Abstract
Background
Obstructive sleep apnea (OSA) predisposes individuals to cardiovascular morbidity, and cardiopulmonary exercise test (CPET) markers prognostic for cardiovascular disease have been found to be abnormal in adults with OSA. Due to the persistence of OSA and its cardiovascular consequences, whether the cardiovascular adaptations normally conferred by exercise are blunted in adults not utilizing established OSA treatment is unknown. The aims of this study were to document whether OSA participants have abnormal CPET responses and determine whether exercise modifies these CPET markers in individuals with OSA.
Methods
The CPET responses of 43 sedentary, overweight adults (body mass index [BMI]>25) with untreated OSA (apnea-hypopnea index [AHI]≥15) were compared against matched non-OSA controls (n=9). OSA participants were then randomized to a 12-week exercise training (n=27) or stretching control treatment (n=16), followed by a post-intervention CPET. Measures of resting, exercise, and post-exercise recovery heart rate (HRR), blood pressure, and ventilation, as well as peak oxygen consumption (VO2peak), were obtained.
Results
OSA participants had blunted HRR compared to non-OSA controls at 1 (P=.03), 3 (P=.02), and 5 min post-exercise (P=.03). For OSA participants, exercise training improved VO2peak (P=.04) and HRR at 1 (P=.03), 3 (P<.01), and 5 min post-exercise (P<.001) compared to control. AHI change was associated with change in HRR at 5-min post-exercise (r=−.30, P<.05), but no other CPET markers.
Conclusions
These results suggest that individuals with OSA have autonomic dysfunction, and that exercise training, by increasing HRR and VO2peak, may attenuate autonomic imbalance and improve functional capacity independent of OSA severity reduction.
Keywords: obstructive sleep apnea, exercise testing, exercise training, heart rate recovery, peak oxygen consumption
INTRODUCTION
Obstructive sleep apnea (OSA) imposes numerous consequences on the cardiovascular system and, without treatment, predisposes to significant cardiovascular disease (CVD) morbidity and mortality [1–3]. The cardiovascular consequences of OSA are thought to be primarily due to the intermittent hypoxia and arousals resulting from recurring apneic and hypopneic events [4]. The subsequent sympathetic nervous system (SNS) hyperactivity, systemic inflammation, and endothelial dysfunction are believed to be integral intermediate mechanisms of CVD pathology in OSA [5,6].
Cardiopulmonary exercise testing (CPET) provides important prognostic information regarding future cardiovascular health, even in apparently healthy individuals [7–10]. In adults with untreated OSA, many CPET responses have been found to be abnormal when compared with age-, activity- and weight-matched non-OSA controls [11]. These abnormal CPET responses have included reduced cardiorespiratory fitness (VO2peak) [12–14], chronotropic impairment [15], exaggerated systolic (SBP) and/or diastolic blood pressure (DBP) [13,16], impaired ventilatory efficiency (VE/VCO2 slope) [17], and blunted heart rate recovery (HRR) [14,18].
Exercise training has well-established benefits for cardiovascular health [19]. In populations with elevated CVD risk, exercise has elicited increases in VO2peak [20] and HRR [21,22] and reductions in ventilatory inefficiency [23]. However, the effect of exercise on cardiovascular adaptations in OSA patients is largely unknown. Without amelioration of the frequent night-time SNS surges and intermittent hypoxia by OSA treatment (e.g., continuous positive airway pressure [CPAP]), persistent SNS hyperactivity and systemic inflammation may blunt adaptations normally conferred by exercise [24,25]. Indeed, in physically active young adults, two weeks of chronic intermittent hypoxia during sleep, similar to what would be experienced in OSA, has recently been shown to result in cardiovascular and ventilatory impairments during exercise [26].
The aims of this study were: (1) to compare the baseline CPET responses of participants with OSA to a control group of age- and body mass index (BMI)-matched non-OSA adults (who were not subsequently randomized to an intervention), and (2) to evaluate whether CPET responses were altered in participants with OSA following exercise training versus a stretching control treatment. The outcomes presented here were part of a parent randomized controlled trial of exercise training on the severity of OSA, the results of which have been previously published [27].
MATERIALS AND METHODS
Participant Recruitment and Screening
Adults with at least moderate-severity OSA (apnea-hypopnea index [AHI] ≥ 15) but without current treatment for OSA were eligible to participate. Inclusion criteria included being ages 18–55 yr, overweight/obese (BMI ≥ 25), sedentary (< 2 exercise bouts/week), and being at a stable weight (i.e., weight ± 2 kg over last 3 months, not engaged in weight loss efforts). Individuals were excluded for known or suspected significant cardiovascular, pulmonary or metabolic disease [28], uncontrolled hypertension (> 159/99 mmHg), use of beta-blocker medication, pregnancy, or health problems that contraindicated exercise. Due to the high prevalence of hypertension in this population [2], use of antihypertensive medication was not a reason for exclusion provided that the dose remained stable during the study and the medication was not known to alter the chronotropic response to exercise.
Recruitment occurred through sleep medicine clinic referrals and media advertisements. Following a phone screening to determine initial eligibility, individuals were mailed additional screening materials that included the Berlin Questionnaire [29] and questionnaires pertaining to health history, medication use and OSA-specific symptoms. Individuals previously diagnosed with OSA or classified as “high risk” for OSA based on the Berlin Questionnaire and otherwise eligible were initially enrolled in the study. Prior to participation, all participants were briefed about the study protocol and signed an informed consent form approved by the Institutional Review Boards of the WJB Dorn VA Medical Center and the University of South Carolina. Following initial enrollment, 75 individuals were scheduled for one night of laboratory polysomnography (PSG) to screen for OSA.
Individuals with a screening AHI < 15 (n = 24) were excluded from the intervention portion of the study. However, a subset of these individuals with AHI < 10 and similar age and BMI to participants with OSA (n = 9) was invited to continue undergoing the baseline assessments and serve as non-OSA controls for Aim 1 of the present study.
Baseline cardiopulmonary exercise testing (CPET) and body composition assessments were conducted on separate days over a 7–10 day period for the remaining 51 prospective participants with a screening AHI ≥ 15. In addition to serving as a baseline assessment, the CPET also served as a final screen for possible adverse responses to exercise. Individuals with CPET responses suggestive of significant CVD (e.g., ST-segment changes, severe DBP hypertension) were excluded from further participation and referred to cardiologist care (n = 3, all OSA participants). Five additional participants withdrew from the study prior to the completion of baseline assessments.
Participants with significant OSA (i.e., a screening AHI ≥ 15) underwent another night of laboratory PSG, which served as their baseline measure of sleep quality and OSA severity. Once baseline assessments were complete, the OSA participants (n = 43) were randomized to either a 12-week stretching control or an exercise training treatment. After completion of the intervention, OSA participants completed the same assessments as performed at baseline following a day of non-exercise. The subset of non-OSA controls did not participate in the 12-week intervention and were not assessed following baseline measurements.
Polysomnographic Assessment
A standard recording montage was used for the laboratory PSG [30]. Time in bed was fixed at 8 h and initiated according to the participant's usual bedtime. Sleep stage and respiratory event scoring were performed according to standard criteria [30] by a registered polysomnographic technician blinded to participant status. The AHI (calculated as the number of apneas and hypopneas per hour of sleep), oxygen desaturation index (ODI; the number of oxyhemoglobin saturation [SaO2] reductions ≥ 4% per hour of sleep), and the percentage of total sleep time with SaO2 < 90% (SaO290) were retained as markers of OSA severity.
Cardiopulmonary Exercise Testing
At baseline for non-OSA controls and pre- and post-intervention for OSA participants, a physician-supervised CPET was completed on a day separate from PSG testing. Participants were asked to abstain from caffeine and other stimulants on the day of testing; however, regular medications were allowed provided they did not have a known chronotropic effect. Testing always occurred between 10:00–14:00 h. Following a 5-min seated rest, resting HR and blood pressure (BP) were assessed [28]. Participants were then prepared for testing with 12-lead ECG (Quinton Q-Stress, Bothell, WA), which was monitored continuously.
The Bruce treadmill protocol [28], with an additional introductory stage of 1.7 mph and 5% incline, was utilized for testing. Participants exercised until volitional fatigue, then recovered for 5 min at 1.7 mph and 0% incline. Heart rate was recorded via ECG each minute, and BP was assessed via manual sphygmomanometry every 3 min during the test, at immediate post-exercise, and 3 min and 5 min into recovery. Respiratory gas exchange measurements were collected on a breath-by-breath basis and condensed to 15-s averages using a computerized metabolic cart (Cosmed Quark PFT Ergo, Rome, Italy), calibrated before each test.
Peak oxygen consumption (VO2peak) was determined by the highest 15-sec VO2 value obtained. The chronotropic response to exercise was assessed by the percentage of HR reserve achieved, calculated as [(HRpeak – HRrest)/(age-predicted maximum HR [APMHR] – HRrest)], where APMHR was estimated as (220 – age) [10]. Heart rate recovery (HRR) was defined as the difference in beats/min between HRpeak and HR at 1 min, 3 min and 5 min into recovery (HRR-1, HRR-3, HRR-5, respectively) [9]. Recovery SBP and DBP were calculated as the percentage of SBP and DBP at 3 min into recovery divided by SBPpeak and DBPpeak, respectively [18]. As an index of ventilatory efficiency, the VE/VCO2 slope was calculated as the slope of the relationship between ventilation (VE) and carbon dioxide output (VCO2) plotted from exercise onset to maximal exercise [23].
Body Composition and Lifestyle Behaviors
Height and weight measurements were obtained using standardized procedures [28]. Total body dual energy x-ray absorptiometry (DXA; Lunar Prodigy, GE Medical Systems, Madison, WI) measured body fat percentage. One technician conducted and analyzed all DXA scans. Quality assurance tests and phantom scans were performed prior to all measurement sessions. Because one OSA participant exceeded the weight limit for the DXA machine, n = 42 was used for all DXA analyses involving OSA participants.
Daily steps were recorded with daily use of a piezoelectric pedometer (NL1000, New Lifestyles Inc., Lees Summit, MO). The Rapid Eating Assessment for Participants-Short Version questionnaire (REAP-S) [31] assessed dietary habits.
Treatments
Following baseline assessment, the OSA participants were randomized by a 3:2 ratio to exercise and stretching control treatments, respectively. Randomization was stratified based upon sex and severity of OSA (AHI 15–30 vs. AHI > 30).
Exercise Training
Participants assigned to the exercise training treatment met 4 times per week for 12 weeks under supervised conditions. The aerobic exercise dose, which consisted of treadmill and elliptical exercise, was gradually increased during the initial 4 weeks of the intervention. Aerobic activity, which began at 50 min distributed over 4 sessions during the initial week of the intervention, was increased by 25 min/week through week 4. For weeks 5–12 of the intervention, the aerobic exercise dose was 150 min/week distributed over 4 sessions. Aerobic exercise intensity was at 60% of HR reserve and monitored with telemetry (FS2, Polar Electro Oy, Kempele, Finland). A 5-min warm-up and cool-down preceded and followed each aerobic exercise session, not included in the prescribed duration.
Resistance exercise followed aerobic activity on 1–2 days/week. As with aerobic activity, the dose of resistance exercise was gradually increased during the initial 4 weeks of the intervention. During weeks 1 through 4, 1 set of 10–12 repetitions was performed for 8 different resistance exercises, either once per week (weeks 1 and 2) or twice per week (weeks 3 and 4). For weeks 5–12 of the intervention, 2 sets of 10–12 repetitions for 8 exercises were performed twice per week. The specific exercises that were performed (along with their targeted muscle groups) included the shoulder press (deltoids), lat pulldown (latissimus dorsi), leg extension/curl (alternated between sessions; quadriceps and hamstrings, respectively), chest press (pectorals), upright row (trapezius), seated leg press (gluteus maximus, hamstrings, quadriceps, gastrocnemius, soleus), biceps curls/triceps extension (alternated between sessions; biceps and triceps, respectively) and abdominal curls (rectus abdominis). Resistance was increased on an exercise once 12 repetitions could be performed on the second set with proper form.
Stretching Control
Participants assigned to the stretching treatment met twice/week for 12 weeks for supervised flexibility training sessions. At each visit, participants performed 2 sets of 12–15 stretches, each held for 15–30 s, that focused on whole-body flexibility. No change in OSA severity was expected from this control treatment.
Statistical Power
This study was ancillary to a parent study that evaluated the effects of exercise training on OSA severity [27], and was originally powered to detect changes in AHI. However, the primary outcome of interest for these analyses was HRR-1. For Aim 1, prior research indicated a HRR-1 of 16.2 ± 6.5 beats for adults with OSA and 23.2 ± 6.8 beats for non-OSA adults [13,14,18,32]; statistical power was 80% to detect an identical difference between 40 OSA and 9 non-OSA adults. For Aim 2, statistical power was 72% to detect a HRR-1 improvement of 4.5 beats/min following exercise training, assuming a HRR-1 change standard deviation of 5.5 beats/min and no HRR-1 change in the control condition, with a total of 40 participants randomized by a 3:2 ratio.
Statistical Analysis
Comparisons between non-OSA controls and OSA participants on baseline characteristics and CPET responses were made with independent t-tests or χ2 tests as appropriate. Associations between CPET responses and markers of OSA severity were evaluated with Pearson product-moment correlations (r). For OSA participants, analyses were based on an intent-to-treat plan, with the last observation carried forward in the case of drop-outs or missing data. Changes in CPET responses were evaluated by analysis of covariance of post-intervention CPET values with control for baseline CPET values. Pearson correlations (r) between changes in CPET responses and changes in OSA severity were also performed.
All analyses were completed using SAS version 9.2 (SAS Institute, Cary, NC). Unless otherwise specified, data are presented as mean ± standard error. All tests were two-tailed, with statistical significance set at P < .05.
RESULTS
Baseline Characteristics
Table 1 provides a summary of the baseline characteristics of the participants. No significant differences in age, BMI, body fat, or daily activity were noted between non-OSA and OSA participants. As expected, OSA participants had significantly higher AHI (t50 = 2.73, P < .01), ODI (t50 = 2.71, P < .01), and SaO290 (t50 = 2.06, P = .04) than non-OSA participants.
Table 1.
Baseline Characteristics of Non-OSA and OSA Participants.
| Non-OSA (n = 9) | OSA (n = 43) | |
|---|---|---|
| Age | 46.5 (2.9) | 46.9 (1.2) |
| Sex, M/F | 5/4 | 24/19 |
| BMI | 32.5 (1.2) | 34.8 (0.9) |
| DXA total BF %1 | 41.6 (2.3) | 41.6 (1.4) |
| Pedometer steps/day | 5887.5 (543.8) | 5579.8 (408.6) |
| REAP-S | 26.0 (2.1) | 26.3 (0.7) |
| AHI | 4.7 (0.9) | 29.3 (4.1)* |
| ODI | 3.2 (0.7) | 21.6 (3.1)* |
| SaO290 | 0.5 (0.2) | 4.6 (0.9)* |
All data are presented as mean (standard error) unless otherwise noted. AHI: apnea-hypopnea index; BMI = body mass index; DXA: dual x-ray absorptiometry; MVPA: moderate- to vigorous-intensity physical activity; ODI: oxygen desaturation index; OSA: obstructive sleep apnea; REAP-S: Rapid Eating Assessment for Participants-Short version; SaO290: percentage of total sleep time with SaO2 < 90%.
n = 42 for OSA participants.
Significantly different between OSA and non-OSA participants (P < .05).
CPET Responses Between OSA and Non-OSA
A summary of the responses to CPET is given in Table 2 (left panel). Non-OSA participants had a significantly lower HRrest than participants with OSA (t50 = 2.63, P = .01). Chronotropic impairment was not apparent in the OSA participants, as no between-group difference was found for HRpeak or % HR reserve attained. However, despite similar HRpeak, HRR was significantly different between groups at each recovery time-point. HRR-1 (t50 = −2.23, P = .03), HRR-3 (t50 = −2.32, P = .02), and HRR-5 (t50 = −2.30, P = .03) were each significantly blunted in participants with OSA compared to non-OSA participants. No other between-group differences in CPET responses were noted.
Table 2.
CPET Responses between OSA and non-OSA Participants and Associations with OSA Severity.
| Correlations |
|||||
|---|---|---|---|---|---|
| CPET Variable | Non-OSA (n = 9) | OSA (n = 43) | AHI | SaO290 | ODI |
| HRrest, bpm | 69.8 (3.6) | 80.1 (1.6)* | .33† | .09 | .30† |
| HRpeak, bpm | 166.1 (6.1) | 163.9 (2.2) | −.25 | −.24 | −.27 |
| % Heart Rate Reserve | 95.0 (4.7) | 89.9 (2.0) | −.40† | −.30† | −.42† |
| HRR-1, bpm | 24.3 (2.5) | 17.9 (1.2)* | −.45† | −.25 | −.37† |
| HRR-3, bpm | 48.7 (3.1) | 38.8 (1.8)* | −.59† | −.29† | −.51† |
| HRR-5, bpm | 53.7 (1.5) | 43.3 (4.2)* | −.61† | −.32† | −.54† |
| SBPrest, mmHg | 125.1 (2.7) | 129.6 (1.7) | .06 | −.01 | .02 |
| SBPpeak, mmHg | 188.9 (7.4) | 186.7 (3.6) | −.18 | .01 | −.09 |
| Recovery SBP3, % peak | 89.3 (3.0) | 89.2 (1.0) | −.05 | −.13 | −.14 |
| DBPrest, mmHg | 78.9 (2.8) | 82.5 (1.4) | −.13 | −.28† | −.20 |
| DBPpeak, mmHg | 79.8 (2.3) | 85.7 (1.9) | −.14 | −.22 | −.15 |
| Recovery DBP3, % peak | 98.2 (1.2) | 99.5 (1.2) | .09 | −.09 | −.02 |
| Peak RER | 1.16 (0.07) | 1.19 (0.02) | .17 | .14 | .11 |
| VO2peak, mL/kg/min | 22.1 (2.3) | 21.6 (0.8) | −.19 | −.05 | −.13 |
| VE/VCO2 slope | 27.8 (1.1) | 27.2 (0.7) | −.07 | −.10 | −.11 |
Left: data are presented as mean (standard error); right: data are presented as Pearson product-moment correlations. AHI: apnea-hypopnea index; CPET: cardiopulmonary exercise test; DBP: diastolic blood pressure; DBP3: DBP at 3-min active recovery, divided by peak DBP; HR: heart rate; HRR: heart rate recovery at 1, 3, and 5 min into active recovery (HRR-1, HRR-3, HRR-5, respectively); ODI: oxygen desaturation index; OSA: obstructive sleep apnea; RER: respiratory exchange ratio; SBP: systolic blood pressure; SBP3: SBP at 3-min active recovery, divided by peak SBP; SaO290: percentage of total sleep time with SaO2 < 90%; VO2peak: peak oxygen consumption.
Significantly different between non-OSA and OSA participants (P < .05).
Statistically significant correlation (P < .05).
Associations Between CPET Responses and Markers of OSA Severity
A summary of the associations between CPET responses and markers of OSA severity is provided in Table 2 (right panel). Markers of OSA severity were significantly correlated with each of the HR markers; as OSA severity increased, HRrest increased, HRpeak decreased, and HRR decreased. In addition, a significant association between DBPrest and SaO290 was observed (r = −.28, P < .05). However, no other associations between OSA severity and SBP, DBP, or respiratory parameters were noted.
Intervention Summary
Forty-three participants with OSA ≥ 15 at screening were randomized to exercise training (n = 27) or stretching control (n = 16). Three exercise training participants and 2 stretching control participants discontinued participation before study completion. As previously reported[27], adherence did not differ between the exercise training (87.0 ± 3.7%) and the control treatment (79.7 ± 5.2%).
Exercise training resulted in a significant (~25%) reduction in AHI (exercise: 32.2 ± 5.6 to 24.6 ± 4.4, stretching: 24.4 ± 5.6 to 28.9 ± 6.4; F1,40 = 9.54, P < .01), as previously described [27]. Exercise training also resulted in a significant decrease in ODI (exercise: 24.5 ± 4.2 to 21.5 ± 3.7, stretching: 16.8 ± 4.2 to 23.2 ± 5.8; F1,40 = 5.05, P = .03), though no between-treatment changes were found for SaO290. Aside from a significant improvement in the percentage of total sleep time that was N3 sleep (i.e., slow-wave sleep) relative to control (exercise: 12.8 ± 1.2 to 13.2 ± 1.1%, stretching: 12.4 ± 1.7 to 9.2 ± 1.5%; F1,40 = 5.75, P = .02), PSG sleep parameters were not significantly improved following exercise training [27]. No significant difference in body weight reduction between the two treatments was observed (exercise: 105.6 ± 3.0 to 104.7 ± 3.1 kg, stretching: 99.3 ± 5.1 to 98.7 ± 5.0 kg) [27].
Changes in CPET Responses Following Exercise Training
Table 3 presents a summary of the changes in CPET responses between treatments. Compared to stretching control, exercise training did not significantly change HRrest or any HRpeak measure. However, exercise training elicited significant improvements in cardiorespiratory fitness (F1,40 = 4.46, P = .04) and in HRR-1 (F1,40 = 4.81, P = .03), HRR-3 (F1,40 = 8.12, P < .01), and HRR-5 (F1,40 = 17.78, P < .01) compared to control (Figure 1). However, no between-treatment changes in the VE/VCO2 slope or any BP measure (resting, peak, or recovery SBP or DBP) were noted.
Table 3.
CPET Responses in OSA Participants Before and Following the Intervention.
| Stretching Control (n = 16) |
Exercise Training (n = 27) |
|||
|---|---|---|---|---|
| CPET Variable | BL | POST | BL | POST |
| HRrest, bpm | 78.3 (3.1) | 77.4 (2.6) | 81.2 (1.8) | 75.4 (1.7) |
| HRpeak, bpm | 168.0 (3.1) | 167.7 (3.5) | 161.5 (2.9) | 159.9 (2.9) |
| % Heart rate reserve | 93.3 (2.5) | 93.2 (3.5) | 87.9 (2.8) | 86.7 (2.4) |
| SBPrest, mmHg | 132.4 (3.7) | 125.1 (3.1) | 136.4 (3.1) | 129.7 (2.2) |
| SBPpeak, mmHg | 182.6 (5.5) | 187.8 (5.3) | 189.1 (4.7) | 195.9 (3.9) |
| Recovery SBP3, % peak | 89.1 (1.6) | 85.4 (1.4) | 89.2 (1.3) | 86.4 (1.3) |
| DBPrest, mmHg | 84.7 (2.3) | 82.0 (2.3) | 81.3 (1.8) | 81.3 (1.4) |
| DBPpeak, mmHg | 91.1 (2.5) | 84.5 (2.1) | 82.5 (2.6) | 83.0 (2.2) |
| Recovery DBP3, % peak | 96.8 (1.7) | 99.1 (1.7) | 101.1 (1.6) | 95.1 (1.8) |
| Peak RER | 1.18 (0.03) | 1.20 (0.03) | 1.20 (0.03) | 1.21 (0.03) |
| VO2peak, mL/kg/min | 23.2 (1.3) | 23.4 (1.4) | 20.6 (1.0) | 22.9 (1.2)* |
| VE/VCO2 slope | 25.8 (0.9) | 26.8 (1.1) | 28.0 (1.0) | 27.4 (0.8) |
BL: baseline; CPET: cardiopulmonary exercise testing;; HR: heart rate; OSA: obstructive sleep apnea; POST: post-intervention; RER: respiratory exchange ratio;; VO2peak: peak oxygen consumption.
denotes a statistically significantly difference between stretching control and exercise training participants (P < .05).
Figure 1.
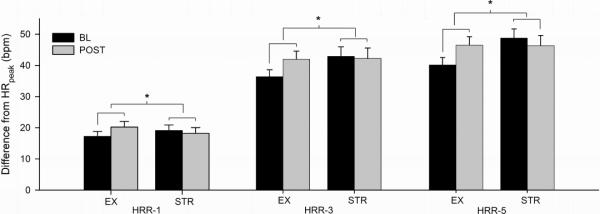
Between-treatment changes in heart rate recovery following the intervention. Data are presented as mean ± standard error. * indicates significant difference between stretching control and exercise training participants (P < .05).
Associations Between Changes in OSA Severity and CPET Responses
Pre- to post-intervention change in AHI was significantly correlated with change in HRR, but only at 5 min into recovery (r = −.30, P < .05). No other significant associations were noted between changes in CPET responses and changes in OSA severity.
DISCUSSION
Participants with OSA had a blunted HRR compared to matched non-OSA controls at baseline, and exercise training significantly improved HRR and VO2peak in participants with OSA. However, improvements in CPET responses were not strongly associated with reduction in OSA severity.
Compared with BMI- and age-matched participants without OSA, OSA participants had a significantly blunted HRR at all time-points. Furthermore, lower HRR was significantly correlated with higher OSA severity. The blunted HRR is consistent with most prior studies that have evaluated HRR responses in individuals with OSA [13,14,18,33,34]. As HRR is a recognized marker of parasympathetic reactivation and SNS withdrawal, these findings agree with previous demonstrations of autonomic dysfunction in OSA [35].
Similar HRpeak values were observed between non-OSA and OSA participants in the current study. Whereas some studies have failed to show a blunted HR response to exercise in OSA [12,18,33,36], others have reported significant chronotropic impairment during exercise [13–15,32,34], and it has been hypothesized that the impairment may be due to downregulated β-adrenergic receptors consequent to SNS hyperactivity [37]. Despite a lack of between-group differences in HRpeak, modest correlations were observed between measures of HRpeak and OSA severity (r = −.24 to −.42), suggesting a possible dose-response association between the chronotropic response to exercise and OSA severity [15].
We also found no evidence of reduced cardiorespiratory fitness in OSA, which is in line with some [18,32,38], but not all prior research [12–14,34]. In studies that have reported reduced VO2peak with OSA, it has been speculated that this impairment may be due to hypoxia- or SNS-induced deficits in oxidative capacity [36,39,40], increased reliance on anaerobic metabolism [36], and/or reduced cardiac output secondary to ventricular dysfunction and increased afterload [38]. Alternatively, impaired VO2peak in OSA may not necessarily be due to underlying cardiac or metabolic dysfunction, but rather to increased weight and sedentary lifestyle, exacerbated by fatigue and sleepiness [12,16,18,40]. Our results were consistent with this alternative interpretation, since the OSA and non-OSA participants did not differ in body weight or sedentary lifestyle.
Prior findings of an exaggerated SBP and/or DBP response to maximum exercise [13,15,16,32,36] or blunted post-exercise SBP recovery [13,32] in adults with OSA were not replicated by our results, which are instead in agreement with other studies in which the exercise BP response [18,32] and BP recovery [18] were found to be unaltered in individuals with OSA. Although allowing anti-hypertensive medication for the CPET may have reduced the likelihood of finding differences in the BP response between the two groups, a similar proportion of OSA and non-OSA participants were on medication (OSA: 13 of 43; non-OSA: 3 of 9), and it was deemed unsafe to have the participants undergo CPET without these medications.
The ventilatory response during exercise was also similar between OSA and non-OSA groups in the current study. Two studies have noted an exaggerated ventilatory response to exercise [17,32] for OSA patients, which is consistent with the known heightened chemoreflex response in OSA [41]. However, our results agree with most prior research that has failed to observe exaggerated ventilation in OSA [12,14,33,34,38].
Following the 12-week intervention, exercise training resulted in a significant improvement in HRR at all three recovery time-points as well as an 11% improvement in VO2peak compared to control treatment. The significant improvement in HRR with exercise training represents improved autonomic balance, which is an important adaptation given that autonomic dysfunction is a strong predictor of cardiovascular morbidity and mortality [42]. Moreover, the magnitude of improvement in VO2peak following exercise training is consistent with other recent exercise trials that have involved relatively healthy yet sedentary adult populations [20,43].
Previous exercise training studies in adults with OSA provided equivocal results regarding whether normal cardiovascular adaptations occurred with exercise training. Norman and colleagues, who observed a 50% reduction in AHI following a 6-month exercise program, reported an 18% improvement in exercise capacity along with 9 and 8 mmHg reductions in resting SBP and DBP, respectively [44]. Similarly, Ueno and colleagues found that exercise training resulted in a 26% increase in VO2peak and significant decrease in muscle SNS activity following a 4-month exercise regimen in adults with chronic heart failure and OSA [45]. However, two other studies failed to observe significant improvements in exercise performance or VO2peak following exercise training that reduced AHI by 25% [24,25]. Although it is possible that the lack of significant cardiovascular improvements from exercise in the latter two studies was due to insufficient reduction of OSA severity, it is more likely that the results were limited by small sample sizes (n ≤ 10 exercise participants in either study) and/or insufficient exercise dose. In our sample, despite an AHI reduction of only 25%, significant improvements in HRR and VO2peak were observed following a dosage of exercise that was designed to comply with public health physical activity recommendations [46]. Moreover, we found minimal associations between improvements in HRR and VO2peak and changes in OSA severity, suggesting that improvements in these CPET markers were largely independent of reductions in OSA severity.
By minimizing the occurrence of night-time respiratory events, CPAP has been shown to address many of the mechanisms that could cause the abnormal CPET responses seen in adults with untreated OSA (e.g., SNS hyperactivity, cardiac dysfunction) [38,47]. However, few studies have documented increases in VO2peak [48,49] and HRR [49] following CPAP treatment similar to the present study's findings, and others have failed to note VO2peak improvements with CPAP use [38,50]. As the effects of CPAP on SNS hyperactivity are dependent upon compliance [51] and lost with CPAP withdrawal [52], exercise training may be a beneficial adjunct therapy to promote cardiovascular health in adults with OSA.
A study limitation was the lack of randomization of non-OSA participants into the 12-week intervention. Exercise training in these participants would have permitted direct comparison of whether the adaptations conferred from exercise were similar between OSA and non-OSA groups. An additional limitation was a lack of SNS activity or cardiac function measurement, which limited our ability to interpret the mechanisms underlying the results. Although elucidation of the possible physiological mechanisms behind abnormal CPET responses [12,38] was not the purpose of the present study, future studies should endeavor to investigate the mechanisms behind the blunted HRR response in OSA and how exercise may improve autonomic function in OSA.
In summary, this study provides evidence of blunted HRR in individuals with OSA compared with matched non-OSA adults. Moreover, exercise training significantly improved HRR among participants with OSA, and also resulted in increased VO2peak. Importantly, these improvements were generally not associated with changes in OSA severity. Thus, in light of the significant cardiovascular risk in this population, these data suggest that exercise training may be an important therapy for improving the cardiovascular health profile in adults with OSA, regardless of whether significant AHI reduction is achieved.
ACKNOWLEDGMENTS
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [53].
This work was supported by Public Health Dissertation Grant 1R36CD000695-1 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. Additional funding support for this work was provided by NHLBI T32 HL082610-05.
The authors gratefully acknowledge SleepMed of South Carolina and the WJB Dorn VA Sleep Laboratory for their assistance with recruitment and data collection, respectively. The authors also are indebted to Kelli Giles, Graham Jones, Ashley Reluzco and Elizabeth Rowell for their assistance with data collection.
Financial Support: CDC 1R36CD000695-1, NHLBI T32 HL082610-05
Trial registration: Clinicaltrials.gov identification number NCT00956423.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Blair receives book royalties from Human Kinetics, has received honoraria for service on the scientific/medical advisory boards for Alere, Technogym, Santec, Clarity, and Jenny Craig, and has received honoraria for lectures and consultations from scientific, educational, and lay groups. No other authors have a conflict of interest to report.
REFERENCES
- [1].Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- [2].Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- [3].Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131–6. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- [4].Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- [5].Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–8. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- [6].Baguet JP, Hammer L, Levy P, et al. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407–12. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- [7].Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–10. [PubMed] [Google Scholar]
- [8].McHam SA, Marwick TH, Pashkow FJ, Lauer MS. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34:754–9. doi: 10.1016/s0735-1097(99)00269-7. [DOI] [PubMed] [Google Scholar]
- [9].Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- [10].Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–9. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- [11].Aron A, Zedalis D, Gregg JM, Gwazdauskas FC, Herbert WG. Potential clinical use of cardiopulmonary exercise testing in obstructive sleep apnea hypopnea syndrome. Int J Cardiol. 2009;132:176–86. doi: 10.1016/j.ijcard.2008.11.014. [DOI] [PubMed] [Google Scholar]
- [12].Lin CC, Hsieh WY, Chou CS, Liaw SF. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol. 2006;150:27–34. doi: 10.1016/j.resp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [13].Vanhecke TE, Franklin BA, Zalesin KC, et al. Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest. 2008;134:539–45. doi: 10.1378/chest.08-0567. [DOI] [PubMed] [Google Scholar]
- [14].Nanas S, Sakellariou D, Kapsimalakou S, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clin Cardiol. 2010;33:46–51. doi: 10.1002/clc.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grote L, Hedner J, Peter JH. The heart rate response to exercise is blunted in patients with sleep-related breathing disorder. Cardiology. 2004;102:93–9. doi: 10.1159/000077911. [DOI] [PubMed] [Google Scholar]
- [16].Tryfon S, Stanopoulos I, Dascalopoulou E, Argyropoulou P, Bouros D, Mavrofridis E. Sleep apnea syndrome and diastolic blood pressure elevation during exercise. Respiration. 2004;71:499–504. doi: 10.1159/000080635. [DOI] [PubMed] [Google Scholar]
- [17].Hargens TA, Guill SG, Aron A, et al. Altered ventilatory responses to exercise testing in young adult men with obstructive sleep apnea. Respir Med. 2009;103:1063–9. doi: 10.1016/j.rmed.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [18].Hargens TA, Guill SG, Zedalis D, Gregg JM, Nickols-Richardson SM, Herbert WG. Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep. 2008;31:104–10. doi: 10.1093/sleep/31.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–16. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- [20].Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- [21].Legramante JM, Iellamo F, Massaro M, Sacco S, Galante A. Effects of residential exercise training on heart rate recovery in coronary artery patients. Am J Physiol Heart Circ Physiol. 2007;292:H510–5. doi: 10.1152/ajpheart.00748.2006. [DOI] [PubMed] [Google Scholar]
- [22].Kim MK, Tanaka K, Kim MJ, Matsuo T, Ajisaka R. Exercise training-induced changes in heart rate recovery in obese men with metabolic syndrome. Metab Syndr Relat Disord. 2009;7:469–76. doi: 10.1089/met.2008.0086. [DOI] [PubMed] [Google Scholar]
- [23].Anaya SA, Church TS, Blair SN, Myers JN, Earnest CP. Exercise dose-response of the VE/VCO2 slope in postmenopausal women in the DREW study. Med Sci Sports Exerc. 2009;41:971–6. doi: 10.1249/MSS.0b013e3181930009. [DOI] [PubMed] [Google Scholar]
- [24].Giebelhaus V, Strohl KP, Lormes W, Lehmann M, Netzer N. Physical exercise as an adjunct therapy in sleep apnea—an open trial. Sleep Breath. 2000;4:173–6. doi: 10.1007/s11325-000-0173-z. [DOI] [PubMed] [Google Scholar]
- [25].Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath. 2011;15:49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- [26].Tonini J, Michallet AS, Flore P, et al. Effect of chronic intermittent hypoxia on exercise adaptations in healthy subjects. Respir Physiol Neurobiol. 2011;179:287–93. doi: 10.1016/j.resp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- [27].Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34:1631–40. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 8th ed. Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- [29].Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- [30].Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; Westchester, Illinois: 2007. [Google Scholar]
- [31].Segal-Isaacson CJ, Wylie-Rosett J, Gans KM. Validation of a short dietary assessment questionnaire: the Rapid Eating and Activity Assessment for Participants short version (REAP-S) Diabetes Educ. 2004;30:774, 776, 778. doi: 10.1177/014572170403000512. [DOI] [PubMed] [Google Scholar]
- [32].Kaleth AS, Chittenden TW, Hawkins BJ, et al. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007;8:160–8. doi: 10.1016/j.sleep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [33].Maeder MT, Munzer T, Rickli H, et al. Association between heart rate recovery and severity of obstructive sleep apnea syndrome. Sleep Med. 2008;9:753–61. doi: 10.1016/j.sleep.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [34].Chien MY, Lee P, Tsai YF, Yang PC, Wu YT. C-reactive protein and heart rate recovery in middle-aged men with severe obstructive sleep apnea. Sleep Breath. doi: 10.1007/s11325-011-0549-2. in press. [DOI] [PubMed] [Google Scholar]
- [35].Wiklund U, Olofsson BO, Franklin K, Blom H, Bjerle P, Niklasson U. Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin Physiol. 2000;20:234–41. doi: 10.1046/j.1365-2281.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- [36].Vanuxem D, Badier M, Guillot C, Delpierre S, Jahjah F, Vanuxem P. Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med. 1997;91:551–7. doi: 10.1016/s0954-6111(97)90089-5. [DOI] [PubMed] [Google Scholar]
- [37].Nelesen RA, Dimsdale JE, Mills PJ, Clausen JL, Ziegler MG, Ancoli-Israel S. Altered cardiac contractility in sleep apnea. Sleep. 1996;19:139–44. doi: 10.1093/sleep/19.2.139. [DOI] [PubMed] [Google Scholar]
- [38].Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Obstructive sleep apnoea-hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–15. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- [39].Bonanni E, Pasquali L, Manca ML, et al. Lactate production and catecholamine profile during aerobic exercise in normotensive OSAS patients. Sleep Med. 2004;5:137–45. doi: 10.1016/j.sleep.2003.08.009. [DOI] [PubMed] [Google Scholar]
- [40].Ucok K, Aycicek A, Sezer M, et al. Aerobic and anaerobic exercise capacities in obstructive sleep apnea and associations with subcutaneous fat distributions. Lung. 2009;187:29–36. doi: 10.1007/s00408-008-9128-0. [DOI] [PubMed] [Google Scholar]
- [41].Mateika JH, Ellythy M. Chemoreflex control of ventilation is altered during wakefulness in humans with OSA. Respir Physiol Neurobiol. 2003;138:45–57. doi: 10.1016/s1569-9048(03)00174-5. [DOI] [PubMed] [Google Scholar]
- [42].Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–33. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- [43].Larose J, Sigal RJ, Boule NG, et al. Effect of exercise training on physical fitness in type II diabetes mellitus. Med Sci Sports Exerc. 2010;42:1439–47. doi: 10.1249/MSS.0b013e3181d322dd. [DOI] [PubMed] [Google Scholar]
- [44].Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- [45].Ueno LM, Drager LF, Rodrigues AC, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32:637–47. doi: 10.1093/sleep/32.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- [47].Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131:1406–13. doi: 10.1378/chest.06-2580. [DOI] [PubMed] [Google Scholar]
- [48].Lin CC, Lin CK, Wu KM, Chou CS. Effect of treatment by nasal CPAP on cardiopulmonary exercise test in obstructive sleep apnea syndrome. Lung. 2004;182:199–212. doi: 10.1007/s00408-004-2502-7. [DOI] [PubMed] [Google Scholar]
- [49].Maeder MT, Ammann P, Munzer T, et al. Continuous positive airway pressure improves exercise capacity and heart rate recovery in obstructive sleep apnea. Int J Cardiol. 2009;132:75–83. doi: 10.1016/j.ijcard.2007.10.040. [DOI] [PubMed] [Google Scholar]
- [50].Pendharkar SR, Tsai WH, Eves ND, Ford GT, Davidson WJ. CPAP increases exercise tolerance in obese subjects with obstructive sleep apnea. Respir Med. 2011;105(10):1565–71. doi: 10.1016/j.rmed.2011.06.007. [DOI] [PubMed] [Google Scholar]
- [51].Waradekar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996;153:1333–8. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- [52].Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217–25. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- [53].Coats AJS, Shewan LG. Statement on authorship and publishing ethics in the International Journal of Cardiology. Int J Cardiol. 2011;153:239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]


