SUMMARY
There is a greater prevalence of neuroinflammatory diseases in females than males. Microglia, the major immunocompetent cells of the central nervous system, play a key role in neuroinflammation. We aimed to determine if inherent differences in toll-like receptor 4 mediated pro-inflammatory response in glia could possibly contribute to the skewed female prevalence of neuroinflammatory disorders. In addition, in order to identify if estradiol (E2), the major female sex steroid contributes to a heightened pro-inflammatory response, estradiol was added both in vivo and in vitro. Microglia and astrocytes were isolated from neonatal pups and stimulated with lipopolysaccharide (LPS) in the presence and absence of E2. Hippocampal microglia were isolated from adult male and female rats and stimulated ex vivo with LPS. Male neonatal microglia and astrocytes produced greater IL-1β mRNA than females. However, when co-incubated with varying doses of estradiol (E2), the E2 produced anti-inflammatory effects in the male microglia but a pro-inflammatory effect in female microglia. LPS-induced IL-1β mRNA was attenuated by E2 in female but not male adult hippocampal microglia. However, females supplemented with E2 in vivo produced a potentiated IL-1β mRNA response. TLR4 mRNA was decreased by LPS in both microglia and astrocytes but was not affected by sex or E2. CD14 mRNA was increased by LPS and may be elevated more in females than males in microglia but not astrocytes. Therefore, sexual dimorphic differences do occur in both neonatal and adult microglia though maturity of the microglia at the time of isolation influences the pro-inflammatory response.
Keywords: microglia, sex steroid, cytokines, toll-like receptor 4
Introduction
Microglia are the predominant immunocompetent cells within the central nervous system. They release pro-inflammatory mediators in response to injury or inflammation. Microglia have been implicated in many neurodegenerative and neuroinflammatory diseases including multiple sclerosis, Alzheimer’s disease and chronic pain (Watkins et al. 2007; Perry et al. 2010; Streit 2010). Females have a greater prevalence than males in all of these pathologies, (Streit et al. 2005; Achiron and Gurevich 2009; Fillingim et al. 2009; Amor et al. 2010; Streit 2010). Therefore, the question arises as to whether microglia, which contribute to neuroinflammation, also contribute to the difference in pro-inflammatory response between males and females.
There is recent evidence to suggest that morphological and phenotypic differences exist between male and female microglia, but that the differences change with age. For example, neonatal male microglia display an amoeboid morphology and have a more classically activated phenotype compared to neonatal female microglia (Schwarz et al. 2011). However, unstimulated microglia isolated from naive female 60 day old rats present with a more classically activated state with higher levels of IL-1 expression and lower IL-10 expression (Schwarz et al. 2011). Purine receptor expression on microglia, known to be involved in microglial activation, also appears to vary between sex and age (Crain et al. 2009). However, no studies have investigated whether these sexually dimorphic differences remain when the microglia are stimulated with an immune challenge.
It is becoming increasingly recognized that toll-like receptors (TLR) are involved not only in the recognition of pathogens but also in the recognition of endogenous danger signals (Erridge 2010). TLR4 is a pattern recognition receptor that has been implicated, within the central nervous system (CNS), as importantly contributing to the pathological processes underlying neuropathic pain, multiple sclerosis, and Alzheimer’s disease through the release of neuroinflammatory and neuroexcitatory substances as a consequence of TLR4 activation (Marta 2009; Morales et al. 2010; Nicotra et al. 2011). Therefore, in this series of studies we investigated whether there is a potential sex difference in changes in mRNA expression in neonatal microglia following an immune challenge using lipopolysaccharide, the classic TLR4 ligand. CD14 and MD2 are co-receptors for the TLR4 complex and are shown to be involved in neuroinflammatory conditions (Nadeau and Rivest 2002; Cao et al. 2009; Loram et al. 2011). Therefore, the subsequent changes to TLR4 and its co-receptors will be investigated.
The second aim of this study was to identify whether this difference in phenotype of the microglia between neonates and adults arises from changes in estrogen levels. Estrogens are not only critically involved in reproduction, but can also modulate neuronal function, acting as a neurosteroid, and can attenuate or potentiate inflammatory responses in immune cells both in vivo and in vitro (Compagnone and Mellon 2000; Vegeto et al. 2006; Calippe et al. 2010). Neonatal cells are not yet exposed to the cyclical fluctuations of estrus seen in adults. Therefore, microglia from both P0/1 pups and microglia isolated from adult rats were investigated to compare these two states. We have selected to test the influence of 17β-estradiol (E2), as it is the predominant circulating estrogen. The effect of E2 on glial cells was tested both in vitro and in vivo to identify if differences exist between the two conditions.
While microglia are the major immunocompetent cell within the CNS, astrocytes also have the capacity to produce pro-inflammatory mediators and are now implicated in a number of neuroinflammatory diseases (Pineau et al. 2010). Astrocytes have been identified to have sexually dimorphic differences in vivo (Suarez et al. 1991; Johnson et al. 2008). However, only one study has investigated the immune responses of isolated astrocytes in vitro (Santos-Galindo et al. 2011). Therefore, we investigated whether astrocytes from male and female neonatal pups responded differently to LPS in vitro in the presence and absence of E2.
Materials and methods
Subjects
10–12 wk old male (325–350 g) and female (200–225 g) pathogen-free Sprague-Dawley rats (Harlan Lab, Madison, WI, USA) were used in this study. All rats had a 12 h light cycle in temperature-controlled rooms (lights on at 0700h, 25°C). The rats were acclimatized to the colony rooms for 1 wk before experimentation. The male and female rats were housed in the same room but were housed 2–3 per cage as separate sexes. Separate female Sprague-Dawley rats were bred and the neonatal P0/1 day old male and female pups were used in the neonatal cell culture experiments. Standard rat chow and tap water were available ad libitum. All experimental procedures were conducted in accordance with protocols approved by the University of Colorado Institutional Animal Care and Use Committee.
Drugs
Lipopolysaccharide (serotype 0111:B4) was purchased from Sigma (St. Louis, MO, USA). E2 (E0950-000, Steraloid, Newport, RI, USA), comparable to endogenous rat E2, capsules were made as described previously (Stern and McDonald 1989). Briefly, silastic tubing (Dow Corning 0.058 in i.d., 0.077 in o.d. Midland, MI, USA) was cut into 15 mm sections, wooden dowel sticks were used to plug the one end and secured with Silastic Medical adhesive (Dow-Corning, Midland, MI, USA). 4 mm of crystalline E2 was packed into the silastic tubing. The other end of the tubing was plugged with another piece of dowel stick and secured with adhesive. The capsules were covered to prevent light exposure to the E2 and the glue was allowed to dry overnight. The capsules were washed in ethanol and incubated for at least 10 min in phosphate-buffered saline (pH 7.0) before implantation. Empty capsules were implanted as controls.
17β-Estradiol (E2) Radioimmunoassay
Intracardiac blood was collected at the time of saline perfusion and allowed to stand for 30 min before being centrifuged at 14,000 rpm for 10 min at room temperature. Serum was collected and stored at −80°C until further analysis. E2 concentration was evaluated in the serum of rats receiving E2 supplementation, using a double antibody radioimmunoassay according to the manufacturer’s guidelines (Coat-a-Count, Siemens Medical Solutions Diagnostics. Los Angeles, CA, USA).
Neonatal cell isolation
Brain cortices from P0/1 neonatal male and female Sprague-Dawley rat pups were separately and carefully dissected and the overlying meninges removed. The cortical tissue was then minced with a scalpel blade and digested for 30 min in Liberase Blendzyme III (1.4 mWunsch Units per brain, Roche Applied Science, Mannheim, Germany) and DNAse (0.1 U per brain, Sigma, St Louis, MO, USA) at 37°C with agitation. The cells were triturated with a 21 Gauge and a 23 Gauge hypodermic needle. Phenol red-free MEM (100 U/ml penicillin, 100 μg/ml streptomycin, 0.6% glucose and 2 mM l-glutamine, Invitrogen, Carlsbad, CA, USA) was added and the cells centrifuged at 250 × g for 5 min at RT. The supernatant was discarded and the cells resuspended in 10 ml of MEM media with supplements per 4 brains. The cells were filtered through a 70 μm and then a 40 μm filter. The cells were plated in 75 cm2 tissue culture flasks at 4 brains per flask. Cells were incubated at 37 °C and 5% CO2 until confluence was reached (about 10 days). Media was changed every 3–4 days, with the first change being a complete media change and subsequent changes being a 50% media change. Once confluence was reached, microglial cells were shaken from the remaining astrocytes for 90 min on an orbital shaker at 160 rpm. The media containing the microglia was removed and centrifuged at 300 × g for 5 min at RT. The supernatant was discarded and the pellet resuspended in 1 ml fresh MEM media. A small aliquot of cells was counted with trypan exclusion and plated in 96-well v-bottom tissue culture plates at 40,000–50,000 cells per well in 100 μl media. 24 h after plating, the drugs were added. Once the microglia were removed from the flask, the astrocytes were treated for 2 h with 10 ml phenol red free DMEM (10% FBS and 0.6% glucose) containing 5mM L-leucine methyl ester to deplete any remaining microglia. The cells were washed twice with DPBS. A single cell suspension was obtained by 5 min incubation with 0.05% trypsin/EDTA. The cells were centrifuged for 10 min at 200 × g. The pellet was reconstituted with phenol red free DMEM/F12 (100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM l-glutamine) at 100,000 cells per 1ml in a 24 well tissue culture plate. The cells were incubated for 24 h before drug administration.
Surgery
Ovariectomy and hormone supplementation
Ovariectomies (OVX) in adult female rats were conducted under isoflurane anesthesia (Webster Veterinary, Sterling, MA, USA). A small cut through the skin, subcutaneous tissue, and muscle on each side of the shaved abdomen was made 3 cm below the rib cage. The ovaries were isolated, exteriorized and the uterine tube ligated using 2-0 silk, before removal of the ovary. The abdominal wall and overlying muscle was sutured using 3-0 silk and the skin stapled with wound clips. The males received the same incision and wound closing. The rats were allowed two weeks recovery before experimentation. Two weeks after surgery, while the rats were under isoflurane anesthesia, an incision was made on the left flank. A pouch was made between the skin and fat layer by blunt dissection, and an E2 or control capsule inserted. The incision was stapled with wound clips.
Adult hippocampal microglial isolation
Hippocampal microglia were rapidly isolated from adult rats as previously described (Frank et al. 2006). The hippocampal microglia were selected as they have been demonstrated to be microglia and not other CNS macrophages and the isolation procedure has been demonstrated in hippocampal microglia to maintain the in vivo phenotype (Frank et al. 2006). Briefly, rats were deeply anesthetized with sodium pentobarbital (IP), cardiac blood collected and then transcardially perfused with ice-cold saline. The brain was removed and the whole hippocampus dissected and placed into 2 ml of 0.2% glucose Dulbecco’s Phosphate Buffered Saline (DPBS). The tissue was homogenized in Bellco glass hand homogenizers, filtered through 40 μm cell strainers, and centrifuged (350 × g for 10 min at RT). The cells were isolated using a Percoll density gradient (Sigma, St Loius, MO, USA, 50% and 70% gradients) and centrifuged at 1200 × g for 45 min (no additional acceleration or brake). The cells were removed from the interface of the Percoll layers, rinsed in PBS and centrifuged at 1200 × g for 10 min.
RNA extraction and cDNA synthesis
The adult hippocampal microglial cells were lysed for mRNA using a kit optimized for 10,000 cells or less (Cells-Direct III cDNA synthesis kit, Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines as described previously (Loram et al. 2009). Briefly, after washing the cells in 100 μl of Dulbecco’s PBS, the cells were lysed in 10 μl of lysis buffer and lysis solution per 10,000 cells for 10 min on ice. Ten microliters of cell lysate were added to 1μl of RNase inhibitor and incubated at 75°C for 10 min. First-strand cDNA was synthesized by adding 2 μl of oligo dT, 1 μl of dNTP, and 7.8 μl of nuclease-free water to the cell lysate and incubating at 70°C for 5 min. After 2 min on ice, 6 μl of 5 X RTbuffer, 1 μl of RNase inhibitor, 1 μl of Superscript III, and 1 μl of dithiothreitol was added to the cell lysate and incubated at 50°C for 50 min, 5 min for 85°C. Finally, 1 μl of RNase H was added and incubated at 37°C for 20 min. All cDNA was stored at −80°C until real-time PCR (RT-PCR) was performed.
Total RNA from the neonatal cells was extracted using the standard phenol:chloroform extraction with TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. Total RNA was reverse transcribed into cDNA using Superscript II First-Strand Synthesis System (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using total RNA, random hexamer primer (5 ng/μl) and 1mM dNTP mix (Invitrogen, Carlsbad, CA) and incubated at 65 °C for 5 min. Following 2 min incubation on ice, a cDNA synthesis buffer (5 X RT buffer, Invitrogen, Carlsbad, CA) and dithiothreitol (10 mM) was added and incubated at 25 °C for 2 min. Reverse transcriptase (Superscript II, 200 Units, Invitrogen, Carlsbad, CA) was added to a total volume of 20 μl and incubated for 10 min at 25 °C, 50 min at 42 °C and deactivating the enzyme at 70 °C for 15 min. cDNA was stored at −80 °C.
RT-PCR
Primer sequences were obtained from the Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov) and displayed in Table 1. Primer sequences, where delineation is available, were selected to span across an intron to avoid genomic contamination. Amplification of the cDNA was performed using Quantitect SYBR Green PCR kit (Qiagen, Valencia, CA) in iCycler iQ 96-well PCR plates (Bio-Rad, Hercules, CA) on a MyiQ single Color Real-Time PCR Detection System (Bio-Rad). The reaction mixture (26 μl) was composed of QuantiTect SYBR Green (containing fluorescent dye SYBR Green I, 2.5 mM MgCl2, dNTP mix and Hotstart Taq Polymerase), 10 nM fluorescein, 500 nM of each forward and reverse primer (Invitrogen, Carlsbad, CA), nuclease-free water and 1 μl of cDNA from each sample. Each sample was measured in duplicate. The reactions were initiated with a hotstart at 95 °C for 25 min, followed by 40 cycles of 15 s at 94 °C (denaturation), 30 s at 55–60 °C (annealing) and 30 s at 72 °C (extension). Melt curve analyses were conducted to assess uniformity of product formation, primer-dimer formation and amplification of non-specific products. The PCR product was monitored in real-time, using the SYBR Green I fluorescence, using the MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Threshold for detection of PCR product was set in the log-linear phase of amplification and the threshold cycle (CT) was determined for each reaction. The level of the target mRNA was quantified relative to the housekeeping gene (GAPDH) and were expressed as fold change in the gene of interest compared to GAPDH.
Table 1.
Primer sequences for RT-PCR
| Gene | Primer sequence (5′ -3′) | GenBank accession No. |
|---|---|---|
| GAPDH | TCTTCCAGGAGCGAGATCCC (forward) TTCAGGTGAGCCCCAGCCTT (reverse) |
NC_005103.2 |
| IL-1β | CCTTGTGCAAGTGTCTGAAG (forward) GGGCTTGGAAGCAATCCTTA (reverse) |
NM_0315122.2 |
| CD14 | ACCGACCATGAAGCTTATGC (forward) CTGAGAAGTTGCAGTAGCAG (reverse) |
NM_021744.1 |
| TLR4 | TCCCTGCATAGAGGTACTTC (forward) CACACCTGGATAAATCCAGC (reverse) |
NM_019178.1 |
| MD2 | ATCTGAGAGGCAACAGTG (forward) CCTCTTGGAATGAACTCAACA (reverse) |
NM_001024279.1 |
Experimental designs
Experiment 1. LPS dose response in neonatal microglia
Neonatal microglia were incubated with vehicle (saline), 1 ng/ml, 10 ng/ml, or 100 ng/ml LPS for 4 h at 37 C and 5% CO2.. The cells were centrifuged at 1,000 × g for 10 min at 4°C. The supernatant was discarded and cells processed for IL-1β, MD2, TLR4 and CD14 mRNA gene expression.
Experiment 2. Effect of E2 on responses to LPS in neonatal microglia
Neonatal microglia were incubated with 10 ng/ml LPS or vehicle coadministered with 0, 1 nM or 10 nM E2 for 4 h at 37 C and 5% CO2.. The cells were centrifuged at 1,000 × g for 10 min at 4°C. The supernatant was discarded and cells processed for IL-1β, MD2, TLR4 and CD14 mRNA gene expression.
Experiment 3. LPS dose response in neonatal astrocytes
Neonatal astrocytes were incubated with vehicle (saline), 1 ng/ml, 10 ng/ml, or 100 ng/ml LPS for 4 h at 37 C and 5% CO2.. The cells were centrifuged at 1,000 × g for 10 min at 4 °C. The supernatant was discarded and cells processed for IL-1β, MD2, TLR4 and CD14 mRNA gene expression.
Experiment 4. Effect of E2 on responses to LPS in neonatal astrocytes
Neonatal astrocytes were incubated with 10 ng/ml LPS or vehicle coadministered with 0, 1 nM or 10 nM E2 for 4 h at 37 C and 5% CO2.. The cells were centrifuged at 1,000 × g for 10 min at 4 °C. The supernatant was discarded and cells processed for IL-1β, MD2, TLR4 and CD14 mRNA gene expression.
Experiment 5. Effect of E2 ex vivo on LPS in adult hippocampal microglia
Ovariectomized female rats or sham-operated male rats were allowed 2 weeks to recover from surgery. The rats were then terminally anesthetized with sodium pentobarbital and transcardially perfused with ice-cold saline. The hippocampal cells were isolated as described above and plated at 10,000 cells/well in a 96-well v-bottom cell culture plate in 100 μl of phenol red-free DMEM media with 10% FBS (Invitrogen, Carlsbad, CA, USA). The cells were incubated with 100 ng/ml LPS or 0, and 10 nM, or 100 nM E2 for 3 h at 37 C and 5% CO2. After incubation, all cells were centrifuged at 1,000 × g for 10 min at 4°C. The supernatant was discarded and cells processed for IL-1β, MD2, TLR4 and CD14 mRNA gene expression.
Experiment 6. LPS dose response in rapidly isolated adult hippocampal microglia and the effect of in vivo E2
Ovariectomized females and sham-operated males were allowed 2 wk recovery before E2 or control (empty) capsules were implanted. Twelve days after hormone or control capsule implantation, or females in proestrus/estrus, were deeply anesthetized with sodium pentobarbital and transcardially perfused with ice-cold saline. Hippocampal microglia were isolated as described above. The hippocampal microglial cells were plated at 10,000 cells/well in a 96-well v-bottom cell culture plate in 100 μl of phenol red-free DMEM media with 10% FBS (Invitrogen, Carlsbad, CA, USA). The cells were incubated with vehicle (media), 10 ng/ml or 100 ng/ml LPS for 3 h at 37 C and 5% CO2. After incubation, all cells were centrifuged at 1,000 × g for 10 min at 4°C. The supernatant was discarded and cells processed for mRNA gene expression. The cells were processed for IL-1β, MD2, TLR4 and CD14 mRNA gene expression.
Statistical analysis
Data in the text are reported as mean ± SEM. RT-PCR data were analyzed using a two-way ANOVA using Graphpad Prism version 5. Bonferroni post-hoc tests were used where appropriate and P < 0.05 was considered statistically significant.
Results
Serum E2 concentration
The estradiol capsules produced E2 concentrations of 124 ± 9 pg/ml in the females and 113 ± 15 pg/ml in the males. This amount of E2 creates circulating E2 levels at the high end of the range of peak circulating levels in proestrus at 60–120 pg/ml (Smith et al. 1975). In none of the experiments were the E2 serum concentrations different between sexes or between experiments. In ovariectomized females and non-supplemented males, the E2 concentrations were less than 5 pg/ml.
Experiment 1. LPS dose response in male and female neonatal microglia
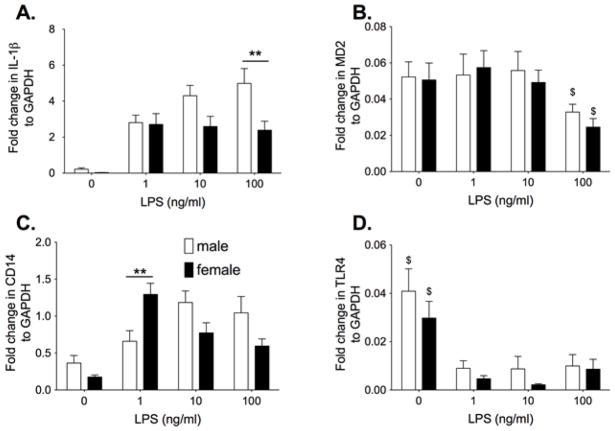
Neonatal cortical microglial cells from male and female P0/P1 rat pups were stimulated with the classic TLR4 ligand, LPS to identify whether there are inherent differences in their pro-inflammatory and TLR4 signaling response. Microglial cells were incubated with 0, 1 ng/ml, 10 ng/ml, or 100 ng/ml LPS in phenol red free media. IL-1β, MD2, CD14 and TLR4 mRNA are presented in Figure 1 as fold expression relative to GAPDH. The GAPDH expression was not significantly different between groups (n=9 per group). The male microglia had a significantly greater IL-1β response to LPS compared to the females (Interaction: F3,83 = 2.87, P<0.05) with significant post-hoc comparisons between males and females at 100 ng/ml LPS (P<0.01). There was also significantly higher CD14 mRNA at 1 ng/ml LPS (P<0.05) in females compared to males (Interaction: F3,84 = 7.12, P<0.0001). There was a significant increase in MD2 mRNA (F1,3 = 3.93, P<0.05) and a significant decrease in TLR4 mRNA (F1,3 = 15.65, P<0.0001) when LPS was administered compared to vehicle. There was no significant effect of sex or interaction between sex and LPS dose on MD2 (sex: F3,84 = 0.25, P=0.62, Interaction: F3,84 = 0.21, P= 0.89) or TLR4 (sex: F3,87 = 2.70, P=0.10, Interaction: F3,87 = 0.33, P= 0.80).
Figure 1.
Neonatal male and female microglia isolated from P0/1 pups were incubated with 0, 1, 10 or 100ng/ml LPS for 4 h. IL-1β mRNA (A), MD2 mRNA (B), CD14 mRNA (C) and TLR4 mRNA (D) was measured. There was a significant LPS dose response on IL-1β mRNA and TLR4 mRNA suppression in male but not female microglia. Males produced a greater IL-1β mRNA response than females while females produced a greater CD14 mRNA response at the lowest dose of LPS. Data are expressed as mean ± SEM (n=9–12/gp). ** P<0.01 between groups. $ P<0.05 compared to other doses of LPS.
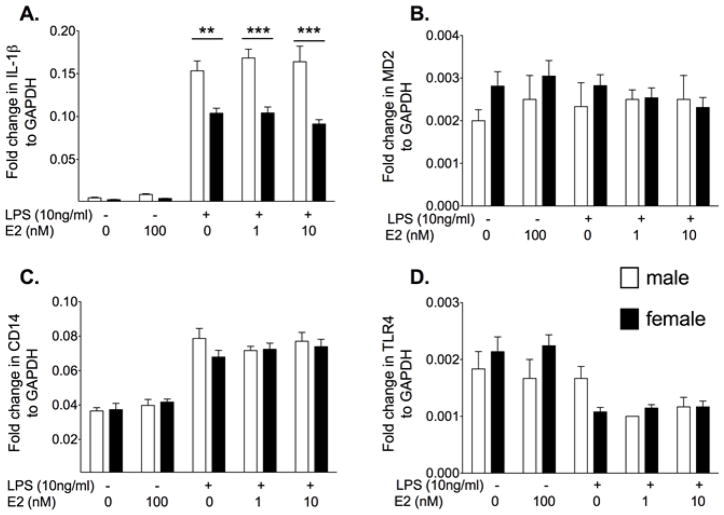
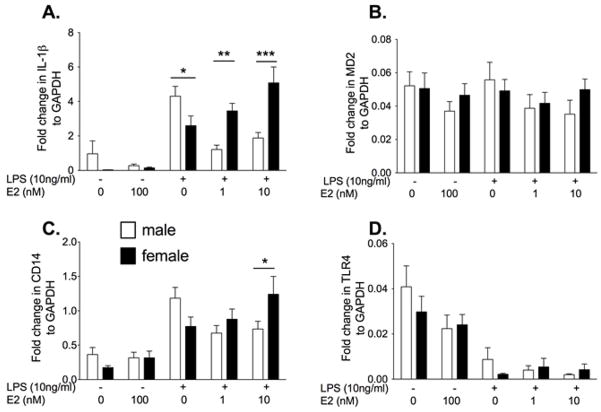
Experiment 2. E2 potentiates IL-1β mRNA expression in female but is anti-inflammatory in male neonatal microglia
Neonatal cells are not yet exposed to fluctuations in circulating sex steroids. Therefore, to determine if the response changes in the presence of E2, the cells were co-incubated with LPS and E2. There was a significantly greater IL-1β mRNA in the females compared to the males in the presence of 1 nM (P<0.01) and 10 nM E2 (F4,107 = 9.54, post hoc: P<0.001, Fig. 1). In the males, E2 suppressed IL-1β mRNA while in females IL-1β mRNA was potentiated by E2. There was also significantly higher CD14 mRNA at the high dose of E2+LPS in females compared to males (F4,109 = 3.51, P<0.01). There was no significant differences in MD2 mRNA between groups (E2: F1,4 = 1.07, P=0.37, sex: F4,103 = 0.57, P=0.45, Interaction: F4,103 = 0.57, P= 0.68). There was a significant decrease in TLR4 mRNA following LPS (F1,4 = 16.73, P<0.0001), but there was no significant difference between the sexes or an effect of E2 (sex: F4,106 = 0.62, P=0.43, Interaction: F4,106 = 0.75, P= 0.56).
Experiment 3. LPS dose response in male and female neonatal astrocytes
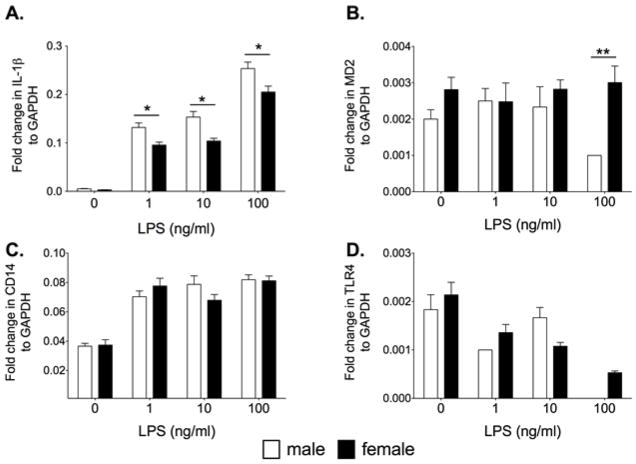
Microglia are the dominant immunocompetent cell within the CNS. However, astrocytes also have immune functions. Therefore, astrocytes were challenged with LPS to identify if sex differences occur in neonatal astrocytes. Neonatal astrocytes were incubated with 0, 1 ng/ml, 10 ng/ml or 100 ng/ml LPS and the mRNA expression for IL-1β, MD2, CD14 and TLR4 mRNA are presented in Figure 3. There was a significant increase in IL-1β mRNA in both sexes with the males presenting with significantly greater IL-1β expression compared to females at all doses of LPS (F3,40=3.17, P<0.05). CD14 mRNA was significantly increased by LPS (F3,40 = 49.15, P<0.0001) regardless of sex. There was no significant difference in CD14 expression between the sexes (F1,3 = 0.09, P=0.76). There was a significant difference in MD2 mRNA between the males and females at 100 ng/ml LPS (F3,30 = 2.94, P<0.05). There was a significant decrease in TLR4 mRNA in the LPS groups compared to vehicle (F3,30 = 3.89, P<0.05) but no significant difference between the sexes (P>0.05).
Figure 3.
Neonatal male and female astrocytes isolated from P0/1 pups were incubated with 0, 1, 10 and 100ng/ml LPS for 4 h and measured for IL-1β mRNA (A), MD2 mRNA(B), CD14 mRNA (C) and TLR4 mRNA (D). There was a significant LPS dose response on IL-1β mRNA and TLR4 mRNA in both male and female astrocytes. Males produced a greater IL-1β mRNA response than females. There was a significant effect of LPS on CD14 mRNA but no difference between the sexes. There was no significant effect on MD2 except in males at the high dose of LPS. Data are expressed as mean ± SEM (n=6/gp). * P<0.05 between groups.
Experiment 4. E2 has no effect on male and neonatal astrocytes
Estrogen receptors are found on all cells within the CNS including astrocytes. Therefore, we tested whether E2 influenced the responses of astrocytes to LPS. When astrocytes were co-incubated with LPS and E2, males had a significantly higher IL-1β mRNA expression with LPS alone (P<0.001), LPS+1 nM E2 (F4,50 = 8.13, P<0.0001) and LPS+10 nM E2 (P<0.0001, Figure 4). CD14 mRNA was significantly increased by LPS (F4,50 = 50.94, P<0.0001) and TLR4 mRNA was significantly decreased by LPS (F4,40 = 8.56, P<0.0001). However, there was no significant difference between the sexes or an effect of E2 (CD14: F1,3 = 0.99, P=0.38, TLR4: F1,4 = 0.74, P=0.41). There was no significant effect of sex or dose on MD2 mRNA. Therefore, astrocytes were non-responsive to E2 in the conditions tested.
Figure 4.
Neonatal astrocytes isolated from male and female pups that were incubated with vehicle or 10 ng/ml LPS and 0, 10 nM or 100 nM 17-β estradiol (E2) for 4 h. IL-1β, MD2, CD14 and TLR4 mRNA was measured. E2 had no significant effect on IL-1β, MD2, CD14 or TLR4 mRNA in either male and female astrocytes. Data are expressed as mean ± SEM (n=6/gp).** P<0.01, *** P<0.001, between groups.
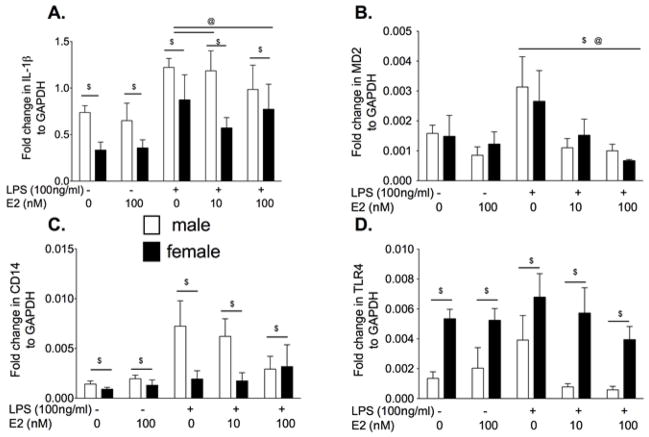
Experiment 5. Acute E2 ex vivo suppresses ex vivo pro-inflammatory responses in adult microglia
Neonatal microglia are considered immature and may be in a more activated state than adult microglia. Therefore, in order to test if E2 in vitro has the same effect on adult microglia, we isolated microglia from adult rats and stimulated them with E2 ex vivo. We stimulated hippocampal microglia from adult OVX females and males with 10 or 100 nM E2 ex vivo in the presence or absence of 100 ng/ml LPS. Previous studies have demonstrated that estradiol attenuates pro-inflammatory cytokines produced by adult microglia in vitro (Vegeto et al. 2001). Therefore, 100 ng/ml LPS was selected over 10 ng/ml LPS to ensure attenuation of pro-inflammatory cytokine mRNA could be detected. There was a significant suppressive effect of E2 on IL-1β mRNA (F4,44 = 5.22, P<0.01), as shown in Fig. 5. Male microglia produced significantly greater IL-1β mRNA than females (F1,4 = 5.22, P<0.05). In addition, male microglia had greater CD14 mRNA than females regardless of the drug administered (F1,4 = 6.90, P<0.05). There was a significant effect of LPS and E2 on MD2 mRNA (F1,4 = 0.01, P<0.01) with LPS increasing MD2 expression and E2 suppression the LPS mediated increase in MD2 expression. There was no significant effect of sex on MD2 mRNA (F4,47 = 0.0.1, P = 0.94). There was significantly greater TLR4 mRNA in the females compared to males (F1,4 = 27.93, P<0.0001) regardless of the dose of LPS or E2 (F4,48 = 2.18, P = 0.09).
Figure 5.
Rapidly isolated hippocampal microglia from adult male and OVX female rats were incubated with 0 or 100 ng/ml LPS and 0, 10 or 100 nM E2 for 3 h. IL-1β, MD2, CD14 and TLR4 mRNA from the cells was measured. There was significantly greater IL-1β and CD14 mRNA in males compared to females. E2 significantly suppressed the IL-1β mRNA response to LPS in female microglia. Females had significantly greater TLR4 mRNA than males. Data are expressed as mean ± SEM (n=6–8 per group). $ P<0.05 for main effect of sex, @ P<0.05 significant effect of LPS/E2 where indicated
Experiment 6. Chronic E2 in vivo potentiates ex vivo proinflammatory responses
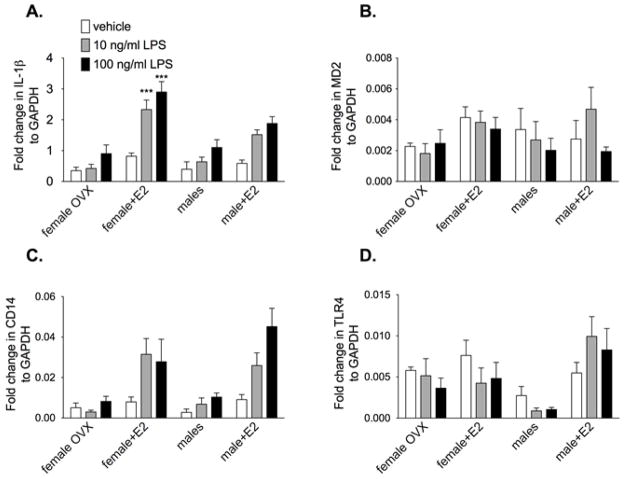
All previous experiments were done with E2 being administered with the LPS in vitro. The aim of this study was to identify if E2 in vivo altered the response of microglia stimulated with LPS ex vivo. We assessed the IL-1β, MD2, CD14 and TLR4 mRNA response from hippocampal microglia rapidly isolated from adult male rats and OVX female rats supplemented with and without E2. The cells were incubated with 0, 10 or 100 ng/ml LPS. There was significantly greater IL-1β mRNA (F6,84 = 2.48, P<0.05) in OVX females supplemented with E2 compared to groups not supplemented with E2 at both 10 ng/ml and 100 ng/ml LPS but not vehicle, as demonstrated in Fig. 6. In addition, females with E2 had significantly greater IL-1β mRNA than males with E2 at the 100 ng/ml LPS. LPS significantly increase CD14 mRNA (F3,6 = 6.98, P<0.0001) and E2 significantly increased CD14 mRNA (F3,76 = 6.09, P<0.0001). The males and females supplemented with E2 had the greatest CD14 mRNA expression. There was a no significant difference between groups on MD2 mRNA at any dose of LPS. There was significantly lower TLR4 mRNA in males compared to all other group (F2,8 = 12.91, P<0.0001) but no significant effect of LPS (F2,6 = 0.44, P=0.26).
Figure 6.
Rapidly isolated hippocampal microglia from adult male and OVX ± E2 female rats were incubated with 0, 10 or 100 ng/ml LPS for 3 h. IL-1β, MD2, CD14 and TLR4 mRNA was measured. LPS significantly increased IL-1β and CD14 mRNA in all groups. Supplemented E2 in the OVX females significantly increased IL-1β mRNA compared to all other groups at both doses of LPS. There was a significant LPS dose response on CD14 mRNA, with males and females supplemented with E2 having the greatest CD14 mRNA expression. Females and males with E2 had significantly greater TLR4 mRNA in response to LPS compared to those not supplemented with E2. Data are expressed as mean ± SEM (n=6–10 per group). *** P<0.001 compared to all other groups at the same dose of LPS.
Discussion
The present series of studies document, for the first time, that male neonatal microglia and astrocytes demonstrate a greater pro-inflammatory response to LPS than female cells. However, in the presence of E2, the LPS mediated pro-inflammatory response is attenuated in the male microglia but potentiated in the female microglia. Interestingly, E2 does not change the pro-inflammatory response of male or female neonatal astrocytes. Adult microglia from ovariectomized (OVX) females respond to LPS in a similar fashion to that of intact males. However, microglia from OVX adult females stimulated with LPS and E2 ex vivo displayed a suppressed IL-1β mRNA response compared to LPS alone. In contrast, microglia isolated from intact males are not influenced by E2 when stimulated with LPS ex vivo. When males and females are supplemented in vivo with high doses of E2, microglial IL-1β mRNA response is potentiated. Similar trends of potentiation by E2 occur in intact females but the changes are more subtle. CD14 mRNA is upregulated in the presence of LPS in both neonatal and adult microglia, regardless of sex. CD14 mRNA is also upregulated by LPS in astrocytes but it is not dependent on the dose of LPS or influenced by E2. MD2 mRNA throughout the experiments was variable with no clear pattern. LPS predominantly suppressed TLR4 mRNA except in males supplemented with E2, where no significant change in TLR4 mRNA was detected.
It has been identified that the morphology and gene expression of unstimulated microglia is dynamic throughout the early phases of development (Schwarz et al. 2011). In addition, neonatal microglia are not functionally mature (de Groot et al. 1992; Schwarz et al. 2011) and mixed sex microglia express a partially activated phenotype (Carson et al. 1998; Aloisi 1999). It is possible that this partially activated phenotype in neonatal cells may explain the apparent conflicting results seen presented here between neonatal and adult microglia cells. Purine receptors, known to induce microglial pro-inflammatory activation, also present with changes in basal expression between the sexes and age (Crain et al. 2009). It is possible that the extent of changes during development and the differences between the sexes can be extended to other key receptors and function of microglia such as TLR4. We have demonstrated that not only is the basal expression more pro-inflammatory in male neonatal microglia compared to female microglia (Schwarz et al. 2011), but also subsequent to immune stimulation neonatal microglia from male pups produced a greater pro-inflammatory response compared to neonatal female microglia. Therefore, caution may be required for future studies translating results obtained from neonatal glial cells to understanding microglial responses in adults in the context of sex differences and sex steroids.
An alternative microglia population can be obtained using a recognized method of microglia cell isolation from adult hippocampus. This method has been used previously to explore the sensitization of prior glucocorticoids on microglia stimulated with LPS ex vivo (Frank et al. 2006; Frank et al. 2010). This rapid microglial isolation approach allows us to manipulate the sex steroids both in vivo and in vitro in adult rats such that the combinations can be investigated. In vivo experiments provide information regarding system interactions but do not provide cell specificity. Here, we have demonstrated that while intact cycling females do display a potentiated pro-inflammatory response compared to OVX females, exogenous administration of high doses of E2 exaggerated the TLR4 mediated response. A caveat within the experimental design lies in the males being gonadally intact. It is possible that different effects would be identified in gonadectomized males.
Our results demonstrate that sexual dimorphic differences exist in microglia. Previous studies have shown that the timing and dosing of exogenous E2 may be critical (Craft et al. 2008). Immune cells including mouse peritoneal cells, and splenocytes from OVX females exposed to chronic in vivo E2 have potentiated pro-inflammatory responses, such as increased IL-1β mRNA, NFκB activation and nitric oxide following an LPS challenge compared to OVX females without E2 and to that of males (Calippe et al. 2008; Dai et al. 2008). Females with their ovaries removed behave and respond in a similar fashion to that of males (Craft et al. 2008). However, OVX females supplemented with acute E2 mimicking the cycling of normal females respond comparably to that of intact females (Craft et al. 2008). It remains to be elucidated in the studies presented here and previous studies whether it is the steady state of exogenous E2 or the dose of E2 that resulted in the potentiation of IL-1β mRNA. In addition, why the in vivo administration of E2 results in conflicting responses to that of in vitro E2 administration remains to be fully elucidated. It is possible that E2 in vivo exerts indirect effects on microglia through cell interactions resulting in a mounted pro-inflammatory response. Alternatively, the duration of exposure to E2 for a week compared to a few hours in vitro may demonstrate a biphasic response. The underlying mechanism for these apparent conflicting responses is worthy of further investigation.
Prior studies have demonstrated potent anti-inflammatory effects of E2 on mixed sex neonatal microglial responses in vitro (Vegeto et al. 2000; Vegeto et al. 2001). The effect of E2 on LPS-stimulated neonatal microglia with no sex specified, produced the same response as demonstrated here in the male neonatal microglia (Bruce-Keller et al. 2000; Vegeto et al. 2001). However, when separating out the sexes, we have discovered that the reverse occurs in female neonatal cells where pro-inflammatory responses are potentiated by E2. No prior studies have investigated the effect of E2 on microglia from male versus female adult rats stimulated ex vivo with LPS. Interestingly, in microglial cells from OVX females when stimulated ex vivo with E2, E2 suppressed IL-1β expression, while male microglia were less responsive to the E2 (Johnson and Sohrabji 2005). Therefore, it appears that E2 had the opposite effect on microglial cells rapidly isolated from female adult rats to that of female neonatal cells.
It is well recognized that regional specificity exists with neurons. But, it also appears that microglia may have regional specificity also. Some differences have been identified in morphology and density, proliferative rate and expression of immunoregulatory proteins. It is possible that the differences noted between microglia from neonates and adults arise from regional differences where cortical cells were used from the neonates and hippocampal cells from the adults. However, regional differences were not identified, at least in morphology in P60 rats between the cortex and the hippocampus (Schwarz et al. 2011).
TLR4 activation requires a complex formation of the TLR4 receptor, MD2, CD14 and LPS binding protein (McGettrick and O’Neill 2010). Most studies have investigated the effect of TLR4 activation on changes in TLR4 mRNA expression with changes in the co-receptors such as CD14 and MD2 being less studied. In the absence of any immune challenge, TLR4 surface receptor, but not CD14 cell surface expression, on peritoneal macrophages was decreased in OVX females compared to intact females (Rettew et al. 2009). Elevated CD14, as observed following chronic E2, makes TLR4 more sensitive to subsequent binding, thereby producing greater NFκB activation (Regen et al. 2011). When OVX mice were supplemented with chronic E2, CD14 and TLR4 cell surface expression significantly increased (Rettew et al. 2009). However, another study demonstrated that both CD14 and TLR4 protein were unaffected by E2 in the presence and absence of LPS in a macrophage RAW264.7 cell line (Vegeto et al. 2004). We have identified in both neonatal microglia and astrocytes that CD14 and TLR4 mRNA expression was not significantly affected by E2 but was by LPS. However, in adult rats, both males and females had greater CD14 mRNA when supplemented with E2. Also, the TLR4 mRNA expression, though decreased by LPS was potentiated by E2 in vivo, regardless of sex. But, in vitro E2 on adult microglia had no effect on TLR4 mRNA on females but decreased TLR4 mRNA in males. TLR4 mediated responses, as occur in infection, show intact females, both clinically and in rodent models, having a more robust peripheral inflammatory response than males (Kahlke et al. 2000; Marriott et al. 2006; Choudhry et al. 2007; Rettew et al. 2009; Rettew et al. 2010). This heightened pro-inflammatory response in females may be mediated by estrogens acting on estrogen receptors, increasing NFκB activation resulting in subsequent pro-inflammatory cytokine release (Soucy et al. 2005; Rettew et al. 2009; Calippe et al. 2010). This potentiation in TLR4 signaling may correspond with the elevated CD14 and TLR4 mRNA expression seen with E2 supplementation.
All cells within the CNS possess both estrogen receptor alpha (ERα) and beta (ERβ) (Sierra et al. 2008; Brown et al. 2010). In addition, a novel G-protein coupled receptor activated by estrogen, GPR30, has been identified on astrocytes, macrophages and microglia (Blasko et al. 2009; Bondar et al. 2009; Kuo et al. 2010). We did not see any effect of E2 on neonatal astrocytes. However, previous studies have found potent anti-inflammatory effect of E2 on astrocytes in vivo (Mong et al. 1996). Interestingly, ERα deletion in astrocytes using CRE-loxP demonstrated that the neuroprotective effects of E2 arose from ERα activation on astrocytes but not neurons (Spence et al. 2011). ERα on microglia was not investigated. E2 is anti-inflammatory in astrocytes in both in vivo and in vitro studies in both adult cells and neonatal cells (Chaban et al. 2004; Azcoitia et al. 2010; Cerciat et al. 2010; Kuo et al. 2010). However, regional brain differences have been identified in astrocyte response to LPS stimulation ex vivo (Kipp et al. 2008) and astrocytes are critically involved in early brain development (McCarthy et al. 2002). Therefore, it is possible that the neonatal astrocytes isolated from the cortex are non responsive to estradiol.
Activated microglia, and in certain diseases astrocytes, are hallmarks of neurodegenerative diseases and contribute to neuronal cell death (Cheepsunthorn et al. 2001; Perry et al. 2010). While the role of sexual dimorphisms and sex steroids in neuroinflammatory disorders remain far from clear, they do appear to influence the severity of the disease state. Further studies are required to elucidate the complex endocrine and neuroimmune interactions in neuroinflammatory diseases.
Figure 2.
Neonatal microglia were isolated from male and female P0/1 pups and incubated with vehicle or 10ng/ml LPS and 0, 10 nM or 100 nM 17-β estradiol (E2) for 4 h. IL-1β, MD2, CD14 and TLR4 mRNA were measured. E2 had a significant effect on IL-1β mRNA in both male and female microglia. E2 potentiated the IL-1β mRNA response to LPS in females but attenuated the IL-1β mRNA response in the male microglia. Similar trends were identified in CD14 mRNA expression. There was no significant effect on MD2 mRNA expression, but TLR4 mRNA expression was suppressed by LPS in both sexes. Data are expressed as mean ± SEM (n=9–12/gp).* P<0.05, ** P<0.01, *** P<0.001 between groups on post-hoc comparisons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achiron A, Gurevich M. Gender effects in relapsing-remitting multiple sclerosis: correlation between clinical variables and gene expression molecular pathways. J Neurol Sci. 2009;286:47–53. doi: 10.1016/j.jns.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv Exp Med Biol. 1999;468:123–133. doi: 10.1007/978-1-4615-4685-6_10. [DOI] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci. 2010;32:1995–2002. doi: 10.1111/j.1460-9568.2010.07516.x. [DOI] [PubMed] [Google Scholar]
- Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors alpha and beta. Endocrinology. 2010;151:4916–4925. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, Pipy B, Bayard F, Arnal JF, Guery JC, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, Guery JC, Bayard F, Arnal JF, Gourdy P. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180:7980–7988. [Google Scholar]
- Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158:896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 2010;58:93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response--Effect of gender differences. Injury. 2007;38:1382–1391. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Craft RM, Ulibarri C, Leitl MD, Sumner JE. Dose- and time-dependent estradiol modulation of morphine antinociception in adult female rats. Eur J Pain. 2008;12:472–479. doi: 10.1016/j.ejpain.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J Neuroinflamm. 2009;6:24. doi: 10.1186/1742-2094-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R, Phillips RA, Zhang Y, Khan D, Crasta O, Ahmed SA. Suppression of LPS-induced Interferon-gamma and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: a novel mechanism of immune modulation. Blood. 2008;112:4591–4597. doi: 10.1182/blood-2008-04-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot CJ, Huppes W, Sminia T, Kraal G, Dijkstra CD. Determination of the origin and nature of brain macrophages and microglial cells in mouse central nervous system, using non-radioactive in situ hybridization and immunoperoxidase techniques. Glia. 1992;6:301–309. doi: 10.1002/glia.440060408. [DOI] [PubMed] [Google Scholar]
- Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukocyte Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Johnson AB, Sohrabji F. Estrogen’s effects on central and circulating immune cells vary with reproductive age. Neurobiol Aging. 2005;26:1365–1374. doi: 10.1016/j.neurobiolaging.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol. 2008;511:599–609. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlke V, Angele MK, Ayala A, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH. Immune dysfunction following trauma-haemorrhage: influence of gender and age. Cytokine. 2000;12:69–77. doi: 10.1006/cyto.1999.0511. [DOI] [PubMed] [Google Scholar]
- Kipp M, Norkute A, Johann S, Lorenz L, Braun A, Hieble A, Gingele S, Pott F, Richter J, Beyer C. Brain-region-specific astroglial responses in vitro after LPS exposure. J Mol Neurosci. 2008;35:235–243. doi: 10.1007/s12031-008-9057-7. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, et al. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun. 2011;25:1408–1415. doi: 10.1016/j.bbi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott I, Bost KL, Huet-Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol. 2006;71:12–27. doi: 10.1016/j.jri.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Marta M. Toll-like receptors in multiple sclerosis mouse experimental models. Ann N Y Acad Sci. 2009;1173:458–462. doi: 10.1111/j.1749-6632.2009.04849.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Amateau SK, Mong JA. Steroid modulation of astrocytes in the neonatal brain: implications for adult reproductive function. Biol Reprod. 2002;67:691–698. doi: 10.1095/biolreprod.102.003251. [DOI] [PubMed] [Google Scholar]
- McGettrick AF, O’Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr Opin Immunol. 2010;22:20–27. doi: 10.1016/j.coi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- Morales I, Farias G, Maccioni RB. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation. 2010;17:202–204. doi: 10.1159/000258724. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Endotoxemia prevents the cerebral inflammatory wave induced by intraparenchymal lipopolysaccharide injection: role of glucocorticoids and CD14. J Immunol. 2002;169:3370–3381. doi: 10.4049/jimmunol.169.6.3370. [DOI] [PubMed] [Google Scholar]
- Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2011 doi: 10.1111/j.1365–2249.2010.04196.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature Rev. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Regen T, van Rossum D, Scheffel J, Kastriti ME, Revelo NH, Prinz M, Bruck W, Hanisch UK. CD14 and TRIF govern distinct responsiveness and responses in mouse microglial TLR4 challenges by structural variants of LPS. Brain Behav Immun. 2011;25:957–970. doi: 10.1016/j.bbi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150:3877–3884. doi: 10.1210/en.2009-0098. [DOI] [PubMed] [Google Scholar]
- Rettew JA, McCall SHt, Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;26:609–617. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Diff. 2011;2:7. doi: 10.1186/2042-6410-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2011 doi: 10.1111/j.1471–4159.2011.07630.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, et al. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Nat Ac Sci USA. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM, McDonald C. Ovarian hormone-induced short-latency maternal behavior in ovariectomized virgin Long-Evans rats. Hormones Behav. 1989;23:157–172. doi: 10.1016/0018-506x(89)90057-3. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Frontiers Aging Neurosci. 2010;2:22. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurological research. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Rubio M, Fernandez B. Sexual dimorphism in the distribution of glial fibrillary acidic protein in the supraoptic nucleus of the hamster. J Anat. 1991;178:79–82. [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147:2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Ghisletti S, Meda C, Etteri S, Belcredito S, Maggi A. Regulation of the lipopolysaccharide signal transduction pathway by 17beta-estradiol in macrophage cells. J Steroid Biochem Mol Biol. 2004;91:59–66. doi: 10.1016/j.jsbmb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Pollio G, Ciana P, Maggi A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35:1309–1316. doi: 10.1016/s0531-5565(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]