Abstract
Background
In our institutional experience treating pediatric choledochal cysts over the last 12 years, we noted 7/32 patients (21.9%) had comorbid congenital cardiac anomalies. This association has not been previously described other than in isolated case reports. We aimed to quantify this association on a national level.
Materials and Methods
We queried the 2009 Healthcare Cost and Utilization Project Kids' Inpatient Database. Patients with a diagnosis of choledochal cyst (ICD-9-CM 75169, 75162, 75160) or biliary atresia (75161) were identified. Cardiac anomalies were defined using the Clinical Classification Software code (CCS 213). Comorbid choledochal cysts or biliary atresia and congenital cardiac anomalies were quantified in both infant (<12 mos) and child (1–18 yrs) subpopulations.
Results
Of 1,646 estimated discharges for patients with choledochal cysts, 506 (30.7%) were for patients who also had congenital cardiac anomalies, compared to 2.6% in the general hospitalized population (χ2, p<0.0001). The frequency of congenital cardiac anomalies was lower in 1,973 hospitalizations for biliary atresia (13.8%) than in those for patients with choledochal cysts (χ2, p<0.0001). Cardiac anomalies were detected in 44.9% of choledochal cyst hospitalizations for infants <12 months (vs. 3.44% general hospitalized population; χ2, p<0.0001), but in 6.9% of for children ages 1–18 yrs (vs. 1.3% general hospitalized population; χ2, p<0.0001).
Conclusions
A strong association was observed between pediatric choledochal cysts and congenital cardiac anomalies that more commonly manifests in infancy. When choledochal cysts are diagnosed either prenatally or in infancy, we suggest echocardiographic screening for cardiac anomalies, which may impact timing of surgery and anesthetic planning.
Keywords: Choledochal cyst, biliary atresia, congenital cardiac anomalies, HCUP KID database
Introduction
The major structural diseases responsible for neonatal hyperbilirubinemia requiring surgical intervention are choledochal cysts and biliary atresia.(1) Choledochal cysts, which have an incidence of 1:100,000 to 1:150,000, are congenital dilatations of the extra and/or intrahepatic bile ducts and are postulated to arise secondary to pancreaticobiliary malunion, predisposing to reflux of pancreatic digestive enzymes into the biliary tree.(2, 3) Over time, chronic exposure of the biliary epithelium to an admixture of digestive enzymes results in variable cystic dilatation of the extra and intrahepatic bile ducts and often leads to obstruction of this ductal network within the duodenum.(4) The most common types of choledochal cysts are corrected by surgical resection and Roux-en-Y hepaticoenterostomy.(3) Biliary atresia, arising in 1:10,000 to 1:16,700 live births, is a congenital obliteration of the extrahepatic bile ducts, results in neonatal or infantile jaundice, and typically necessitates either Kasai portoenterostomy or liver transplantation.(1)
In a review of our institutional experience treating pediatric choledochal cysts over the last 12 years, we noted 7/32 patients (21.9%) had comorbid congenital cardiac anomalies. Outside isolated case reports, this association has not been previously described in detail.(5–7) Congenital cardiac anomalies have been reported to occur in between 3 and 8% of cases of biliary atresia, but are thought to occur in only 0.06–0.75% of the general population.(8, 9) Given these anecdotal observations and a potential link between childhood biliary disorders and congenital cardiac anomalies in published case series, we aimed to characterize the association between choledochal cysts or biliary atresia and congenital cardiac anomalies using a large national database.
Materials and Methods
We performed a retrospective study using prospectively collected national discharge data from the 2009 Healthcare Cost and Utilization Project (HCUP) Kids' Inpatient Database (KID), sponsored by the Agency for Healthcare Research and Quality (ARHQ; Rockville, MD). The HCUP KID is a dataset of hospital use, outcomes, and charges specifically designed to study utilization of pediatric hospital services in the United States.(10) Each edition contains individual level discharge data containing approximately 100 variables collected in the previous calendar year. The KID performs an 80% sampling of complicated in-hospital births and other pediatric cases from each hospital contained within its sampling frame, enabling national estimates using provided discharge weights.
The KID provides a primary diagnosis for each discharge using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes with up to 24 secondary diagnoses. Clinical Classification Software (CCS) codes, a diagnostic categorization system, permit the grouping of related conditions into broader clinical entities. Using this system, multiple ICD-9-CM codes are collected into a smaller number of categories that are often more useful than individual ICD-9CM codes. For example all ICD-9-CM codes for cardiac congenital anomalies are grouped together under one CCS code (213).
Case selection for patients with choledochal cysts was based upon the fulfillment of one of three ICD-9-CM codes for choledochal cyst, 75160 (unspecified anomaly of gallbladder, bile ducts, and liver), 75169 (other anomalies of gallbladder, bile ducts, and liver), or 75162 (congenital cystic disease of liver). Biliary atresia was defined by the ICD-9-CM code of 75161 (biliary atresia). The presence of congenital cardiac anomalies was determined by fulfillment of the CCS code of 213 (cardiac and circulatory congenital anomalies). To designate a more specific population we created a category that would only include patients receiving surgical intervention for a congenital cardiac anomaly during that admission. These patients were defined by the CCS of 213 plus Procedure Clinical Classification Software (PRCCS) code of 43 (heart valve procedure), or 49 (other heart OR procedure). Likewise, to limit potential recounting of patients corresponding to multiple hospital admissions, we designated a population of patients receiving surgical intervention for their biliary anomaly during a given admission. These patients were defined using the above listed ICD-9-CM codes for either choledochal cysts or biliary atresia plus any of the following ICD-9-CM procedure codes: 5411 exploratory laparotomy, 5136 choledochoenterostomy, 5137 anastomosis of hepatic duct to gastrointestinal tract, 5139 other bile duct anastomosis, 5163 other excision of common duct, 5169 excision of other bile duct, 5172 choledochoplasty, and 5122 cholecystectomy.
Data Analysis
We collected information regarding patient demographics (age, sex, race, and family income) and payer type. Descriptive data were reported using weighted means with 95% confidence intervals. Chi-square test was used to test for associations between categorical variables. The general hospitalized population refers to all patient discharges (ages 1–18 yrs) contained in the 2009 HCUP KID database that did not have a diagnosis of either choledochal cyst or biliary atresia. When subgroup analyses were performed on infants (age < 1yr) and children (age 1–18 yrs) they were compared only to the general hospitalized population with identical age restrictions. Values of p<0.05 were considered to be statistically significant. All statistical analysis was performed using Stata version 11.2 (Stat Corp; College Station, TX). The Vanderbilt University Institutional Review Board approved this work as an exempted study.
Results
Of 7.4 million hospital discharge records predicted by the 2009 HCUP KID database, 1646 hospitalizations were identified for patients with the diagnosis of choledochal cyst. Among these choledochal cyst hospitalizations, 506 (30.7%) were also associated with the diagnosis of a congenital cardiac anomaly, compared to 2.6% in the general hospitalized population (χ2, p<0.0001; Table 1). The frequency of congenital cardiac anomalies was lower in 1973 hospitalizations for biliary atresia (13.8%) than in those for patients with choledochal cysts (χ2, p<0.0001), but was also significantly higher than the rate of congenital cardiac anomalies in the general hospitalized population (χ2, p<0.001; Table 1).
Table 1.
Comparison of overall and surgical congenital cardiac anomalies among hospital discharges for patients with choledochal cysts (CDC), biliary atresia (BA), and in the general hospitalized population.
| Overall hospitalizations for CDC or BA patients in HCUP database | ||||||
|---|---|---|---|---|---|---|
| Total | Cardiac anomalies | No cardiac anomalies | % cardiac anomalies | p-value (χ2) | ||
| Choledochal Cyst | 1646 | 506 | 1140 | 30.7% | 0.0001† | <0.0001§ |
| Biliary Atresia | 1973 | 272 | 1701 | 13.8% | ||
| General Hospitalized Population | 7,400,000 | 200,000 | 7,200,000 | 2.6% | <0.0001‡ | |
| Hospitalizations for CDC or BA patients undergoing cardiac surgery during that admission | ||||||
|---|---|---|---|---|---|---|
| Total | With cardiac surgery | Without cardiac surgery | % requiring surgery | p-value (χ2) | ||
| Choledochal Cyst | 1646 | 24 | 1622 | 1.45 | <0.001† | <0.001§ |
| Biliary Atresia | 1973 | 16 | 1957 | 0.81 | ||
| General Hospitalized Population | 7,400,000 | 20,000 | 7,380,000 | 0.27 | 0.002‡ | |
| Hospitalizations for infants (<1 yr) with CDC or BA undergoing biliary surgery during that admission | ||||||
|---|---|---|---|---|---|---|
| Total | Cardiac anomalies | No cardiac anomalies | % cardiac anomalies | p-value (χ2) | ||
| Choledochal Cyst | 71 | 11 | 60 | 15.4% | 0.587† | <0.0001§ |
| Biliary Atresia | 302 | 38 | 264 | 12.6% | ||
| General Hospitalized Population (<1yr) | 4,600,000 | 160,000 | 4,440,000 | 3.5% | 0.0001‡ | |
p-value Pearson Chi Squared choledochal cyst vs. biliary atresia
p-value biliary atresia vs. general hospitalized population
p-value choledochal cyst vs. general hospitalized population
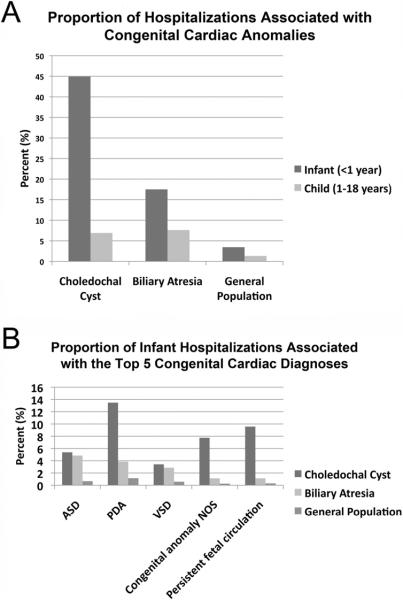
To determine if the association between choledochal cysts, or biliary atresia, and cardiac anomalies is more frequently detected during infancy, we repeated the above analysis on infants (< 12 months of age) and in children (1–18 years of age). Cardiac anomalies were detected in 44.9% of choledochal cyst hospitalizations for infants < 12 months months (vs. 3.44% general hospitalized population <12 months; p <0.0001), and in 6.9% of choledochal cyst hospitalizations for children ages 1–18 yrs (vs. 1.3% general hospitalized population 1–18 yrs; p <0.0001); (Figure 1A). Cardiac anomalies were detected in 17.5% of biliary atresia hospitalizations for infants < 12 months (vs. 3.45% general hospitalized population <12 months; p<0.0001), and in 7.6% of biliary atresia hospitalizations for children ages 1–18 yrs (vs. 1.3% general hospitalized population 1–18 yrs; p<0.0001); (Figure 1A).
Figure 1.
(A) Proportion of hospitalizations associated with a diagnosis of congenital cardiac anomalies in patient admissions with choledochal cysts, biliary atresia, and in the general hospitalized population. Infant (age < 1 year) and child (age 1–18 years) admissions are represented separately. (B) Proportion of Infant (age < 1 year) hospitalizations with biliary anomalies and the top 5 coded congenital cardiac abnormalities (atrial septal defect (ASD), patent ductus arteriosus (PDA), ventricular septal defect (VSD), congenital cardiac anomaly not otherwise specified (NOS), and persistent fetal circulation. Rates of congenital cardiac anomalies in the general hospitalized population (<1yr) are also represented.
To determine if choledochal cysts or biliary atresia associated specifically with a particular congenital cardiac anomaly, we independently tested for an association with each of the top five most common congenital cardiac anomalies included in the HCUP KID database (atrial septal defect (ASD), patent ductus arteriosus (PDA), ventricular septal defect (VSD), cardiac congenital anomaly not otherwise specified (NOS), and persistent fetal circulation). For infants, both choledochal cysts and biliary atresia independently associated with each of the top five congenital cardiac anomalies greater than the general hospitalized population <12 months (p<0.0001; Figure 1B). For children ages 1–18, both choledochal cysts and biliary atresia independently associated with ASD, PDA, VSD, and congenital anomaly NOS with greater frequency than the general hospitalized population ages 1–18 yrs (p<0.0001) however no hospitalizations existed for persistent fetal circulation in children > 1 year of age due to the nature of this diagnosis.
To minimize the potential error associated with multiple hospital readmissions for the same patient, we performed a similar analysis on patients with either a diagnosis of choledochal cyst or biliary atresia who underwent biliary surgery during a given hospital admission. For biliary atresia, this analysis was limited to infants < 1 year because biliary atresia patients undergoing an abdominal operation after this age were likely receiving a liver transplant, and biliary atresia patients awaiting liver transplant are known to have cardiac structural anomalies by echocardiography secondary to liver failure.(11) Of 71 infants (<1yr) undergoing an operation for a choledochal cyst captured by this analysis, 11 (15.5%) had a comorbid congenital cardiac anomaly compared to 3.5% of the general hospitalized population < 1 year (p<0.0001; Table 1). Of 160 children (1–18 yrs) undergoing an operation for a choledochal cyst captured by this analysis, 6 (3.82%) had a comorbid congenital cardiac anomaly compared to 1.3% in the general hospitalized population ages 1–18 yrs (p=0.0301). Of 302 infants (<1yr) undergoing an operation for biliary atresia, 38 (12.7%) had a comorbid congenital cardiac anomaly compared to 3.5% of the general hospitalized population < 1 year (p<0.0001; Table 1). In this analysis, the rates of congenital cardiac anomalies in infants (<1yr) undergoing an operation for a choledochal cyst (15.3%) or biliary atresia (12.7%) were not statistically different (p=0.587; Table 1).
To examine the association between choledochal cysts, or biliary atresia, and structural congenital cardiac anomalies, we conducted a review of the association between choledochal cysts, or biliary atresia, and congenital cardiac anomalies requiring surgical or procedural intervention during a given hospitalization. Of 1646 hospitalizations examined for patients with choledochal cysts, 24 (1.45%) were for patients with congenital cardiac anomalies requiring surgical intervention during that hospitalization, compared to 0.81% in biliary atresia and 0.27% in the general hospitalized population (χ2, p<0.0001 CDC vs. general hospitalized population; p<0.0001 biliary atresia vs. general hospitalized population; Table 1).
Discussion
These data support a strong association between choledochal cysts and cardiac anomalies that most often manifests in infancy, suggesting a potential congenital link between these two maladies. This association appears to be at least as strong as the link between biliary atresia and cardiac anomalies, however, from this study, both choledochal cysts and biliary atresia have a greater association with congenital cardiac anomalies than does the general hospitalized population.
The association between choledochal cysts and cardiac anomalies has not been previously described other than in isolated case reports. Our institutional review of choledochal cyst cases over the last 12 years revealed 7/32 (21.9%) patients had congenital cardiac disease (data not shown). Kim et. al. reported a case of a choledochal cyst in a patient with Simpson-Golabi-Behmel syndrome, which is characterized by overgrowth, cleft lip and palate, cardiac and skeletal abnormalities, supernumerary nipples, diaphragmatic hernia, and polydactyly. This syndrome has also been associated in isolated cases with other gastrointestinal abnormalities including Meckel diverticulum, omphalocele, and malrotation.(5) However, no unifying genetic cause of these varying associations has been identified. Rayamajhi et. al reported a case of a type IVa choledochal cyst in an infant with a subaortic ventricular septal defect.(6) Tantemsapya et. al. reported two cases of pediatric heart transplant recipients, one of whom had a history of hypoplastic left heart syndrome, and each were diagnosed with choledochal cysts between 4 and 9 years after their transplants, however this relationship is potentially confounded by chronic immunosuppression.(7)
Biliary atresia, which shows a greater association with congenital cardiac anomalies than the general hospitalized population, but potentially similar to the association between cardiac anomalies and choledochal cysts, has also been previously linked to cardiac disease. A review of the Canadian-wide biliary atresia database in 2009 revealed 26 of 328 (7.9%) biliary atresia patients born between 1985 and 2002 had major structural congenital cardiac anomalies.(8) In a Japanese series, Tanano et. al report a rate of 3 of 87 (3.4%) biliary atresia patients with congenital cardiac disease.(9) Biliary atresia is associated with extrahepatic malformations in a minority of patients (10–15%), most commonly involving congenital cardiac abnormalities, splenic malformations, abdominal situs abnormalities, malrotation, and a range of circulatory defects including the presence of a preduodenal portal vein and abnormalities of the inferior vena cava. This constellation of findings has been termed BASM for Biliary Atresia Structural Malformation syndrome. Although the genetic basis of this syndrome has not yet been clarified, patients with biliary atresia and situs abnormalities have been found to harbor mutations in the left-right axis patterning genes CFC1 and ZIC3.(8)
Choledochal cysts, interestingly, have also been associated with malrotation in clinical case reports, but otherwise have not been linked to anomalies typical of BASM (with the exception of the current report and cardiac anomalies).(12, 13) Furthermore, choledochal cysts and biliary atresia are sometimes found simultaneously, a disorder termed biliary cystic malformations.(14) In our study, the association between choledochal cysts or biliary atresia and congenital cardiac anomalies was most robust in infancy, supporting a unifying embryologic or genetic cause. Alagile syndrome, an autosomal dominant, congenital syndromic paucity of the interlobular bile ducts which leads to cholestasis in the first year of life, also includes branch pulmonary artery hypoplasia or stenosis as one if its main features.(15, 16) Coupled with data from the current study, which suggests a congenital link between choledochal cysts, biliary atresia, and cardiac anomalies, these findings are suggestive of a potential embryonic link between these disorders, which could occur at the inductive signaling interface between the hepatic diverticulum and cardiac mesoderm during embryogenesis.
Due to the rare nature of choledochal cysts and biliary atresia, large datasets are necessary to determine comorbid associations that, while important clinically, exist in a minority of cases. The HCUP KID database used for this investigation, while robust and inclusive, has several inherent limitations that must be considered in the interpretation of these data. First, individual patient identifiers are not included in this database, and thus patients that share the diagnosis of a biliary disorder and congenital cardiac anomalies and are hospitalized multiple times (which is a very likely scenario) may be recounted in the analysis. We attempted to minimize this effect by performing two subgroup analyses of hospitalizations requiring biliary and cardiac surgical intervention, therefore only counting those hospitalizations that required surgery and minimizing the number of times an individual patient is recounted in the data. Even after this subgroup analysis, the association between congenital biliary anomalies and cardiac disease persists. Second, as with any study involving a large database, clinical diagnoses and procedures performed are only as accurate as the diagnostic coding information included. The findings of this study would most ideally be confirmed and associated with long-term outcomes in a multicenter study with access to more specific and longitudinal clinical information beyond patient discharge.
In summary, these data support a link between congenital biliary disorders and cardiac anomalies, which is most likely to manifest in infancy. Our study supports previously published case reports signifying a link between choledochal cysts and congenital cardiac anomalies and also supports case series indicating an increased incidence of these anomalies in biliary atresia patients. When choledochal cysts are diagnosed either prenatally, in infancy, or in the evaluation of neonatal jaundice, we suggest that echocardiography be performed to evaluate for the presence of congenital cardiac anomalies, which may impact timing of surgery and anesthetic planning. Likewise, patients with biliary atresia should be considered for echocardiography as part of their preoperative workup before a Kasai procedure or orthotopic liver transplantation.
Acknowledgements
The authors would like to thank Cathy Carney for her assistance with the review of our institutional choledochal cyst experience. Work was supported in part by National Cancer Institute (NCI) grant 5T32CA106183-06A1 (AJM and JA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman RP, Buchmiller TL. The Jaundiced Infant: Biliary Atresia. In: Grosfeld JL, O'Neill JA Jr, Fonkalsrud EW, Coran AG, editors. Pediatric Surgery. 2 v. Mosby/Elsevier; Philadelphia: 2006. p. xxix, 2146. [Google Scholar]
- 2.Babbitt DP, Starshak RJ, Clemett AR. Choledochal cyst: a concept of etiology. Am J Roentgenol Radium Ther Nucl Med. 1973;119:57–62. doi: 10.2214/ajr.119.1.57. [DOI] [PubMed] [Google Scholar]
- 3.de Vries JS, de Vries S, Aronson DC, Bosman DK, Rauws EA, et al. Choledochal cysts: age of presentation, symptoms, and late complications related to Todani's classification. J Pediatr Surg. 2002;37:1568–1573. doi: 10.1053/jpsu.2002.36186. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Ando H, Nagaya M, Sugito T. Congenital dilatation of the common bile duct in children.--The etiologic significance of the narrow segment distal to the dilated common bile duct. Z Kinderchir. 1984;39:40–45. doi: 10.1055/s-2008-1044167. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Idowu O, Chen E. Choledochal cyst in Simpson-Golabi-Behmel syndrome. Am J Med Genet. 1999;87:267–270. [PubMed] [Google Scholar]
- 6.Rayamajhi A, Singh R, Prasad R, Basnet NB. An unusual case of Type IV(A) choledochal cyst with subaortic ventricular septal defect. Pediatr Int. 2006;48:187–189. doi: 10.1111/j.1442-200X.2006.02187.x. [DOI] [PubMed] [Google Scholar]
- 7.Tantemsapya N, Pahl E, Melin-Aldana H. Superina R Choledochal cyst in two pediatric heart transplant patients. Pediatr Transplant. 2009;13:645–649. doi: 10.1111/j.1399-3046.2008.01011.x. [DOI] [PubMed] [Google Scholar]
- 8.Guttman OR, Roberts EA, Schreiber RA, Barker CC. Ng VL Biliary atresia with associated structural malformations in Canadian infants. Liver Int. 2011;31:1485–1493. doi: 10.1111/j.1478-3231.2011.02578.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanano H, Hasegawa T, Kawahara H, Sasaki T. Okada A Biliary atresia associated with congenital structural anomalies. J Pediatr Surg. 1999;34:1687–1690. doi: 10.1016/s0022-3468(99)90645-0. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. 2011. AHRQ HCUP-US KID Overview. [Google Scholar]
- 11.Desai MS, Zainuer S, Kennedy C, Kearney D, Goss J, et al. Cardiac structural and functional alterations in infants and children with biliary atresia, listed for liver transplantation. Gastroenterology. 2011;141:1264–1272. 1272, e1261–1264. doi: 10.1053/j.gastro.2011.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbell D, Orkin B, Naveh Y, Gur I. Udassin R Duodenojejunal atresia with absent dorsal mesentery, choledochal cyst, and malrotation in a premature newborn--a case report. J Pediatr Surg. 2006;41:e11–13. doi: 10.1016/j.jpedsurg.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 13.George CD, Patton MA, el Sawi M, Sharland M, Adam EJ. Abdominal ultrasound in Noonan syndrome: a study of 44 patients. Pediatr Radiol. 1993;23:316–318. doi: 10.1007/BF02010926. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka N, Ueno T, Takama Y, Fukuzawa M. Diagnosis and management of biliary cystic malformations in neonates. J Pediatr Surg. 2010;45:2119–2123. doi: 10.1016/j.jpedsurg.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 15.Alagille D Cholestasis in the first three months of life. Prog Liver Dis. 1979;6:471–485. [PubMed] [Google Scholar]
- 16.Alagille D, Estrada A, Hadchouel M, Gautier M, Odievre M, et al. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110:195–200. doi: 10.1016/s0022-3476(87)80153-1. [DOI] [PubMed] [Google Scholar]