Abstract
Contemporary methods for evaluation and treatment of arrhythmia are increasingly dependent upon characterization of the underlying myocardial substrate. Cardiovascular magnetic resonance offers unsurpassed soft tissue resolution capable of visualizing detailed cardiac anatomic features and intra-myocardial barriers to conduction. Non-invasive visualization of such anatomic detail has the potential to improve methods to diagnose, risk stratify, and treat patients with arrhythmia. This review describes a brief overview of the current knowledge on the applications of cardiac magnetic resonance for evaluation and treatment of patients with arrhythmia.
Due to its wide availability, echocardiography is the most frequently utilized diagnostic modality for diagnosis of the underlying substrate for cardiac arrhythmia. However, echocardiography is unable to visualize intra-myocardial substrates for reentrant arrhythmia such as fat or scar fibrosis. Replacement of myocytes with non-viable tissue results in hypertrophy of the remaining viable cells and yields altered ion channel and gap junction expression. These changes affect myocardial mechanical function as well as promoting arrhythmia.1 Due to its soft tissue resolution and multi-planar imaging capabilities, cardiac magnetic resonance (CMR) offers significant advantages compared to echocardiography. Specialized CMR techniques are uniquely suited for diagnosis of various structural changes,2 and can be applied to identify arrhythmic substrates.
Ischemic cardiomyopathy is left ventricular dysfunction resulting from coronary artery disease and myocardial infarction and is a common substrate of ventricular arrhythmia. Techniques to visualize infarcted myocardium in the acute3–7 and chronic8–11 settings have been well described and rely primarily upon steady state free precession (SSFP) cine CMR for evaluation of function, and late gadolinium enhancement (LGE) imaging to assess scar burden and distribution. The SSFP cine technique relies on short repetition times and electrocardiographic gating to provide dynamic visualization of the heart during the full cardiac cycle. The LGE technique utilizes an inversion recovery gradient echo sequence with an optimal inversion time set to null the signal of normal myocardium. In the setting of ischemic cardiomyopathy, images are acquired 10–15 minutes after intravenous administration of 0.2 mmol/kg gadolinium chelate. Gadolinium chelates are hydrophilic and have low molecular weights thereby concentrating into the extracellular fluid space. The extent of LGE is primarily determined by expansion of the extracellular space in fibrotic tissue which slows washout of the gadolinium chelates.12 This “delayed” time period from contrast administration to scan acquisition allows clearance of the contrast medium from the normal myocardium, while non-viable myocardium shows LGE due to enhanced relaxivity of excited protons adjacent to retained gadolinium which increases the signal on T1 weighted images.
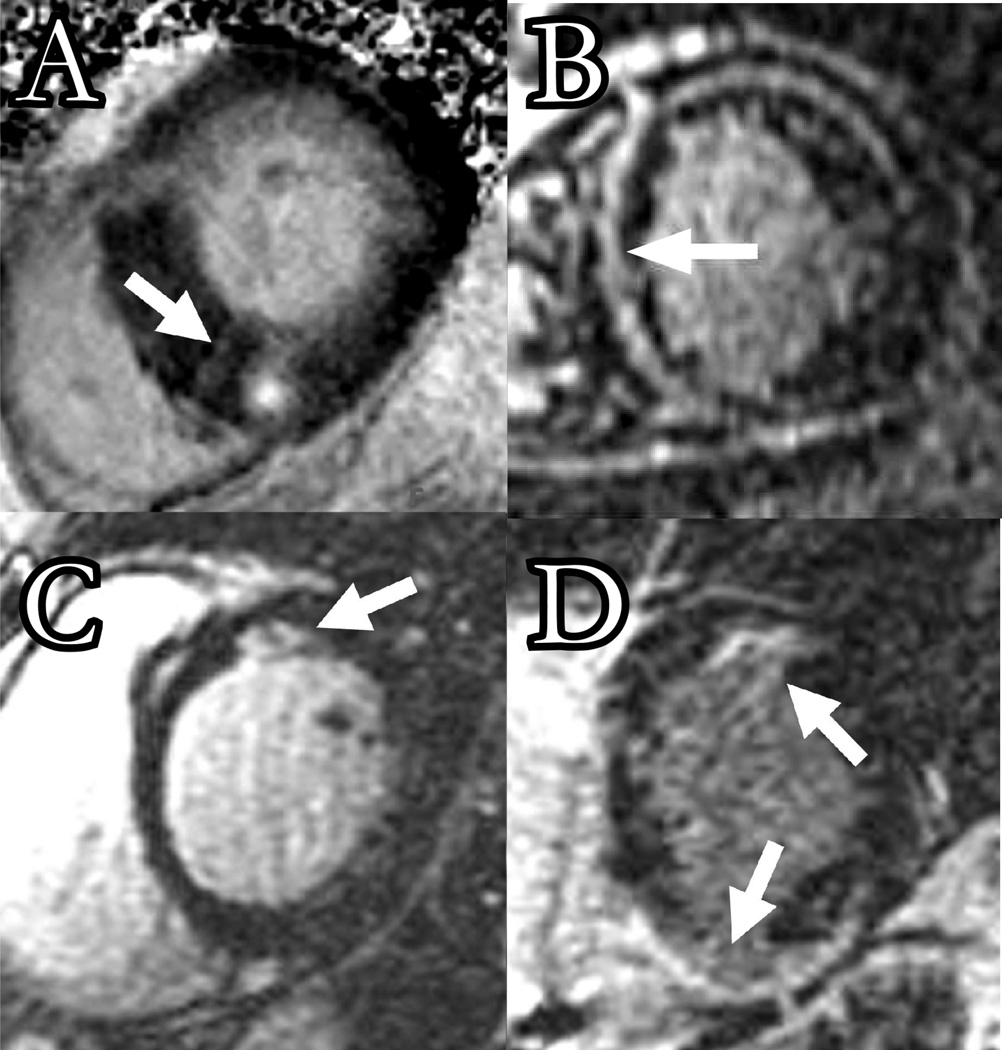
Hypertrophic cardiomyopathy is characterized by left and/or right ventricular hypertrophy resulting from an inherited defect in the protein components of the cardiac sarcomere. In cases where hypertrophy occurs at the basal septum, obstruction of the left ventricular outflow tract and mitral regurgitation due to systolic anterior motion of the anterior leaflet of the mitral valve can be present. Echocardiography is the standard technique for evaluation of hypertrophic cardiomyopathy.13 CMR is an appropriate alternative to confirm the diagnosis or identify atypical cases,14, 15 and is most useful when the echocardiography acoustic window is limited.16 LGE can detect midwall and patchy scar in regions with hypertrophy (Figure 1A).17 Necropsy studies have revealed good correlation between the LGE pattern of enhancement and the distribution of scar.16, 18
Figure 1.
The figure illustrates LGE images in cardiomyopathies and delayed enhancement patterns (arrows). A) Hypertrophic cardiomyopathy, B) idiopathic cardiomyopathy, C) sarcoidosis, D) ARVD.
Non-Ischemic cardiomyopathy is characterized by left ventricular or biventricular dilatation and impaired contraction in the absence of flow limiting coronary disease. While some cases are due to viral, genetic, toxic, or immune causes, many are of unknown etiology. The anatomic and functional abnormalities of non-ischemic cardiomyopathy are readily assessed by cine CMR.19 As demonstrated in Figure 1B, LGE can be used in the evaluation of patients with non-ischemic cardiomyopathy. Although absence of hyper-enhancement is the most common finding in non-ischemic cardiomyopathy, midwall striae or patches of enhancement can be identified in approximately one third of cases.20–22 Compared to ischemic cardiomyopathy, the pattern and location of LGE in non-ischemic cardiomyopathy is often atypical, making it difficult to distinguish artifact from true scar. The presence of scar should therefore be verified by use of multiple image planes and optimized inversion times.
Sarcoidosis with cardiac involvement is relatively uncommon (less than 5% of patients with pulmonary sarcoidosis). Currently used techniques, including echocardiography,23 scintigraphy,24 and myocardial biopsy25 are often inadequate for early diagnosis. In patients with systemic sarcoidosis suspected of cardiac involvement, CMR may provide a diagnostic alternative and a method by which disease activity can be followed. Several authors have reported the occurrence of CMR abnormalities in patients with ongoing systemic sarcoidosis.26–29 Due to increased T2 relaxation time, inflammatory sarcoid granules present as high signal intensity regions on T2 weighted images. Focal areas of hyper-enhancement likely representing fibrosis, can also be noted on LGE images (Figure 1C), most commonly in the basal segments of the left ventricle.30
Arrhythmogenic right ventricular dysplasia (ARVD) is characterized by enlargement, dysfunction, and fibro-fatty infiltration of the right ventricle. Clinical manifestations include ventricular tachycardia and symptoms of right heart failure. There are many methods to assess the right ventricle, but techniques like CMR that facilitate comprehensive coverage of the right ventricle are essential.31 Combined with its ability to characterize fibro-fatty infiltration of the right ventricle, CMR has rapidly evolved into the diagnostic standard for identifying ARVD.32–34 CMR abnormalities in ARVD can be divided into functional and morphologic abnormalities.35 Functional abnormalities include regional wall motion abnormalities, focal aneurysms, right ventricular dilation and/or systolic and diastolic dysfunction, and are best evaluated via SSFP cine imaging of the entire right ventricle in axial or long-axis stacks. Furthermore, substantial normal right ventricular variations, including reduced wall motion near the moderator band, variable trabeculation, and fat deposits surrounding the coronary vessels and epicardium can limit interpretation for the non-experienced observer.36–38 Morphologic abnormalities include intramyocardial fatty infiltration, focal wall thinning, wall hypertrophy, trabencular hypertrophy and disarray, moderator band hypertrophy, and right ventricular outflow tract enlargement. LGE can show areas of delayed enhancement in both the right and left ventricles (Figure 1D). These findings are best identified on a series of long axis images of the right ventricle. Importantly, LGE for assessment of scar in the right ventricle must be performed with optimization of the inversion time for myocardial signal suppression for the right ventricle which is often substantially different than that optimized for the left ventricle.39 Intramyocardial fatty infiltration can be observed as an area of high signal intensity on T1 weighted images. However, the normal presence of fat in the atrioventricular groove and anteroapical right ventricular epicardium, and artifacts due to motion, arrhythmia, and surface coil proximity can substantially reduce the specificity of high T1 signal intensity for the presence of intramyocardial fat INCLUDE REFERENCE: Macedo R, Prakasa K, Tichnell C, Marcus F, Calkins H, Lima JA, Bluemke DA. Marked lipomatous infiltration of the right ventricle: MRI findings in relation to arrhythmogenic right ventricular dysplasia. AJR Am J Roentgenol. 2007 May;188(5):W423–7. PMID: 17449737. It is also important to emphasize that identification of right ventricular fat signal by imaging is not unique to ARVD, or a recognized criterion for its diagnosis. The contribution of CMR to ARVD diagnostic criteria are primarily through functional assessments such as regional right ventricular wall motion abnormalities, dilatation, and aneurysms, and are summarized in the first row of Table 1.40
Table 1.
The table provides a summary of current diagnostic criteria for ARVD. The diagnosis of ARVD would be fulfilled by the presence of two major, or one major plus two minor criteria or four minor criteria from different groups.40
| I. Global and/or Regional Dysfunction and Structural Alterations on echocardiography, angiography, CMR, or radionuclide scintigraphy. | |
| Major |
|
| Minor |
|
| II. Tissue Characterization of Wall | |
| Major |
|
| III. Repolarisation Abnormalities | |
| Minor |
|
| IV. Depolarization/Conduction Abnormalities | |
| Major |
|
| Minor |
|
| V. Arrhythmias | |
| Minor |
|
| VI. Family History | |
| Major |
|
| Minor |
|
Acute inflammatory myocarditis can accompany systemic immune dysfunction or result from exposure to pathogens and toxins. Endomyocardial biopsy, the gold-standard for diagnosing myocarditis, is limited by inadequate sensitivity and specificity.41 LGE with early imaging (1–2 minutes) can show relative myocardial enhancement compared with skeletal muscle and is likely due to the loss of cellular membrane integrity which allows accumulation of gadolinium chelates.42 Abnormal myocardial signal may also be present with T2-weighted spin-echo CMR images, and is a result of interstitial edema which increases the T2 relaxation time. Normalization of signal intensity occurs with healing, unless cell death has occurred, in which case LGE may show patchy enhancement. LGE 2–4 weeks after the onset of symptoms can predict functional and clinical long-term outcomes.42–44 Enhanced contrast is often observed in the epicardium of the lateral free wall, a finding that is consistent with postmortem studies.45
Chagas disease is an inflammatory disease caused by the parasitic protozoan Trypanosome cruzi. Although most patients survive the acute phase of the disease and remain asymptomatic for many years, 20% eventually present with heart failure. CMR can accurately assess morphological and functional aspects of cardiac involvement in Chagas disease.46
Surgical scar can serve as an anatomic barrier for arrhythmic reentry. CMR is capable of delineating cardiac structure and function post cardiac surgery, a setting where echocardiography is often hindered due to chest wall changes that diminish the acoustic window. LGE has been shown to identify fibrous tissue in the post surgical myocardium.47
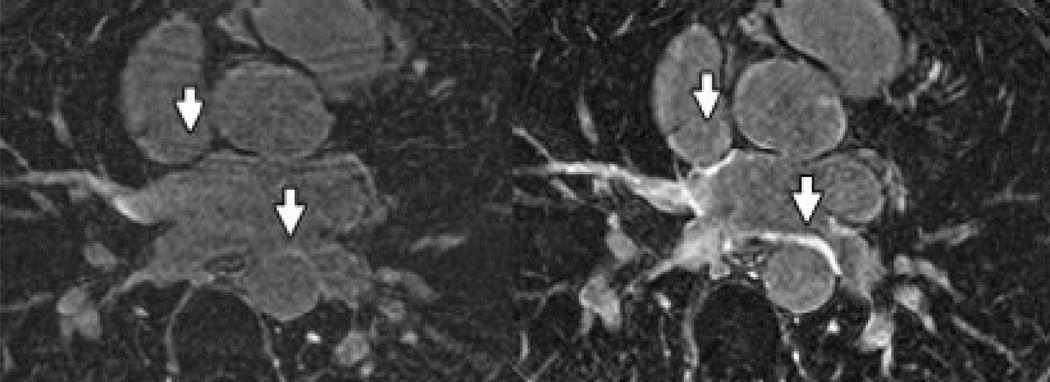
Atrial scar may occur in the setting of any of the above myopathies or in isolation.48 While LGE imaging has been well established for detection of fibrosis in ventricular myocardium, imaging of scar in the atrium has proved challenging due to reduced wall thickness and the resulting requirement for higher spatial resolution. Recent studies have suggested a potential role for detection of atrial scar utilizing LGE after pulmonary vein isolation procedures for atrial fibrillation (Figure 2, from Peters et al).49, 50
Figure 2.
Transverse inversion recovery gradient echo images of the left atrium obtained by Peters et al,50 showing no LGE pre-ablation (left panel), in comparison to post ablation images (right panel) with LGE noted in the pulmonary vein ostial region where radiofrequency energy was delivered.
Applications of CMR for Arrhythmic Risk Assessment
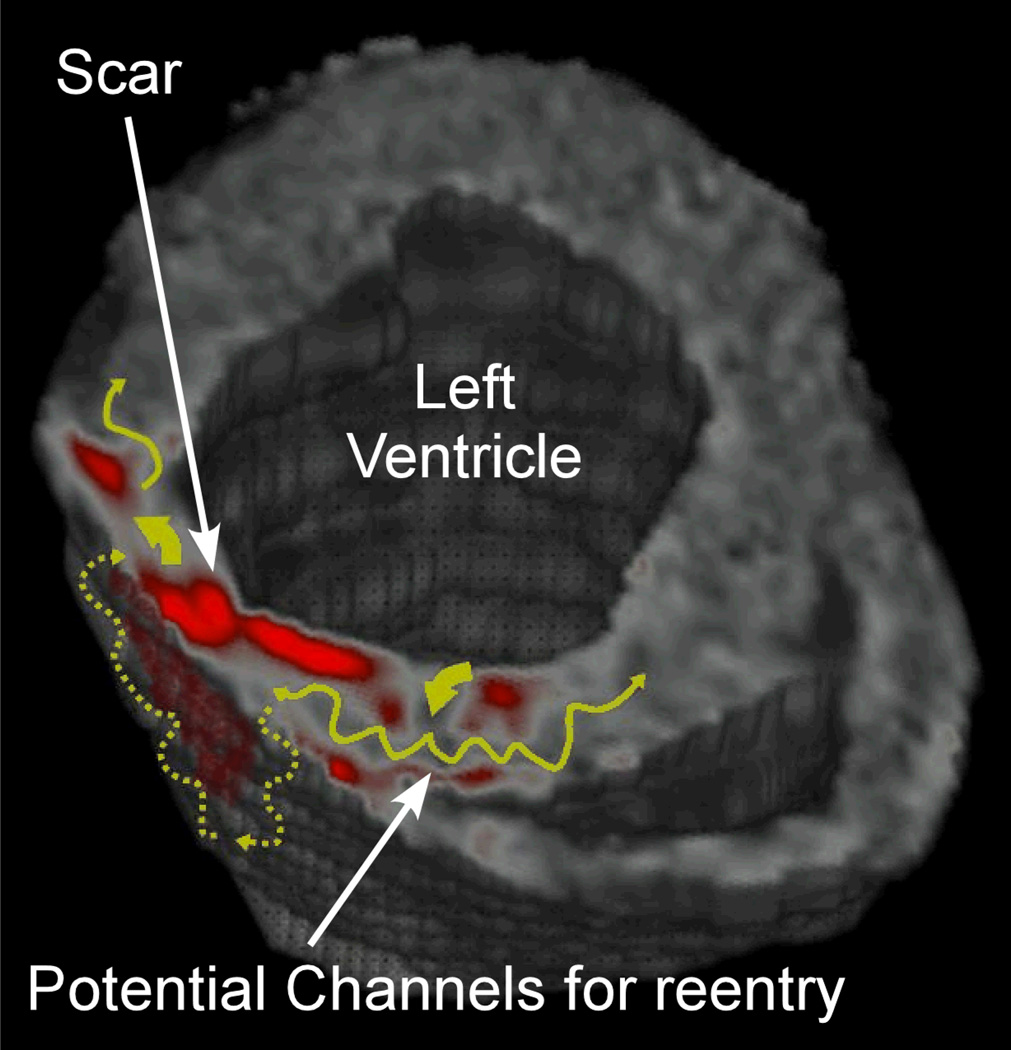
Traditional techniques for arrhythmic risk assessment primarily rely upon the clinical history, electrocardiographic features, morphologic evaluation of ejection fraction or wall thickness by echocardiography, and electrophysiology study results. The mechanism of arrhythmia is often reentry or the propagation of activation around a barrier to conduction. This reentrant circuit is often complicated, involving parts or combinations of viable tissue channels delineated by scar islands (Figure 3). Since CMR is capable of imaging non-viable tissue, it stands to reason that it would be capable of identifying potential substrates for reentry. Several recent studies have highlighted a potential role for CMR to complement traditional approaches for risk stratification of ventricular arrhythmia. These studies have been summarized below and also in Table 2.
Figure 3.
The figure is a 3 dimensional processed LGE image of a patient with ischemic cardiomyopathy. Scar has been highlighted in red. The yellow curved arrows show potential pathways for reentry.
Table 2.
Summary of studies of the relation of LGE with arrhythmia
| Source | Number of Patients, study design |
Finding | |
|---|---|---|---|
| Ischemic Cardiomyopathy | Bello et al 54 | 48, prospective cohort | Infarct size and surface area was greater in patients with monomorphic ventricular tachycardia inducible at electrophysiology study. |
| Yan et al 55 | 144, retrospective cohort | Mortality was higher in patients with greater peri-infarct zone extent to infarct extent ratio. | |
| Schmidt et al 56 | 47, prospective cohort | Higher LGE defined tissue heterogeneity at the infarct periphery was predictive of induciblity at electrophysiology study. | |
| Hypertrophic Cardiomyopathy | Teraoka et al 60 | 59, prospective cohort | Presence and extent of LGE were directly correlated with the presence of non sustained ventricular tachycardia on Holter monitoring. |
| Dimitro et al99 | 47, prospective cohort | Patients with non-sustained ventricular tachycardia on Holter monitoring were more likely to exhibit LGE. The extent of scar was not significantly different between the two groups. | |
| Adabag et al 61 | 177, prospective cohort | Non sustained ventricular tachycardia was more common in patients with LGE, and patients with LGE had greater numbers of non sustained ventricular tachycardia episodes. The extent of scar was not significantly different between the two groups. | |
| Non-Ischemic Cardiomyopathy | Nazarian et al 64 | 26, prospective cohort | Predominance of scar distribution involving 26–75% of wall thickness predicted inducible ventricular tachycardia at electrophysiology study. |
| Assomull et al65 | 101, prospective cohort | Midwall fibrosis was present in 35% of patients and predicted the combined endpoint of all cause death or hospitalization. | |
| Wu et al 66 | 65, prospective cohort | The presence of LGE predicted the composite endpoint of hospitalization for heart failure, appropriate ICD firing, and cardiac death. | |
| ARVD | Tandri et al 38 | 30, prospective cohort | The presence of LGE predicted inducible ventricular tachycardia at electrophysiology study. |
| Chagas Disease | Rochitte et al80 | 51, retrospective cohort | LGE was present in 100% of patients with documented history of ventricular tachycardia. |
Ischemic cardiomyopathy patients suffer from a significant risk of ventricular arrhythmia, and have been shown to derive survival benefit from implantable cardioverter defibrillators (ICD).51–53 Bello et al showed that out of 48 patients referred for electrophysiology study, the 18 with monomorphic ventricular tachycardia had larger infarcts than the 21 patients with no inducible arrhythmia. Interestingly, the 9 patients with inducible polymorphic ventricular tachycardia or ventricular fibrillation had intermediate infarct mass. The authors also showed that in logistic regression models including both infarct mass and left ventricular ejection fraction, or both infarct surface area and left ventricular ejection fraction, infarct mass and surface area were the only significant predictors of inducible ventricular tachycardia.54 Yan et al examined the extent of peri-infarct zone quantified by LGE as an independent predictor of mortality in patients with history of myocardial infarction. The authors found that out of 144 patients with coronary artery disease and LGE, those with above median peri-infarct zone extent to infarct extent ratios (defined by ratio of the extent of LGE region with intensity 2–3 standard deviations above null myocardium over extent of LGE region with intensity 3 standard deviations above null myocardium) had higher mortality. After adjusting for age and left ventricular ejection fraction, peri-infarct zone extent to infarct extent ratio remained predictive of all cause and cardiovascular mortality.55 Schmidt et al also studied the utility of an LGE measure of peri-infarct tissue heterogeneity (defined as the myocardium with signal intensity > peak remote signal intensity but <50% of maximal signal intensity of the manually contoured high signal intensity myocardium) in 47 patients referred for prophylactic ICD implantation for ischemic cardiomyopathy. The authors found that higher tissue heterogeneity at the infarct periphery was predictive of induciblity at electrophysiology study and that it was the only significant predictor of induciblity in a stepwise logistic regression model containing infarct location and core extent, and left ventricular ejection fraction and end-diastolic volume.56 These studies provide evidence that LGE may provide additional benefit for risk stratification of patients with ischemic cardiomyopathy. A prospective study to determine the benefits of ICD implantation in patients stratified by infarct morphology identified by LGE is currently underway.57
Hypertrophic cardiomyopathy patients are at significant risk for sudden death, therefore accurate and early risk stratification is essential in this condition.13 Ventricular arrhythmia occurrence rates of 5% per year for primary events and double that for secondary events have been reported.58 The degree of fibrosis in hypertrophic cardiomypathy appears to correlate with arrhythmia risk. Pathology studies in which hearts of patients with HCM were examined after death or transplantation have shown greater extent of fibrosis in patients with history of ventricular arrhythmia.59 Therefore it is plausible that the extent of myocardial fibrosis in this disease may correlate with the occurance of ventricular arrhythmia. Teraoka et al performed CMR examinations in 59 patients with hypertrophic cardiomyopathy and noted that the presence and extent of LGE were directly correlated with the presence of nonsustained ventricular tachycardia on Holter monitoring.60 Dimtrow et al also assessed LGE imaging in patients with hypertrophic cardiomyopathy and found lower likelihood of LGE in patients without non sustained ventricular tachycardia compared with those with non sustained ventricular tachycardia on Holter monitoring. However, the extent of scar was not significantly different between the two groups in Dimitrow et al’s study. Later, Adabag et al performed LGE imaging on 177 patients with hypertrophic cardiomyopathy and found that nonsustained ventricular tachycardia was more common in patients with LGE, and that patients with LGE had greater numbers of non sustained ventricular tachycardia episodes. Similar to the findings of Dimitrow et al, however, the extent of LGE was similar in patients with and without non sustained ventricular tachycardia.61 These findings suggest a potential utility of CMR for risk stratification of ventricular arrhythmia in patients with hypertrophic cardiomyopathy.
Non-ischemic cardiomyopathy commonly presents with atrial and ventricular arrhythmias. However, syncope and sudden death are rarely the initial manifestations of the disease.62 Current guidelines propose ICD implantation for prevention of sudden death in non-ischemic cardiomyopathy patients with left ventricular ejection fraction less than 35% and symptoms of congestive heart failure.63 We performed LGE imaging prior to electrophysiology study in 26 patients with non-ischemic left ventricular dysfunction. We found that the predominance of scar distribution involving 26–75% of wall thickness was predictive of inducible ventricular tachycardia after adjusting for left ventricular ejection fraction.64 Assomull and colleagues enrolled 101 patients with non-ischemic dilated cardiomyopathy and found that midwall fibrosis, which was present in 35% of patients, predicted the combined endpoint of all cause death or hospitalization. Midwall fibrosis was also predictive of the combined endpoint of sudden death and ventricular tachycardia after adjusting for left ventricular ejection fraction.65 Similarly, Wu and colleagues found that the presence of LGE in the setting of non-ischemic left ventricular dysfunction predicts the composite endpoint of hospitalization for heart failure, appropriate ICD firing, and cardiac death.66 Identification of scar fibrosis may assist non-ischemic cardiomyopathy patient selection for ICD implantation,67 and help direct ventricular tachycardia ablation mapping efforts toward the site of scar related reentry.68
Sarcoidosis involving the heart is uncommon, but sudden death due to arrhythmia may be its initial clinical presentation. Accurate diagnosis is essential as ICD implantation and early immunosuppressive therapy may improve prognosis.69 The capability of LGE to identify cardiac sarcoidosis suggests utility for risk stratification in this condition.
Arrhythmogenic right ventricular cardiomyopathy may be responsible for 10–20% of sudden cardiac deaths among certain populations.70 Tandri et al performed LGE imaging and electrophysiology testing in 30 patients being evaluated for ARVD. The authors found sustained ventricular tachycardia inducible in 6 of 8 patients with LGE, whereas none of the patients without LGE had inducible ventricular tachycardia at electrophysiology study.38 While the long-term prognostic significance of this finding remains to be validated, it suggests that LGE has a potential role in risk stratification of patients with ARVD.
Acute inflammatory myocarditis can present with refractory ventricular tachycardia, torsade de pointes, or sudden cardiac death.71–73 Refractory atrial fibrillation has also been associated with inflammatory infiltrates in the atrium.74 The role of CMR for arrhythmia management has not been assessed in acute myocarditis.
Chagas disease is commonly associated with ventricular arrhythmia75–77 often presenting during or after exercise.75, 78 Importantly, ventricular arrhythmia may develop before cardiomegaly and heart failure are detected.75, 79 Atrial arrhythmias, including atrial fibrillation have also been reported.75 Rochitte et al performed LGE imaging in 51 patients with Chagas disease and found that 100% of those with previously documented ventricular tachycardia had fibrosis detectable by LGE.80 This study suggests a potential role for use of CMR for prospective risk stratification in Chagas disease.
Surgical Scar can be associated with reentrant arrhythmias including atrial tachycardia status post atriotomy scars81–83 and ventricular tachycardia after ventriculotomy or patch repair.84, 85 CMR can be used to characterize the anatomy and assess scar burden thus aiding risk stratification and pre-procedural planning, and is particularly useful in the case of complex post surgical congenital heart disease.
Atrial scar extent and distribution detected by LGE has been associated with atrial arrhythmias.48 Recent studies have also suggested that post pulmonary vein isolation LGE may detect the extent of radiofrequency induced damage in the atria.50 The extent of atrial scar appears to be inversely correlated with left atrial function86 and appears to be directly correlated to the success of atrial fibrillation ablation. The role of CMR in detecting atrial scar remains experimental, and future studies to determine its potential utility toward management of atrial arrhythmia are warranted.
Applications of CMR for Catheter Ablation
Introduction of electro-anatomic mapping systems with the capability to merge pre-acquired images with procedural catheter positions and intra-cardiac voltage and activation sequence maps has led to rapid integration of CMR and computed tomography images into the clinical electrophysiology laboratory. Pre-acquired CMR images are commonly used to provide a “shell” of the endocardial/epicardial boundaries of cardiac chambers and other anatomic regions of interest such as the aortic root and coronary vessels. This technology has been particularly useful in atrial fibrillation ablation, where knowledge of the pulmonary vein anatomy can help avoid complications due to inadvertent ablation too far inside the vein or at the ostium of smaller variant branches.87 Additionally, knowing the location of the left atrial appendage can help avoid perforation due to catheter advancement in this area. By providing an anatomic reference of the pre-procedural location of the esophagus, image integration may also help reduce the potential of collateral damage by ablating in close proximity to this structure.88 However, this technique may be limited by potential movement of the esophagus compared to the time the pre-acquired image was obtained.89 Software upgrades to enable integration of myocardial scar images into the electro-anatomic system are under development and will likely reduce procedural time devoted to voltage mapping in ventricular tachycardia. Importantly, such a capability may also allow the delivery of lesions targeted near midwall scar not otherwise identifiable via endocardial or epicardial voltage mapping.
Ultimately, pre-acquired image integration may be replaced by real-time CMR guidance of electrophysiology procedures. However, catheter guidance by real-time CMR may be limited by catheter heating,90 current induction,91 image distortion,92 and electromagnetic signal interference.93 We have recently reported the feasibility of performing electrophysiology studies with a custom electrophysiology system compatible with real-time CMR guidance.94 In our study, heating, current induction and signal interference were mitigated by using non-ferromagnetic catheters, radiofrequency filters, and limiting the specific absorption rate of CMR sequences. Image distortion was minimized by shortening of the echo time, and the use of spin echo and fast spin echo CMR sequences. In the study we demonstrated successful anatomic targeting of catheters and comprehensive electrophysiology studies with recording of intracardiac electrograms and pacing in the CMR environment. The capabilities of real-time CMR guidance for superior resolution of anatomic soft tissues, identification of scar arrhythmia substrates, and monitoring of lesion formation within linear sets and with respect to surrounding structures may improve the safety and efficacy of complex electrophysiology procedures.
Safety Considerations
Patients with cardiac arrhythmia often have high acuity of disease associated with decreased renal function, or ferromagnetic implants as potential CMR contraindications. Recent studies have raised the possibility of gadolinium induced nephrotoxicity, and nephrogenic systemic fibrosis (progressive and severe fibrosis of the skin and other organs) in patients with advanced kidney disease and exposure to gadolinium chelates.95 Current guidelines recommend avoidance of gadolinium chelates in patients with estimated glomerular filtration rate < 30 mL/min/1.73 m2. Ferromagnetic materials in a magnetic field are subject to force and torque. The radio-frequency and pulsed gradient magnetic fields in the MRI environment may induce electrical currents in leads and other ferromagnetic wires within the field. Radio-frequency pulses may also lead to implant heating and tissue damage at the device-tissue interface. Additionally, sophisticated electronic implants, such as those in neuro-stimulators, pacemakers and implantable cardioverter defibrillators have the potential for receiving electromagnetic interference in the MRI environment, resulting in programming changes or loss of function. However, techniques for safe imaging with MRI in the setting of certain permanent pacemaker and implantable cardioverter defibrillator systems have been developed.96, 97 Familiarity with CMR contraindications and implantable device classes with potential for electro-magnetic interaction are essential for radiologists and cardiologists performing examinations in this population of patients. The reader is encouraged to consult web sites that provide more specific information regarding individual devices (e.g., www.mrisafety.com) for specific device testing details. Additionally, current guidelines recommend avoidance of CMR during the first three months of pregnancy due to potential tissue heating, acoustic fetal damage, and teratogenic effects of gadolinium.98 The decision to perform CMR in patients with potential contraindications is frequently made by considering the potential benefit of CMR relative to the attendant risks. Given the potential risks, it is important to conduct a systematic review of the patient’s condition, implanted devices, and safety for CMR. At our institution all patients are asked to review and answer a safety questionnaire (Table 3).
Table 3.
Sample patient safety questionnaire.
|
This section is to be filled out by the PATIENT: The MRI room contains a very strong magnet. Before you go into the room, we must know if you have any metal in your body. Please complete the following: | |||
| Pacemaker/Wires | □ Yes □ No | Recent Stent Placement | □ Yes □ No |
| Aneurysm Clips | □ Yes □ No | Shunt | □ Yes □ No |
| Surgical Clips | □ Yes □ No | Ear Implant | □ Yes □ No |
| Eye Implant | □ Yes □ No | IUD | □ Yes □ No |
| Implantable Defibrillator | □ Yes □ No | Blood Vessel Coil | □ Yes □ No |
| Bullets, Pellets, BBs | □ Yes □ No | Heart Valve | □ Yes □ No |
| Stimulator/Wires | □ Yes □ No | Artificial Limb | □ Yes □ No |
| Infusion Pump | □ Yes □ No | Cochlear Device | □ Yes □ No |
| Penile Prosthesis | □ Yes □ No | Tracheostomy | □ Yes □ No |
| Do you have kidney disease? Have you ever been a machinist or metal worker? Have you ever had a facial injury from metal? Have you ever had metal removed from your eyes? Are you pregnant? Last menstrual period_____________________________ Do you have any allergies? If yes, please specify_________________________ Current medications______________________________________________ ______________________________________________________________ Weight________________________________________________________ Patient signature_____________________________ Date _______________ |
□ Yes □ No □ Yes □ No □ Yes □ No □ Yes □ No □ Yes □ No □ Yes □ No |
||
| This section is to be filled out by the RADIOLOGY STAFF: | |||
| Operations | □ Yes □ No | ||
| Orbit Films | □ Yes □ No | ||
| Anyone attending the patient in the MRI room has been cleared for MRI safety requirements. | □ Yes □ No | ||
| Radiology staff signature/Title_______________________________________________ Date ___________________ | |||
Conclusion
CMR is increasingly recognized as an important imaging adjunct for the diagnosis of arrhythmogenic myocardial substrates. Advances in CMR, electroanatomic mapping technologies with image integration, and real-time CMR guidance of electrophysiology procedures will likely facilitate patient selection and catheter ablation.
Footnotes
Disclosures
Dr Halperin serves as scientific advisor for Boston Scientific Inc. and holds a patent on MRI compatible catheter technology. Dr Bluemke has received honoraria from General Electric Healthcare for lectures. The Johns Hopkins University Advisory Committee on Conflict of Interest manages all commercial arrangements.
References
- 1.Spector PS. Diagnosis and management of sudden cardiac death. Heart. 2005;91:408–413. doi: 10.1136/hrt.2003.024026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macedo R, Schmidt A, Rochitte CE, Lima JA, Bluemke DA. MRI to assess arrhythmia and cardiomyopathies. J Magn Reson Imaging. 2006;24:1197–1206. doi: 10.1002/jmri.20739. [DOI] [PubMed] [Google Scholar]
- 3.Eichstaedt HW, Felix R, Danne O, Dougherty FC, Schmutzler H. Imaging of acute myocardial infarction by magnetic resonance tomography (MRT) using the paramagnetic relaxation substance gadolinium-DTPA. Cardiovasc Drugs Ther. 1989;3:779–788. doi: 10.1007/BF01857631. [DOI] [PubMed] [Google Scholar]
- 4.de Roos A, van Rossum AC, van der Wall E, Postema S, Doornbos J, Matheijssen N, van Dijkman PR, Visser FC, van Voorthuisen AE. Reperfused and nonreperfused myocardial infarction: diagnostic potential of Gd-DTPA--enhanced MR imaging. Radiology. 1989;172:717–720. doi: 10.1148/radiology.172.3.2772179. [DOI] [PubMed] [Google Scholar]
- 5.Van Rossum AC, Visser FC, Van Eenige MJ, Sprenger M, Valk J, Verheugt FW, Roos JP. Value of gadolinium-diethylene-triamine pentaacetic acid dynamics in magnetic resonance imaging of acute myocardial infarction with occluded and reperfused coronary arteries after thrombolysis. Am J Cardiol. 1990;65:845–851. doi: 10.1016/0002-9149(90)91425-6. [DOI] [PubMed] [Google Scholar]
- 6.Holman ER, van Jonbergen HP, van Dijkman PR, van der Laarse A, de Roos A, van der Wall EE. Comparison of magnetic resonance imaging studies with enzymatic indexes of myocardial necrosis for quantification of myocardial infarct size. Am J Cardiol. 1993;71:1036–1040. doi: 10.1016/0002-9149(93)90569-x. [DOI] [PubMed] [Google Scholar]
- 7.Lima JA, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation. 1995;92:1117–1125. doi: 10.1161/01.cir.92.5.1117. [DOI] [PubMed] [Google Scholar]
- 8.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 9.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 10.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 11.Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5:505–514. doi: 10.1081/jcmr-120022267. [DOI] [PubMed] [Google Scholar]
- 12.Ordovas KG, Reddy GP, Higgins CB. MRI in nonischemic acquired heart disease. J Magn Reson Imaging. 2008;27:1195–1213. doi: 10.1002/jmri.21172. [DOI] [PubMed] [Google Scholar]
- 13.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, 3rd, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–1713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 14.Devlin AM, Moore NR, Ostman-Smith I. A comparison of MRI and echocardiography in hypertrophic cardiomyopathy. Br J Radiol. 1999;72:258–264. doi: 10.1259/bjr.72.855.10396215. [DOI] [PubMed] [Google Scholar]
- 15.Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90:645–649. doi: 10.1136/hrt.2003.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, Weil J, Zenovich AG, Maron BJ. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855–861. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 17.Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 18.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–1567. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 19.Strohm O, Schulz-Menger J, Pilz B, Osterziel KJ, Dietz R, Friedrich MG. Measurement of left ventricular dimensions and function in patients with dilated cardiomyopathy. J Magn Reson Imaging. 2001;13:367–371. doi: 10.1002/jmri.1052. [DOI] [PubMed] [Google Scholar]
- 20.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 21.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–748. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann O, Grebe O, Merkle N, Nusser T, Kochs M, Bienek-Ziolkowski M, Hombach V, Torzewski J. Myocardial biopsy findings and gadolinium enhanced cardiovascular magnetic resonance in dilated cardiomyopathy. Eur J Heart Fail. 2005 doi: 10.1016/j.ejheart.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Lewin RF, Mor R, Spitzer S, Arditti A, Hellman C, Agmon J. Echocardiographic evaluation of patients with systemic sarcoidosis. Am Heart J. 1985;110:116–122. doi: 10.1016/0002-8703(85)90524-1. [DOI] [PubMed] [Google Scholar]
- 24.Saeki M, Kitazawa H, Kodama M, Izumi T, Shibata A, Kido S, Masani F. Images in cardiovascular medicine. Cardiac sarcoidosis. 67Ga imaging and histology. Circulation. 1995;91:2497–2498. doi: 10.1161/01.cir.91.9.2497. [DOI] [PubMed] [Google Scholar]
- 25.Ratner SJ, Fenoglio JJ, Jr, Ursell PC. Utility of endomyocardial biopsy in the diagnosis of cardiac sarcoidosis. Chest. 1986;90:528–533. doi: 10.1378/chest.90.4.528. [DOI] [PubMed] [Google Scholar]
- 26.Kurashima K, Shimizu H, Ogawa H, Ohka T, Nobata K, Ueno K, Rikimaru S, Fujimura M, Matsuda T. MR and CT in the evaluation of sarcoid myopathy. J Comput Assist Tomogr. 1991;15:1004–1007. doi: 10.1097/00004728-199111000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Vignaux O, Dhote R, Duboc D, Blanche P, Devaux JY, Weber S, Legmann P. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast-enhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr. 2002;26:762–767. doi: 10.1097/00004728-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Vignaux O, Dhote R, Duboc D, Blanche P, Dusser D, Weber S, Legmann P. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest. 2002;122:1895–1901. doi: 10.1378/chest.122.6.1895. [DOI] [PubMed] [Google Scholar]
- 29.Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol. 2005;184:249–254. doi: 10.2214/ajr.184.1.01840249. [DOI] [PubMed] [Google Scholar]
- 30.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Cheriex EC, Gorgels AP, Crijns HJ. The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest. 2005;128:1629–1637. doi: 10.1378/chest.128.3.1629. [DOI] [PubMed] [Google Scholar]
- 31.Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 32.Blake LM, Scheinman MM, Higgins CB. MR features of arrhythmogenic right ventricular dysplasia. AJR Am J Roentgenol. 1994;162:809–812. doi: 10.2214/ajr.162.4.8140995. [DOI] [PubMed] [Google Scholar]
- 33.Midiri M, Finazzo M. MR imaging of arrhythmogenic right ventricular dysplasia. Int J Cardiovasc Imaging. 2001;17:297–304. doi: 10.1023/a:1011628121778. [DOI] [PubMed] [Google Scholar]
- 34.Tandri H, Friedrich MG, Calkins H, Bluemke DA. MRI of arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Magn Reson. 2004;6:557–563. doi: 10.1081/jcmr-120030583. [DOI] [PubMed] [Google Scholar]
- 35.Jain A, Tandri H, Calkins H, Bluemke DA. Role of cardiovascular magnetic resonance imaging in arrhythmogenic right ventricular dysplasia. J Cardiovasc Magn Reson. 2008;10:32. doi: 10.1186/1532-429X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine G, Fontaliran F, Zenati O, Guzman CE, Rigoulet J, Berthier JL, Frank R. Fat in the heart. A feature unique to the human species? Observational reflections on an unsolved problem. Acta Cardiol. 1999;54:189–194. [PubMed] [Google Scholar]
- 37.Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation. 1998;97:1571–1580. doi: 10.1161/01.cir.97.16.1571. [DOI] [PubMed] [Google Scholar]
- 38.Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, Rosen B, Lima JA, Calkins H, Bluemke DA. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 39.Desai MY, Gupta S, Bomma C, Tandri H, Foo TK, Lima JA, Bluemke DA. The apparent inversion time for optimal delayed enhancement magnetic resonance imaging differs between the right and left ventricles. J Cardiovasc Magn Reson. 2005;7:475–479. doi: 10.1081/jcmr-200053534. [DOI] [PubMed] [Google Scholar]
- 40.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanes JG, Ghali J, Billingham ME, Ferrans VJ, Fenoglio JJ, Edwards WD, Tsai CC, Saffitz JE, Isner J, Furner S, et al. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation. 1987;75:401–405. doi: 10.1161/01.cir.75.2.401. [DOI] [PubMed] [Google Scholar]
- 42.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 43.Scott RL, Ratliff NB, Starling RC, Young JB. Recurrence of giant cell myocarditis in cardiac allograft. J Heart Lung Transplant. 2001;20:375–380. doi: 10.1016/s1053-2498(00)00314-4. [DOI] [PubMed] [Google Scholar]
- 44.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 45.Shirani J, Freant LJ, Roberts WC. Gross and semiquantitative histologic findings in mononuclear cell myocarditis causing sudden death, and implications for endomyocardial biopsy. Am J Cardiol. 1993;72:952–957. doi: 10.1016/0002-9149(93)91113-v. [DOI] [PubMed] [Google Scholar]
- 46.Kalil Filho R, de Albuquerque CP. Magnetic resonance imaging in Chagas' heart disease. Sao Paulo Med J. 1995;113:880–883. doi: 10.1590/s1516-31801995000200022. [DOI] [PubMed] [Google Scholar]
- 47.Harris MA, Johnson TR, Weinberg PM, Fogel MA. Delayed-enhancement cardiovascular magnetic resonance identifies fibrous tissue in children after surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2007;133:676–681. doi: 10.1016/j.jtcvs.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 48.Massumi A, Rasekh A, Saeed M, Flam S, Cheong B, Mojibian H, Razavi M. Organized incessant atrial arrhythmias in the setting of severe, isolated biatrial scarring. Pacing Clin Electrophysiol. 2008;31:666–675. doi: 10.1111/j.1540-8159.2008.01069.x. [DOI] [PubMed] [Google Scholar]
- 49.Peters D, Wylie J, Kissinger K, Hauser T, Botnar R, Essebag V, Josephson M, Manning W. Detection of pulmonary vein ablation with high resolution MRI. J Cardiovasc Magn Reson. 2006;8:4–5. [Google Scholar]
- 50.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 51.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 52.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 53.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 54.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, Kadish AH, Goldberger JJ. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 55.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The DETERMINE Trial (DEfibrillators To REduce Risk by MagnetIc ResoNance Imaging Evaluation) doi: 10.1111/j.1540-8167.2009.01503.x. Available at: http://determinetrialcom/. [DOI] [PMC free article] [PubMed]
- 58.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 59.Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol. 2001;88:275–279. doi: 10.1016/s0002-9149(01)01640-x. [DOI] [PubMed] [Google Scholar]
- 60.Teraoka K, Hirano M, Ookubo H, Sasaki K, Katsuyama H, Amino M, Abe Y, Yamashina A. Delayed contrast enhancement of MRI in hypertrophic cardiomyopathy. Magn Reson Imaging. 2004;22:155–161. doi: 10.1016/j.mri.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 62.Sugrue DD, Rodeheffer RJ, Codd MB, Ballard DJ, Fuster V, Gersh BJ. The clinical course of idiopathic dilated cardiomyopathy. A population-based study. Ann Intern Med. 1992;117:117–123. doi: 10.7326/0003-4819-117-2-117. [DOI] [PubMed] [Google Scholar]
- 63.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 64.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JA, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 66.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marchlinski FE, Jessup M. Timing the implantation of implantable cardioverter-defibrillators in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:2483–2485. doi: 10.1016/j.jacc.2006.01.075. [DOI] [PubMed] [Google Scholar]
- 68.Stevenson WG, Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115:2750–2760. doi: 10.1161/CIRCULATIONAHA.106.655720. [DOI] [PubMed] [Google Scholar]
- 69.Shammas RL, Movahed A. Sarcoidosis of the heart. Clin Cardiol. 1993;16:462–472. doi: 10.1002/clc.4960160603. [DOI] [PubMed] [Google Scholar]
- 70.Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339:364–369. doi: 10.1056/NEJM199808063390602. [DOI] [PubMed] [Google Scholar]
- 71.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. Jama. 1996;276:199–204. [PubMed] [Google Scholar]
- 72.Badorff C, Zeiher AM, Hohnloser SH. Torsade de pointes tachycardia as a rare manifestation of acute enteroviral myocarditis. Heart. 2001;86:489–490. doi: 10.1136/heart.86.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theleman KP, Kuiper JJ, Roberts WC. Acute myocarditis (predominately lymphocytic) causing sudden death without heart failure. Am J Cardiol. 2001;88:1078–1083. doi: 10.1016/s0002-9149(01)02000-8. [DOI] [PubMed] [Google Scholar]
- 74.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 75.Hagar JM, Rahimtoola SH. Chagas' heart disease. Curr Probl Cardiol. 1995;20:825–924. [PubMed] [Google Scholar]
- 76.Bellotti G, Bocchi EA, de Moraes AV, Higuchi ML, Barbero-Marcial M, Sosa E, Esteves-Filho A, Kalil R, Weiss R, Jatene A, Pileggi F. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas' heart disease. Am Heart J. 1996;131:301–307. doi: 10.1016/s0002-8703(96)90358-0. [DOI] [PubMed] [Google Scholar]
- 77.Bestetti RB, Muccillo G. Clinical course of Chagas' heart disease: a comparison with dilated cardiomyopathy. Int J Cardiol. 1997;60:187–193. doi: 10.1016/s0167-5273(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 78.de Paola AA, Gomes JA, Terzian AB, Miyamoto MH, Martinez Fo EE. Ventricular tachycardia during exercise testing as a predictor of sudden death in patients with chronic chagasic cardiomyopathy and ventricular arrhythmias. Br Heart J. 1995;74:293–295. doi: 10.1136/hrt.74.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carrasco HA, Parada H, Guerrero L, Duque M, Duran D, Molina C. Prognostic implications of clinical, electrocardiographic and hemodynamic findings in chronic Chagas' disease. Int J Cardiol. 1994;43:27–38. doi: 10.1016/0167-5273(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 80.Rochitte CE, Oliveira PF, Andrade JM, Ianni BM, Parga JR, Avila LF, Kalil-Filho R, Mady C, Meneghetti JC, Lima JA, Ramires JA. Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas' disease: a marker of disease severity. J Am Coll Cardiol. 2005;46:1553–1558. doi: 10.1016/j.jacc.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 81.De Ponti R, Verlato R, Bertaglia E, Del Greco M, Fusco A, Bottoni N, Drago F, Sciarra L, Ometto R, Mantovan R, Salerno-Uriarte JA. Treatment of macro-re-entrant atrial tachycardia based on electroanatomic mapping: identification and ablation of the mid-diastolic isthmus. Europace. 2007;9:449–457. doi: 10.1093/europace/eum055. [DOI] [PubMed] [Google Scholar]
- 82.Abrams DJ, Earley MJ, Sporton SC, Kistler PM, Gatzoulis MA, Mullen MJ, Till JA, Cullen S, Walker F, Lowe MD, Deanfield JE, Schilling RJ. Comparison of noncontact and electroanatomic mapping to identify scar and arrhythmia late after the Fontan procedure. Circulation. 2007;115:1738–1746. doi: 10.1161/CIRCULATIONAHA.106.633982. [DOI] [PubMed] [Google Scholar]
- 83.Seiler J, Schmid DK, Irtel TA, Tanner H, Rotter M, Schwick N, Delacretaz E. Dual-loop circuits in postoperative atrial macro re-entrant tachycardias. Heart. 2007;93:325–330. doi: 10.1136/hrt.2006.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chinushi M, Aizawa Y, Kitazawa H, Takahashi K, Washizuka T, Shibata A. Clockwise and counter-clockwise circulation of wavefronts around an anatomical obstacle as one mechanism of two morphologies of sustained ventricular tachycardia in patients after a corrective operation of tetralogy of Fallot. Pacing Clin Electrophysiol. 1997;20:2279–2281. doi: 10.1111/j.1540-8159.1997.tb04250.x. [DOI] [PubMed] [Google Scholar]
- 85.Perloff JK, Middlekauf HR, Child JS, Stevenson WG, Miner PD, Goldberg GD. Usefulness of post-ventriculotomy signal averaged electrocardiograms in congenital heart disease. Am J Cardiol. 2006;98:1646–1651. doi: 10.1016/j.amjcard.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 86.Wylie JV, Jr, Peters DC, Essebag V, Manning WJ, Josephson ME, Hauser TH. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm. 2008;5:656–662. doi: 10.1016/j.hrthm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 87.Dong J, Dickfeld T, Dalal D, Cheema A, Vasamreddy CR, Henrikson CA, Marine JE, Halperin HR, Berger RD, Lima JA, Bluemke DA, Calkins H. Initial experience in the use of integrated electroanatomic mapping with three-dimensional MR/CT images to guide catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:459–466. doi: 10.1111/j.1540-8167.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 88.Bunch TJ, Day JD. Novel ablative approach for atrial fibrillation to decrease risk of esophageal injury. Heart Rhythm. 2008;5:624–627. doi: 10.1016/j.hrthm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 89.Daoud EG, Hummel JD, Houmsse M, Hart DT, Weiss R, Liu Z, Augostini R, Kalbfleisch S, Smith MC, Mehta R, Gangasani A, Raman SV. Comparison of computed tomography imaging with intraprocedural contrast esophagram: implications for catheter ablation of atrial fibrillation. Heart Rhythm. 2008;5:975–980. doi: 10.1016/j.hrthm.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 90.Nitz WR, Oppelt A, Renz W, Manke C, Lenhart M, Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001;13:105–114. doi: 10.1002/1522-2586(200101)13:1<105::aid-jmri1016>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 91.Peden CJ, Collins AG, Butson PC, Whitwam JG, Young IR. Induction of microcurrents in critically ill patients in magnetic resonance systems. Crit Care Med. 1993;21:1923–1928. doi: 10.1097/00003246-199312000-00021. [DOI] [PubMed] [Google Scholar]
- 92.Wacker FK, Hillenbrand CM, Duerk JL, Lewin JS. MR-guided endovascular interventions: device visualization, tracking, navigation, clinical applications, and safety aspects. Magn Reson Imaging Clin N Am. 2005;13:431–439. doi: 10.1016/j.mric.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Laudon MK, Webster JG, Frayne R, Grist TM. Minimizing interference from magnetic resonance imagers during electrocardiography. IEEE Trans Biomed Eng. 1998;45:160–164. doi: 10.1109/10.661264. [DOI] [PubMed] [Google Scholar]
- 94.Nazarian S, Kolandaivelu A, Zviman MM, Meininger GR, Kato R, Susil RC, Roguin A, Dickfeld TL, Ashikaga H, Calkins H, Berger RD, Bluemke DA, Lardo AC, Halperin HR. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation. 2008;118:223–229. doi: 10.1161/CIRCULATIONAHA.107.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perazella MA. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr Drug Saf. 2008;3:67–75. doi: 10.2174/157488608783333989. [DOI] [PubMed] [Google Scholar]
- 96.Sommer T, Naehle CP, Yang A, Zeijlemaker V, Hackenbroch M, Schmiedel A, Meyer C, Strach K, Skowasch D, Vahlhaus C, Litt H, Schild H. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114:1285–1292. doi: 10.1161/CIRCULATIONAHA.105.597013. [DOI] [PubMed] [Google Scholar]
- 97.Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, Weiss RG, Berger RD, Bluemke DA, Halperin HR. Clinical Utility and Safety of a Protocol for Noncardiac and Cardiac Magnetic Resonance Imaging of Patients With Permanent Pacemakers and Implantable-Cardioverter Defibrillators at 1.5 Tesla. Circulation. 2006;114:1277–1284. doi: 10.1161/CIRCULATIONAHA.105.607655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen MM, Coakley FV, Kaimal A, Laros RK., Jr Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol. 2008;112:333–340. doi: 10.1097/AOG.0b013e318180a505. [DOI] [PubMed] [Google Scholar]
- 99.Dimitrow PP, Klimeczek P, Vliegenthart R, Pasowicz M, Oudkerk M, Podolec P, Tracz W, Dubiel JS. Late hyperenhancement in gadolinium-enhanced magnetic resonance imaging: comparison of hypertrophic cardiomyopathy patients with and without nonsustained ventricular tachycardia. Int J Cardiovasc Imaging. 2007 doi: 10.1007/s10554-007-9209-9. [DOI] [PubMed] [Google Scholar]