Abstract
We employed brain-behavior analyses to explore the relationship between performance on tasks measuring phonological awareness, pseudoword decoding, and rapid auditory processing (all predictors of reading (dis)ability) and brain organization for print and speech in beginning readers. For print-related activation, we observed a shared set of skill-correlated regions, including left hemisphere temporoparietal and occipitotemporal sites, as well as inferior frontal, visual, visual attention, and subcortical components. For speech-related activation, shared variance among reading skill measures was most prominently correlated with activation in left hemisphere inferior frontal gyrus and precuneus. Implications for brain-based models of literacy acquisition are discussed.
Keywords: fMRI, individual differences, reading, phonological awareness, decoding, rapid auditory processing, thalamus
Reading disability (RD) is a brain-based difficulty in acquiring fluent reading skills, typically associated with phonological deficits, which affects significant numbers of children (Lyon, Shaywitz, & Shaywitz, 2003). Depending, in part, on definitional criteria applied (i.e., achievement, discrepancy, or Response to Intervention) prevalence estimates for RD vary from 5–20% (Fletcher et al., 2007; Katustic et al., 2001; Shaywitz & Shaywitz, 2003). Definitional questions and related decisions about cut-offs for diagnosis are further complicated by evidence from epidemiological population-based studies that have suggested that RD symptomology reflects normally-distributed behavioral (Jorm et al., 1986; Shaywitz et al., 1992; Stevenson, 1988) and genetic (Plomin & Kovas, 2005) variation, and thus might be more accurately viewed as a dimensional, rather than a discrete, developmental disorder (Fletcher, 2009). The current study, which seeks to gain new insights into the neurobiology of RD, adopts the dimensional perspective and, with a large cohort of beginning readers (ranging from impaired to highly skilled), examines the relationship between variation on behavioral measures of reading-relevant skills and brain activation for print and speech. To motivate the specific tasks chosen here, we begin by considering findings from behavioral research on reading acquisition and on those cognitive skills that are most associated with variable outcomes in reading acquisition.
Behavioral research on typical and atypical reading development
The overwhelming majority of children with RD have pronounced problems in utilizing phonological structures of language and with phonological awareness (PA) in particular (Ball & Blachman, 1991; Vellutino et al., 2004). PA refers to the metalinguistic understanding that spoken words are made up of smaller units such as syllables and phonemes (Liberman et al., 1974). For pre-literate children and beginning readers, individual differences in PA ability (often measured by tasks that examine phoneme deletion or blending skills) are strongly predictive of word reading outcomes over the first few years of schooling (Ball & Blachman, 1991; Foorman et al., 1998; Johnson et al., 2009). Moreover, research indicates that the training of PA skills for high-risk pre-school children can have beneficial effects on subsequent reading trajectories (Byrne et al., 2008; Foorman et al., 1998; Torgesen et al., 1992). Findings of this type have been taken to suggest a causal relationship between PA and reading acquisition (Byrne et al., 2008), although it should be noted that PA is also influenced by reading skills during the first few years of reading instruction, which implies a complex reciprocal relationship between PA and reading (Castles & Coltheart, 2004).
The canonical view of how PA comes to impact the development of visual word recognition skills is that it instills in the learner a sensitivity to component features of spoken words, which creates the metacognitive foundation necessary for learning to associate visual representations (graphemes) with the phonemes they represent. The process of learning these relations has been referred to as mastering the alphabetic principle (Liberman et al., 1974; Liberman & Shankweiler, 1985). Deficits in PA and the consequent failure to master the alphabetic principle impede the development of efficient grapheme-to-phoneme decoding routines. These decoding skills are typically assessed by pseudoword reading tests. Pseudoword reading performance is highly correlated with PA and, like PA, is also strongly predictive of word reading outcomes in developing readers (Torgesen et al., 1999; Vellutino et al., 2004). These results all suggest that initial phonological processing deficits restrict the development of high quality lexical representations for print, where lexical quality depends upon adequate integration and binding of orthographic with phonological and semantic features (Harm & Seidenberg, 1999; Perfetti & Hart, 2001). Thus, PA and pseudoword decoding are key skills in reading acquisition, and the current study includes measures of these skills in order to uncover key brain-behavior relationships that exist across the continuum of early reading ability.
In seeking to uncover the cause(s) of PA deficits, many investigators have focused on those neurocognitive systems that encode phonological representations (Elbro, 1996; Fowler, 1991; Goswami & Ziegler, 2006) on the assumption that these deficits are specific to this component of language. Others, motivated by the idea that phonological processing deficits might be reducible to abnormalities in basic sensory or sensorimotor processing, have used tasks that measure visual motion processing deficits (Demb et al., 1998; Stein & Walsh, 1997), or auditory processing deficits, at both shorter (Tallal, 1980; Ahissar et al., 2004) and longer (Goswami et al., 2010) time scales; differences between typically developing (TD) and RD readers have been reported for each of these tasks (although some researchers argue that auditory and visual deficits may be present only in subsets of RD children; cf., Ramus, White, & Frith, 2006). Sperling and colleagues (2005, 2006) have argued that observed deficits in performance on visual or auditory sensory tasks might arise from attentional mechanisms that impact signal-noise discrimination, resulting in what are termed “noise exclusion” deficits (see Ziegler et al., 2009 for a similar proposal). At present, the question of whether phonological deficits are language specific or not is still a topic of some debate (Castles, McLean & McArthur, 2010; Ramus et al., 2006; Snowling & Hulme, 2011). The current study employed exemplars of both language and non-language predictor tasks that have been linked to RD (see below for details) to map out important brain-behavior relations in beginning readers.
Brain research on typical and atypical reading development
Much of what is known about systems-level neurobiological differences that discriminate typically from atypically developing readers has come from neuroimaging studies of older children or adults who have either mastered, or failed to master, basic word reading skills (see Pugh et al., 2010, for a review). Functional neuroimaging studies have consistently shown differences between TD and RD readers at those left hemisphere (LH) regions that compose a distributed circuitry for word reading (Brunswick et al., 1999; Meyler et al., 2007; Pugh et al., 2000a; Rumsey et al., 1997; Salmelin et al., 1996; Shaywitz et al., 1998; 2002, Temple et al., 2003). The most common finding is that RD readers tend to under-activate LH posterior areas, especially temporoparietal (TP) and occipitotemporal (OT) networks. This disruption is also evinced as reduced functional connectivity among these regions (Hampson et al., 2004; Horwitz et al., 1998; Pugh et al., 2000b). In addition, RD readers often, but do not always, show evidence of two apparently compensatory responses to their LH posterior dysfunction: an increased functional role for right hemisphere (RH) posterior regions (Sarkari et al., 2002; Shaywitz et al., 1998; Simos et al., 2002) and increased bi-hemispheric frontal lobe activation (Brunswick et al., 1999; Shaywitz et al., 1998; 2002).
Structural neuroimaging studies have identified coarse-grained anatomic differences, such as reduced grey matter volumes in RD, at those regions with reported functional anomalies, including TP (Brambati et al., 2004; Brown & O’Regan, 2001) and OT (Kronbichler et al., 2008; Silani et al., 2005). Diffusion tensor imaging studies also indicate that individuals with RD have anomalous white matter tracts connecting LH reading-relevant cortical networks, possibly reflecting reduced myelination in RD (Beaulieu et al., 2005; Klingberg et al., 2000; Niogi & McCandliss, 2006).
Although extant findings with older children or adults reveal a strong association between reading abilities and the structural and functional integrity of LH posterior cortical systems (especially TP and OT) that support word reading, only a few studies to date have examined these relationships in emergent readers. In one such study, Raschle and colleagues (2011) used structural imaging methods and identified reduced gray matter volume at both TP and OT regions in high-risk kindergarten pre-readers; because these anatomical differences predate reading experience the authors suggest that neurobiological anomalies may be causally related to later reading difficulties rather than a result of them. Functional activation differences at TP and OT sites have also been observed in low and high-risk kindergarten children (Specht et al., 2009). In a longitudinal study of children (from 7–12 years of age at onset) of varying reading levels, Ben-Shachar and colleagues (2011) report that a region at the left OT sulcus develops increasing specialization for words over the first few years of reading instruction. Moreover, a recent study by Blau et al. (2010) examining high-risk beginning readers (age 6) who were undergoing a training program that reinforced grapheme-to-phoneme mapping skills showed that activation of the left OT depended on these trained skills (see Brem et al., 2010 for similar findings). In another recent study Yamada et al. (2011) examined print processing during a one-back task for letters versus false font stimuli in typically developing and high-risk kindergarten children at the beginning and middle of the school year. High-risk children, relative to typically developing children, showed reduced LH parietal activation at the first session and greater frontal lobe and RH involvement at the second session, indicating that these regions may play an important role in discriminating TD from RD learners at early stages of reading development. Thus, extant studies of young children reinforce the importance of those same LH posterior networks that come to support skilled word reading in older children and adults.
However, it seems reasonable to speculate that the early learning circuitry must include a more widely distributed set of cortical and subcortical networks to support the difficult work of learning to bind orthographic with phonological and semantic codes as children cope with the cognitive and linguistic demands of learning of becoming fluent decoders. With respect to this learning circuitry, we (Pugh et al., 2000a; 2010) have put forward a general neurodevelopmental hypothesis which posits that distributed LH and RH temporal and parietal (dorsal) networks, operating in conjunction with frontal lobe networks (especially inferior frontal gyrus, IFG), are doing the computational work of initially developing PA (Katzir et al., 2005) and then encoding relations among orthographic, phonological, morphological, and semantic features of words. Over time this relational knowledge will shape the computational organization (Dehaene & Cohen, 2011) and connectivity (Price & Devlin, 2011) of LH ventral cortex (especially the LH OT region, which includes the putative Visual Word Form Area, VWFA) that will come to support fluent word recognition in older readers (McCandliss, Cohen, & Dehaene, 2003; Pugh et al., 2000a). Therefore, for beginning readers, we anticipate that along with TP and OT, an array of bilateral posterior and anterior cortical networks (c.f., Shaywitz et al., 2002; Turkeltaub et al., 2003) will show strong associations with individual differences in reading and reading-relevant cognitive skills.
More generally, learning to decode printed words fluently and automatically is a prototypical example of cognitive skill acquisition or expertise learning. This would suggest that, along with those cortical regions described above, cortical-basal ganglia-thalamic pathways implicated in procedural learning (Ullman & Pierpoint, 2005) would also discriminate TD and RD beginning readers as they learn to automate word recognition. Involvement of the basal ganglia or thalamus in neuroimaging studies of word reading (Binder et al., 2005; Preston et al., 2010, Seghier et al., 2009, Turkeltaub et al., 2002) and print word learning (Pugh et al., 2008) have been reported; moreover, lesion studies also suggest that damage to subcortical foci, including the basal ganglia and thalamus, can negatively impact language and reading performance (Crosson, 1999). A role for thalamic nuclei in the development of reading (and their variable functioning in good and poor readers) might also be expected given post-mortem studies of RD adults that found abnormal cellular organization in the lateral geniculate nucleus (LGN) and medial geniculate nucleus (MGN) of the thalamus (Galaburda et al., 1985, 2006). Finally, greater cerebellar involvement in emergent readers might be important for early development given the association of this region with skill acquisition (Nicolson, Fawcett, & Dean. 2001; Shankweiler et al., 2008). In sum, along with LH posterior networks including TP and OT, we hypothesize that the learning circuitry will consist of a widely distributed array of cortical and subcortical networks operating in support of mastery of word decoding.
Present study
The aim of the current study is to gain a deeper understanding of those brain pathways that mediate individual differences in learning to read. A primary focus of the study is to explore whether individual differences in reading-related skills among early readers will be associated not only with relative involvement of well-established LH posterior regions such as OT and TP, but also with more broadly distributed bi-hemispheric networks and with subcortical regions associated with general skill learning. A second focus is on the relations among varied predictor tasks. Given that sensory and phonological deficits correlate with one another and with reading, the current study uses indices of both in relation to each other and to the neurobiological pathways for reading. Although these analyses will not adjudicate debates about causality (for which longitudinal data are required; cf. Johnson et al., 2009), examining relations among diverse predictors of reading is important for understanding the extent to which they influence the developing reading circuitry via shared or distinct brain pathways (Tallal & Gaab, 2006), and this is an important first step toward causal models.
In summary, we employ a brain-behavior analysis framework to identify those cortical and subcortical networks that best discriminate children with better or worse reading readiness skills (assessed with multiple measures). Although differences between some brain regions are expected (e.g., LH TP and OT), the precise topology of this skill-correlated circuitry is still largely unknown at present given the relative paucity of studies with young emergent readers.
Methods
Participants
We examined a cohort of beginning readers, whose reading abilities range along a continuum from conventionally RD to superior readers. Data was taken from the initial testing visits of an ongoing longitudinal study that includes multiple behavioral measures and fMRI data. Sixty-two speakers of English (37 males; 25 females) participated in the experiment in exchange for payment. Participants were recruited through the Yale Reading Center. All participants had normal or corrected-to-normal vision, normal hearing, no history of neurological impairment or psychiatric disorder, and a full-scale IQ of at least 80. Participants ranged in age from 5.47 to 8.89 years at time of testing (mean: 7.7). Informed assent was obtained in compliance with Yale University’s human subjects protection guidelines. Examination of the scores on our reading battery reveals that approximately 16% (10 of 62) of the sample had averaged standard scores of 90 or less (at or below the 25th percentile) on a composite TOWRE score (based on word and pseudoword reading subtests), which falls into conventional RD range, and five children had composite scores < 95, including one participant who had a previous diagnosis of RD based on clinical evaluation.
Behavioral Testing
Prior to functional imaging, participants completed a behavioral battery to characterize their reading, language, and general cognitive skills. Measures were obtained from five standardized test batteries: the Woodcock-Johnson III Tests of Achievement (WJ; Woodcock, McGrew, & Mather, 2001); the Tests of Word Reading Efficiency (TOWRE; Torgesen, Wagner & Rashotte, 1999); the Comprehensive Test of Phonological Processing (CTOPP; Wagner, Torgesen, & Rashotte, 1999); the Peabody Picture Vocabulary Test (PPVT III; Dunn & Dunn, 1997); and the Wechsler Abbreviated Scale of Intelligence (WASI; The Psychological Corporation, 1999). For the purposes of the current study we were interested in the phonological measures: a PA task (Elision from CTOPP) and a timed measure of pseudoword decoding efficiency (PDE from the TOWRE).
We also included a Temporal Order Judgment (TOJ) task developed by Tallal and colleagues (Tallal et al., 1980). This task measures perception of perception of rapid successions of tones and is typically interpreted as an index of auditory sensory processing. As discussed above, the inclusion of the TOJ task in the current analysis is motivated by the finding that performance on this non-language task is often correlated with phonological and reading skills (Tallal, 1980; Tallal & Gaab, 2006; but see Ramus, 2003 for data suggesting that TOJ deficits hold only for subsets of RD readers). Participants are trained to indicate via a button press whether they hear a high tone (305 Hz) or a low tone (100 Hz). Participants are then presented 75 msec tone pairs, separated by a 425 msec interstimulus interval (ISI), and asked to press buttons to indicate the correct order of the two tones (low-low; low-high; high-low; high-high). During these training trials, feedback is provided to indicate a correct response (smiley face) or an incorrect response (sad face). After training, participants receive a rapid perception test during which tone pairs are presented without feedback at each of six ISIs (8, 15, 30, 60, 150 or 305 msec).
Although these three tasks (PA, PDE, TOJ) make very different demands on cognitive, language, and metacognitive processes, all have been linked to the cognitive phenotype of RD.
Summary statistics for performance on the standardized tests and TOJ task are provided in Table 1. Note that we present age-adjusted standard scores for describing the sample, which are easily compared to other samples; however, raw scores are used in all analyses because our interest is in correlations with skill, not skill relative to children of the same age.
Table 1.
Descriptive statistics for the sample (N=62) on age, standardized behavioral measures, temporal order judgment, and fMRI task performance.
| Mean | SD | Range | |
|---|---|---|---|
| Age | 7.70 | 0.69 | 5.47–8.89 |
|
| |||
| Age-normed standard scores | |||
|
| |||
| CTOPP Elision | 12.08 | 3.17 | 5–18 |
| TOWRE Sight Word Efficiency | 109.79 | 15.46 | 81–145 |
| TOWRE Phonemic Decoding Efficiency | 106.33 | 15.21 | 79–145 |
| WJ-III Letter-Word ID | 114.98 | 15.48 | 88–142 |
| WJ-III Word Attack | 112.85 | 12.40 | 86–138 |
| WJ-III Passage Comprehension | 108.32 | 13.18 | 87–134 |
| PPVT | 113.90 | 13.33 | 84–154 |
| WASI Performance IQ | 111.43 | 17.45 | 80–151 |
|
| |||
| Temporal Order Judgment | |||
|
| |||
| Accuracy | 66.00 | 22.80 | 16.67–100 |
| Reaction Time | 1288 | 343 | 742–2124 |
|
| |||
| fMRI Task Performance | |||
|
| |||
| Sensitivity (A-prime) | 0.88 | 0.11 | .48–.99 |
| Bias (B″D) | 0.15 | 0.24 | −.64–.70 |
| Speech Accuracy | 85.16 | 10.23 | 54–99 |
| Print Accuracy | 83.64 | 14.26 | 43–99 |
Notes: Standard scores mean = 100, SD = 15 except for CTOPP, for which mean = 10, SD = 3. CTOPP = Comprehensive Test of Phonological Processing; TOWRE = Tests of Word Reading Efficiency; WJ-III = Woodcock-Johnson Tests of Achievement; PPVT-III = Peabody Picture Vocabulary Test; WASI = Wechsler Abbreviated Scales of Intelligence.
fMRI paradigm
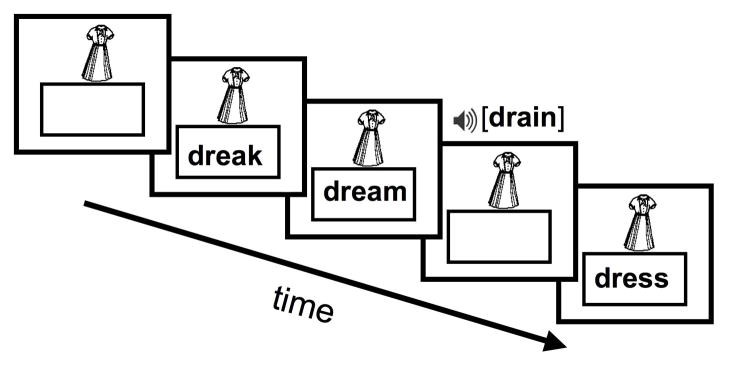
We employed a cue-target identity task with an event-related protocol that required a match/mismatch judgment on each trial via a button press (see Frost et al., 2009 and Preston et al., 2010 for recent studies using this paradigm; see Figure 1 for a schematic of the task). Participants viewed a picture of an animal or common object (e.g., a picture of a dress) in the upper central portion of the display with an empty box beneath, followed by a series of trials on which word and pseudowords were presented to eye or ear. Each picture remained on the screen through approximately a quarter of the run after which it was replaced by another picture. Prior to the experiment participants were shown the pictures to ensure familiarity and name agreement. Pictures were initially presented on the screen alone, allowing sufficient time to model separately the evoked responses to processing of the picture cues and for participants to encode the picture for comparison to the stimuli on subsequent trials. The majority of trials (80%) consisted of mismatches between the picture and print or speech target; only data from mismatch trials were included in analyses so that brain responses were compared on a common mismatch decision. Conditions considered in this report are auditory and printed monosyllabic words (e.g., DREAM) and pseudowords (e.g. DREAK). Print stimuli were displayed in the box beneath the picture cue in 18-point Verdana font and speech stimuli were presented through MR compatible headphones. Stimulus presentation and response collection was controlled by a PC running E-prime 1.2 (Psychology Software Tools Inc., Pittsburgh, PA). Participants completed training in a mock scanner prior to fMRI in which they were played examples of the task and scanner noise while receiving feedback on movement.
Figure 1.
Schematic of the fMRI paradigm. A picture cue is displayed and participants make a series of identity match/mismatch judgments to print and speech tokens. Figure is reprinted from Frost et al., 2009 with permission.
fMRI Acquisition and Analysis
fMRI data were acquired using a Siemens 1.5 Tesla Sonata scanner at the Yale School of Medicine. Participants’ heads were immobilized in a circularly polarized head coil with a neck support, foam wedges, and a restraining band drawn across the forehead. Prior to imaging, 20 axial-oblique anatomic images (TE (echo time) 11 ms; TR (repetition time) 420 ms; FOV (field of view) 20 × 20 cm; 6 mm slice thickness, no gap; 256 × 256 × 1 NEX (number of excitations)) were prescribed parallel to the intercommissural line. Activation images were collected using single shot, gradient echo, echo-planar acquisitions (FA (flip angle) 80 degrees; TE 50 ms; TR 2000 ms; FOV 20 × 20 cm; 6 mm slice thickness, no gap; 64 × 64 × 1 NEX) at the same 20 slice locations used for anatomic images. High-resolution anatomical images were gathered for 3D reconstruction (sagittal MPRAGE acquisition, FA, 8 degrees; TE, 3.65 ms; TR, 2000 ms; FOV, 25.6 × 25.6 cm; 1 mm slice thickness, no gap; 256 × 256 × 1 NEX; 160 slices total). Trials were presented at jittered intertrial intervals (ITIs) of 4, 5, 6, and 7s durations with occasional longer ITIs (i.e., null trials); visual targets remained onscreen for 2s. A maximum of ten imaging runs of 3:46 each (3:38 plus 8s for image stabilization) was obtained for each participant (median = 8), with all conditions represented in each run.
Data analysis was performed using software written in MATLAB (Mathworks, Natick, MA). Images were sinc-interpolated to correct for slice acquisition time, motion-corrected with SPM-99 (Friston et al., 1995) and spatially smoothed with a 5.15 mm FWHM Gaussian filter. Images exceeding 2 mm displacement or 2 degrees rotation from the first image in the entire functional series were discarded, as well as images that exceeded an image-to-image change of 1 mm displacement or 1 mm rotation. Using this criteria all subjects had usable data from at least 6 functional runs. Single-subject event-related analysis used a regression-based method for direct estimation of the hemodynamic response for each trial type, at each voxel separately, without prior specification of a reference function (Miezin et al., 2000). Parameters from this regression model were used to uniquely estimate the mean response for each condition from −3 to +15 seconds relative to stimulus onset. Subject activation maps were created using the regression estimates to calculate the mean difference in activity for an activation period (3–8 seconds post trial onset) relative to a baseline period (0–3 seconds prior to trial onset) for each condition. Linear contrasts for effects of interest were applied to these regression estimates to obtain contrast images for each participant. Prior to across-subjects analysis, a nonlinear transformation was obtained for each participant using BioImage Suite (www.bioimagesuite.org), mapping between the subject-space high-resolution anatomic and the standard brain space defined by the Montreal Neurological Institute (MNI) “Colin” brain (www.bic.mni.mcgill.ca), and this transformation was applied to the single-subject activation maps, with trilinear interpolation, into 2 mm isotropic MNI space.
Results
fMRI Task Performance
Given the 80–20 ratio of mismatch to match responses, non-parametric measures of sensitivity (A′) and bias (B″D) were calculated to assess performance and reported in Table 1, along with accuracy levels for speech and print targets. Potential values for A′ range from 0 (no sensitivity) to 1 (perfect sensitivity) and for B″D from −1 (complete Match bias) to +1 (complete Mismatch bias); thus the observed values of 0.88 and 0.15 respectively show high sensitivity and little bias, indicating that the task was developmentally appropriate for our participants.
Behavioral Intercorrelations and Word Reading Predictors
As discussed earlier, performance on measures of PA, pseudoword reading, and rapid auditory processing are typically correlated, both with each other and with word reading skill. As shown in Table 2, this was true in the current sample: Pearson (r) correlations among raw scores for our measures of PA (CTOPP Elision), timed printed pseudoword decoding (PDE), and rapid auditory processing (TOJ1) were all significant, and the measures were all correlated with our timed measure of word reading skill (TOWRE Sight Word Efficiency) (r range from 0.387 to 0.824; below the diagonal). This finding of mid-to-high level intercorrelations in performance suggests the tasks are providing partially redundant information about the reading-related skills. This motivates analyses discussed below examining both shared and unshared influences on reading performance and brain activation during functional scanning. Because our primary analyses utilize raw scores and because general maturational influences are of interest to many language researchers Table 2 also shows the correlation values of each skill measure with participant’s age at time of testing. Although the correlation values are quite weak (likely a consequence of the intentionally restricted age range in the study), we also considered whether variation in age might be an important determinant of the intercorrelations in task performance. As shown in Table 2 (above the diagonal), examination of semi-partial correlations in which variance linearly related to age was regressed out of each task variable did not qualitatively change the correlational structure among the tasks; therefore age has little impact on the shared influences among performance measures in this sample.
Table 2.
Correlations among raw scores for measures of PA (CTOPP Elision), timed print decoding (TOWRE Phonemic Decoding Efficiency), sensorimotor processing (Temporal Order Judgment accuracy), and time word reading (TOWRE Sight Word Efficiency) and their correlation with age at time of testing. Values above the diagonal are partial correlations with the linear effects of age removed from each measure.
| TOWRE PDE | CTOPP ELISION | TOJ ACCURACY | TOWRE SWE | |
|---|---|---|---|---|
| TOWRE PDE | ---- | .618** | .443** | .813** |
| CTOPP ELISION | .637** | ---- | .374* | .543** |
| TOJ ACCURACY | .485** | .416** | ---- | .311‡ |
| TOWRE SWE | .824** | .570** | .387* | ---- |
|
| ||||
| AGE | .237 | .212 | .342* | .323‡ |
p<.05;
p<.01;
p<.001
Brain-behavior analytic approach
Given the observed intercorrelations among PA, PDE, and TOJ, we used Principal Components Analysis (PCA) to reduce the dimensionality of our data and gain a better understanding of how the shared variation relates to individual differences in brain activation during our functional tasks.2 Raw scores from the all three tasks were entered into PCA using SPSS (version 18.0.3) with Varimax rotation to force orthogonal factors3. Results of the PCA found a single underlying dimension of common variation among the three variables (identified based on eigenvalues > 1). This principal component accounted for 67% (eigenvalue = 2.01) of the total variance among the three input variables. Loadings for TOJ, PDE, and PA were within the same range [.75, .87, .84, respectively], indicating that each of the three variables contributed similarly to the component.
Principal component scores were extracted for each individual and then correlated with TOWRE sight word reading scores, showing a strong and significant relationship (r = .733, p<.001), thus confirming that this shared variance is relevant to reading performance in this sample. Next, the principal component scores were used as predictors of whole brain activation by computing the Pearson correlation coefficient across subjects, between each subject’s principal component score and the regression parameter estimate for print processing (combination of printed words and pseudowords) and speech processing (the combination of spoken words and pseudowords) at each voxel separately (Pugh et al., 1997). Finally, we used partial correlation techniques to examine dimensions of individual difference that are uniquely associated with each measure (PA, PDE, TOJ). When the unique variance in a measure was significantly related to behavioral word reading skill, we conducted brain-behavior analyses using residual scores to identify brain pathways that might mediate this unique influence.
fMRI analyses
Brain-behavior analyses for print
Analyses of baseline activation that measure activation “on average” indicate that the cue-target identity task produces robust activations in well-established pathways for both print and speech (see Supplementary Table 1); however our primary analyses targeted those brain pathways where print (or speech) related activation varies as function of individual differences in component scores, reflecting the underlying dimension of common variance among PA, PDE, and TOJ. Figure 2 shows the results of the primary brain-behavior analysis for print. PC scores were positively correlated with print-related activation levels in bilateral posterior thalamus (implicating the pulvinar with greater LH than RH involvement), LH Brodmann Areas 41 and 22 within the superior temporal gyrus (STG), LH visual cortex (cuneus), LH OT/fusiform gyrus extending into LH parahippocampal gyrus, LH angular gyrus (AG), LH inferior frontal gyrus (IFG), RH inferior and middle temporal gyri (ITG/MTG), precuneus, anterior cingulate, bilateral prefrontal cortex (SFC), and RH inferior parietal lobule (IPL) (see Table 3 for full list of regions sorted by significance levels). To illustrate that the PC score reflects shared variance across the three predictor tasks, we present the scatterplots of the simple correlations between print-related activation and TOJ, PA, and PDE in Figure 3. This figure shows correlations in three representative regions: left superior temporal gyrus, left fusiform gyrus, and left thalamus, thus reinforcing the implication from the PCA of common influences.
Figure 2.
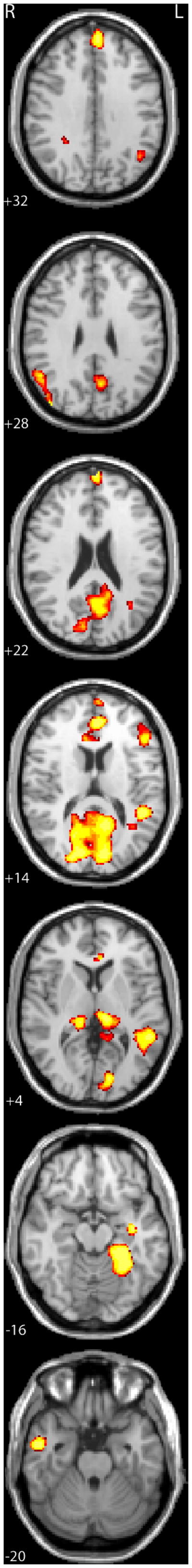
Areas showing a correlation between activation levels for print (words and pseudowords) and component scores reflecting shared variance among behavioral predictors of reading skill. Images are presented in radiological convention with the left hemisphere on the right side of the images at a univariate threshold of p < 0.01, corrected for mapwise false discovery rate (FDR; Genovese et al. 2002) with a cluster threshold of 10 contiguous significant voxels. Numbers of the lower left side of each slice corresponds to the position along the Z-axis in MNI space.
Table 3.
Regions showing a correlation between the component scores and activation levels for print (words and pseudowords).
| Region | volume (mm3) | MNI coordinates (peak voxel) | |||
|---|---|---|---|---|---|
| X | Y | Z | r-value | ||
| L Superior Temporal Gyrus | 7776 | −56 | −44 | 7 | 0.556 |
| R Inferior Parietal Lobule | 1560 | 40 | −88 | 24 | 0.513 |
| R Inferior Temporal Gyrus | 936 | 48 | −10 | −44 | 0.512 |
| Cuneus/Precuneus | 23128 | −14 | −86 | 9 | 0.504 |
| L Fusiform/Parahippocampal Gyrus | 4120 | −30 | −50 | −18 | 0.504 |
| L Thalamus | 1856 | −12 | −26 | 8 | 0.483 |
| Anterior Cingulate | 3752 | −2 | 66 | 20 | 0.473 |
| R Middle/Inferior Temporal Gyrus | 2384 | 54 | −4 | −28 | 0.466 |
| R Lingual Gyrus | 1776 | 12 | −62 | −3 | 0.463 |
| L Superior Frontal Gyrus | 1312 | −4 | 52 | 34 | 0.462 |
| Posterior Cingulate | 2240 | −14 | −52 | 10 | 0.461 |
| R Thalamus | 1632 | 18 | −32 | 2 | 0.442 |
| L Parahippocampal Gyrus | 256 | −36 | −12 | −16 | 0.435 |
| L anterior Superior Temporal Gyrus | 592 | −48 | −2 | −6 | 0.418 |
| Superior Frontal Gyrus | 936 | 22 | 54 | 18 | 0.405 |
| L Inferior Frontal Gyrus | 856 | −48 | 28 | 15 | 0.402 |
| Anterior Cingulate | 376 | −6 | 34 | 2 | 0.401 |
| R Fusiform Gyrus | 256 | 36 | −52 | −18 | 0.381 |
| L Angular Gyrus | 264 | −44 | −62 | 32 | 0.366 |
| Precuneus | 288 | −10 | −50 | 38 | 0.352 |
r-values greater than +/− 0.33 significant at p< 0.01
r-values values greater than +/− 0.41 significant at p < 0.001.
Figure 3.
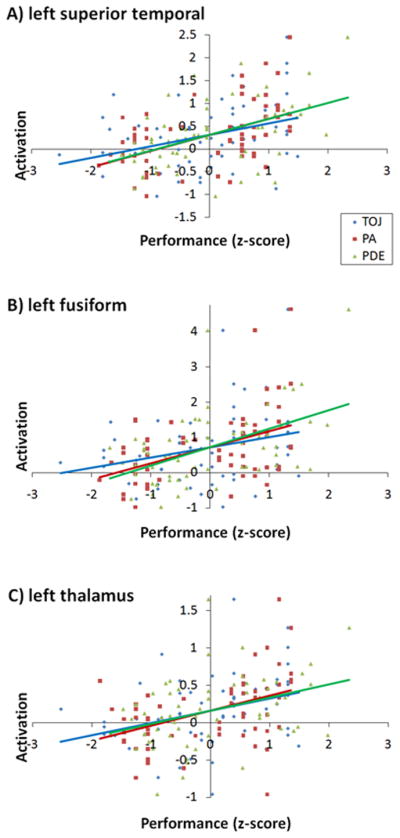
Scatterplots and regression lines showing correlations of rapid temporal order judgment (blue), elision (red), and phonemic decoding efficiency (green) measures with standardized activation values for print in the left superior temporal gyrus (A), left fusiform gyrus (B), and left thalamus (C).
We also conducted analyses to determine whether the residuals of each behavioral measure were associated with unique variance in TOWRE sight word reading. The residuals of PDE were associated with unique variance in TOWRE sight word reading scores (r=.575, p<.001); however, neither PA nor TOJ residuals showed a unique relationship with reading (both p>.6). Thus, individual regressions of PA and TOJ residual scores onto brain activation data are of little interest here, given the goal of explicating brain-behavior relationships that influence reading. For the PDE score, two RH foci (thalamus and fusiform gyrus) and a few small foci in LH fusiform and IPL were associated with this measure. With the exception of RH fusiform region, these findings appear to reflect increased spatial extent for areas seen in the PCA analyses and may simply reflect the higher strength of correlation of PDE (the only predictor task involving print tokens) relative to PA or TOJ on reading scores in revealing the same general brain pathways. In short, these findings suggest shared as opposed to unique influences of these varied skill measures on reading and brain.
Brain-behavior analyses for speech
As a secondary question in this investigation, we also explored the relation of this PC measure on individual differences in activation for speech. As shown in Table 4, foci in LH IFG and the precuneus (extending to posterior cingulate gyrus) were most prominent, with additional, smaller foci observed in aspects of STG and RH thalamus and RH fusiform gyrus. The strong IFG association may suggest a feedback effect of reading-related skills on listening (Castro-Caldas et al., 1998); in short, children with better reading-related skills appear to engage attentional and speech motor systems more than those with poor skills when processing simple spoken words and pseudowords.
Table 4.
Regions showing a correlation between the component scores and activation levels for speech (words and pseudowords).
| Region | volume (mm3) | MNI coordinates (peak voxel) | |||
|---|---|---|---|---|---|
| X | Y | Z | r-value | ||
| L Inferior Frontal Gyrus | 3576 | −38 | 24 | 17 | 0.475 |
| Precuneus | 1608 | 20 | −54 | 36 | 0.458 |
| Posterior Cingulate/Precuneus | 7744 | −6 | −58 | 26 | 0.438 |
| R Medial occipito-temporal sulcus | 696 | 40 | −38 | −12 | 0.435 |
| R Thalamus | 1392 | 22 | −28 | 2 | 0.428 |
| R anterior Middle Temporal Gyrus | 448 | 40 | 4 | −32 | 0.397 |
| L Paracentral Lobule | 496 | −8 | −44 | 54 | 0.396 |
| R Precentral Gyrus | 408 | 16 | −23 | 60 | 0.39 |
| L Superior Temporal Gyrus | 384 | −62 | −48 | 11 | 0.382 |
| R Supramarginal Gyrus | 232 | 54 | −54 | 24 | 0.359 |
r-values greater than +/− 0.33 significant at p< 0.01
r-values values greater than +/− 0.41 significant at p < 0.001.
Discussion
The primary goal of the current study was to identify brain pathways that are most strongly associated with individual differences on measures of three reading-relevant skills (phonological awareness, decoding, and rapid auditory processing abilities) at the point in time when the mature circuitry that will come to support fluent reading is, to a large extent, still coming online. As anticipated, relative to findings from studies of older cohorts of good and poor readers (where brain-behavior analyses implicate LH TP and OT), the skilled-correlated circuitry in beginning readers appears to be more broadly distributed. We regressed a principal component score that reflects shared variance among PA, PDE, and TOJ tasks on activation for print tokens. This brain-behavior analysis revealed strong positive correlations4 between variation on this cognitive composite score, and neural responses in LH TP, OT, and IFG, along with visual cortex (cuneus), precuneus, posterior thalamus (centered in pulvinar), prefrontal cortex, and RH parietal and temporal networks. Analysis of residual scores for TOJ and PA revealed no unique contributions to reading above and beyond those shared with one another. At a general level, these results are consistent with the argument that, at least for children in this age range, individual differences in diverse skill measures on reading and on the neural pathways that support reading is through shared neurocognitive mechanisms. Finally, although our primary focus in this study was on examining brain-behavior relations in the early reading circuitry, when PC scores were correlated with activation for auditory tokens, the most prominent associations were seen at LH IFG and precuneus. We consider the implications of these findings in the following sections.
Along with well-etsablished LH regions, our PCA analyses identified several RH regions that are positively correlated with reading skill, including ITG/MTG and parietal loci. RH contributions to reading are strong early on, and have been shown to diminish with age and experience in TD but not RD cohorts (Shaywitz et al., 2002; see also Turkeltaub et al., 2003 for similar findings). The current findings reinforce the idea that the initial learning circuitry for TD children contains important but as yet unspecified contributions from RH networks. We speculate that the RH ITG/MTG reflect semantic contributions and RH IPL, along with the anterior cingulate and prefrontal networks, reflects greater attentional and cognitively controlled processing in emergent readers as they progress toward increased fluency and automaticity, with corresponding increases in LH OT specialization for processing of printed langauge.
The strong association between PC scores and activation of posterior thalamus (including a strong foci in pulvinar) implicates subcortical contributions to early reading. Although not often focused on in developmental research on reading, there are many findings that make the current finding unsurprising. A role for the thalamus in online print learning with older readers has also been observed in our lab (Pugh et al., 2008). In that study we used fMRI to examine online repetition learning for printed tokens in TD and RD adolsecents and found that, across repeated exposures, changes in brain activation in LH thalamus discriminated TD from RD learners. Gaab et al. (2007) implicated the same pulvinar coordinates found here as showing reduced activation in older RD, relative to TD, children performing a variant of the TOJ task; moreover, they found increased activation in this same region for RD children on this task after an intervention (see also Temple et al., 2000 for similar results with adult RD learners).
At the structural level of analysis, early histology studies reported abnormal magnocelluar organization of the lateral and medial geniculate nuclei (LGN, MGN) of the thalamus, which is argued to impact the quality of both visual and auditory sensorimotor processing (Stein & Walsh, 1997; Galaburda et al., 1985). The current brain-behavior analysis implicates a large swath of thalamus, consisting of multiple functionally disasociable components, and does not provide the granularity necessary to assess relative involvement of specific nuclei; clearly further studies are needed more precisely determine whether, for example, LGN or MGN loci are differentially implicated in this brain-behavior relationship. The strong involvement of the pulvinar, however, is clear and robust, and we next consider current understanding on the function of pulvinar and its anatomical and functional connections.
Animal work shows rich structural connectivity between dorsal aspects of pulvinar and distributed cortical systems, including fronto-parietal, superior temporal, and precuneus (Baleydier & Morel, 1992; Cavanna & Trimble, 2006). Ventral pulvinar has extensive bidirectional connections with visual areas ranging from primary visual cortex to the fusiform gyrus, via cortico-thalamo-cortico loops (Casanova, 2004). In the current study, skill-related brain-behavior correlations were found with each of these regions, along with pulvinar; the speculation that this subcortical component might play a key role in mediating interactions between widely distributed visual, language, and attentional regions is not unreasonable given known pulvinar connectivity (Goldman-Rakic, 1988).
In terms of the functional role of the pulvinar in reading and language, activation in LH pulvinar has been shown to vary with attentional and language processing demands for printed words (Lockwood, Murphy, & Khalak, 1997), and links to acquired reading deficits with lesions specific to pulvinar have been reported (Crosson, 1999). In terms of more general attentional roles, research on humans and primates indicates that the pulvinar is retinotopically organized in its ventral aspect (Fischer & Whitney, 2009) and implicated in the control of visually-guided attention to specific features in the visual array, especially under experimental conditions where distractors must be ignored (Desimone et al., 1990; LaBerge & Buschbaum, 1990; Posner & Raichle, 1995). Moreover, the pulvinar appears to play a role in learning to bind visual features during learning (Ward & Jackson, 2002). Following our speculation above regarding a role for the pulvinar in mediating interactions between visual language and attentional regions, we suggest that in the context of orthographic learning the connectivity between pulvinar and ventral visual networks may allow for the selection (or attentional enhancement) of those visual features that will come to shape the functional organization of the ventral visual pathways for orthographic form learning. Because orthographic learning is not simply a visual pattern learning process, but is fundamentally relational and constrained by phonological, morphological, and possibly semantic knowledge, we further suggest that as the pulvinar mediates selective attention to features that shape orthographic learning, it does so with computational input from regions sensitive to these linguistic forms, including regions within TP and IFG networks. That attentional processing controlled by pulvinar might influence specialization for ventral visual regions via resonance established with frontal and parietal cortices has been put forward for visual processing in general (Serences & Yantis, 2006); however, an extension to orthographic learning will require more detailed experimental study.
The present findings trace a distributed, multimodal, attentionally-controlled, learning circuit for reading that appears to be operating more efficiently in children with better reading readiness skills, which will come to shape print expertise in LH ventral regions. These findings are broadly consistent with our dorsal/ventral developmental model (Pugh et al., 2000a; 2010), which posited that training the ventral visual pathway for print expertise is under the control of language-related dorsal TP and inferior frontal systems. However, that speculative account was underspecified with respect to exact component cortical networks in early reading development, and no consideration of subcortical involvement was made. Along with TP and IFG, the current findings implicate visually tuned regions, including cuneus and fusiform gyrus, along with cortical (precuneus) and subcortical (pulvinar) visual attention networks, general attention regions including RH IPL and anterior cingulate gyrus, and RH ventral regions, which are also associated with individual differences in learning to read proficiently.
The behavioral findings from the current study also indicate that PA, PDE, and TOJ tasks all correlate with reading ability and have shared influences on reading scores. Futhermore, brain-behavior analyses suggest that the influence of these measures on the learning circuitry for reading appear to be via common brain pathways. It need not have turned out this way - it might well have been the case that residual analyses would reveal independent influences across these measures, particularly for the two tasks that do not directly involve decoding (TOJ and PA). However, the data indicate that at least in the young learners in this study, each of these skills taps a common mechanism associated with systems-level differences in organization of the emergent reading circuitry5. The identification of this common influence on reading circuits constrains how we think about these measures in relation to each other and skill learning more broadly, and suggests value added for brain-level analyses. As discussed in the introduction, rapid auditory processing is only one of several proposed deficits in RD related to sensorimotor processing; others include visual motion deficits (Stein & Walsh, 1997), amplitude modulation deficits (Goswami et al., 2010), and problems with noise exclusion (Sperling et al., 2005; 2006). We suggest that future studies might contrast these with PA and pseudoword decoding, as we have done here, to examine whether they show common or independent influences on reading and reading circuits.
As discussed earlier, there is an active ongoing debate as to whether PA difficulties are casually related to rapid auditory processing deficits, as well as sensory deficits more broadly. Because we examined reading at a single time point, data from the current study do not provide definitive evidence for or against causal relationships between rapid auditory processing and phonological abilities, for which longitudinal design are more suitable. However, the neurobiological findings are generally consistent with a set of requirements for a causal account for rapid auditory processing on PA laid out by Tallal and Gaab (2006). These requirements are 1) that the neurocircuitry associated with individual differences in both rapid auditory processing and phonological skills overlaps, and 2) that these overlapping brain regions show differences between RD and TD. In the current study, we did observe overlapping brain regions associated for TOJ and PA skills, and these regions are less activated in poor reader. PA and TOJ are also moderately correlated with one another behaviorally (although regression analysis shows that the link from TOJ to reading is weaker than from PA to reading6). Moreover, abnormal organization of thalamus, particularly MGN, has been speculated to be a major factor in rapid auditory processing deficits (Galaburda et al., 2006), and the current findings cetrainly implicate the thalamus, although we cannot be sure whether the nuclei implicated the current study included MGN. However, we must also note that a number of findings from the behavioral literature favor a non-causal account, either failing to find an association between rapid auditory deficits and phonological processing when controlling for factors like attention (Breier et al., 2003; Landerl & Willburger, 2010) or finding that this relationship holds only for subsets of RD learners, with similar skill-related distributions seen in non-RD cohorts (Ramus et al., 2003). Moreover, at least one recent longitudinal study failed to find support for a causal path from rapid auditory deficits to PA over the early stages of learning to read (Johnson et al., 2009). The current findings of shared brain pathways do not demand a causal model, of course, because it may simply be the case that rapid auditory and phonological processing depend on common cortical and subcortical networks and that these are less than optimally organized in RD learners (see Ramus, 2006 for a similar argument). Clearly, longitudinal studies with intergated brain-behavior designs will be key for resolving this debate going forward.
Finally, our finding that individual differences in PC scores, when regressed on activation during spoken word and pseudoword processing, implicate speech motor systems in IFG is intriguing in that it may be indicative of a feedback effect of reading-readiness on speech perception. Previous research that has found that degree of speech motor (supplementary motor and IFG) involvement during speech perception tasks is higher when processing demands greater segmental processing or attention to phonetic details (Peschke et al., 2011; Zatorre et al., 1996). It may be the case that our data indicate a feedback from learning to read on processing speech such that children further along the literacy curve are more focused on componential features in general, and thus listen to words with greater attention to phonetic features. This is speculative of course, but should be examined in future studies aimed at exploring how learning to read affects spoken language abilities.
Conclusions
The present findings identify reading relevant subcortical and cortical regions that we can target in the future to challenge contrasting accounts of RD more directly. In addition, whatever the causal relations between rapid auditory processing, phonological processing, and reading skills, the findings from the current study point to common neurocognitive influences on reading development and the brain pathways that underlie it, and suggest that all of these indices are serving as general assays of skill. At a more detailed level, our findings suggest the need to include greater focus on subcortically-mediated reorganization of ventral visual pathways via attentionally-mediated links to distributed LH and RH language networks as children learn to read more fluently. A proper neurobiological theory of early reading difficulties will need to further specify the computational mechanisms that, via these identified cortical and subcortical pathways, constrain progress in learning to decode printed words. Finally, prospective longitudinal research with neurobiological and behavioral measures will be critical to address which early factors within the developing brain best predict whether reading will or will not eventually become fully automated.
Supplementary Material
We correlated sensory and phonological skills with functional activation for reading.
Skill level was correlated with differences in cortical and subcortical activations.
Language and non-language measures influenced reading via common brain pathways.
Language, skill consolidation and sensori-motor integration regions were involved.
Acknowledgments
This study is supported by National Institute of Child Health and Human Development (NICHD) P01 HD 001994 to Haskins Laboratories (Carol Fowler, PI) and NICHD R01 HD 048830 to Yale University (Ken Pugh, PI). We thank Bethany Eaton and Annie Stutzman for behavioral assessment and Hedy Serofin and Teri Hickey for help with imaging participants.
Footnotes
For the temporal order judgment task, we utilized the total number of errors collapsed across the six ISIs as our dependent measure for analyses. We conducted analyses in this manner because each ISI only yields four data points, and because the data were analyzed the same way in Tallal’s (1980) original study showing a correlation between TOJ and reading skill.
We utilized Principal Components Analysis because it is the most commonly used extraction method for dimensionality reduction; however, we also explored other extraction methods (i.e., maximum likelihood, principal axis factoring, generalized least squares). Correlations among values for these extraction methods were 0.976 and above, and the set of significant regions obtained in brain-behavior correlation analyses were identical.
To further examine whether a general maturational factor might have any influence on commonality among our behavioral predictors and thus influence our brain-behavior analyses, we conducted an analogous PCA on the 3 task variables in which we first regressed away the variance in each measure that was related to age. A single PC was obtained that accounted for 65% (eigenvalue = 1.96) of the variance among the three age-residualized measures. The PCs identified with and without age related variance were extremely similar (r = .949, p<.001), which again suggests that age is not a strong determinant of the behavioral or brain-behavioral correlational structure in this dataset. Thus, all additional analyses simply utilized the PC identified via the raw metrics.
Poorer readers are likely processing with greater general effort, but effort-related effects would be expected to produce negative corrleations with skill (i.e., higher skill should produce less effort and less effort produces lower activation levels). That we observed only positive correlations between activation and skill is consistent with the idea that these regions come online as a function of skills.
Note that scores on PA and PDE may be confounded with experiential factors (good readers tend to read more), whereas scores on TOJ arguably should not (we do not practice or learn such tasks directly). That individual differences on TOJ share a link to brain patterns that discriminate reading skill level in this study is consistent with the conclusion that the anomalous activation of these regions in poor readers is not simply a reflection of differential experience with print, but likely reflect systems-level differences in the integrity of those subcortical and cortical networks involved in learning to read.
We regressed the two non-print related measures, PA (Elision), and TOJ scores, on word reading scores from the TOWRE Sight Word Efficiency subset. This model accounted for 35% of the variance in timed word reading. The beta weight for PA was significant (β = 1.88, p < .001), but the beta weight for TOJ was not (p > .10). This indicates that PA was more strongly associated with reading than TOJ in this cohort.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kenneth R. PUGH, Email: pugh@haskins.yale.edu.
Nicole LANDI, Email: landi@haskins.yale.edu.
Jonathan L. PRESTON, Email: preston@haskins.yale.edu.
W. Einar MENCL, Email: einar@haskins.yale.edu.
Alison C. AUSTIN, Email: austin@haskins.yale.edu.
Daragh SIBLEY, Email: sibley@haskins.yale.edu.
Robert K. FULBRIGHT, Email: robert.fulbright@yale.edu.
Mark S. SEIDENBERG, Email: seidenberg@wisc.edu.
Elena L. GRIGORENKO, Email: elena.grigorenko@yale.edu.
R. Todd CONSTABLE, Email: todd.constable@yale.edu.
Peter MOLFESE, Email: pmolfese@haskins.yale.edu.
Stephen J. FROST, Email: frosts@haskins.yale.edu.
References
- Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends in Cognitive Sciences. 2004;8:457–464. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ball EW, Blachman BA. Does phoneme awareness training in kindergarten make a difference in early word recognition and developmental spelling? Reading Research Quarterly. 1991;26:49–66. [Google Scholar]
- Baleydier C, Morel A. Segregated thalamocortical pathways to inferior parietal and inferotemporal cortex in macaque monkey. Visual Neuroscience. 1992;8:391–405. doi: 10.1017/s0952523800004922. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25(4):1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. The development of cortical sensitivity to visual word forms. Journal of Cognitive Neuroscience. 2011;23(9):2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Blau V, Reithler J, van Atteveldt N, Seitz J, Gerretsen P, Goebel R, Blomert L. Deviant processing of letters and speech sounds as proximate cause of reading failure: A functional magnetic resonance imaging study of dyslexic children. Brain. 2010;133:868–879. doi: 10.1093/brain/awp308. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proceedings of the National Academy of Sciences. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Fletcher JM, Foorman BR, Klass P, Gray LC. Auditory temporal processing in children with specific reading disability with and without attention deficit/ hyperactivity disorder. Journal of Speech, Language, and Hearing Research. 2003;46:31–42. doi: 10.1044/1092-4388(2003/003). [DOI] [PubMed] [Google Scholar]
- Brown JK, O’Regan ME. The neurology of speech and language disorders in children. Part I: Disorders of comprehension and inner language. Clinics in Developmental Medicine. 2001;156:1–32. [Google Scholar]
- Brunswick N, McCrory E, Price C, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Byrne B, Coventry WL, Olson RK, Hulslander J, Wadsworth S, DeFries JC, Samulesson S. A behavior-genetic analysis of orthographic learning, spelling, and decoding. Journal of Research in Reading. 2008;31:8–21. [Google Scholar]
- Casanova C. The visual functions of the pulvinar. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge, USA: The MIT Press; 2004. pp. 592–608. [Google Scholar]
- Castles A, Coltheart M. Is there a causal link from phonological awareness to success in learning to read? Cognition. 2004;91:77–111. doi: 10.1016/s0010-0277(03)00164-1. [DOI] [PubMed] [Google Scholar]
- Castles A, McLean GMT, McArthur G. Dyslexia (neuropsychological) WIREs Cognitive Science. 2010;1:426–432. doi: 10.1002/wcs.16. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Petersson KM, Reis A, Stone-Elander S, Ingvar M. The illiterate brain. Learning to read and write during childhood influences the functional organization of the adult brain. Brain. 1998;121:1053–1063. doi: 10.1093/brain/121.6.1053. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: Lexical–semantic mechanisms and the thalamus. Brain and Cognition. 1999;40:414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Best M, Heeger DJ. Psychophysical evidence for a magnocellular pathway deficit in dyslexia. Vision Research. 1998;38:1555–1559. doi: 10.1016/s0042-6989(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Desimone R, Wessinger M, Thomas L, Schneider W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harbor Symposia on Quantitative Biology. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Elbro C. Early linguistic abilities and reading development: A review and hypothesis. Reading and Writing. 1996;8(6):453–485. [Google Scholar]
- Fischer J, Whitney D. Attention narrows position tuning of population responses in V1. Current Biology. 2009;19(16):1356–1361. doi: 10.1016/j.cub.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Fuchs LS, Barnes MA. Learning disabilities: From identification to intervention. New York: Guilford; 2007. [Google Scholar]
- Fletcher J. Dyslexia: The evolution of a scientific concept. Journal of the International Neuropsychological Society. 2009;15:501–508. doi: 10.1017/S1355617709090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foorman BR, Francis D, Fletcher JK, Schatschneider C, Mehta P. The role of instruction in learning to reading: Preventing reading failure in at-risk children. Journal of Educational Psychology. 1998;90:37–55. [Google Scholar]
- Fowler AE. How early phonological development might set the stage for phoneme awareness. In: Brady S, Shankweiler DP, editors. Phonological processes in literacy: A tribute to Isabelle Y Liberman. Hillsdale NJ: Erlbaum; 1991. pp. 97–118. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: Levels of inference and power. NeuroImage. 1995;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Frost SJ, Landi N, Mencl WE, Sandak R, Fulbright RK, Tejada ET, Jacobsen L, Grigorenko EL, Constable RT, Pugh KR. Phonological awareness predicts activation patterns for print and speech. Annals of Dyslexia. 2009;59:78–97. doi: 10.1007/s11881-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JDE, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Restorative Neurology and Neuroscience. 2007;25:295–310. [PubMed] [Google Scholar]
- Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nature Neuroscience. 2006;9(10):1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Annals of Neurology. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: paralleled distribution networks in primate association cortex. Annual Review of Neuroscience. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goswami U, Fosker T, Huss M, Mead N, Szűcs D. Rise time and formant transition duration in the discrimination of speech sounds: the Ba-Wa distinction in developmental dyslexia. Developmental Science. 2010;14:34–43. doi: 10.1111/j.1467-7687.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- Goswami U, Ziegler JC. A developmental perspective on the neural code for written words. Trends in Cognitive Sciences. 2006;10:142–143. doi: 10.1016/j.tics.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. NeuroReport. 2004;15:1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychological Review. 1999;106:491–528. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy Sciences. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EP, Pennington BF, Lee NR, Boada R. Directional effects between rapid auditory processing and phonological awareness in children. Journal of Child Psychology and Psychiatry. 2009;50:902–910. doi: 10.1111/j.1469-7610.2009.02064.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Share DL, Maclean R, Matthews R. Cognitive factors at school entry predictive of specific reading retardation and general reading backwardness: A research note. Journal of Child Psychology and Psychiatry. 1986;27:45–54. doi: 10.1111/j.1469-7610.1986.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort, 1976–1982, Rochester, Minn. Mayo Clinic Proceedings. 2001;76:1081–1092. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- Katzir T, Misra M, Poldrack RA. Imaging phonology without print: Assessing the neural correlates of phonemic awareness using fMRI. NeuroImage. 2005;27:106–115. doi: 10.1016/j.neuroimage.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JDE, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Human Brain Mapping. 2008;29:613–625. doi: 10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. Journal of Neuroscience. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K, Willburger E. Temporal processing, attention, and learning disorders. Learning and Individual Differences. 2010;20:393–401. [Google Scholar]
- Liberman IY, Shankweiler D. Phonology and the problems of learning to read and write. Remedial and Special Education. 1985;6:8–17. [Google Scholar]
- Liberman IY, Shankweiler D, Fischer W, Carter B. Explicit syllable and phoneme segmentation in the young child. Journal of Child Psychology. 1974;18:201–212. [Google Scholar]
- Lockwood AH, Murphy BW, Khalak H. Attentional systems and the allocation of cerebral resources in reading and grammatical tasks. International Journal of Neuroscience. 1997;91:241–52. doi: 10.3109/00207459708986380. [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA. A definition of dyslexia. Annals of Dyslexia. 2003;53:1–14. [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, Gabrieli JDE, Just MA. Brain activation during sentence comprehension among good and poor readers. Cerebral Cortex. 2007;10(17):2780–2787. doi: 10.1093/cercor/bhm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Hart L. The lexical basis of comprehension skill. In: Gorfein D, editor. The consequences of meaning selection. Washington, DC: American Psychological Association; 2001. pp. 67–86. [Google Scholar]
- Peschke C, Ziegler W, Eisenberger J, Baumgaertner A. Phonological manipulation between speech perception and production activates a parieto-frontal circuit. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.07.025. In press. [DOI] [PubMed] [Google Scholar]
- Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychological Bulletin. 2005;131:592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Posner MI, Raichle ME. Precis of images of mind. Behavioral and Brain Sciences. 1995;18:327–383. [Google Scholar]
- Preston JL, Frost SJ, Mencl WE, Fulbright RK, Landi N, Grigorenko E, Jacobsen L, Pugh KR. Early and late talkers: School-age language, literacy and neurolinguistic differences. Brain. 2010;133:2185–2195. doi: 10.1093/brain/awq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The Interactive Account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Moore D, Della Porta G, Rueckl JG, Mencl WE. The neural basis of reading. In: Cornelissen PL, Hansen PC, Kringelbach ML, Salmelin R, editors. Mapping the word reading circuitry in skilled and disabled readers. New York: Oxford University Press; 2010. pp. 281–305. [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Rueckl JG, Constable RT, Seidenberg MS, Fulbright RK, Katz L, Mencl WE. Effects of stimulus difficulty and repetition on printed word identification: An fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. Journal of Cognitive Neuroscience. 2008;20:1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation & Developmental Disabilities Research Reviews. 2000a;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Skudlarski P, et al. The angular gyrus in developmental dyslexia: Task-specific differences in functional connectivity in posterior cortex. Psychological Science. 2000b;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Predicting reading performance from neuroimaging profiles: The cerebral basis of phonological effects in printed word identification. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:299–318. doi: 10.1037//0096-1523.23.2.299. [DOI] [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction. Current Opinion in Neurobiology. 2003;13:212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ramus F, White S, Frith U. Weighing the evidence between competing theories of dyslexia. Developmental Science. 2006;9:265–269. doi: 10.1111/j.1467-7687.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57(3):742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Service E, Kiesila P, Uutela K, Salonen O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Annals of Neurology. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- Sarkari S, Simos PG, Fletcher JM, Castillo EM, Breier JI, Papanicolaou AC. The emergence and treatment of developmental reading disability: Contributions of functional brain imaging. Seminars in Pediatric Neurology. 2002;9:227–236. doi: 10.1053/spen.2002.35506. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Reading aloud boosts connectivity through the putamen. Cerebral Cortex. 2010;20:570–582. doi: 10.1093/cercor/bhp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends in Cognitive Sciences. 2006;10(1):38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Shankweiler D, Mencl WE, Braze D, Tabor W, Pugh KR, Fulbright RK. Reading differences and brain: cortical integration of speech and print in sentence processing varies with reader skill. Developmental Neuropsychology. 2008;33:745–775. doi: 10.1080/87565640802418688. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Escobar MD, Shaywitz BA, Fletcher JM, Makuch RW. Evidence that dyslexia may represent the lower tail of a normal distribution of reading ability. New England Journal of Medicine. 1992;326:145–150. doi: 10.1056/NEJM199201163260301. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fullbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. The science of reading and dyslexia. Journal of AAPOS. 2003;7(3):158–166. doi: 10.1016/s1091-8531(03)00002-8. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E. Brain abnormalities underlying altered activation in dyslexia: A voxel based morphometry study. Brain. 2005;128(10):2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, Davis RN, Fitzgerald M, Papanicolaou AC. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, Hulme C. Annual Research Review: The nature and classification of reading disorders: a commentary on proposals for DSM-5. Journal of Child Psychology and Psychiatry. 2011 doi: 10.1111/j.1469-7610.2011.02495.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht K, Hugdahl K, Ofte S, Nygard M, Bjornerud A, Plante E, Helland T. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year old children. Scandanavian Journal of Psychology. 2009;50(1):79–91. doi: 10.1111/j.1467-9450.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu ZL, Manis FR, Seidenberg MS. Deficits in perceptual noise exclusion in developmental dyslexia. Nature Neuroscience. 2005;8:862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu ZL, Manis FR, Seidenberg MS. Motion-perception deficits and reading impairment: It’s the noise, not the motion. Psychological Science. 2006;17:1047–1053. doi: 10.1111/j.1467-9280.2006.01825.x. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read: The magnocellular theory of dyslexia. Trends in Neurosciences. 1997;20:147–151. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Stevenson J. Which aspects of reading disability show a “hump” in their distribution? Applied Cognitive Psychology. 1988;2:77–85. [Google Scholar]
- Studdert-Kennedy M, Mody M. Auditory temporal perception deficits in the reading impaired: A critical review of the evidence. Psychonomic Bulletin and Review. 1995;2:508–514. doi: 10.3758/BF03210986. [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain & Language. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Gaab N. Dynamic auditory processing, musical experience and language development. Trends in Neurosciences. 2006;29(7):382–390. doi: 10.1016/j.tins.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Protopapas A, Nagarajan S, Salz T, Tallal P, Merzenich MM, Gabrieli JD. Disruption of the neural response to rapid acoustic stimuli in dyslexia: evidence from functional MRI. Proceedings of the National Academy of Sciences. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Morgan ST, Davis C. Effects of two types of phonological awareness training on word learning in kindergarten children. Journal of Educational Psychology. 1992;84:364–370. [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA, Rose E, Lindamood P, Conway T, Garvan C. Preventing reading failure in young children with phonological processing disabilities: Group and individual responses to instruction. Journal of Educational Psychology. 1999;91(4):579–593. [Google Scholar]
- Turkeltaub P, Eden G, Jones K, Zeffiro T. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–73. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pierpont EI. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): What have we learned in the past four decades? Journal of Child Psychology and Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Ward R, Jackson SR. Visual attention in blindsight: sensitivity in the blind field increased by targets in the sighted field. NeuroReport. 2002;13:301–304. doi: 10.1097/00001756-200203040-00011. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Yamada Y, Stevens C, Dow M, Harn BA, Chard DJ, Neville HJ. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: An fMRI study. Neuroimage. 2011;57(3):704–713. doi: 10.1016/j.neuroimage.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC. PET studies of phonetic processing of speech: review, replication, and reanalysis. Cerebral Corex. 1996;6(1):21–30. doi: 10.1093/cercor/6.1.21. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Pech-Georgel C, George F, Lorenzi C. Speech-perception-in-noise deficits in dyslexia. Developmental Science. 2009;12:732–745. doi: 10.1111/j.1467-7687.2009.00817.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.