different concentrations (0, 50, 100, and 500 μM) were prepared first. These concentrations were within the range of those which have been used to elicit physiological responses of H2S (10–600 μM).[19–22] These plasma solutions were then diluted and incubated with probe 1. After 1 hour, the mixture was diluted again with PBS buffer and fluorescence signals were measured. As expected, strong fluorescence was observed in plasma solutions in the presence of NaHS (Figure 4). We noticed that the fluorescence intensity response to certain H2S concentration obtained in plasma was lower than the signal obtained in pure buffer solutions. This is likely due to the fact that H2S can be quickly scavenged by proteins present in plasma.[18] Nevertheless we conclude that probe 1 can be used for the selective detection of H2S in complex biological systems like plasma.
Figure 4.
Fluorescence response of probe 1 to H2S in plasma. 1) probe 1 only, 2) probe 1 + NaHS (50 μMa, 2 μMb), 3) probe 1 + NaHS (100 μMa, 4 μMb), 4) probe 1 + NaHS (500 μMa, 21 μMb). aoriginal concentration in plasma, bdiluted concentration when fluorescence was recorded.
We also used cultured COS7 cells to investigate the potential of 1 for use in the detection of H2S in cells. As shown in Figure 5, COS7 cells were incubated with compound 1 (100 μM) for 30 min and we did not observe any fluorescent cells. Strong fluorescence in the cells was induced after treatment with sodium sulfide (250 μM). Thus we conclude that probe 1 can be used for the detection of H2S in cultured cells.
Figure 5.
Fluorescence images of H2S detection in COS7 cells using probe 1. COS7 cells on glass coverslips were incubated with 1 (100 μM) for 30 min, and then subjected to different treatments. Top row was control (no sodium sulfide was added); bottom row was treated with sodium sulfide (250 μM).
In summary, we reported in this study a H2S-mediated benzodithiolone formation under mild conditions. This reaction proved to be selective for H2S and it did not proceed with other biological thiols such as cysteine and glutathione. Based on this reaction, a fluorescent probe, i.e. compound 1, was developed for the detection of H2S. The efficiency of this probe was demonstrated in aqueous buffers and plasma, as well as in cells. Using this strategy, the concentration of H2S can not only be measured by the fluorescence signal, but also be assessed from the analysis of the benzodithiolone product. We are now actively pursuing more specific H2S fluorescent probes based on this new benzodithiolone formation and related reactions.
Supplementary Material
Footnotes
We thank Prof. Jeanne McHale for her help with fluorescence measurement. This work is supported in part by NIH (R01GM088226 to M.X.).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- [1].Li L, Rose P, Moore PK. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- [2].Szabo C. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- [3].Lowicka E, Beltowski J. Pharmacol. Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- [4].Kimura H. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- [5].Stipanuk MH, Ueki I. J. Inherit. Metab. Dis. 2011;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Whiteman M, Moore PK. J. Cell. Mol. Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leffler CW, Parfenova H, Jaggar JH, Wang R, R. J. Appl. Physiol. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jacob C, Anwar A, Burkholz T. Planta Med. 2008;74:1580–1592. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- [9].Kabil O, Banerjee R. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Olson KR. Biochim. Biophys. Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- [11].Furne J, Saeed A, Levitt MD. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- [12].a) Han Y, Qin J, Chang X, Yang Z, Du Z. Cell. Mol. Neurobiol. 2006;26:101–107. doi: 10.1007/s10571-006-8848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ubuka T. Analyt. Technol. Biomed. Life Sci. 2002;781:227–249. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]; c) Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, Dieken FP. Biochem. Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- [13].Tangerman A. J. Chromatogr. B. 2009;877:3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- [14].Ubuka T. J. Chromatogr. B. 2002;781:227–249. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]
- [15].Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR., Jr. Anal. Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- [16].Nagata T, Kage S, Kimura K, Kudo K, Noda M. J. Forensic. Sci. 1990;35:706–712. [PubMed] [Google Scholar]
- [17].a) Dasgupta PK, Zhang G, Li J, Boring CB, Jambunathan S, Al-Horr R. Anal. Chem. 1999;71:1400–1407. doi: 10.1021/ac981260q. [DOI] [PubMed] [Google Scholar]; b) Toda K, Dasgupta PK, Li J, Tarver GA, Zarus GM. Anal. Chem. 2001;73:5716–5724. doi: 10.1021/ac010602g. [DOI] [PubMed] [Google Scholar]; c) Trettnak W, Wolfbeis OS. Fresenius' J. Anal. Chem. 1987;326:547–550. [Google Scholar]; d) Wolfbeis OS, Trettnak W. Spectrochimica Acta A. 1987;43A:405–408. [Google Scholar]
- [18].Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Free Radic. Biol. Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].A fluorescent probe of H2S was recently reported: Lippert AR, New EJ, Chang CJ. J. Am. Chem. Soc. 2011;133:10078–10080. doi: 10.1021/ja203661j.
- [20].Yang G, Wu L, Jiang B, Yang B, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Proc. Natl. Acad. Sci. USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abe K, Kimura H. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.