Abstract
The subsynovial connective tissue (SSCT) in the carpal tunnel may participate in the origin of carpal tunnel syndrome (CTS), yet material properties of the SSCT have not been well-characterized. We investigated the response of the SSCT to repeated ramp stretch tests. Eight human cadaver wrists were used. The physiological excursion of the flexor digitorum superficialis of the third digit (FDS 3) was measured, starting from a neutral position to maximal flexion of the metacarpophalangeal and proximal interphalangeal joints. The FDS 3 tendon was pulled to 40, 60, 90, and 120% of the physiological excursion. Two ‘ramp stretch’ cycles were performed at every excursion level, except for 120% of excursion, where 3 cycles were performed. The ratio of energy absorbed between the second (E2) and first (E1) ramp-stretch was 0.94 (Std. Dev. = 0.07) for 60%, 0.84 (Std. Dev. = 0.11) for 90%, and 0.68 (Std. Dev. = 0.11) for 120% of the physiological excursion. A significant decrease occurred in energy absorbed after the first ramp-stretch cycle at 90% and 120% of the physiological excursion, which was not seen at 60%. Our data are consistent with a stepwise damage occurring in the SSCT. Furthermore, the damage seems to initiate within the physiological range of tendon excursion. This finding may be important in understanding the pathophysiology of conditions that are associated with SSCT pathology, such as carpal tunnel syndrome.
Keywords: Carpal Tunnel, Subsynovial Connective Tissue, Biomechanics, Human Cadaver, Ramp Stretch Test
INTRODUCTION
Carpal tunnel syndrome (CTS) is a common compression neuropathy of the median nerve in the wrist, with an annual incidence of 3.5 per 1000 people.1 The NIH determined that in the U.S., CTS results in an average lifetime cost of ~$30,000 to each affected working citizen.2 It is widely accepted that forceful and repetitive moments contribute to the development of carpal tunnel syndrome,3–9 but the actual etiology of CTS is still unknown.
In the carpal tunnel, flexor tendons and the median nerve are surrounded by subsynovial connective tissue (SSCT), which consists of multiple layers of fibrous tissue that are interconnected by collagenous fibers.10–13 One hypothesis of CTS etiology is that a certain amount of excursion causes damage to the SSCT, which could initiate fibrosis and then cause CTS.10; 14–18
Yamaguchi et al. previously studied the mechanical properties of the SSCT in a rabbit carpal tunnel model, examining the excursion of the flexor digitorum superficialis of the middle digit (FDS 3) and the SSCT failure load.19 Morizaki et al.20 also analyzed the mechanical properties of the rabbit SSCT using repeated stress relaxation tests. The mechanical properties of the human SSCT have never been investigated.
Our aim was to define the displacement threshold that causes irreversible damage of the human SSCT by investigating changes in the SSCT mechanical response resulting from varying levels of displacement. Furthermore, we wanted to investigate whether such damage was proportional to the amount of displacement, and whether the observed difference could be described in the context of the structural model of the SSCT as a multi-layered structure. The experimental design allowed for separation of damage and viscoelastic effects, by allowing for a rest period between successive tests.
MATERIALS AND METHOD
Specimen Preparation and Setup
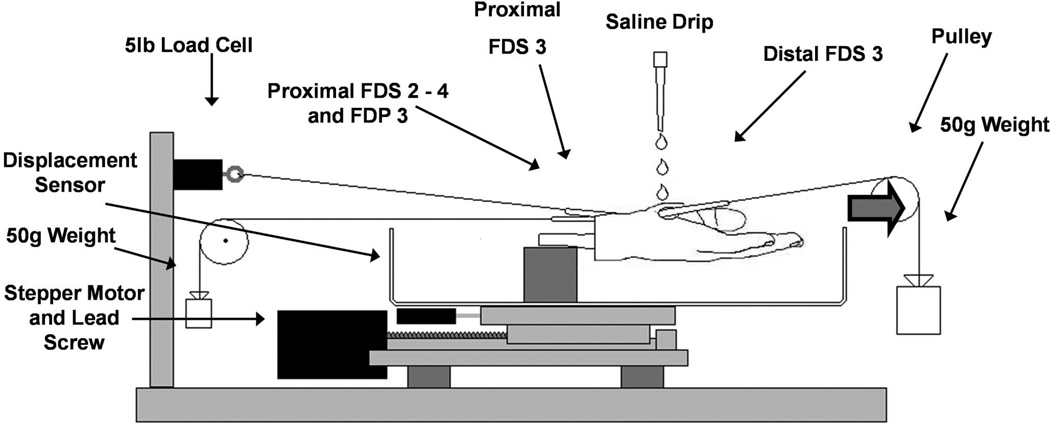
8 fresh frozen human cadaver wrists (6 male, 2 female) were mounted onto a custom test device with the wrist joint fixed in a neutral position (Fig. 1). The mean age of the cadavers was 60 yrs (range = 40 to 88 yrs). Cadavers with a known history of carpal tunnel syndrome or wrist fractures were excluded. The FDS tendons of the index, middle, and ring fingers (FDS 2 to 4) and the middle finger flexor digitorum profundus (FDP 3) tendon were exposed proximal to the proximal wrist crease. To measure the physiological excursion, all digits were initially extended. The tendons were then connected proximally to a 2.0 N weight to maintain tension. The FDS 3 and 4 tendons were marked with a suture at the same level. The middle finger was then fully flexed at the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints, with the other digits held in extension. After full flexion, the difference in displacement between the two sutures was measured. This distance was considered to be the physiological excursion (Fig. 2).
Figure 1.
Test setup. During the ramp stretch test, the hand was displaced distally at 2 mm/s to a predetermined excursion, at which point it was released with the same velocity back to the original neutral position.
Figure 2.
Measuring the physiological excursion. A) All fingers are extended. FDS 2 to 4 are marked at the same level. A reference point on the skin is marked at the carpal tunnel. The distance between all the markings is measured. B) FDS 3 is fully flexed at the MCP and PIP joints while all other fingers are extended. The difference in distance between markings is measured. Eventually, the difference between the markings on the FDS 3 and 4, with the FDS 3 held in flexion, was used as the physiological excursion.
Next, the proximal end of the FDS 3 tendon was connected to a 25 N load cell (MDB-5, Transducer Techniques, Temecula, CA). After exposure of the distal end of the FDS 3, the tendon was connected to a 0.5 N weight to maintain tension during testing. A displacement sensor (TR-50, Novotechnik, Southborough, MA) was connected to a custom stepper motordriven test device to register the movement for every cycle. In each specimen, the carpal tunnel area was kept intact over a 4 cm length. Motion of the actuator with the specimen mounted generated relative motion between the tendon and carpal tunnel. The specimen was held at room temperature and kept moist with saline for the duration of all testing.
Repeated Ramp Stretch Test
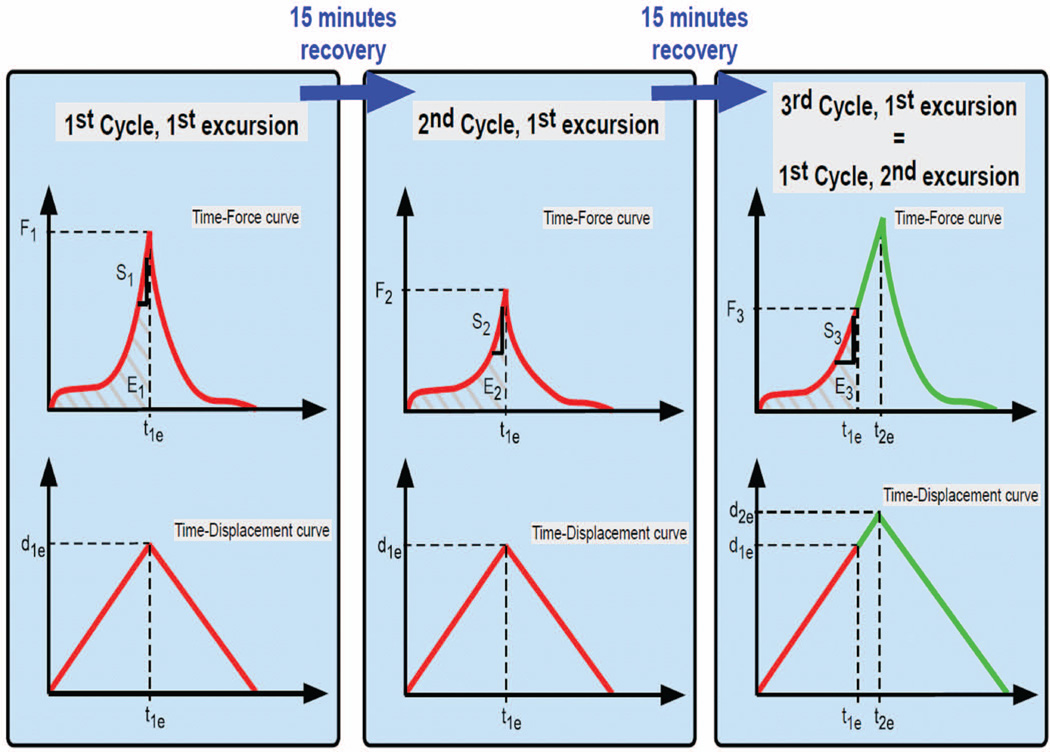
A K-wire was inserted through the MCP, PIP, and distal interphalangeal joints of digits 2 to 5 to keep them extended throughout the test. The specimens were tested in a cycle consisting of ramp-stretch and recovery components. During the test, the hand was displaced in the distal direction at a speed of 2 mm/s until a predetermined excursion was reached, at which point it was released with the same velocity back to the original neutral position (0 mm displacement). This test had the effect of creating a relative displacement between the FDS 3 and the carpal tunnel in the neutral position for 15 mins to allow for viscoelastic recovery. This cycle of displacement and recovery was then repeated with the same excursion (Fig. 3). After the second cycle, the excursion level was increased, and the procedure was repeated. In total, 2 cycles were performed at 40, 60, and 90% of the physiological excursion, and 3 cycles at 120% of the physiological excursion. The 1st cycle at 60, 90, and 120% was also considered to be 3rd cycle of, respectively, 40, 60, and 90% excursion, with data recorded both as the excursion reached the final excursion of the previous cycle and again at the final excursion of the new cycle. For example, after two cycles at 60% excursion, the hand was displaced to 90% excursion. During this displacement, data were recorded as the excursion passed 60%, to provide data for cycle 3 at 60% excursion, and again when displacement reached 90% excursion, as the data for the first cycle at 90%. In this way, we collected data for 3 cycles at every excursion level (Fig. 4).
Figure 3.
Two ‘Ramp Stretch’ cycles were performed at every excursion level: approximately 40, 60, 90, and 120% of the physiological excursion. The 1st cycle at 60, 90, and 120% was also seen as the 3rd cycle of respectively 40, 60, and 90% up to that excursion. Energy-area under the curve (E), Peak Force (F), and 13 Stiffness (S) were calculated for every cycle. d1e and t1e: Displacement and time for the 1st excursion. d2e and t2e: Displacement and time of the subsequent excursion.
Figure 4.
Example of the force-displacement curves for all Ramp Stretch cycles in 1 specimen. The curve is shown up to the point of maximal excursion. Region 1 of the curve was due to friction of the FDS 3 tendon within the carpal tunnel; Region 2 was generated by stretch of the SSCT.
The data obtained from the load cell and the movement sensor were collected at a sample rate of 20 Hz and used to create load-excursion curves for each ramp-stretch. The terms of analysis were defined as follows: the excursion energy absorption (E) was the area under the curve up to the maximum displacement; the peak force (F) was the highest force observed while testing; and the stiffness (S) was the slope of the linear region just before the peak force was reached (Fig. 3).
For each excursion level, ratios between the first and second cycles for each of these parameters were calculated, as were the ratios between the first and third cycles. A custom MATLAB (Mathworks, Natick MA) program was developed to analyze the force-excursion data, providing the ratios for energy, peak force and stiffness results.
Statistical Considerations
All data were reported as ratios. Our primary aim was to examine the difference between the ratios of each parameter for each excursion magnitude (E2/E1 and E3/E1; F2/F1 and F3/F1; S2/S1 and S3/S1). The results were analyzed using a repeated measures ANOVA. A P-value <.05 was considered significant.
RESULTS
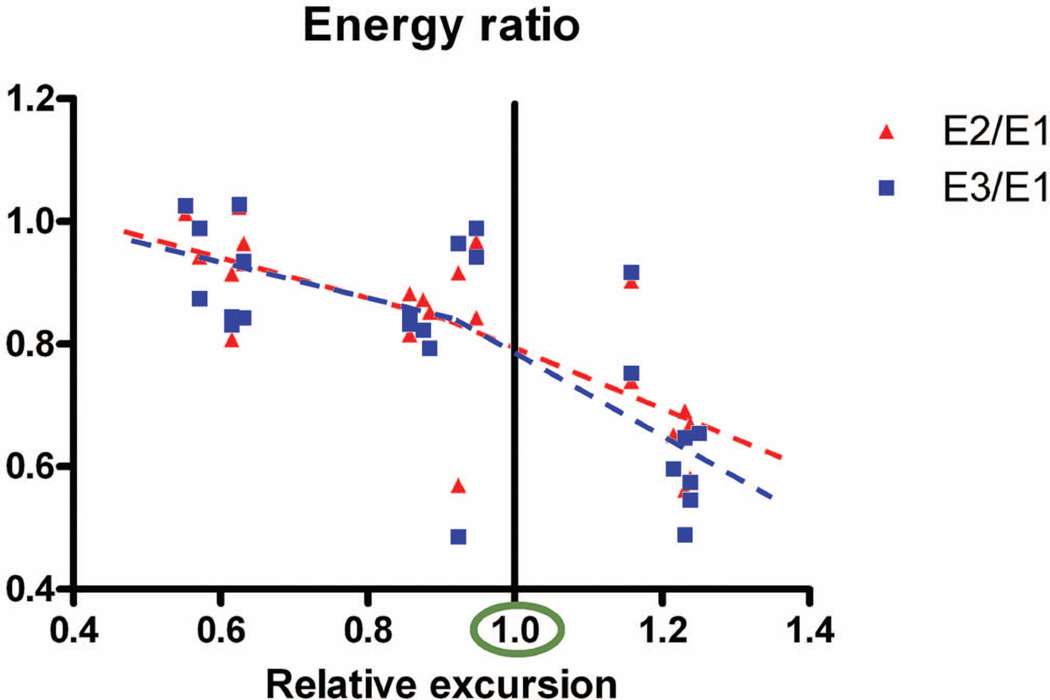
The data are displayed in tabular form in Table 1 and graphically in Figures 5 and 6. The ratio of E2 to E1 (Fig. 7) in the 90% excursion level group was significantly lower compared to the 60% excursion level group. This ratio was also significantly lower in the 120% excursion level group, compared to the 60% and 90% excursion level groups. The ratio of E3 to E1 was not significantly different from the ratio of E2 to E1 at any excursion level.
Table 1.
Parameter ratios for different excursions presented as a percent of physiological excursion.
| Percent of Physiological Excursion | ||||
|---|---|---|---|---|
| 60% | 90% | 120% | ||
| Energy | E2/E1 | 0.94 (SD: 0.07) | 0.84 (SD: 0.11) | 0.68 (SD: 0.11) |
| E3/E1 | 0.92 (SD: 0.08) | 0.83 (SD: 0.16) | 0.65 (SD: 0.13) | |
| Force | F2/F1 | 0.95 (SD: 0.03) | 0.89 (SD: 0.05) | 0.84 (SD: 0.03) |
| F3/F1 | 0.93 (SD: 0.05) | 0.89 (SD: 0.14) | 0.75 (SD: 0.02) | |
| Stiffness | S2/S1 | 1.32 (SD: 0.19) | 1.34 (SD: 0.53) | 1.56 (SD: 0.33) |
| S3/S1 | 1.43 (SD: 0.40) | 1.36 (SD: 0.39) | 1.43 (SD: 0.36) | |
Figure 5.
Graphic display of the energy ratio. Note the trend line decreasing mildly from 60 to 90%, and then more steeply between 90 and 120%.
Figure 6.
Graphic display of force ratio. Note the trend line decreasing mildly from 60 to 90%, and then more steeply between 90 and 120%.
Figure 7.
Energy ratio. Note the significant decrease in the E2/E1 ratio as displacement increased, suggesting progressive damage. Note the lack of difference between E2/E1 and E3/E1, a reflection that the values of E2 and E3 are similar, and that therefore no further damage happened with additional displacement to the same distance.
The ratio of F2 to F1 (Fig. 8) was significantly lower, compared to the 60% excursion level group. This ratio was also significantly lower in the 120% excursion level group, compared to the 60% and 90% excursion level group. The ratio of F3 to F1 was not significantly different to the ratio of F2 to F1 at the 60% or 90% excursion level, but it was significantly different at the 120% excursion level.
Figure 8.
Force ratio. Note the significant decrease in the F2/F1 ratio as displacement increased, suggesting progressive damage. Note the lack of difference between F2/F1 and F3/F1, a reflection that the values of F2 and F3 are similar, and that therefore no further damage happened with additional displacement to the same distance.
No significant difference occurred between the ratios of S2 to S1 at any excursion level. The ratio of S3 to S1 was also not significantly different from the ratio of S2 to S1 at any excursion level.
DISCUSSION
One of the first descriptions of the multilayered structure of the SSCT was made by Guimberteau.21 As the tendon moves, the layers are successively engaged, while the interconnecting fibrils begin to stretch.11; 14; 22 Once they reach their maximum length, the load will deform the fibrils irreversibly or rupture them. Morizaki et al. more recently studied the mechanical properties of the rabbit SSCT. They found a displacement threshold at which irreversible damage occurred to the SSCT. The displacement threshold was situated within the normal excursion of the tendon20.
In this study we generated data for 3 ‘ramp-stretch’ cycles at every excursion level from 40 to 120% (Energy: E1, E2 and E3; Peak Force: F1, F2 and F3; Stiffness: S1, S2 and S3). Because soft biological tissues are viscoelastic, a difference in energy or peak force between cycles at a given excursion level can be caused either by temporary viscoelastic stretching or permanent damage to the SSCT. We allowed 15 minutes of recovery between tests. Subsequent displacement loads were similar to the initial ones at 60% excursion, suggesting that sufficient recovery had indeed occurred without damage to the SSCT. Had sufficient recovery not occurred, we would have expected that the displaced structures would retain some elongation, and that lower loads would be needed to achieve the second displacement, with an exaggerated ‘toe’ region to the load displacement curve and increased stiffness in the linear portion of he curve. If tissue had been damaged, we would have expected the structure to provide less resistance on the second displacement, but without an exaggerated toe region, or an increase in stiffness. The fact that we observed no differences with repeated loading at 40 and 60% displacement, small but significant reductions in the measured loads at the second and third cycles compared to the first cycle at 90% displacement, and even larger significant reductions at 120% displacement, suggests that damage was indeed occurring at the higher displacement levels. The lack of difference between the loads and energy of the second and third cycles is evidence that damage occurred only after the initial displacement, and that subsequent displacement to the same excursion did not create additional damage. Excursions at 40% of the physiological excursion fell completely within the toe region of the force-excursion curve (Region 1 in Fig. 4) producing noisy data. Therefore these data were not used for analysis
The peak force ratios showed a similar trend with one notable difference: a significant difference between the F2/F1 ratio and the F3/F1 ratio at 120% of the physiological excursion. This may show an increase in the SSCT damage at higher excursion levels.
In contrast to the differences in energy and peak force, no significant difference occurred between the ratio of S2 to S1 and the ratio of S3 to S1. Morizaki et al. had similar findings, which may be explained by the structure of the SSCT. The SSCT consists of different layers that engage sequentially as the tendon moves. If each layer has similar mechanical properties, the stiffness, a reflection of the newly engaged, remaining intact interconnections, should not change with higher excursions, even if the previously engaged interconnecting fibrils are damaged.
We sought a displacement threshold that caused irreversible damage to the human SSCT, similar to Morizaki’s findings. Previous studies suggested that repetitive finger motion could cause carpal tunnel syndrome. We found that a stepwise damage to the human SSCT appears to begin within the physiological range, initiating at 90% of the physiological range, and increasing with increasing excursion. The clinical implication rests in our finding that the SSCT is readily injured. This suggests that SSCT injury may be quite common. However, progressive SSCT fibrosis, leading to CTS, is less common, suggesting that there may be important differences between individuals that presuppose some to develop CTS. For example, individual variations in the biological responses of some individuals may encourage a vicious cycle of fibrosis in the SSCT. Indeed, this is the focus of our current research.
Our study also has some limitations. First, the relaxation time and behavior might be different between fresh frozen cadaver and living SSCT. Second, the FDS 3 tendon was fixed during testing while the hand moved, in contrast to the in vivo setting. Finally the displacement speed (2 mm/s), while commonly used in mechanical testing of tendons, is well below the physiological range. The effect of tendon velocity on SSCT mechanics is currently unknown, but, as a viscoelastic structure, it could be expected that the SSCT might become stiffer at higher loading rates, and perhaps even more likely to fail mechanically if the faster loading rate were combined with large excursions. In future projects, we will investigate the effect of different tendon velocities on the mechanical properties of the SSCT. It would also be interesting to study the effect of freezing on SSCT mechanical properties.
In conclusion, stepwise damage occurs in the SSCT. This damage seems to initiate within the physiological range. This finding may have relevance in understanding the pathogenesis of conditions associated with SSCT fibrosis, such as carpal tunnel syndrome.
Acknowledgments
This study was supported by NIH RO1AR49823.
REFERENCES
- 1.Nordstrom DL, DeStefano F, Vierkant RA, et al. Incidence of diagnosed carpal tunnel syndrome in a general population. Epidemiology. 1998;9:342–345. [PubMed] [Google Scholar]
- 2.NINDS. [Accessed on Jun 24];NIoNDaS. http://www.ninds.nih.gov/disorders/carpal_tunnel/Af,detail_carpal_tunnel.htm.
- 3.Amadio PC. Repetitive stress injury. J Bone Joint Surg Am. 2001;83-A:136–137. doi: 10.2106/00004623-200101000-00018. author reply 138-141. [DOI] [PubMed] [Google Scholar]
- 4.English CJ, Maclaren WM, Court-Brown C, et al. Relations between upper limb soft tissue disorders and repetitive movements at work. Am J Ind Med. 1995;27:75–90. doi: 10.1002/ajim.4700270108. [DOI] [PubMed] [Google Scholar]
- 5.Hadler NM. Arm pain in the workplace. A small area analysis. J Occup Med. 1992;34:113–119. doi: 10.1097/00043764-199202000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Latko WA, Armstrong TJ, Franzblau A, et al. Cross-sectional study of the relationship between repetitive work and the prevalence of upper limb musculoskeletal disorders. Am J Ind Med. 1999;36:248–259. doi: 10.1002/(sici)1097-0274(199908)36:2<248::aid-ajim4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Mackinnon SE, Novak CB. Repetitive strain in the workplace. J Hand Surg Am. 1997;22:2–18. doi: 10.1016/S0363-5023(05)80174-1. [DOI] [PubMed] [Google Scholar]
- 8.Saleh SS, Fuortes L, Vaughn T, et al. Epidemiology of occupational injuries and illnesses in a university population: a focus on age and gender differences. Am J Ind Med. 2001;39:581–586. doi: 10.1002/ajim.1057. [DOI] [PubMed] [Google Scholar]
- 9.Szabo RM. Carpal tunnel syndrome as a repetitive motion disorder. Clin Orthop Relat Res. 1998:78–89. [PubMed] [Google Scholar]
- 10.Cobb TK, Dalley BK, Posteraro RH, et al. The carpal tunnel as a compartment. An anatomic perspective. Orthop Rev. 1992;21:451–453. [PubMed] [Google Scholar]
- 11.Ettema AM, Amadio PC, Zhao C, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006;118:1413–1422. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- 12.Ettema AM, Zhao C, An KN, et al. Comparative anatomy of the subsynovial connective tissue in the carpal tunnel of the rat, rabbit, dog, baboon, and human. Hand (N Y) 2006;1:78–84. doi: 10.1007/s11552-006-9009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotman MB, Donovan JP. Practical anatomy of the carpal tunnel. Hand Clin. 2002;18:219–230. doi: 10.1016/s0749-0712(01)00003-8. [DOI] [PubMed] [Google Scholar]
- 14.Ettema AM, Zhao C, Amadio PC, et al. Gliding characteristics of flexor tendon and tenosynovium in carpal tunnel syndrome: a pilot study. Clin Anat. 2007;20:292–299. doi: 10.1002/ca.20379. [DOI] [PubMed] [Google Scholar]
- 15.Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg Br. 1992;17:209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 16.Ugbolue UC, Hsu WH, Goitz RJ, et al. Tendon and nerve displacement at the wrist during finger movements. Clin Biomech (Bristol, Avon) 2005;20:50–56. doi: 10.1016/j.clinbiomech.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 17.van Doesburg MH, Yoshii Y, Villarraga HR, et al. Median nerve deformation and displacement in the carpal tunnel during index finger and thumb motion. J Orthop Res. 2010;28:1387–1390. doi: 10.1002/jor.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, Ettema AM, Osamura N, et al. Gliding characteristics between flexor tendons and surrounding tissues in the carpal tunnel: a biomechanical cadaver study. J Orthop Res. 2007;25:185–190. doi: 10.1002/jor.20321. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Osamura N, Zhao C, et al. The mechanical properties of the rabbit carpal tunnel subsynovial connective tissue. J Biomech. 2008;41:3519–3522. doi: 10.1016/j.jbiomech.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morizaki Y, Vanhees M, Thoreson A, et al. The Response of the Rabbit Subsynovial Connective Tissue to a Stress- Relaxation Test: Effect of Amount of Displacement and Recovery Time. J Orthop Res Submitted. 2011 doi: 10.1002/jor.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guimberteau JC. New Ideas in Hand Flexor Tendon Sugery the Sliding System. Vascularized Flexor Tendon Transfers. Aquitaine Domaine Forestier. 2001 [Google Scholar]
- 22.Ettema AM, Belohlavek M, Zhao C, et al. High-resolution ultrasound analysis of subsynovial connective tissue in human cadaver carpal tunnel. J Orthop Res. 2006;24:2011–2020. doi: 10.1002/jor.20252. [DOI] [PubMed] [Google Scholar]