Abstract
A host:guest-derived gene delivery vector has been developed, based on the self-assembly of cationic β-CD derivatives with a poly(vinyl alcohol)MW27kD (PVA) main chain polymer bearing poly(ethylene glycol)MW750 (PEG) or MW2000 PEG and acid-labile adamantane-modified (Ad) grafts through an acid-sensitive benzylidene acetal linkage. These components were investigated for their ability to promote supramolecular complex formation with pDNA using two different assembly schemes, involving either pre-complexation of the pendant Ad-PVA-PEG polymer with the cationic β-CD derivatives before pDNA condensation (Method A) or pDNA condensation with the cationic β-CD derivatives prior to addition of Ad-PVA-PEG to engage host:guest complexation (Method B). The pendant polymers were observed to degrade under acidic conditions, while remaining intact for more than 5 d at pH 7. HeLa cell culture data shows that these materials have 103-fold lower cytotoxicities than 25 kD bPEI, while maintaining transfection efficiencies that are superior to those observed for this benchmark cationic polymer transfection reagent when the Method A assembly scheme is employed. These findings suggest that degradable cationic polymer constructs employing multivalent host:guest interactions may be an effective and low-toxicity vehicle for delivering nucleic acid cargo to target cells.
Introduction
Safe and efficient delivery of nucleic acid constructs to target cells has great potential for the treatment of genetic diseases1-4, however, the clinical success of this approach greatly depends on the development of effective delivery vehicles with low toxicity. Viral and non-viral vectors both have been studied for this purpose, but suffer from several key limitations. Although efficient and persistent, viral vectors are challenged by issues of large-scale production, immunogenicity and safety, whereas non-viral vectors are limited primarily by lack of efficiency. Nonetheless, non-viral gene delivery has attracted considerable attention due to its scalability and modest host immunogenicity compared to viral vectors5-7. A variety of non-viral vectors have been explored, including cationic lipids, cationic peptides, and cationic polymers such as polyethylenimines (PEI)8,9, poly-(L-lysine)10, PAMAM dendrimers11,12, polyaminoesters13,14, polyamidoamines15, polyphosphoesters16, chitosans17, and cyclodextrin (CD) oligomers4,18-22. These vectors are all capable of condensing DNA at high N:P ratios to form positively charged particles that enter cells via non-specific uptake mechanisms. These particles also protect the genetic material from degradation and enhance their cell permeability, however, they often display significant cytotoxicity at the concentrations needed for effecting nucleic acid cargo bioactivity. Thus, the challenge remains to create non-viral vectors that have high transfection efficiencies while maintaining low toxicity. This requires an integration of various factors such as serum stability, specific binding to target cells, and efficient un-packaging and release of the cargo upon cellular internalization.
β-Cyclodextrin (CD) is a widely used host molecule capable of internalizing guest molecules in water with binding constants in the 100.5 – 105 M−1 range23. CDs have been extensively used for gene delivery due to their ability to stabilize the nucleic acids in biological media, their ability to destabilize and permeate biological membranes, and for obviating undesirable side effects. A variety of CD-based systems such as CD polymers, CD dendrimers, and CD polyrotaxanes are promising materials for non-viral vector development. Davis and coworkers have reported a diverse class of β-CD oligomers coupled via cationic linkers24-27. Recently, one of these derivatives was successfully used as a vector for siRNA delivery in a clinical trial for treatment of melanoma in humans4. Cationic CD polyrotaxanes have also been reported where the cationic substituents have been introduced by post-modification reactions after the macrocycle has been threaded onto the polymer backbone19,28,29. The limited flexibility and highly polycationic nature of these constructs can impede efficient DNA complexation, thus requiring high N:P ratios for nucleic acid compaction that may affect their efficiency.
Herein we report a nucleic acid delivery vector designed to avoid these limitations by employing the self-assembly of cationic β-CD derivatives with a pendant polymer comprised of adamantane-modified (Ad) poly(vinyl alcohol)-poly(ethylene glycol) (PVA-PEG), whose Ad units are linked through an acid-labile acetal motif (Figure 1). It was anticipated that pDNA compaction could be achieved via complexation with self-assembled amino-β-CD+:Ad-PVA-PEG host:guest pendant polymer complexes via multivalent electrostatic interactions between the cationic β-CD derivatives and the nucleic acid cargo. These amino-β-CD units are then held into place via hydrophobic interactions between the β-CD host cavities and the pendant benzylidene acetal-linked Ad groups on the PVA backbone. This design enables the compaction of nucleic acid cargo into stable nanometer-size particles that can then be internalized and degraded within the acidic endosomes of target cells by acid-catalyzed cleavage of the acetal linkage (Scheme 1).
Figure 1.
Structures of amino-β-CDs, Ad-PVA-PEG750 and Ad-PVA-PEG2000.
Experimental Procedures
Materials
All solvents were of reagent grade, purchased from commercial sources, and used without further purification, except DMF and toluene, which were dried over CaH2 under N2, filtered and distilled under reduced pressure. β-CD, NaOH, I2, Ph3P 1-adamantanecarbonyl chloride (Ad-CO-Cl), 4-hydroxybenzaldehyde, Na2CO3, 1,1-carbonyldiimidazole (CDI), β-CD, and p-toluenesulfonyl acid (TSA) and p-toluenesulfonyl chloride were obtained from Aldrich-Sigma, Inc. 1H NMR spectra were recorded on a 300 MHz Varian INOVA 300 NMR spectrometer at 30°C. Chemical shifts were referenced to the residual protonated solvent peak. Qiagen kits were purchased from Qiagen. mhGFP plasmid vector was purchased from Promega. Plasmid DNA was amplified in Escherichia coli and purified according to the supplier's protocol (Qiagen, Hilden, Germany). The purity and concentration of the purified plasmid DNA was determined by absorption at 260 and 280 nm and by agarose gel electrophoresis. The purified plasmid DNA was resuspended in TE buffer (10 mM Tris-Cl, pH 7.5, 1 mM EDTA) and kept in aliquots at a concentration of 0.5 mg/mL.
Synthesis of hepta-6-(2′-aminoethyl)amino-β-CD (1)
Hepta-6-iodo-β-CD (1.0 g, 0.5 mmol) was dissolved in 50 mL 1,2-diaminoethane, then stirred at 60 °C under an atmosphere of N2 for 24 h. The solution was then concentrated under reduced pressure to a few milliliters before pouring into acetone (300 mL). A fine white precipitate was formed and gathered by filtration. The precipitate was washed with acetone and dried under vacuum to yield a stable white powder. Yield = 0.47 g (66%). 1H NMR (270 MHz, D2O): δ = 5.21-5.15 (s, 7H, C1H of CD), 4.10-3.82 (m, 21H, C3H and C5H of CD and NH) 3.77-3.54 (m, 28H, C2H, C4H and C6H of CD), 3.21-2.97 (m, 2H, N1-CH2), 2.97-2.88 (t, 2H, N2-CH2), 2.68-2.56 (b, 14H, NH2). 13C NMR (75 MHz, CDCl3): δ = 102.0 (C(1) of β-CD), 82.3 (C(4) of β-CD), 73.2 (C(3) of β-CD), 72.5 (C(2) of β-CD), 72.1 (C(5) of β-CD), 55.2 (C(6) of β-CD), 53.9 (NH-CH2), 45.2(CH2-NH2).
Synthesis of hepta-6-(2′-hydroxyethyl)amino-β-CD (2)
This was prepared as described for Compound 1, except that 2-aminoethanol was used as nucleophile instead of 1,2-diaminoethane. Yield = 0.51 g (70%). 1H NMR (270 MHz, D2O): δ = 5.10-5.05 (s, 7H, C1H of CD), 4.00-3.85 (m, 14H, C3H and C5H of CD) 3.75-3.42 (m, 28H, C2H, C4H and C6H of CD), 3.01-2.82 (m, 2H, ethanolamine CH2O), 2.78-2.72 (t, 2H, ethanolamine CH2N). 13C NMR (75 MHz, D2O): δ = 104.1 (C(1) of β-CD), 84.6 (C(4) of β-CD), 74.0 (C(3) of β-CD), 73.2 (C(2) of β-CD), 72.5 (C(5) of β-CD), 67.5(CH2-OH), 58.7 (C(6) of β-CD), 57.5 (CH2-N).
Synthesis of hepta-6-hydrazyl-β-CD (3)
This was prepared as described for Compound 1, except that hydrazine was used as nucleophile instead of 1,2-diaminoethane. Yield = 0.52 g (54%). 1H NMR (270 MHz, D2O): δ = 5.13-5.09 (s, 7H, C1H of CD), 4.28-4.02 (b, 7H, NH), 4.02-3.22 (m, 42H, C2H, C3H, C4H, C5H and C6H of CD), 2.00-1.80 (b, 14H, NH2). 13C NMR (75 MHz, D2O): δ = 101.9 (C(1) of β-CD), 81.6 (C(4) of β-CD), 73.0 (C(3) of β-CD), 72.4 (C(2) of β-CD), 72.0 (C(5) of β-CD), 45.9 (C(6) of β-CD).
Synthesis of 4-benzaldehyde adamantanecarboxyl ester (Ad-Ph-CHO)
To a solution of 4-hydroxybenzaldehyde (2.44 g, 20 mmol) in THF (10 mL) was added 3 mL NEt3. The solution was cooled with ice before adding dropwise a solution of AdCOCl (5.94 g, 30 mmol) in THF (10 mL). After 6 h, the THF was removed using a rotary evaporator. The residue was dissolved in 50 mL ether and then washed three times with 1 M Na2CO3 and one time with saturated NaCl solution. The solution was dried over Na2SO4 and the solvent removed using a rotary evaporator to yield a pale yellow solid. Yield = 5.11 g (90%). 1H NMR (400 MHz, CDCl3): δ = 9.99 (s, 1H, CHO), 7.91 (d, J=8.4 Hz, 2H, ph), 7.23 (d, J=8.0 Hz, 2H, ph), 2.09-2.05 (m, 9H, Ad), 1.81-1.74 (m, 6H, Ad).
Synthesis of Ad-PVA
PVA (MW = 27kD) (460 mg, 10 mmol) was dissolved in 10 mL dry DMSO, and then Ad-PhCHO (568 mg, 1.0 mmol) and 50 mg TSA were added. This solution was stirred for 2 d at 50 °C. The solution was then poured into acetone (300 mL). A fine white precipitate was formed and gathered by filtration. The precipitate was washed with acetone and dried under vacuum to yield a stable white solid. Yield = 850 mg (85.2%). 1H NMR (400 MHz, d6-DMSO): δ = 7.42 (w, 2H, Ph), 7.03 (w, 2H, Ph), 5.51 (s, 1H, PhCH), 4.66-4.02 (m, 3H, PVA-OH), 3.96-3.74 (m, 5H, PVA-CH), 2.02-1.95 (m, 9H, Ad), 1.70 (m, 6H, Ad), 1.59-1.21 (m, 10H, PVA-CH2).
Synthesis of Ad-PVA-PEG
A solution of CDI (162 mg, 1 mmol in 10 mL DMSO) was added dropwise to a solution of Ad-PVA (720 mg in 20 mL DMSO). The solution was stirred for 1 d at 50 °C, then processed by addition of 300 mL dry THF three times to precipitate the CDI-activated polymer, which was used directly in the next step after re-dissolving in 10 mL DMSO. After the addition of methoxypolyethylene glycol amine (MW = 750 or 2000) NH2-PEG-OMe (750 mg, 100 eq, 1 mmol of PEG750; or 6.4g, 320 eq, 3.2 mmol of PEG2000) to the solution, the reaction was stirred overnight. The product was dialyzed against DMSO and deionized water three times each (Spectra/Por Membrane, MWCO 6000-8000) to remove low MW impurities. After removal of the solvent, the polymer was redissolved in DMSO and precipitated into acetone. The Ad-PVA-PEG was isolated as a pale yellow solid. Yield = 1.1 g. 1H NMR (400 MHz, H2O): δ = 7.61-7.45 (br, Ph), 7.12-7.01 (br, Ph), 5.2-4.6 (br m, PVA-OH, overlapped with HDO), 4.01-3.05 (br m, PVA-CH and PEG-CH2), 2.2-1.5 (br, Ad). MW Ad-PVA-PEG750 = 112kDa; MW Ad-PVA-PEG2000 = 645kDa.
1H NMR Evidence for Acid Catalyzed Cleavage of Acetal Linker
Ad-PVA (50 mg) was dissolved by sonication in 2 mL of 10 mM β-CD D2O solution for 10 min, followed by removal of undissolved material via centrifugation at 5400 rpm for 1 h. The sample was the transferred to an NMR tube for analysis. Trifluoroacetic acid (TFA) was added to get the desired pH (pH = 4 and pH = 7), and the 1HNMR spectra recorded at 4h and 48h.
Dynamic Light Scattering and Atomic Force Microscopy
The sizes and size distributions of the materials were evaluated by dynamic light scattering using a particle size analyzer (Zetasizer Nano S, Malvern Instruments Ltd.) at room temperature (25°C) with a scattering angle of 90°. AFM imaging of the nanoparticles was conducted in tapping mode (MultiMode, Veeco, USA) using dry samples on mica. The AFM tips (PPP-NCH, Nanoscience Instruments, Inc., USA) had a typical radius of 7 nm or less, and the images were recorded with a scan rate of 0.5 or 1 Hz. Samples were prepared by dropping 2 mL of solution on a mica surface, followed by overnight drying at 20 °C.
Gel Shift Assay
The complexation ability of the systems was studied by gel shift assay. Agarose gels (1% w/v) containing ethidium bromide were made in 1× TE buffer. All transfection complexes were loaded onto the gel at various N:P ratios and 200 ng of DNA added to each well. The gels were run at 50 V for about 1 h and visualized.
Cell Viability Assay
The cytotoxicity of the amino-β-CD+ complexes relative to bPEI (25 kDa) was evaluated using the MTT assay in HeLa cells. The cells were cultured in complete DMEM medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity. The cells were seeded in 96-well microtiter plates (Nunc, Wiesbaden, Germany) at densities of 10,000 cells/well. After 24 h, the culture medium was replaced with serum-supplemented culture medium containing serial dilutions of amino-β-CD+, and the cells incubated for an additional 24 h. Then, 10 μL of sterile-filtered MTT stock solution in PBS (5mg/mL) was added to each well, reaching a final MTT concentration of 0.5 mg/mL. After 5 h, unreacted dye was removed by aspiration. The formazan crystals were dissolved in DMSO (100 μL/well), and the absorbance at 570 nm measured using a microplate reader (Spectra Plus, TECAN). The percent cell viability, compared to control cells cultured in media without polymers, was calculated as [A]test/[A]control × 100%, where [A]test is the absorbance of the wells with polymers and [A]control is the absorbance of the control wells. All experiments were conducted for three samples and averaged. The median lethal dose (LD50) is the dose of a toxic material that kills half (50%) of the cells tested. In this study, LD50 was taken as the concentration of a gene carrier causing a relative cell viability decrease to 50%.
In Vitro Transfection of mhGFP pDNA
HeLa cells were cultured in complete DMEM medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity. Cells were seeded in 24 well plates at a density of 100,000 cells/well. After 48 h, the culture media was replaced with media (serum-free or 10% serum-supplemented) containing the transfection complexes prepared at a 20:1 N:P ratio using mhGFP pDNA. The cells were incubated with the transfection complexes for 24 h, after which the spent media was aspirated and fresh serum-supplemented media was added. After incubation for 24 h, the media was aspirated and the cells washed with PBS, trypsinized and analyzed by FACS using excitation and emission filters of 488 nm and 530 nm, respectively.
Results & Discussion
Synthesis of Amino-β-CD+ and Ad-PVA-PEG Host:Guest Polymer Components
Based on the design shown in Scheme 1, we synthesized three cationic β-CD derivatives (Figure 1) to test the amino-β-CD+:pendant polymer concept, i.e., hepta-6-(1′,2′-diaminoethyl)-β-CD (1), hepta-6-(2′- hydroxyethylamino)-β-CD (2), and hepta-6-(hydrazino)-β-CD (3). The amino-β-CD+ components were synthesized from hepta-6-iodo-β-CD by a simple one step procedure. Ad-PhCHO was prepared from 4-hydroxybenzaldehyde and 1-adamantane carbonyl chloride. Ad-PhCHO was further used to synthesize Ad-PVA and Ad-PVA-PEG from PVA (27 kD). The Ad-PVA was prepared from Ad-PhCHO and PVA in the presence of a catalytic amount of TSA to give the acetal-based pendant polymer. Ad-PVA was isolated by precipitation in acetone. This was further activated by 1,1′-carbonyldiimidazole to give the PEGylated pendant polymer, Ad-PVA-PEG. The Ad-PVA and Ad-PVA-PEG750 were prepared with 19 mol% Ad acetal modifications and 12 mol% PEG750 carbamate modifications (MW = 112kDa), while Ad-PVA-PEG2000 was prepared with 13 mol% Ad acetal modifications and 48 mol% PEG2000 carbamate modifications (MW = 645kDa) as determined by 1H NMR. These pendant polymer constructs then were investigated for their ability to promote pDNA:host:guest complex formation and acid-responsive disassembly.
Characterization of Amino-β-CD+:Ad-PVA-PEG Interaction
Complexation of Ad-PVA and Ad-PVA-PEG750 with the amino-β-CD+ derivatives was confirmed by 1H NMR (Figure 2A-D). The phenyl and Ad resonances of the PVA derivatives showed significant upfield shifts upon the addition of amino-β-CD host ligands in aqueous media, indicating the formation of host:guest polymer complexes via Ad↔CD inclusion. The complexation of the pendant polymer with α-CD and γ-CD as studied by 1H NMR revealed that there was no detectable binding of the α-CD or γ-CD to the polymer (i.e., no upfield shift was observed for the phenyl or Ad resonances upon their addition to the aqueous solution of the pendant polymer; Supplementary Information). Next, we tested the acid-catalyzed cleavage of the acetal bond used to connect the Ad guest ligand to the polymer backbone. 1H NMR spectra of Ad-PVA in D2O in the presence of equimolar unmodified β-CD was measured as a function of pH. At pH = 7, the resonances of the benzylidene acetal pendant group protons were observed at 7.6, 6.8 and 5.6 ppm. After treating this solution at pH = 4 for 48 h, the 1H NMR spectra exhibited three new peaks at 7.0, 8.1, and 9.8 ppm that were attributed to the cleaved benzaldehyde-4-adamantane carboxyl ester unit (Figure 2E-F). Detailed hydrolysis kinetics were studied by a pyrene fluorescence assay to study the time-dependent degradation of the polymer (Supporting Information). Evidence from NMR and the pyrene fluorescence assay of the pendant group cleavage from the polymer main chain at low pH suggests that a similar process may occur upon cellular internalization. Hydrolysis of the pendant groups from the PVA backbone within the acidic endosomal environment would be expected to promote complex disassembly, pDNA un-packaging and escape.
Figure 2.

400 MHz 1H NMR spectra of Ad-PVA-PEG in the absence (A), in the presence (B) of β-CD; Ad-PVA in the absence (C), and presence (D) of β-CD in D2O at 20 °C, and Ad-PVA with β-CD at pH = 7 (E) and pH = 4 (F) in D2O at 20 °C.
Characterization of the Particles Formed by pDNA:Amino-β-CD+:Ad-PVA-PEG Complexation
The ability of these non-covalent pendant polymer assemblies to condense pDNA was then evaluated with respect to particle size and net charge. Two different complexation methods (Scheme 1) were used to evaluate the relative capacity of amino-β-CD+:Ad-PVA-PEG750 host:guest polymer assemblies toward pDNA condensation. In Method A, Ad-PVA-PEG750 was pre-associated with amino-β-CD+s before addition to the pDNA solution. In Method B, the pDNA was first complexed with the respective amino-β-CD+, followed by addition of Ad-PVA-PEG750.
Gel shift assays of pDNA complexes with the amino-β-CD+ and the amino-β-CD+:Ad-PVA-PEG750 pendant polymer assemblies indicate that both methods of formulation had comparable pDNA complexation abilities. Also, in the absence of polymer, much higher N:P ratios were required to condense pDNA effectively. Comparison of the three amino-β-CD+ compounds indicated that 3 has the greatest capacity for condensing pDNA compared to 1 and 2 (Supporting Information). This improved condensation capability of 3 relative to 1 and 2 can be attributed to the availability of both the nitrogens on the hydrazine moiety for interaction with pDNA. In case of 1 and 2, the more basic 2° amines are not as easily accessible to the pDNA, resulting in less effective condensation.
Zeta potentials were measured for both types of complexes with Ad-PVA-PEG750 to determine the surface charge of the resulting transfection particles (Figure 3A). We observed that complexes formed by both methods, as well as polymer-free amino-β-CD+:pDNA complexes, had positive ζ when N:P > 5.
Figure 3.
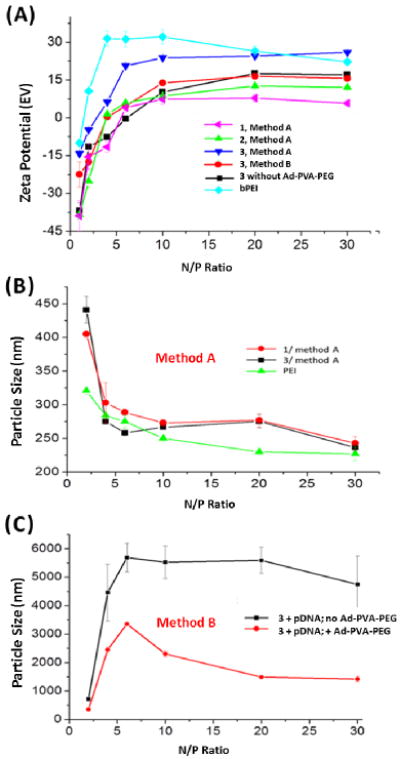
(A) Zeta potential measurements of pDNA:amino-β-CD complexes and pDNA:bPEI complexes. DLS Measurements of (B) pDNA:1:Ad-PVA-PEG750 and pDNA:3:Ad-PVA-PEG750 complexes formulated by Method A and (C) pDNA:3 and pDNA:3:Ad-PVA-PEG750 complexes formulated by Method B.
Hydrazine derivative 3 was an unusual case in that complexes of this species prepared either via Method B or in the absence of Ad-PVA-PEG750 gave similar results, whereas complexes of 3 produced using Method A had a positive charge that was significantly higher and comparable to bPEI:pDNA complexes. Interestingly, we observe that complexes formulated from Ad-PVA-PEG2000 have a surface charge that is slightly negative. Similar trends were observed with Ad-PVA-PEG such complexes formulated with 3 produced nearly neutral surface charges at N:P = 20.
Dynamic light scattering (DLS) was used to determine the transfection complex sizes produced using these different materials and methods. Our data show that both 1 and 3 had plasmid condensation abilities that were similar to bPEI when Method A was used (i.e., the particle diameters were below 300 nm when N:P > 5) (Figure 3B). Compound 2 also condensed pDNA to form particles of ∼400 nm at high N:P ratios (≥ 40) using Method A (Supporting Information). In stark contrast, Method B complexes of 3:pDNA showed a sharp increase in particle size across a narrow N:P ratio range of 2:1→5:1 (500→5500 nm), indicating that extensive aggregation occurs with these complexes in the absence of polymer. Interestingly, the particle sizes of pDNA:3:Ad-PVA-PEG750 complexes initially increased over this same range of N:P ratios, followed by a decrease to below 2000 nm at N:P ≥ 10. We infer from these results that the PEG-grafted polymer helps to sterically stabilize the 3:pDNA particles (Figure 3C). Our findings indicate that Method A is much better than Method B for producing small transfection complexes, presumably due to improved steric stabilization and a reduced propensity for Ad-PVA-PEG750 to promote particle aggregation via host:guest interactions of a single polymer chain between two or more pre-formed amino-β-CD+:pDNA particles. DLS measurements of complexes made with Ad-PVA-PEG2000 showed that the particles were less than 300 nm for all the CD variants. Ad-PVA-PEG complexes prepared by Method B were larger than those prepared by Method A. Another notable observation is that at similar N:P ratios (e.g., 20), complexes formulated from Ad-PVA-PEG2000 are significantly smaller than those prepared from Ad-PVA-PEG750 (225 vs. 295 nm, respectively, for 1). This indicates that the increased PEG MW and grafting density on the polymer backbone sterically stabilizes the complexes and helps condense them into smaller particles (Supporting Information).
AFM images of Ad-PVA-PEG750 samples revealed the presence of spherical particles (Figure 4A) that were formed by aggregation of the pendant Ad units in aqueous media. Upon addition of amino-β-CD+ derivatives, the spherical particles were transformed into granular fibrillar shaped objects (Figure 4B). We infer from these observations that β-CD complexation with the polymer Ad groups via host:guest interaction causes a transformation of the spherical micelle geometry to an elongated, flexible rod structure due to the combined effects of electrostatic repulsion between the neighboring cationic amino-β-CD+s that are appended to the polymer backbone via host:guest inclusion and the excluded volume occupied by the pendant PEG750 segments. When pDNA was added to this system at low N:P ratios, the samples were observed to be a mixture of fibers and particles, presumably due to the presence of both partially-complexed flexible rod structures and more fully compacted pDNA complexes (Figure 4C). When N:P = 6, only particles with diameters of about 100 nm were observed (Figure 4D). Ad-PVA-PEG2000 formed complexes that were smaller than the complexes formed by Ad-PVA-PEG750, but showed similar trends with respect to shape and morphology (Supporting Information). The sizes determined by AFM are smaller than those measured by DLS due to the absence of solvent-swollen polymer in the AFM samples. These results support the conclusion that host:guest complexation of Ad-PVA-PEG with amino-β-CD+ produces a non-covalent assembly that is capable of condensing pDNA into compact, relatively uniform particles that are sufficiently small to be internalized by cells via endocytosis.
Figure 4.
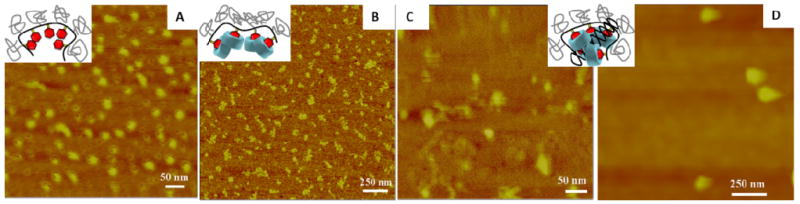
AFM images of (A) Ad-PVA-PEG750 and (B) 1:1 β-CD:Ad-PVA-PEG750. Images of pDNA:3:Ad-PVA-PEG750 prepared by Method B at (C) N:P = 2 and (D) N:P = 6. The insets illustrate the possible structures in these images. The samples were prepared by adding a drop of solution to the mica surface and then slowly evaporating the sample at 25 °C overnight.
Acute Cytotoxicity and Transfection Properties of Amino-β-CD+:Ad-PVA-PEG Complexes
The in vitro cytotoxicity of amino-β-CD+s, Ad-PVA-PEG750, and their host:guest complexes are a highly relevant factor for their long term consideration as a safe non-viral nucleic acid vector. Figure 5 shows that Ad-PVA-PEG750, all of the amino-β-CD+s, and the 1:Ad-PVA-PEG750 host:guest complex were nearly three orders of magnitude less cytotoxic than bPEI, a benchmark reagent for in vitro and in vivo transfections. Specifically, we found that the LD50's of bPEI, 1, 2, 3, Ad-PVA-PEG and 1:1 1:Ad-PVA-PEG750 were 0.01 mM, 4.5 mM, 8.9 mM, 1.6 mM, 1.77 mM and 2 mM, respectively.
Figure 5.
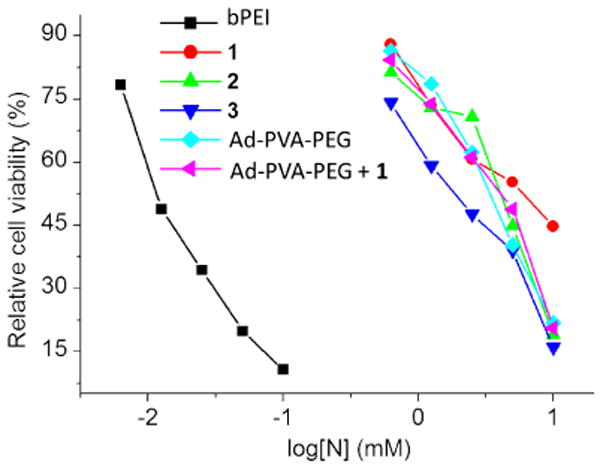
Cytotoxicities of 1, 2, 3, Ad-PVA-PEG750 and 1:Ad-PVA-PEG750 host:guest complexes in HeLa cells using 25kD bPEI as a control. The cells were treated with increasing concentrations of amino-β-CDs, 1:Ad-PVA-PEG750 complexes, and bPEI for 24 h in serum-free media before analysis by MTT assay.
The in vitro performance of the transfection complexes generated by complexation of pDNA (mhGFP) and the amino-β-CD+:Ad-PVA, amino-β-CD+:Ad-PVA-PEG750 or amino-β-CD+:Ad-PVA-PEG2000 host:guest pendant polymer systems were assessed in HeLa cells at N:P = 20 in both serum-free and 10% serum-supplemented media (Figure 6). The transfection efficiencies were calculated using the transfection efficiency of bPEI as 100%. The amino-β-CD+:Ad-PVA complexes showed less than 20% transfection efficiency, with Method A complexes performing marginally better than Method B complexes. We attribute this low level of transfection to the formation of poorly internalized large aggregates that are formed due to the absence of sterically stabilizing PEG segments in this host:guest pendant polymer construct.
Figure 6.
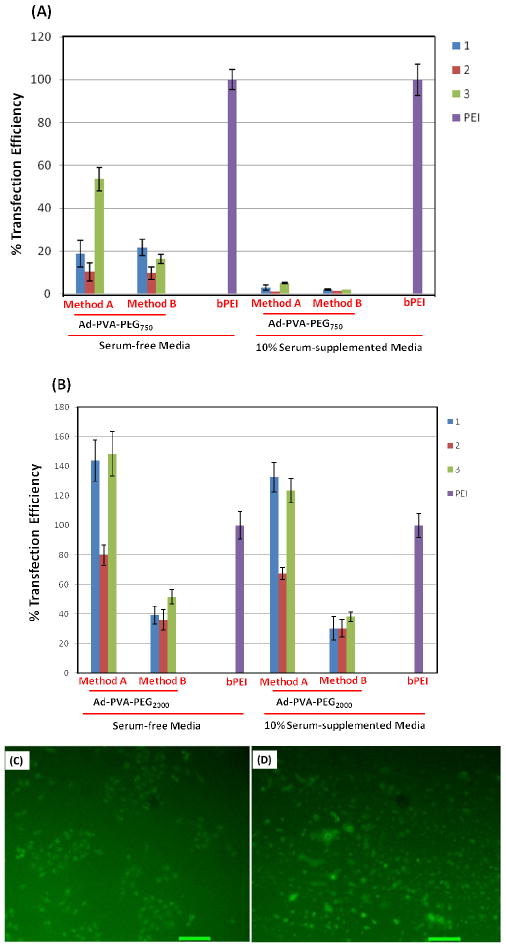
In vitro transfection efficiencies of amino-β-CD host:guest complexes with (A) Ad-PVA-PEG750 and mhGFP pDNA (B) Ad-PVA-PEG2000 and mhGFP pDNA in HeLa cells; with 25kD bPEI (control) considered as 100% transfection efficiency. Fluorescence microscope images of transfected HeLa cells (C) bPEI 25k, (D) 3:Ad-PVA-PEG750 using Method A at N:P = 20 in serum-free media using GFP gene as a reporter gene; scale bar: 100μm.
In the absence of serum, Method A amino-β-CD+:Ad-PVA-PEG750 complexes produced transfection efficiencies in the 15-55% range, depending on the amino-β-CD type, whereas complexes generated using Method B showed transfection efficiencies in the 10-25% range. In the presence of serum, complexes made from Ad-PVA-PEG750 showed less than 10% efficiency. This can be attributed to the low serum stability of the particles due to the relatively low PEG density. Complexes made from Ad-PVA-PEG2000 produced transfection efficiencies in the range of 40-140% of BPEI in the absence of serum and 30-130% of bPEI in the presence of serum. We observed that the presence of serum has little effect on the transfection efficiency of PEG2000 constructs, due to the impact of either higher PEG MW or higher PEG loading on improved serum stability. Complexes made from the hydrazino-modified β-CD (3) and ethylenediamine-modified β-CD (2) showed better transfection efficiency than the ethanolamine (1) derivative. We attribute this finding to the lower charge density on these derivatives (due to the lower pKa expected for 3 and 1 relative to the 2° amines present on 2 that makes them capable of more facile exchange off the pDNA core.
The transfection experimental conditions used were those that had been previously optimized for bPEI in order to provide the most accurate comparison with this widely used transfection reagent. It is important to note, however, that the performance of bPEI is almost twofold lower and nearly 1000 times more toxic than the amino-β-CD+:Ad-PVA-PEG2000 complexes reported here (Figure 5). Indeed, the widely reported dose-limiting toxicity of bPEI has been a major impediment to the further development of gene delivery strategies in vivo using this vector. In view of this limitation, the apparent low toxicity of amino-β-CD+:Ad-PVA-PEG complexes suggests that higher doses may be possible with this vector while still maintaining good cell viability.
Three other observations deserve note for all the amino-β-CD+:Ad-PVA-PEG complexes studied: (i) Method A complexes tend to produce higher transfection efficiencies than the analogous Method B complexes; (ii) Ad-PVA-PEG2000 complexes were significantly more effective than Ad-PVA-PEG750 complexes, which in turn were more effective than Ad-PVA complexes; and (iii) increased PEG loading and use of longer PEG results in complexes that are stable in presence of serum. Smaller transfection complex sizes, improved solubility due to the presence of PEG, and improved steric stabilization are likely to be responsible for these findings. The smaller sizes of the transfection complexes may have led to an increase in extent of cellular internalization of the particles, thereby leading to better transfection efficiencies for Method A. Conversely, the low solubility of the Ad-PVA complexes results in aggregation, giving rise to larger particles that may be too large to be effectively internalized, thus leading to lower transfection efficiencies. Based on our encouraging findings, we plan to evaluate the effect of various material parameters (e.g., pendant group density, pendant group spacing, pendant group hydrolysis rate) on the performance of this novel self-assembled vector strategy in future studies.
Conclusions
In conclusion, a low toxicity and efficient gene delivery system has been developed based on the self-assembly of cationic CD derivatives with adamantane-modified PVA. PVA, linked to Ad via a pH-sensitive acetal linkage, provides a scaffold for binding of cationic amino-β-CD+ that are capable of condensing pDNA into nanoparticles in the <300 nm size range using a multilayer strategy30-32. This system employs reversible host:guest interactions that have also been used to construct cross-linked hydrogels, particles, and transfection complexes33-37. The pendant guest ligands have been attached using acid-sensitive acetal linkages so that they are capable of degrading within acidic endosomes to effect pDNA release. These cationic host:guest pendant polymers are capable of achieving transfections efficiencies that are comparable and superior to those of 25 kD bPEI, while being nearly 1000-fold less toxic than this conventional cationic polymer transfection agent.
Supplementary Material
Acknowledgments
The authors express their special thanks for the support of this work by NIH GM087016, the Showalter Foundation, the Purdue University Center for Cancer Research, and the Purdue University Department of Chemistry.
Footnotes
Supporting Information Available: Characterization of amino-β-CD+s and Ad-PVA-PEG. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M. Gene therapy for red–green colour blindness in adult primates. Nature. 2009;461:784–8. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nature Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DWY, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nature Biotech. 2010;20:172–6. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 4.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayak S, Herzog RW. Progress and Prospects: Immune Responses to Viral Vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li SD, Huang L. Non-viral is superior to viral gene delivery. J Control Rel. 2007;123:181–3. doi: 10.1016/j.jconrel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Anchordoquy T. Drug delivery trends in clinical trials and translational medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J Pharm Sci. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu HJ, Wagner E. Bioresponsive polymers for nonviral gene delivery. Curr Op Mol Ther. 2009;11:165–78. [PubMed] [Google Scholar]
- 9.Vachutinsky Y, Kataoka K. PEG-based polyplex design for gene and nucleotide delivery. Isr J Chem. 2010;50:175–84. [Google Scholar]
- 10.Kawano T, Okuda T, Aoyagi H, Niidome T. Long circulation of intravenously administered plasmid DNA delivered with dendritic poly(L-lysine) in the blood flow. J Control Rel. 2004;99:329–37. doi: 10.1016/j.jconrel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Majoros IJ, Williams CR, Baker JR. Current dendrimer applications in cancer diagnosis and therapy. Curr Top Med Chem. 2008;8:1165–79. doi: 10.2174/156802608785849049. [DOI] [PubMed] [Google Scholar]
- 12.Mintzer MA, Grinstaff MW. Biomedical applications of dendrimers: a tutorial. Chem Soc Rev. 2011;40:173–90. doi: 10.1039/b901839p. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Lynn DM. Multilayered films fabricated from combinations of degradable polyamines: tunable erosion and release of anionic polyelectrolytes. Macromolecules. 2006;39:8928–35. doi: 10.1021/ma061815g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Montañez SI, Jewell CM, Lynn DM. Multilayered films fabricated from plasmid DNA and a side-chain functionalized poly(beta-amino ester): surface-type erosion and sequential release of multiple plasmid constructs from surfaces. Langmuir. 2007;23:11139–46. doi: 10.1021/la702021s. [DOI] [PubMed] [Google Scholar]
- 15.Peng L, Gao Y, Xue YN, Huang SW, Zhuo RX. Cytotoxicity and in vivo tissue compatibility of poly(amidoamine) with pendant aminobutyl group as a gene delivery vector. Biomaterials. 2010;31:4467–76. doi: 10.1016/j.biomaterials.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Huang SW, Zhuo RX. Recent advances in polyphosphoester and polyphosphoramidate-based biomaterials. Phosphorus, Sulfur Silicon Relat Elem. 2008;183:340–348. [Google Scholar]
- 17.Duceppe N, Tabrizian M. Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Exp Op Drug Del. 2010;7:1191–207. doi: 10.1517/17425247.2010.514604. [DOI] [PubMed] [Google Scholar]
- 18.Yui N, Katoono R, Yamashita A. Functional cyclodextrin polyrotaxanes for drug delivery. Adv Polym Sci. 2009;222:55–77. [Google Scholar]
- 19.Yang C, Wang X, Li H, Tan E, Lim CT, Li J. Cationic polyrotaxanes as gene carriers: physicochemical properties and real-time observation of DNA complexation, and gene transfection in cancer cells. J Phys Chem B. 2009;113:7903–11. doi: 10.1021/jp901302f. [DOI] [PubMed] [Google Scholar]
- 20.Mellet CO, Fernandez JMG, Benito JM. Cyclodextrin-based gene delivery systems. Chem Soc Rev. 2011;40:1586–608. doi: 10.1039/c0cs00019a. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasachari S, Fichter KM, Reineke TM. Polycationic beta-cyclodextrin “click clusters”: monodisperse and versatile scaffolds for nucleic acid delivery. J Am Chem Soc. 2011;130:4618–27. doi: 10.1021/ja074597v. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasachari S, Reineke TM. Versatile supramolecular pDNA vehicles via “click polymerization” of beta-cyclodextrin with oligoethyleneamines. Biomaterials. 2009;30:928–38. doi: 10.1016/j.biomaterials.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 23.Rekharsky MV, Inoue Y. Complexation thermodynamics of cyclodextrins. Chem Rev. 1998;98:1875–1918. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconj Chem. 1999;10:1068. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 25.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from charge centers. Bioconj Chem. 2003;14:247–54. doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 26.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconj Chem. 2003;14:255–61. doi: 10.1021/bc025593c. [DOI] [PubMed] [Google Scholar]
- 27.Popielarski SR, Mishra S, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 3 Cyclodextrin type and functionalization. Bioconj Chem. 2003;14:672–8. doi: 10.1021/bc034010b. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita A, Kanda D, Katoono R, Yui N, Ooya T, Maruyama A, Akita H, Kogure K, Harashima H. Supramolecular control of polyplex dissociation and cell transfection: efficacy of amino groups and threading cyclodextrins in biocleavable polyrotaxanes. J Control Rel. 2008;131:137–44. doi: 10.1016/j.jconrel.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Ooya T, Choi HS, Yamashita A, Yui N, Sugaya Y, Kano A, Maruyama A, Akita H, Ito R, Kogure K, Harashima H. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J Am Chem Soc. 128:3852–3. doi: 10.1021/ja055868+. [DOI] [PubMed] [Google Scholar]
- 30.Jewell CM, Lynn DM. Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid-based therapeutics. Adv Drug Del Rev. 2008;60:979–99. doi: 10.1016/j.addr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker AL, Johnston APR, Caruso F. Layer-by-layer-assembled capsules and films for therapeutic delivery. Small. 2010;6:1836–52. doi: 10.1002/smll.201000379. [DOI] [PubMed] [Google Scholar]
- 32.Hatakeyama H, Akita H, Kogure K, Harashima H. A novel nonviral gene delivery system: multifunctional envelope-type nano device. Adv Biochem Engin Biotechnol. 2010;119:197–230. doi: 10.1007/10_2008_40. [DOI] [PubMed] [Google Scholar]
- 33.Charlot A, Auzely-Vélty R. Synthesis of novel supramolecular assemblies based on hyaluronic acid derivatives bearing bivalent β-cyclodextrin and adamantane moieties. Macromolecules. 2007;40:1147–58. [Google Scholar]
- 34.Li L, Guo XH, Wang J, Liu P, Prud'homme RK, May BL, Lincoln SF. Polymer networks assembled by host-guest inclusion between adamantyl and β-cyclodextrin substituents on poly(acrylic acid) in aqueous solution. Macromolecules. 2008;41:8677–81. [Google Scholar]
- 35.Wang H, Wang S, Su H, Chen KJ, Armijo AL, Lin WY, Wang Y, Sun J, Kamei K, Czernin J, Radu CG, Tseng HR. A supramolecular approach for preparation of size-controlled nanoparticles. Angew Chem Int Ed. 2009;48:4344–8. doi: 10.1002/anie.200900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JX, Sun HL, Ma PX. Host-guest interaction mediated polymeric assemblies: multifunctional nanoparticles for drug and gene delivery. ACS Nano. 2010;4:1049–59. doi: 10.1021/nn901213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SK, Park KM, Singha K, Kim J, Ahn Y, Kim K, Kim WJ. Galactosylated cucurbituril-inclusion polyplex for hepatocyte-targeted gene delivery. Chem Comm. 2010;46:692–4. doi: 10.1039/b920753h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



